Rheological, Microstructural and Thermal Properties of Magnetic Poly(Ethylene Oxide)/Iron Oxide Nanocomposite Hydrogels Synthesized Using a One-Step Gamma-Irradiation Method
Abstract
1. Introduction

2. Materials and Methods
2.1. Chemicals for the Synthesis
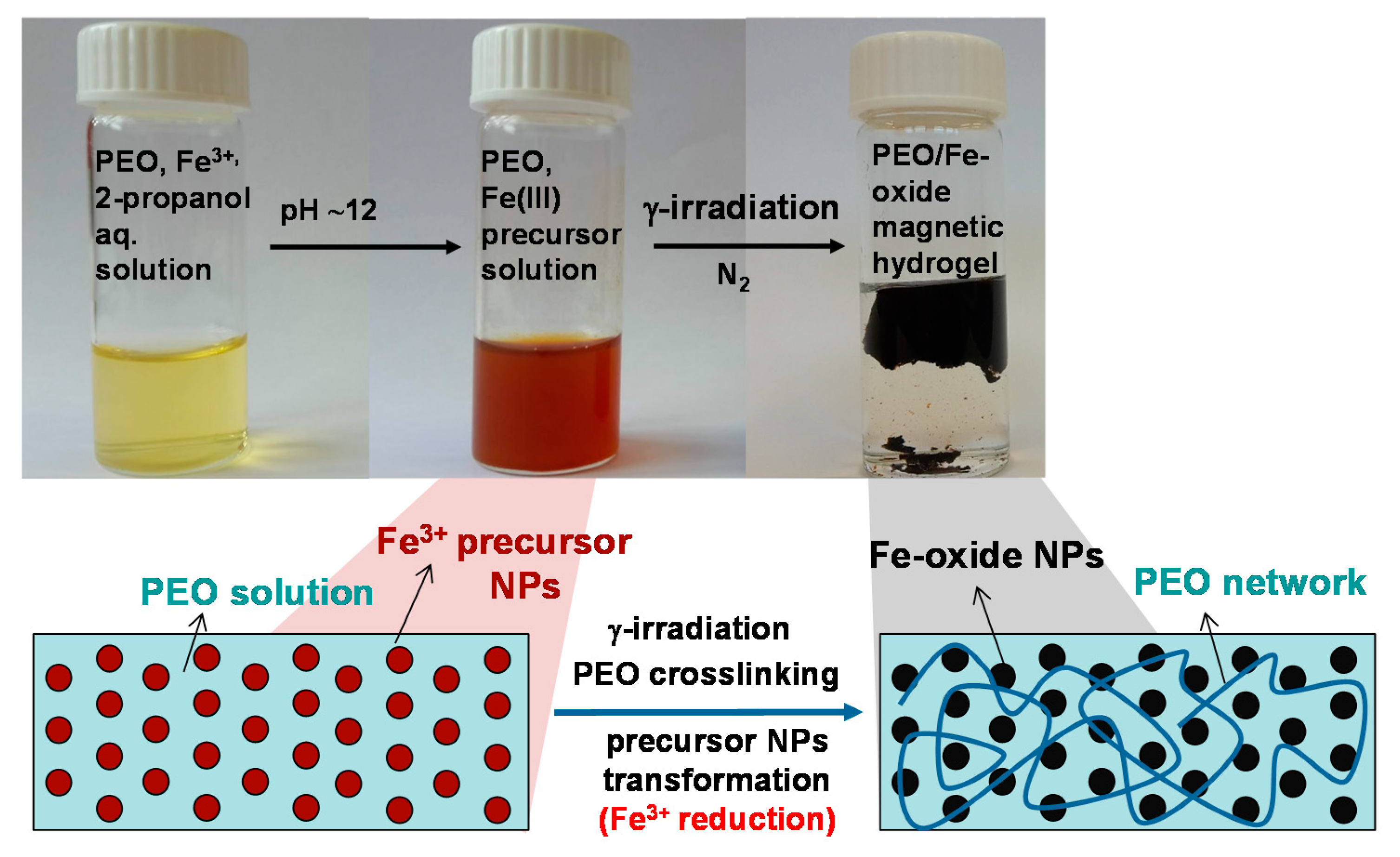
2.2. Synthesis of Samples
2.3. Characterization of Samples
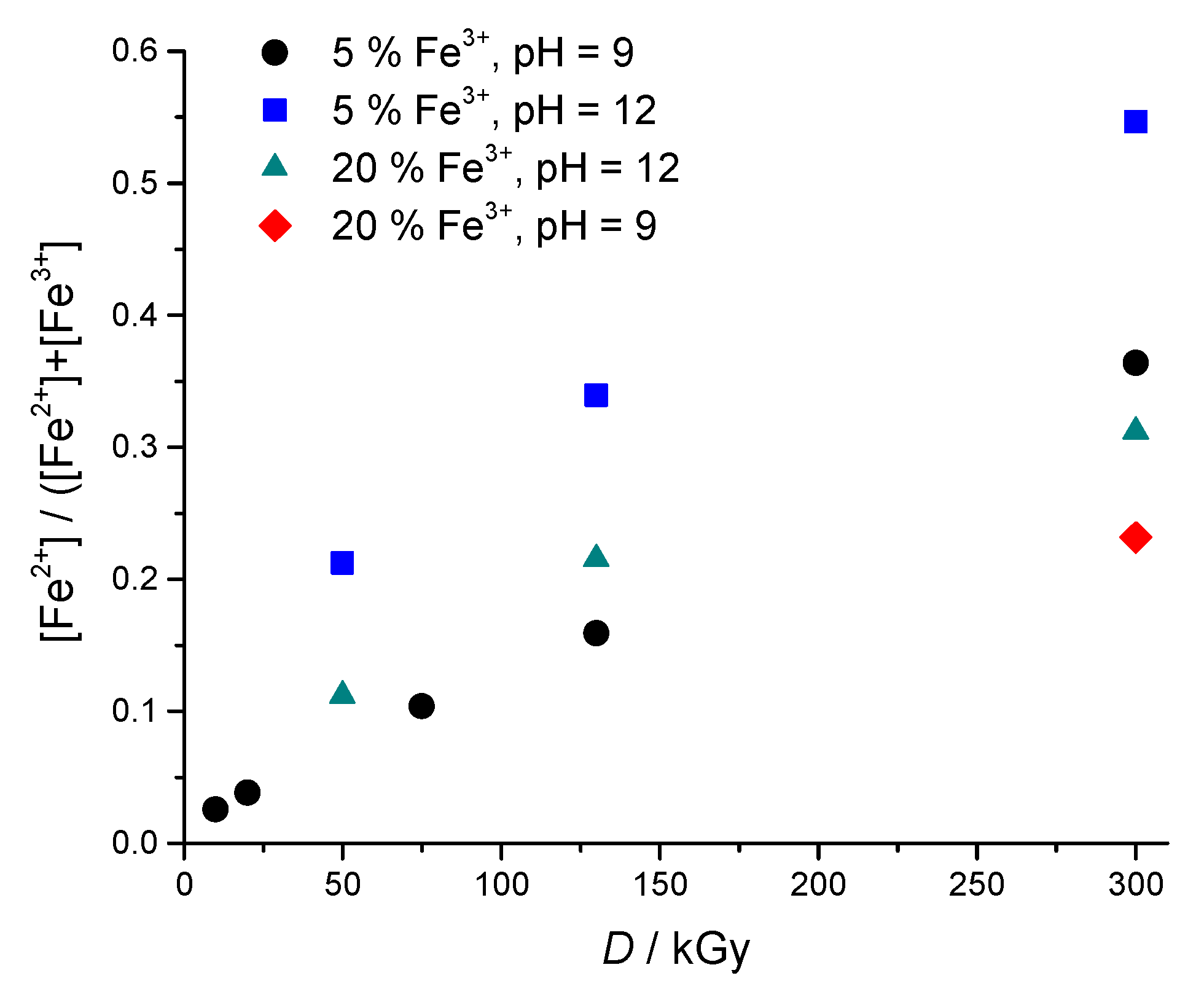
2.4. Quantitative Determination of Fe2+ Using 1,10-Phenanthroline UV-Vis Spectrophotometric Method
2.4.1. Chemicals for Spectrophotometric Determination
2.4.2. Spectrophotometric Determination of Fe2+
3. Results and Discussion
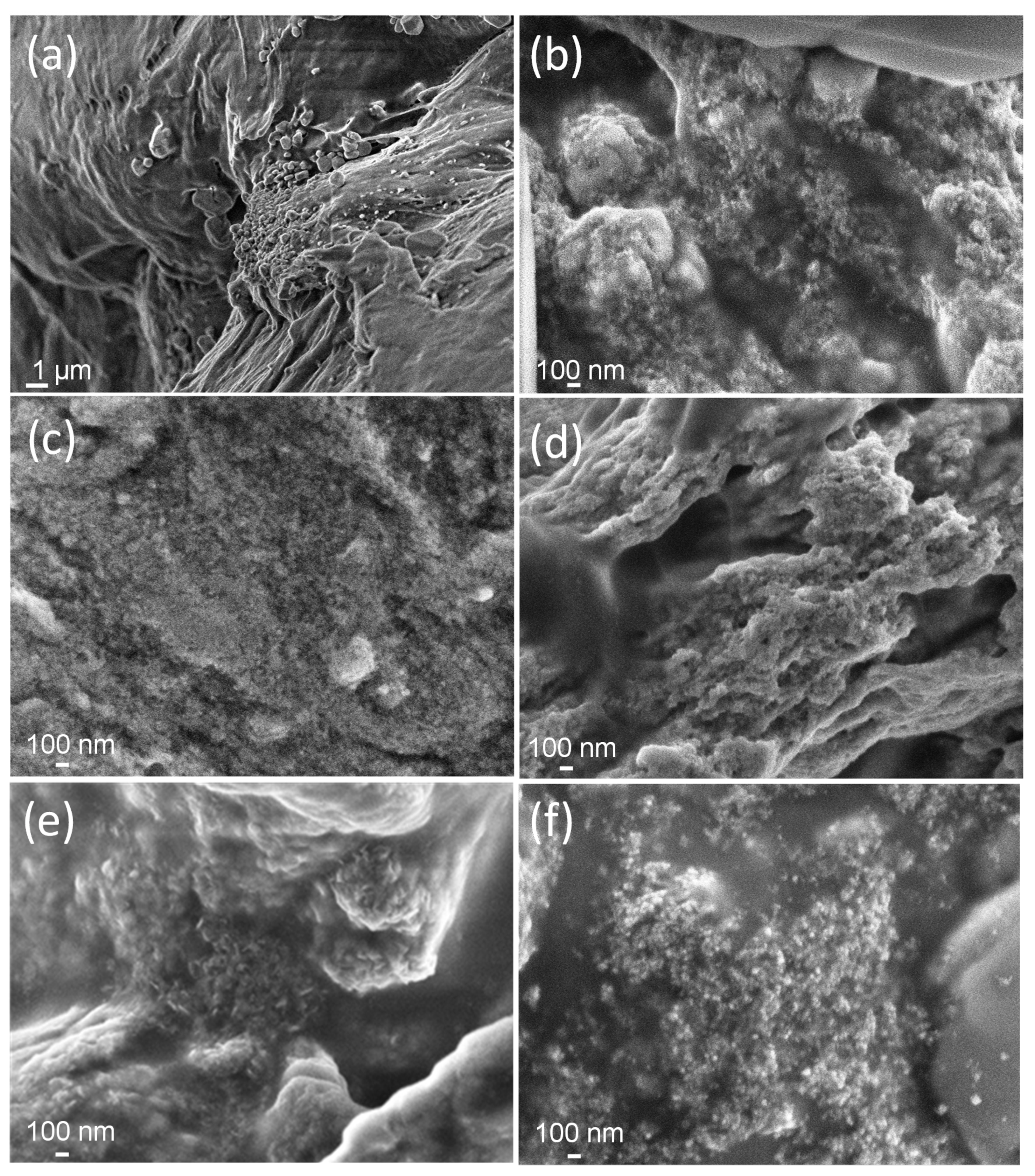
3.1. Microstructural Characterization of Gels (and Suspensions)
