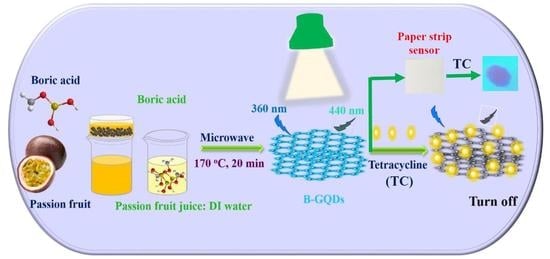
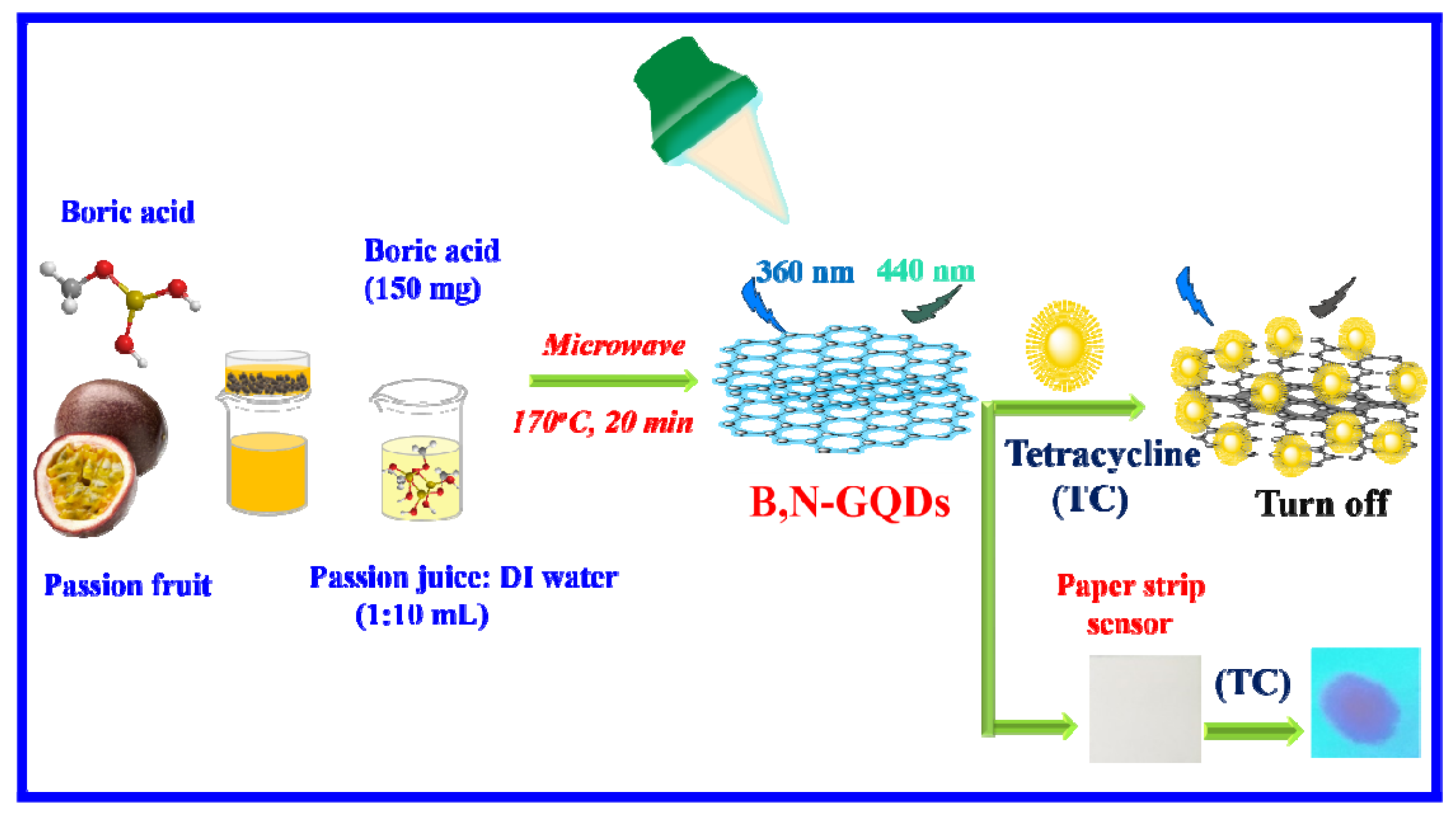
Ultrasensitive Detection of Tetracycline Using Boron and Nitrogen Co-Doped Graphene Quantum Dots from Natural Carbon Source as the Paper-Based Nanosensing Probe in Difference Matrices
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Synthesis of B,N-GQDs
2.3. Surface Characterization
2.4. Detection of TC by B,N-GQDs in PBS, Urine and Human Serum
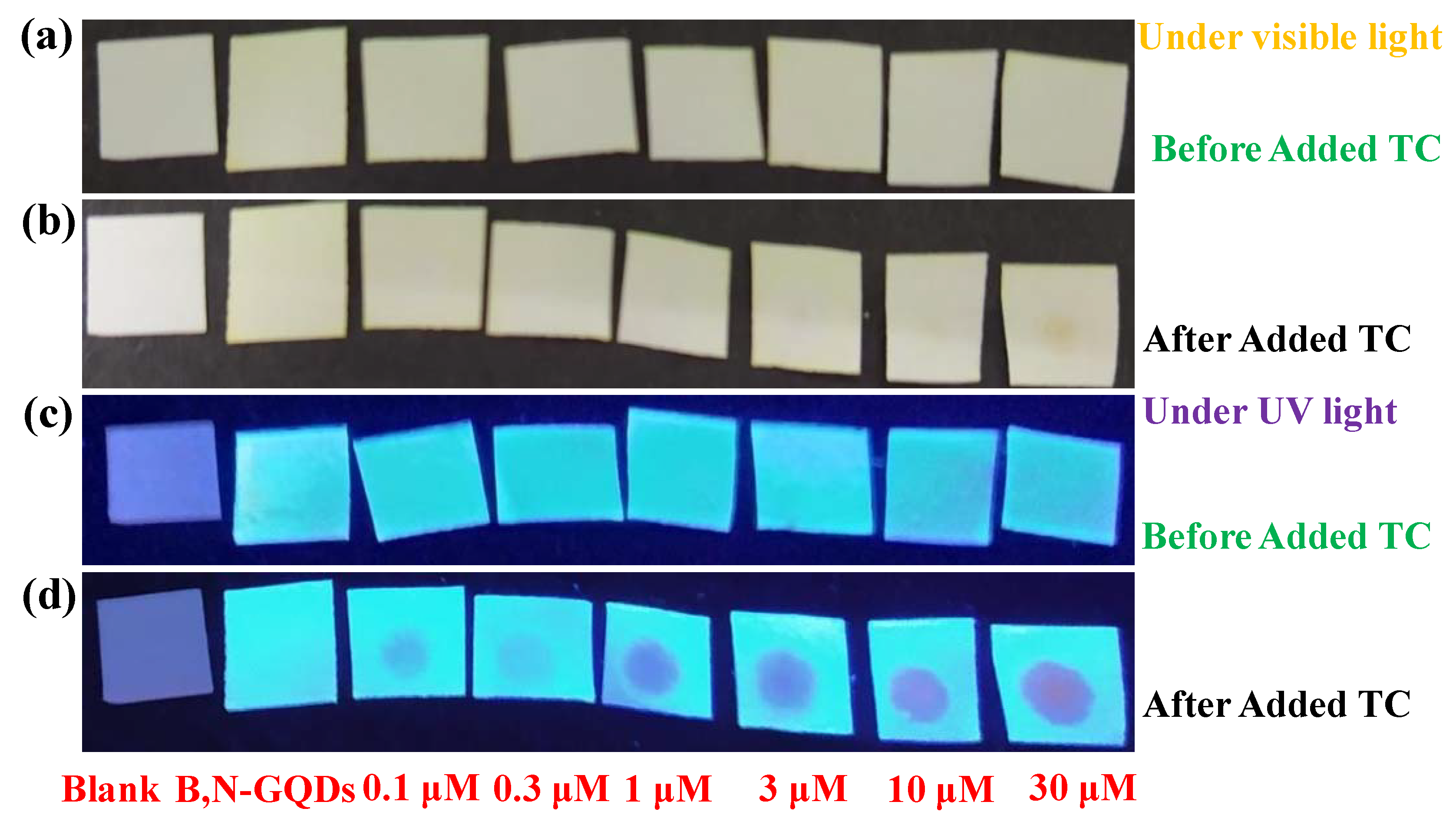
2.5. B,N-GQD Based Paper Strip Nanosensor for TC Detection in Human Serum
2.6. Cytotoxicity Assay of B,N-GQDs
3. Results and discussion
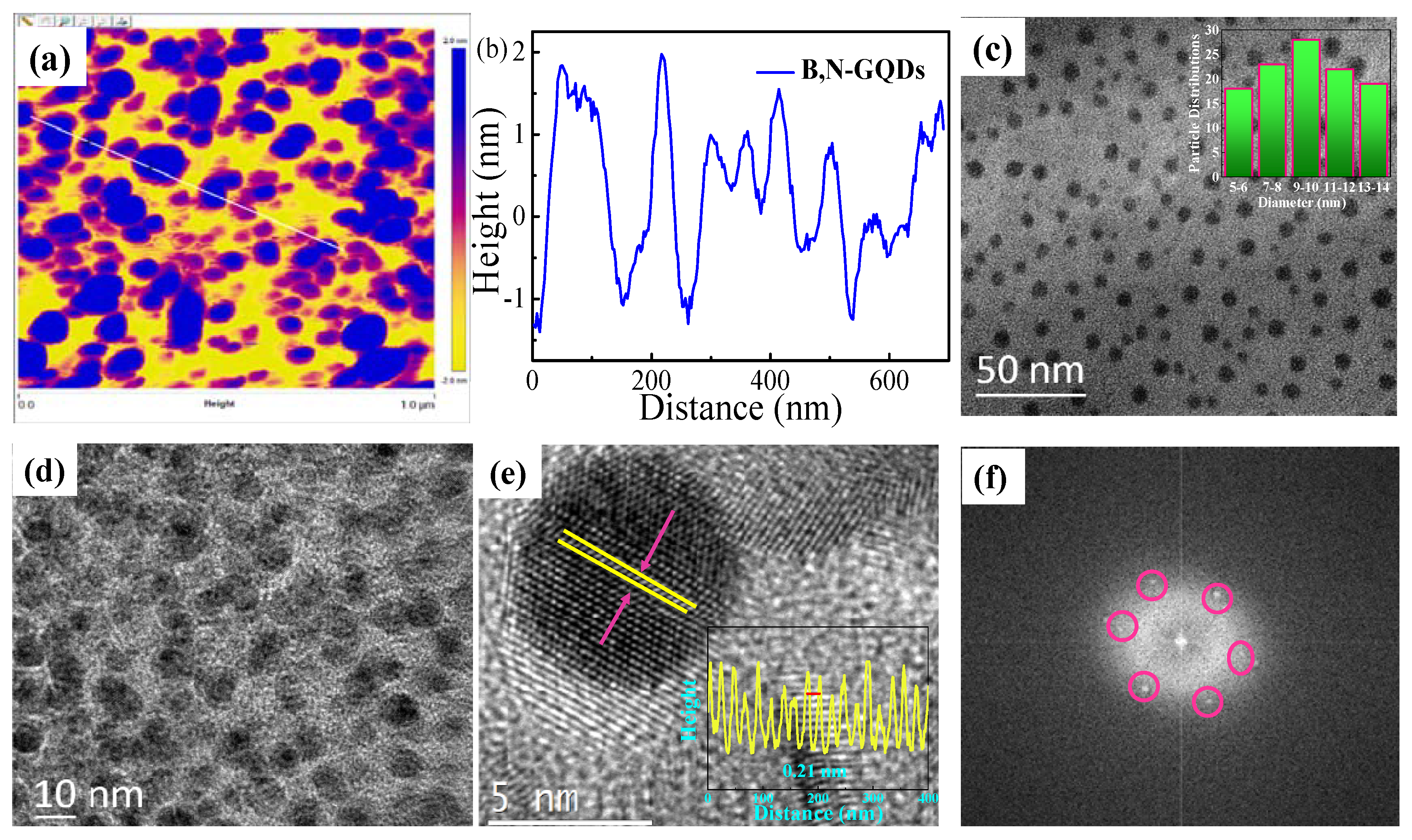
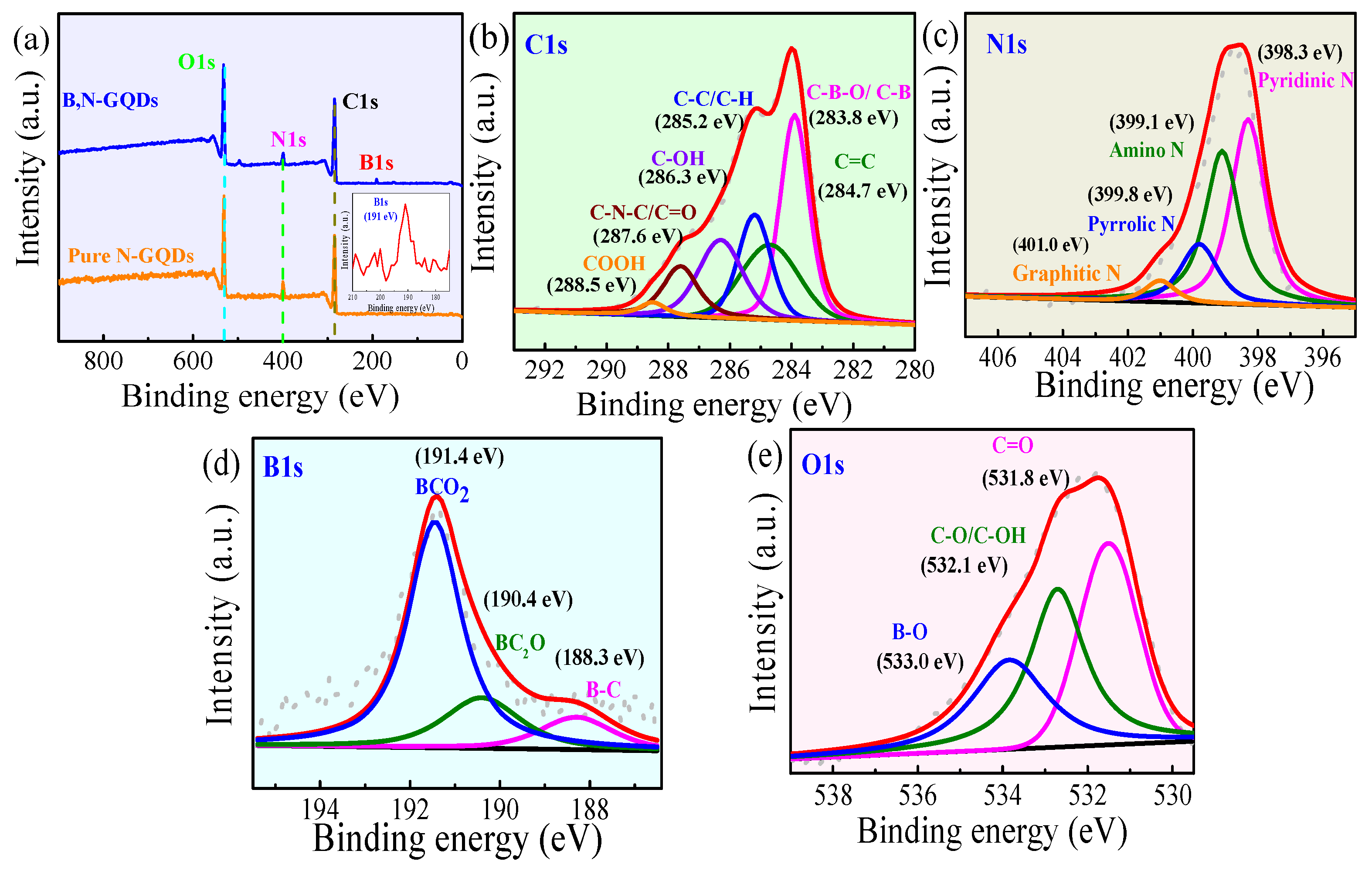
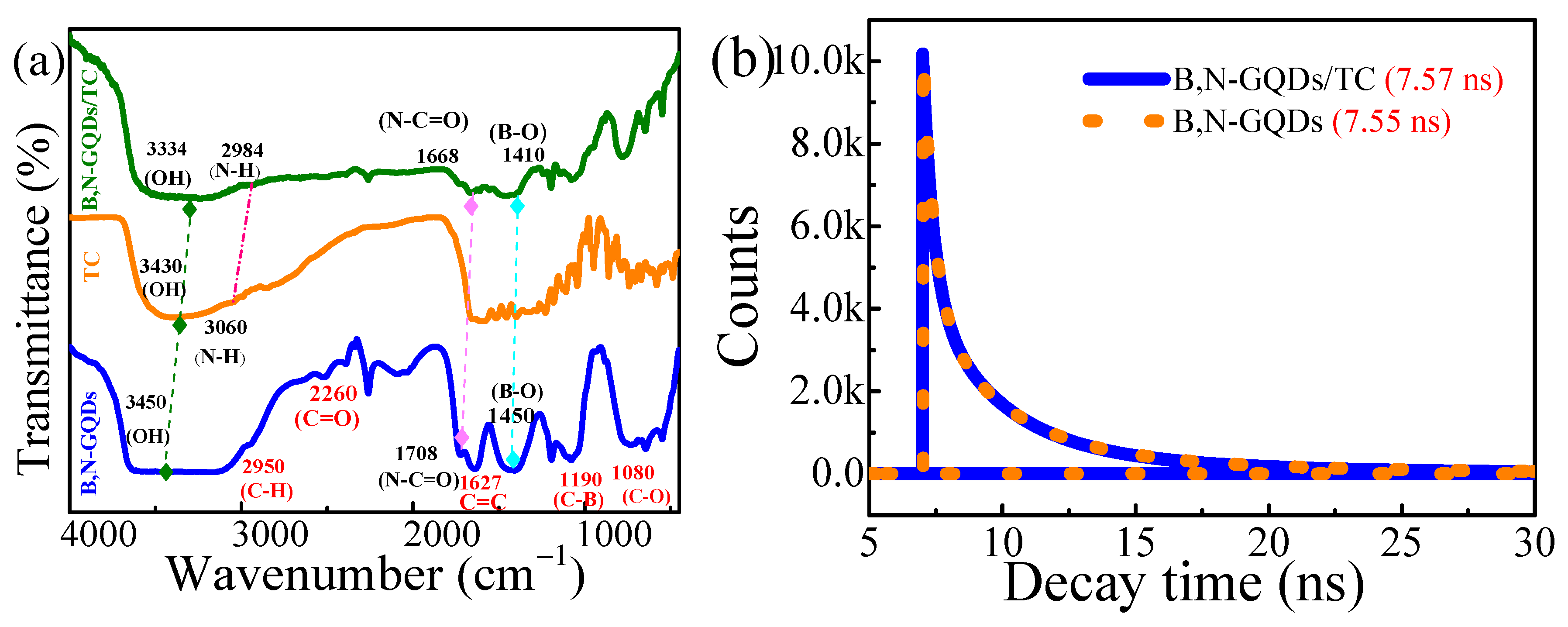
3.1. Characterization of B,N-GQDs
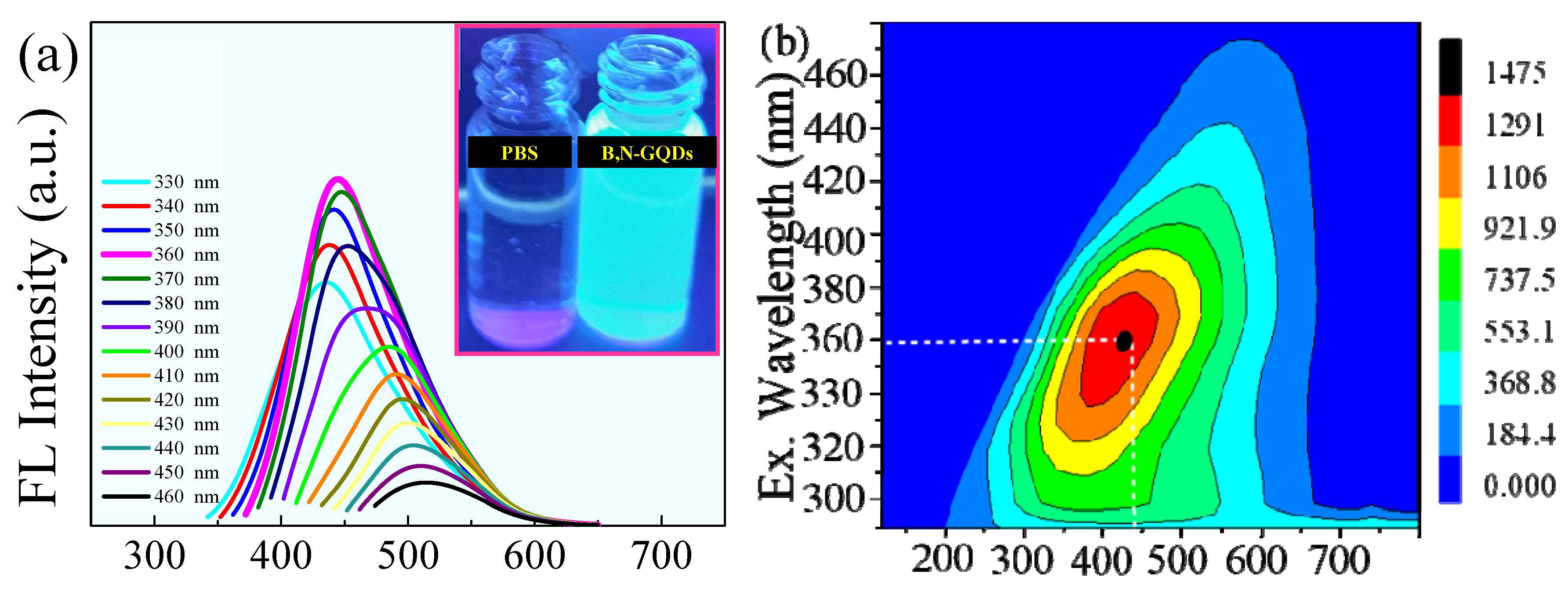
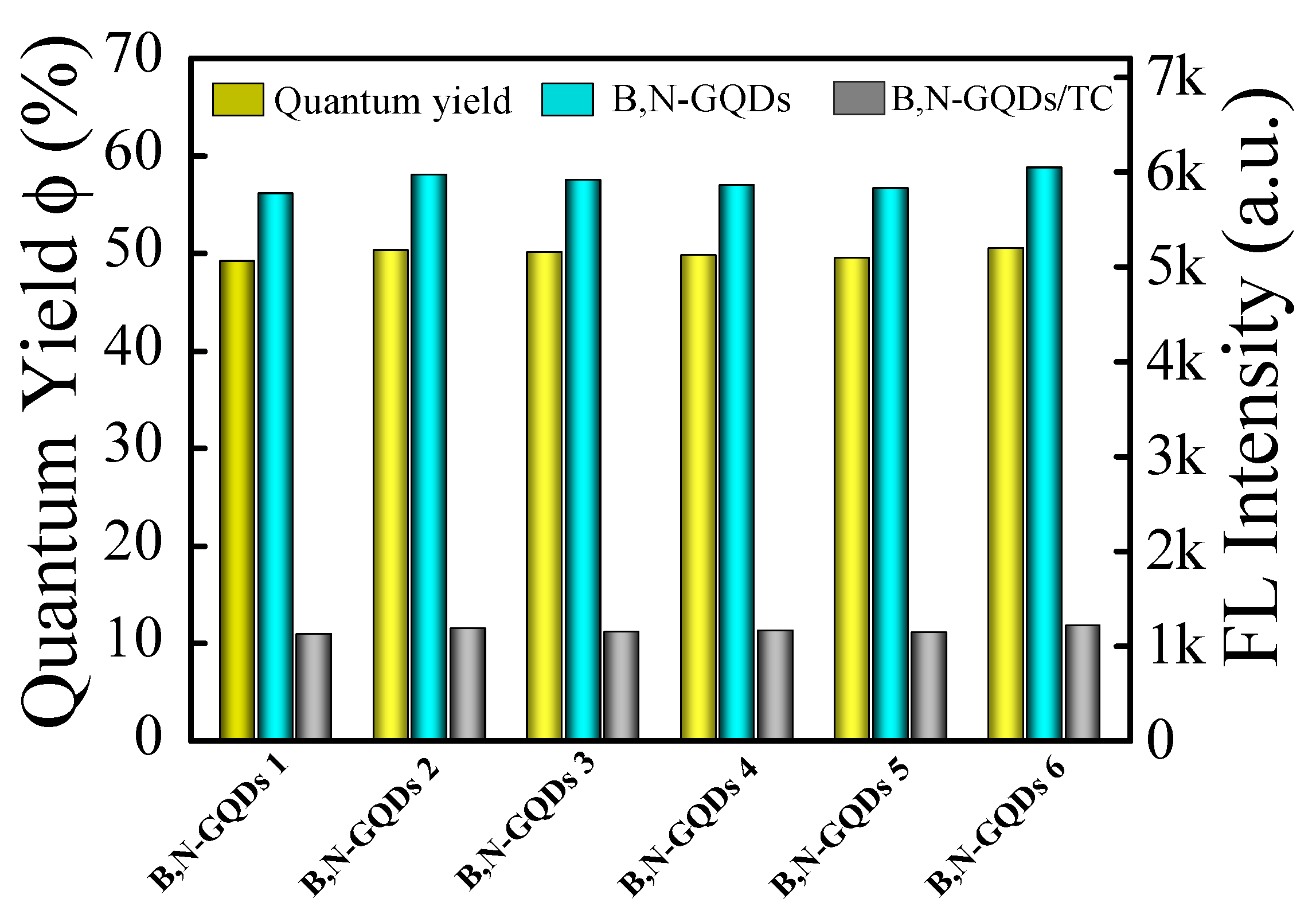
3.2. Optical Property of B,N-GQDs
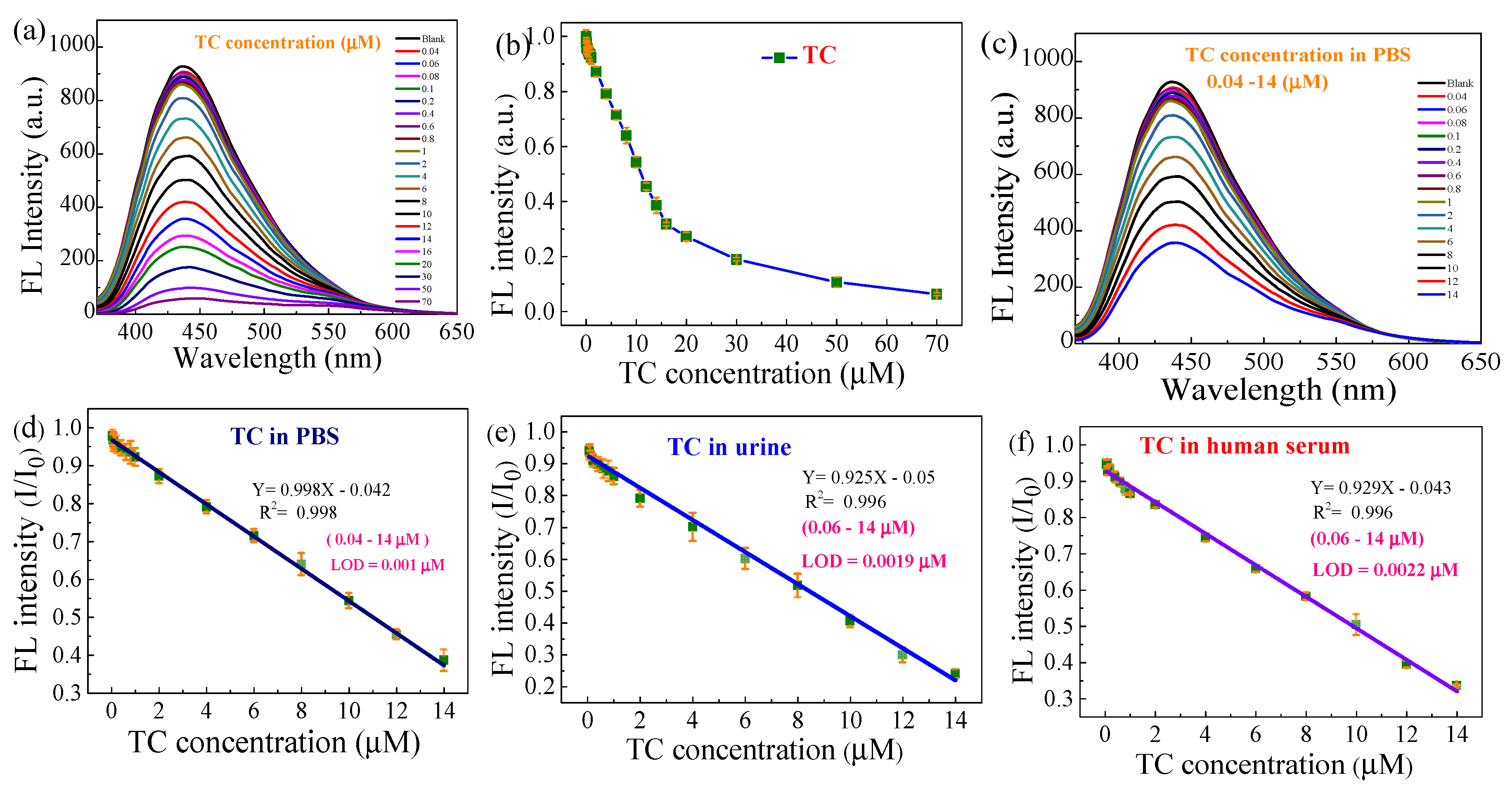
3.3. Detection of TC By B,N-GQDs
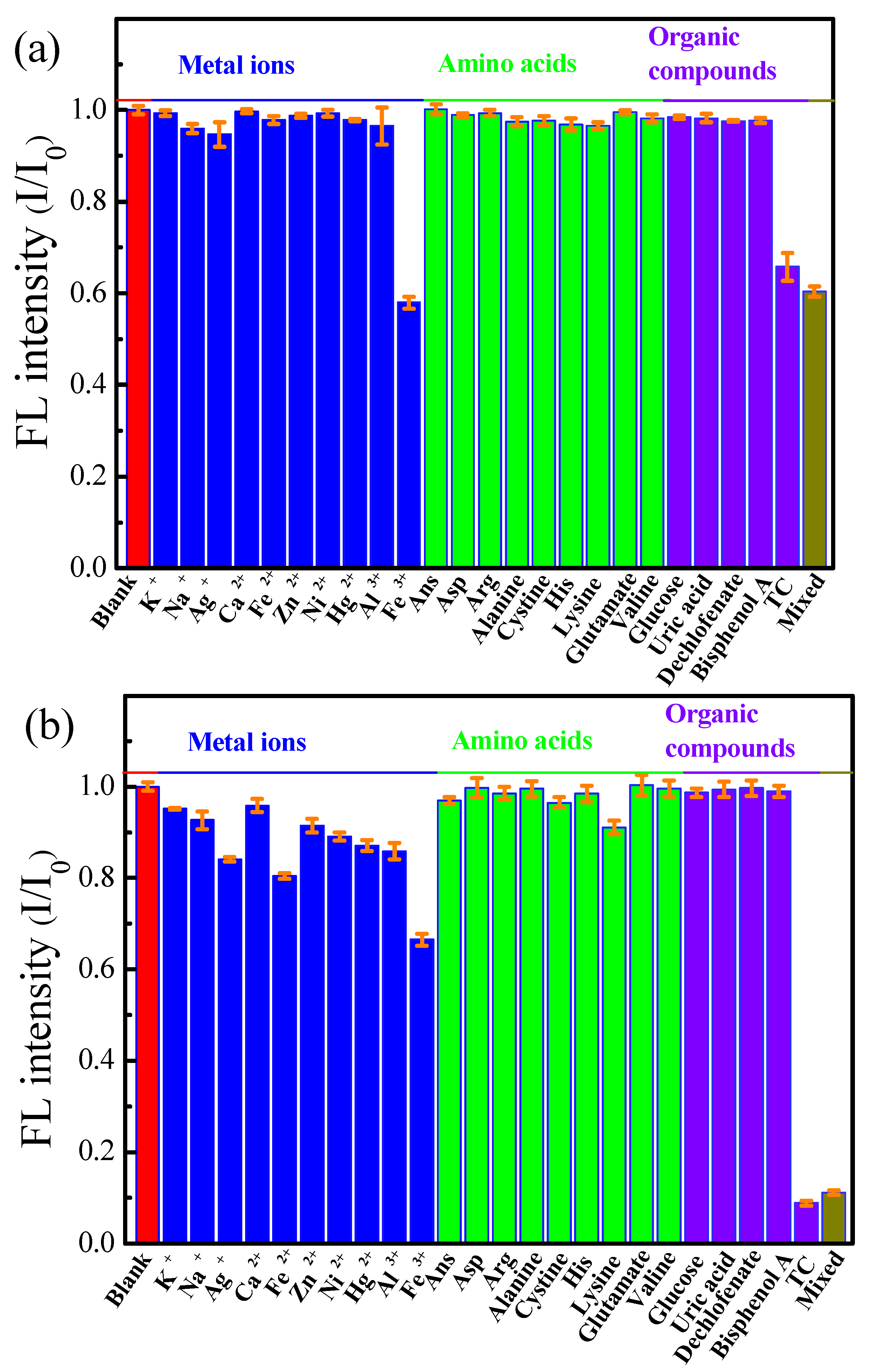
3.4. Selectivity of B,N-GQDs
3.5. Possible Sensing Mechanism for TC Detection by B, N-GQDs
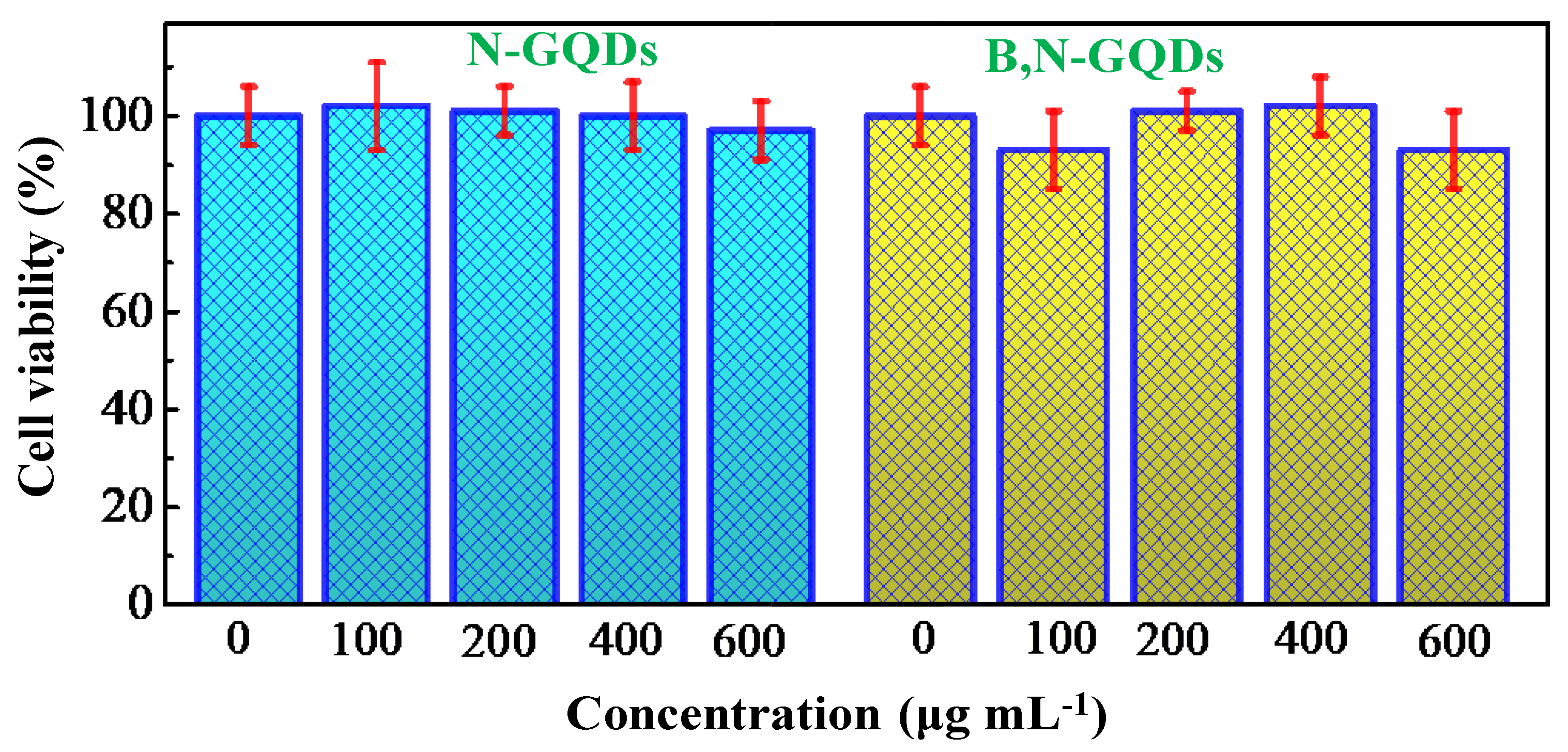
3.6. Cytotoxicity of GQDs Based Nanomaterials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wright, G.D. Solving the Antibiotic Crisis. ACS Infect. Dis. 2015, 1, 80–84. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M.C. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology and Epidemiology of Bacterial Resistance. Microbiol. Mol. Boil. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L. Fluorescence Probe Based on Hybrid Mesoporous Silica/Quantum Dot/Molecularly Imprinted Polymer for Detection of Tetracycline. ACS Appl. Mater. Interfaces 2016, 8, 16248–16256. [Google Scholar] [CrossRef]
- Guo, Q.; Li, X.; Shen, C.; Zhang, S.; Qi, H.; Li, T.; Yang, M. Electrochemical Immunoassay for the Protein Piomarker Mucin 1 and for MCF-7 Cancer Cells Based on Signal Enhancement by Silver Nanoclusters. Microchim. Acta 2015, 182, 1483–1489. [Google Scholar] [CrossRef]
- Kavosi, B.; Salimi, A.; Hallaj, R.; Moradi, F. Ultrasensitive electrochemical immunosensor for PSA biomarker detection in prostate cancer cells using gold nanoparticles/PAMAM dendrimer loaded with enzyme linked aptamer as integrated triple signal amplification strategy. Biosens. Bioelectron. 2015, 74, 915–923. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Ganganboina, A.B.; Tsai, Y.-C.; Chiua, H.-C.; Doong, R.-A. Multifunctional GQDs-Concanavalin A@Fe3O4 nanocomposites for cancer cells detection and targeted drug delivery. Anal. Chim. Acta 2018, 1027, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ganganboina, A.B.; Doong, R.-A. The biomimic oxidase activity of layered V2O5 nanozyme for rapid and sensitive nanomolar detection of glutathione. Sensors Actuators B: Chem. 2018, 273, 1179–1186. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Yang, W.; He, X.; Zhao, S.; Zhang, X.; Tang, W.; Wang, J.; Yue, T.; Li, Z. Amino-Functionalized Al–MOF for Fluorescent Detection of Tetracyclines in Milk. J. Agric. Food Chem. 2019, 67, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Spangler, L.C.; Cline, J.P.; Sakizadeh, J.D.; Kiely, C.J.; McIntosh, S. Enzymatic synthesis of supported CdS quantum dot/reduced graphene oxide photocatalysts. Green Chem. 2019, 21, 4046–4054. [Google Scholar] [CrossRef]
- Chaudhary, N.; Gupta, P.K.; Eremin, S.; Solanki, P.R. One-step green approach to synthesize highly fluorescent carbon quantum dots from banana juice for selective detection of copper ions. J. Environ. Chem. Eng. 2020, 8, 103720. [Google Scholar] [CrossRef]
- Zhao, S.; Song, X.; Chai, X.; Zhao, P.; He, H.; Liu, Z. Green Production of Fluorescent Carbon Quantum Dots Based on Pine Wood and Its Application in the Detection of Fe3+. J. Clean Prod. 2020, 263, 121561. [Google Scholar] [CrossRef]