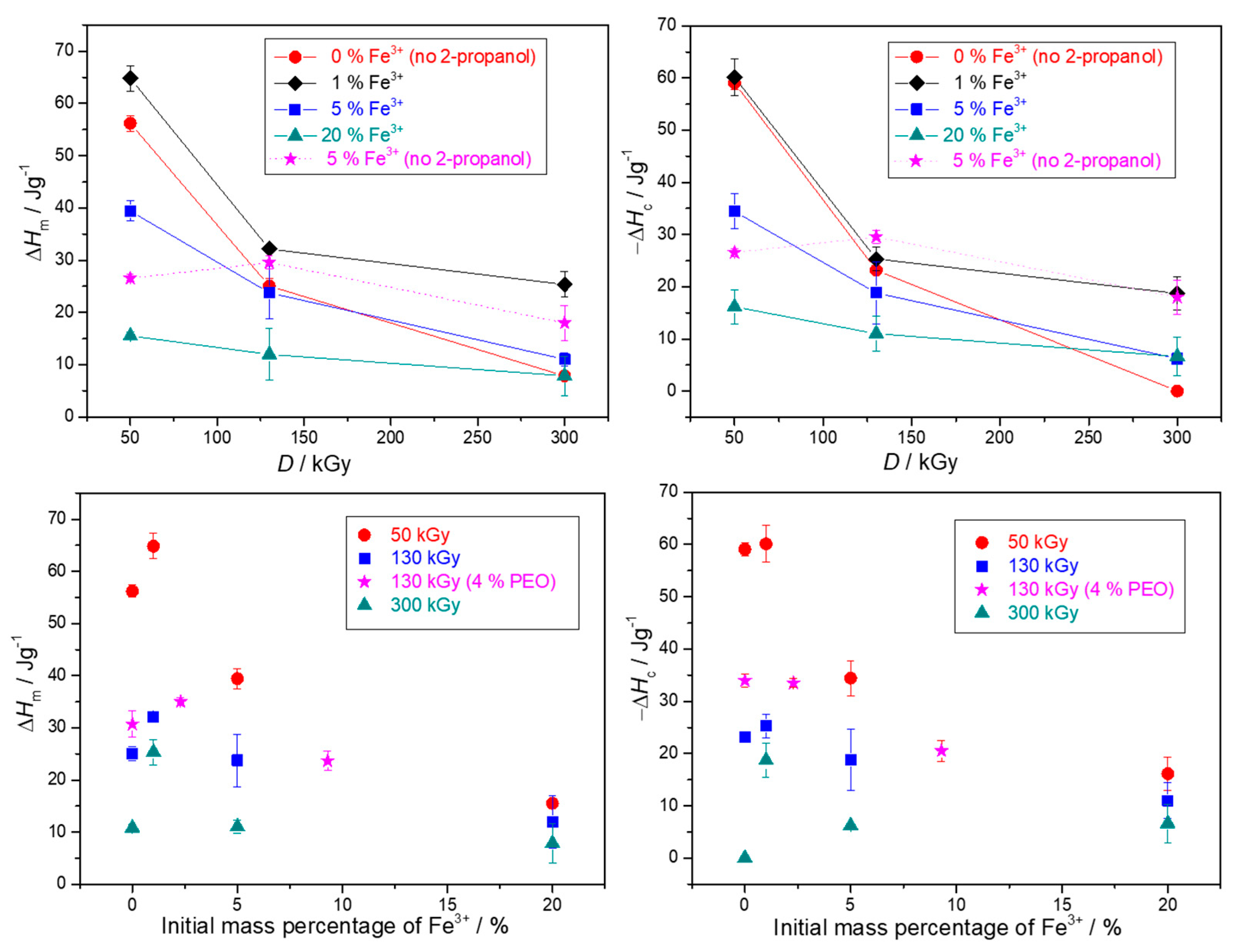
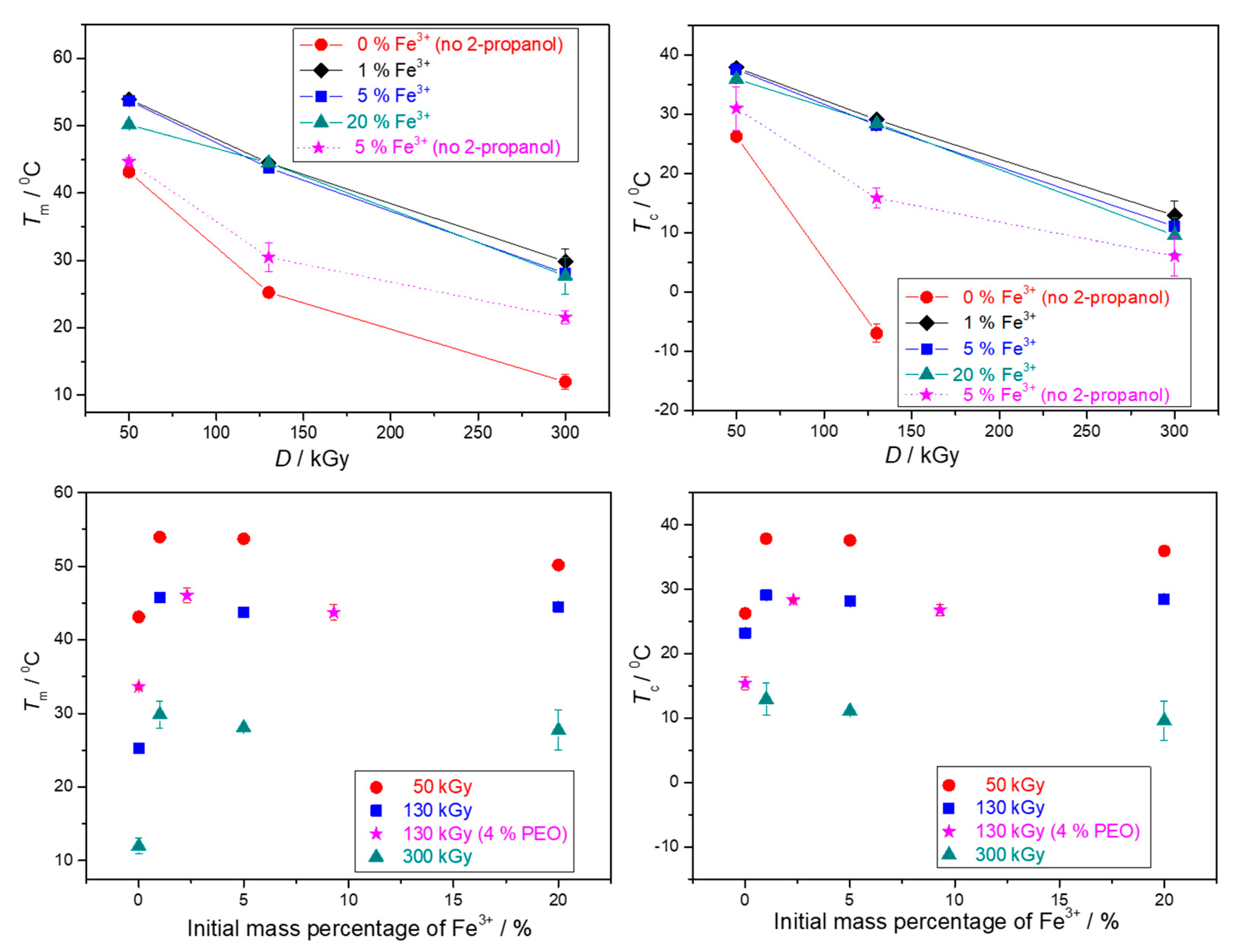
3.2. Thermal Characterization of Gels
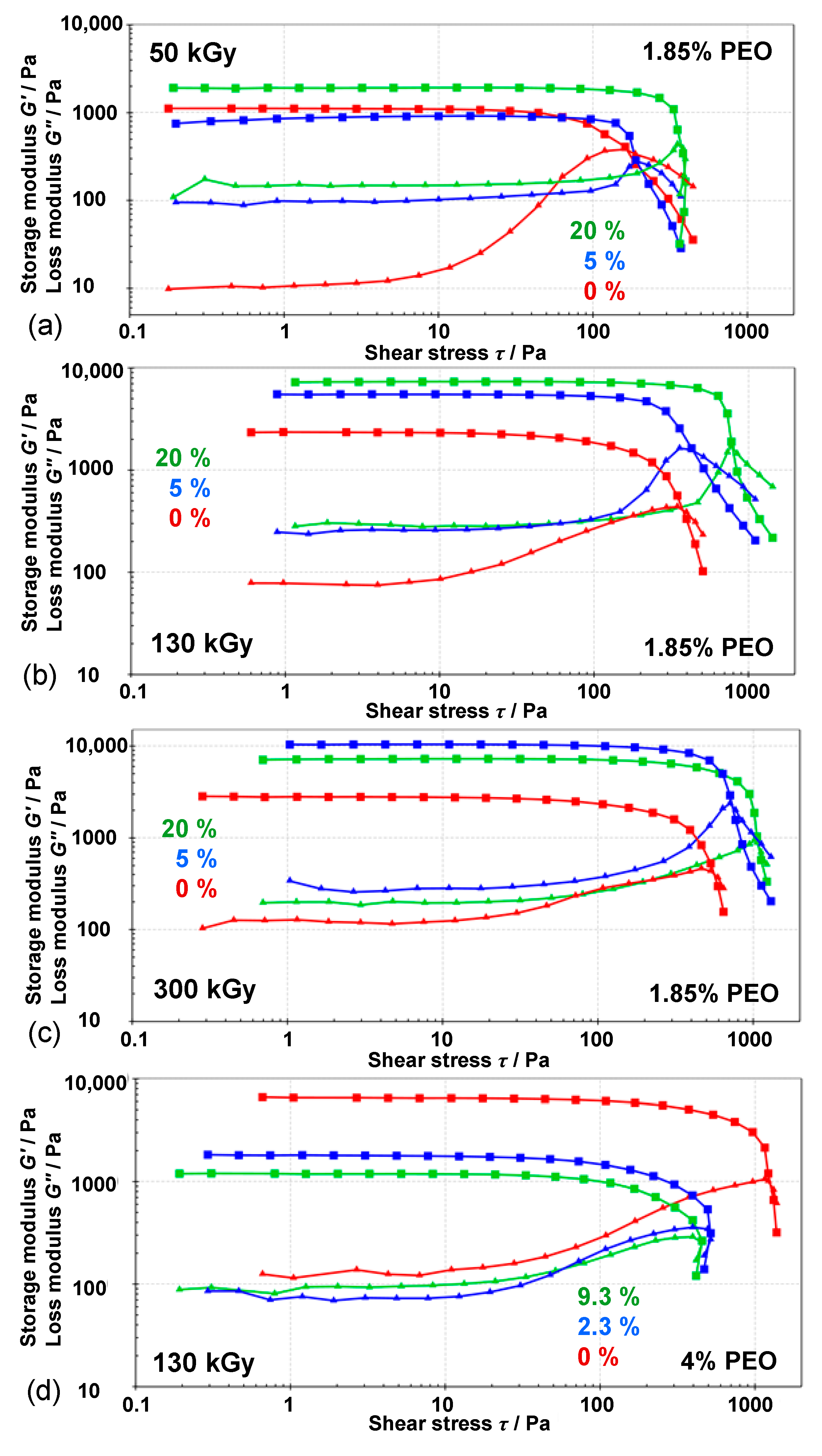
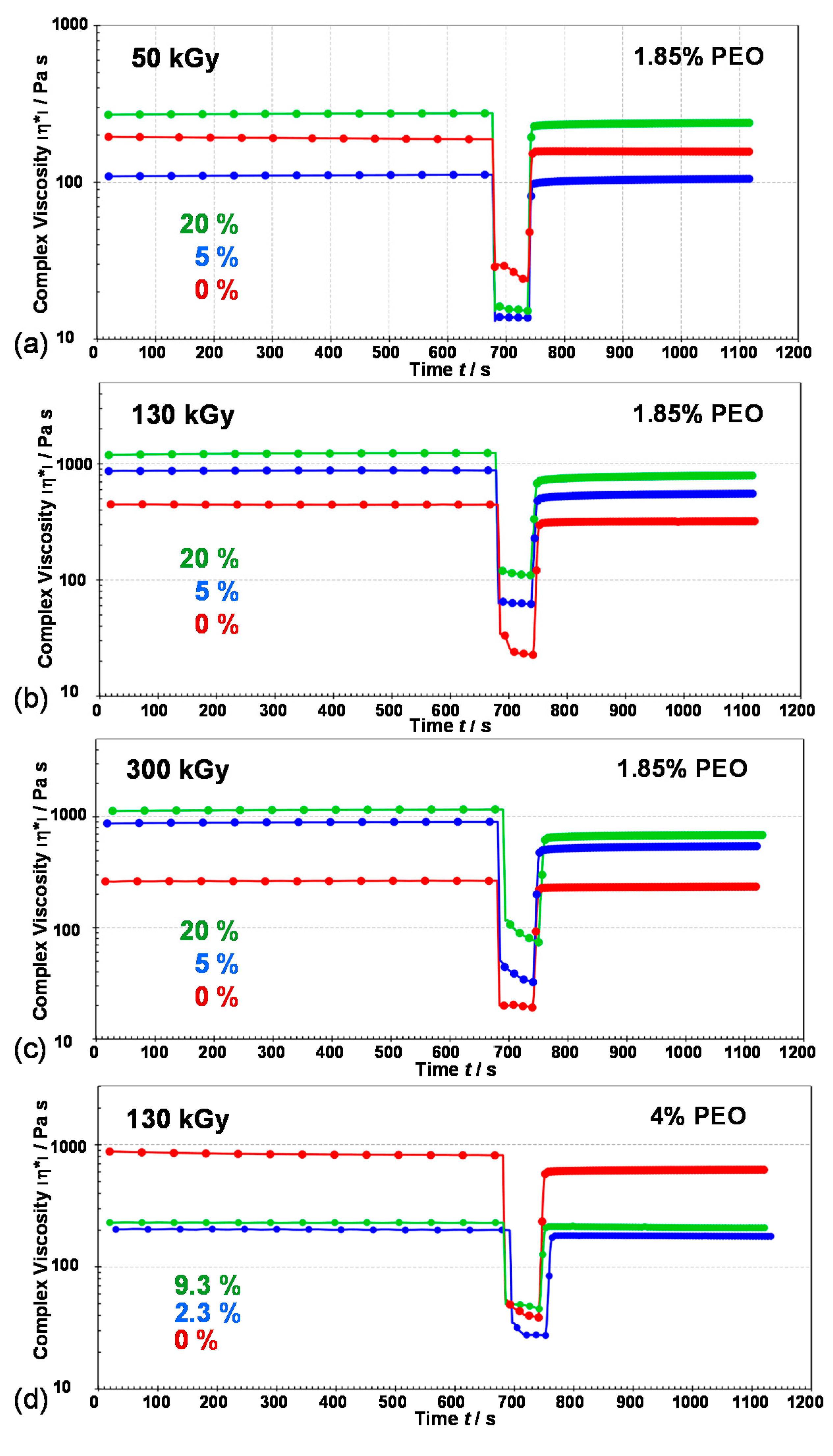
3.3. Rheological Properties of Gels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Blyakhman, F.A.; Makarova, B.E.; Fadeyev, F.A.; Lugovets, D.V.; Safronov, A.P.; Shabadrov, P.A.; Shklyar, T.F.; Melnikov, G.Y.; Orue, I.; Kurlyandskaya, G.V. The contribution of magnetic nanoparticles to ferrogel biophysical properties. Nanomaterials 2019, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Blyakhman, F.A.; Safronov, A.P.; Zubarev, A.Y.; Shklyar, T.F.; Makeyev, O.G.; Makarova, B.E.; Melekhin, V.V.; Larrañaga, A.; Kurlyandskaya, G.V. Polyacrylamide ferrogels with embedded maghemite nanoparticles for biomedical engineering. Results Phys. 2017, 7, 3624–3633. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M.; Zahoranova, A.; Mrlik, M.; Sramkova, P.; Minarik, A.; Sedlacik, M. Poly(2-oxazoline)-based magnetic hydrogels: Synthesis, performance and cytotoxicity. Colloid Surf. B Biointerfaces 2020, 190, 110912. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 2012, 23, 1–13. [Google Scholar] [CrossRef]
- Ramanujan, R.V.; Lao, L.L. The mechanical behavior of smart magnet-hydrogel composites. Smart Mater. Struct. 2006, 15, 952–956. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle-Hydrogel composites: Concept, design, and applications of these promising, multifunctional materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, Y.; Zhao, Y.; Xu, Y.; Zhang, F.; Gu, N.; Ma, J.; Reynolds, M.A.; Xia, Y.; Xu, H.H.K. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018, 12, e2085–e2098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, B.; Ma, F.; Lin, S.; Cao, M.; Li, Y.; Gu, N. Magnetic iron oxide nanoparticles accelerate osteogenic differentiation of mesenchymal stem cells via modulation of long noncoding RNA INZEB2. Nano Res. 2017, 10, 626–642. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Cao, M.; Sun, J.; Wu, H.; Zhao, P.; Xing, J.; Yang, Y.; Zhang, X.; Ji, M.; et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 2016, 86, 11–20. [Google Scholar] [CrossRef]