- Alam, A.-M.; Park, B.; Ghouri, Z.K.; Park, M.; Kim, H.-Y. Synthesis of carbon quantum dots from cabbage with down- and up-conversion photoluminescence properties: Excellent imaging agent for biomedical applications. Green Chem. 2015, 17, 3791–3797. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal Treatment of Grass: A Low-Cost, Green Route to Nitrogen-Doped, Carbon-Rich, Photoluminescent Polymer Nanodots as an Effective Fluorescent Sensing Platform for Label-Free Detection of Cu(II) Ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Wang, Y.; Yangb, X. Carbon dots derived from tobacco for visually distinguishing and detecting three kinds of tetracyclines. Nanoscale 2018, 10, 8139–8145. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Bagherzadeh, M.; Nemati, A. Comparison Between Electrochemical and Photoelectrochemical Detection of Dopamine based on Titania-Ceria-Graphene Guantum Dots nanocomposite. Biosens. Bioelectron. 2020, 151, 111977. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dai, Y.; Chen, H.; Tang, Y.; Chen, X.; Wang, Y.; Zhao, J.; Zhu, X. Integration of fluorescence imaging and electrochemical biosensing for both qualitative location and quantitative detection of cancer cells. Biosens. Bioelectron. 2019, 130, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.D.; Doong, R.-A. Highly Sensitive and Selective Detection of Nanomolar Ferric Ions Using Dopamine Functionalized Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2016, 8, 21002–21010. [Google Scholar] [CrossRef]
- You, F.; Zhu, M.; Ding, L.; Xu, Y.; Wang, K.J.B. Design and Construction of Z-Scheme Bi2S3/Nitrogen-Doped Graphene Quantum Dots: Boosted Photoelectric Conversion Efficiency for High-Performance Photoelectrochemical Aptasensing of Sulfadimethoxine. Biosens. Bioelectron. 2019, 130, 230–235. [Google Scholar] [CrossRef]
- Hassani, N.E.A.E.; Baraket, A.; Boudjaoui, S.; Neto, E.T.T.; Bausells, J.; El Bari, N.; Bouchikhi, B.; Elaissari, A.; Errachid, A.; Zine, N. Development and application of a novel electrochemical immunosensor for tetracycline screening in honey using a fully integrated electrochemical Bio-MEMS. Biosens. Bioelectron. 2019, 130, 330–337. [Google Scholar] [CrossRef]
- Gao, F.; Liu, F.; Bai, X.; Xu, X.; Kong, W.; Liu, J.; Lv, F.; Long, L.; Yang, Y.; Li, M. Tuning the photoluminescence of graphene oxide quantum dots by photochemical fluorination. Carbon 2019, 141, 331–338. [Google Scholar] [CrossRef]
- Ghorbani, F.; Abbaszadeh, H.; Dolatabadi, J.E.N.; Aghebati-Maleki, L.; Yousefi, M. Application of various optical and electrochemical aptasensors for detection of human prostate specific antigen: A review. Biosens. Bioelectron. 2019, 142, 111484. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, Y.; Wang, L.; Kong, W.; Wang, S.; Wang, Z.; Li, Y.; Lu, Q. Photoluminescence of graphene quantum dots doped with different elements. Chin. Sci. Bull. 2019, 64, 411–418. [Google Scholar] [CrossRef]
- Liu, M.L.; Bin Chen, B.; Li, C.M.; Huang, C. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Tran, H.L.; Doong, R.-A. Sustainable fabrication of green luminescent sulfur-doped graphene quantum dots for rapid visual detection of hemoglobin. Anal. Methods 2019, 11, 4421–4430. [Google Scholar] [CrossRef]
- Wang, K.; Dong, L.; Sun, L.; Chen, H. Effects of elemental doping on the photoluminescence properties of graphene quantum dots. RSC Adv. 2016, 6, 91225–91232. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Trivizas, G.; Karakassides, M.A.; Baikousi, M.; Kouloumpis, A.; Gournis, D.; Bakandritsos, A.; Hola, K.; Kozák, O.; Zbořil, R.; et al. Green and simple route toward boron doped carbon dots with significantly enhanced non-linear optical properties. Carbon 2015, 83, 173–179. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, J.H.; Li, R.S.; Li, Y.; Huang, C.; Zhen, S.J. Carbon dots synthesized at room temperature for detection of tetracycline hydrochloride. Anal. Chim. Acta 2019, 1063, 144–151. [Google Scholar] [CrossRef]