- Henstock, J.R.; Rotherham, M.; Rashidi, H.; Shakesheff, K.M.; El Haj, A.J. Remotely activated mechanotransduction via magnetic nanoparticles promotes mineralization synergistically with bone morphogenetic protein 2: Applications for injectable cell therapy. Stem Cells Transl. Med. 2014, 3, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Jurkin, T.; Pucić, I. Irradiation effects in poly(ethylene oxide)/silica nanocomposite films and gels. Polym. Eng. Sci. 2013, 53, 2318–2327. [Google Scholar] [CrossRef]
- Marinović-Cincović, M.T.; Radosavljević, A.N.; Krstić, J.I.; Spasojević, J.P.; Bibić, N.M.; Mitrić, N.M.; Kačarević-Popović, Z.M. Physicochemical characteristics of gamma irradiation crosslinked poly(vinyl alcohol)/magnetite ferrogel composite. Hem. Ind. 2014, 68, 743–753. [Google Scholar] [CrossRef]
- Eid, M. Preparation and characterization of natural polymers as stabilizer for magnetic nanoparticles by gamma irradiation. J. Polym. Res. 2013, 20, 112. [Google Scholar] [CrossRef]
- Jovanović, Ž.; Krklješ, A.; Stojkovska, J.; Tomić, S.; Obradović, B.; Mišković-Stanković, V.; Kačarević-Popović, Z. Synthesis and characterization of silver/poly(N-vinyl-2-pyrrolidone) hydrogel nanocomposite obtained by in situ radiolytic method. Radiat. Phys. Chem. 2011, 80, 1208–1215. [Google Scholar] [CrossRef]
- Krklješ, A.; Nedeljković, J.M.; Kačarević-Popović, Z.M. Fabrication of Ag-PVA hydrogel nanocomposite by γ-irradiation. Polym. Bull. 2007, 58, 271–279. [Google Scholar] [CrossRef]