- Li, W.; Zhu, J.; Xie, G.; Ren, Y.; Zheng, Y.-Q. Ratiometric system based on graphene quantum dots and Eu 3+ for selective detection of tetracyclines. Anal. Chim. Acta 2018, 1022, 131–137. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Chowdhury, A.D.; Doong, R.-A. Highly sensitive and selective detection of mercury ions using N, S-codoped graphene quantum dots and its paper strip based sensing application in wastewater. Sensors Actuators B: Chem. 2017, 252, 1169–1178. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Doong, R.-A. One-Step Synthesis of Size-Tunable Gold@Sulfur-Doped Graphene Quantum Dot Nanocomposites for Highly Selective and Sensitive Detection of Nanomolar 4-Nitrophenol in Aqueous Solutions with Complex Matrix. ACS Appl. Nano Mater. 2018, 1, 2153–2163. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, P.; Wang, F.; Fang, Y. Investigation of the Microstructures of Graphene Quantum Dots (GQDs) by Surface-Enhanced Raman Spectroscopy. Nanomater. 2018, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Van Tam, T.; Kang, S.G.; Babu, K.F.; Oh, E.-S.; Lee, S.G.; Choi, W.M. Synthesis of B-doped graphene quantum dots as a metal-free electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 10537–10543. [Google Scholar] [CrossRef]
- Sangam, S.; Gupta, A.; Shakeel, A.; Bhattacharya, R.; Sharma, A.K.; Suhag, D.; Chakrabarti, S.; Garg, S.K.; Ghosh, S.; Basu, B.; et al. Sustainable synthesis of single crystalline sulphur-doped graphene quantum dots for bioimaging and beyond. Green Chem. 2018, 20, 4245–4259. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Chowdhury, A.D.; Doong, R.-A. N-Doped Graphene Quantum Dots-Decorated V2O5 Nanosheet for Fluorescence Turn Off–On Detection of Cysteine. ACS Appl. Mater. Interfaces 2017, 10, 614–624. [Google Scholar] [CrossRef]
- Sahu, R.S.; Bindumadhavan, K.; Doong, R.-A. Boron-doped reduced graphene oxide-based bimetallic Ni/Fe nanohybrids for the rapid dechlorination of trichloroethylene. Environ. Sci. Nano 2017, 4, 565–576. [Google Scholar] [CrossRef]
- Van Tam, T.; Kang, S.G.; Kim, M.H.; Lee, S.G.; Hur, S.H.; Chung, J.S.; Choi, W.M. Novel Graphene Hydrogel/B-Doped Graphene Quantum Dots Composites as Trifunctional Electrocatalysts for Zn−Air Batteries and Overall Water Splitting. Adv. Energy Mater. 2019, 1900945. [Google Scholar] [CrossRef]
- Bindumadhavan, K.; Chang, P.-Y.; Doong, R.-A. Silver nanoparticles embedded boron-doped reduced graphene oxide as anode material for high performance lithium ion battery. Electrochimica Acta 2017, 243, 282–290. [Google Scholar] [CrossRef]
- Mohammed, L.J.; Omer, K.M. Dual Functional Highly Luminescence B, N Co-doped Carbon Nanodots as Nanothermometer and Fe3+/Fe2+ Sensor. Sci. Rep. 2020, 10, 3028. [Google Scholar] [CrossRef]
- Lin, L.-P.; Song, X.-H.; Chen, Y.; Rong, M.; Zhao, T.; Jiang, Y.; Wang, Y.; Chen, X. One-pot synthesis of highly greenish-yellow fluorescent nitrogen-doped graphene quantum dots for pyrophosphate sensing via competitive coordination with Eu3+ ions. Nanoscale 2015, 7, 15427–15433. [Google Scholar] [CrossRef]
- Rani, P.; Jindal, V.K. Designing band gap of graphene by B and N dopant atoms. RSC Adv. 2013, 3, 802–812. [Google Scholar] [CrossRef]
- Adhikari, S.; Selvaraj, S.; Kim, D.-H. Construction of heterojunction photoelectrode via atomic layer deposition of Fe2O3 on Bi2WO6 for highly efficient photoelectrochemical sensing and degradation of tetracycline. Appl. Catal. B: Environ. 2019, 244, 11–24. [Google Scholar] [CrossRef]
- Ramezani, M.; Danesh, N.M.; Lavaee, P.; Abnous, K.; Taghdisi, S.M. A novel colorimetric triple-helix molecular switch aptasensor for ultrasensitive detection of tetracycline. Biosens. Bioelectron. 2015, 70, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Su, Y.; Shen, Y.; Long, Y.; Li, W. In situ decoration of Au nanoparticles on carbon nitride using a single-source precursor and its application for the detection of tetracycline. J. Colloid Interface Sci. 2019, 536, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Mei, Q.; Tao, Z.; Wu, H.; Zhao, M.; Wang, S.; Liu, Y. A smartphone-integrated ratiometric fluorescence sensing platform for visual and quantitative point-of-care testing of tetracycline. Biosens. Bioelectron. 2019, 148, 111791. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, C.; Yu, X.; Li, J.; Wang, Z.; Zhang, Z.; Liu, B. Microwave-assisted synthesis of cyclen functional carbon dots to construct a ratiometric fluorescent probe for tetracycline detection. J. Mater. Chem. C 2018, 6, 9636–9641. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, R.; Wang, Y.; Sun, L.; Chen, L.; Dai, X.; Pan, J.; Pan, G.; Yan, Y. Surface-imprinted fluorescence microspheres as ultrasensitive sensor for rapid and effective detection of tetracycline in real biological samples. Sensors Actuators B: Chem. 2018, 263, 533–542. [Google Scholar] [CrossRef]
- Dong, G.; Huang, L.; Wu, X.; Wang, C.; Liu, Y.; Liu, G.; Wang, L.; Liu, X.; Xia, H. Effect and Mechanism Analysis of MnO2 on Permeable Reactive Barrier (PRB) System for the Removal of Te tracycline. Chemosphere 2018, 193, 702–710. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Chen, Z. Graphene-related nanomaterials: Tuning properties by functionalization. Nanoscale 2013, 5, 4541–4583. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Ge, L.; Jaroniec, M.; Qiao, S.-Z. Two-Step Boron and Nitrogen Doping in Graphene for Enhanced Synergistic Catalysis. Angew. Chem. Int. Ed. 2013, 52, 3110–3116. [Google Scholar] [CrossRef]
- Larijani, H.; Khorshidian, M. Theoretical insight into the role of pyridinic nitrogen on the catalytic activity of boron-doped graphene towards oxygen reduction reaction. Appl. Surf. Sci. 2019, 492, 826–842. [Google Scholar] [CrossRef]
- Dettlaff, A.; Jakóbczyk, P.; Ficek, M.; Wilk, B.; Szala, M.; Wojtas, J.; Ossowski, T.; Bogdanowicz, R. Electrochemical determination of nitroaromatic explosives at boron-doped diamond/graphene nanowall electrodes: 2,4,6-trinitrotoluene and 2,4,6-trinitroanisole in liquid effluents. J. Hazard. Mater. 2020, 387, 121672. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.D.; Ganganboina, A.B.; Doong, R.-A. Bipyridine- and Copper-Functionalized N-doped Carbon Dots for Fluorescence Turn Off–On Detection of Ciprofloxacin. ACS Appl. Mater. Interfaces 2020, 12, 32247–32258. [Google Scholar] [CrossRef] [PubMed]
| Methods | Materials | LOD (nM) | Linear Range (μM) | Reference |
|---|---|---|---|---|
| Photoelectrochemical | Fe2O3 on Bi2WO6 | 300 | 0.01–25 | [42] |
| Colorimetric aptasensor | THMS/AuNPs | 0.27 | 3 × 10−4–10−2 | [43] |
| Electrochemical | Au/C3N4 | 30 | 0.1–20 20–200 | [44] |
| Electrochemical immunosensor | Bio-MEMS | 1.2 pg mL−1 | 0.1–1000 pg mL−1 | [19] |
| Ratiometric fluorescence | DPA-Ce-GMP-Eu | 6.6 | 0.01–45 | [45] |
| Ratiometric fluorescence | Carbon dot–Eu3+ | 11.6 | 0–7.8 | [46] |
| Ratiometric fluorescence | Eu-GQDs | 300 | 0–20 | [28] |
| Fluorescence | MIPs-AF@SiO2 | 4.26 | 0–0.6 | [47] |
| Fluorescence | Carbon dots | 6,000 | 10–400 | [27] |
| Fluorescence | B,N-GQDs | 1 (PBS) 1.9 (urine) 2.2 (human serum) | 0.04–14 0.06–14 0.06–14 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, H.L.; Darmanto, W.; Doong, R.-A. Ultrasensitive Detection of Tetracycline Using Boron and Nitrogen Co-Doped Graphene Quantum Dots from Natural Carbon Source as the Paper-Based Nanosensing Probe in Difference Matrices. Nanomaterials 2020, 10, 1883. https://doi.org/10.3390/nano10091883
Tran HL, Darmanto W, Doong R-A. Ultrasensitive Detection of Tetracycline Using Boron and Nitrogen Co-Doped Graphene Quantum Dots from Natural Carbon Source as the Paper-Based Nanosensing Probe in Difference Matrices. Nanomaterials. 2020; 10(9):1883. https://doi.org/10.3390/nano10091883
Chicago/Turabian StyleTran, Hai Linh, Win Darmanto, and Ruey-An Doong. 2020. "Ultrasensitive Detection of Tetracycline Using Boron and Nitrogen Co-Doped Graphene Quantum Dots from Natural Carbon Source as the Paper-Based Nanosensing Probe in Difference Matrices" Nanomaterials 10, no. 9: 1883. https://doi.org/10.3390/nano10091883
APA StyleTran, H. L., Darmanto, W., & Doong, R.-A. (2020). Ultrasensitive Detection of Tetracycline Using Boron and Nitrogen Co-Doped Graphene Quantum Dots from Natural Carbon Source as the Paper-Based Nanosensing Probe in Difference Matrices. Nanomaterials, 10(9), 1883. https://doi.org/10.3390/nano10091883