- Spasojević, J.; Radosavljević, A.; Krstić, J.; Jovanović, D.; Spasojević, V.; Kalagasidis-Krušić, M.; Kačarević-Popović, Z. Dual responsive antibacterial Ag-poly(N-isopropylacrylamide/itaconic acid) hydrogel nanocomposites synthesized by gamma irradiation. Eur. Polym. J. 2015, 69, 168–185. [Google Scholar] [CrossRef]
- Abedini, A.; Daud, A.R.; Hamid, M.A.A.; Othman, N.K. Radiolytic formation of Fe3O4 nanoparticles: Influence of radiation dose on structure and magnetic properties. PLoS ONE 2014, 9, e90055. [Google Scholar] [CrossRef]
- Ekoko, G.B.; Lobo, J.K.-K.; Mvele, O.M.; Muswema, J.L.; Yamambe, J.-F.S.; Mangwala, P.K. Gamma irradiation inducing the synthesis of magnetic Fe3O4 nanorod particles in alkaline medium. Int. J. Mater. Sci. Appl. 2014, 3, 339–343. [Google Scholar] [CrossRef][Green Version]
- Gotić, M.; Jurkin, T.; Musić, S. Factors that may influence the micro-emulsion synthesis of nanosize magnetite particles. Colloid Polym. Sci. 2007, 285, 793–800. [Google Scholar] [CrossRef]
- Gotić, M.; Jurkin, T.; Musić, S. From iron(III) precursor to magnetite and vice versa. Mater. Res. Bull. 2009, 44, 2014–2021. [Google Scholar] [CrossRef]
- Jurkin, T.; Zadro, K.; Gotić, M.; Musić, S. Investigation of solid phase upon γ-irradiation of ferrihydrite-ethanol suspension. Radiat. Phys. Chem. 2011, 80, 792–798. [Google Scholar] [CrossRef]
- Jurkin, T.; Štefanić, G.; Dražić, G.; Gotić, M. Synthesis route to δ-FeOOH nanodiscs. Mater. Lett. 2016, 173, 55–59. [Google Scholar] [CrossRef]
- Jurkin, T.; Gotić, M.; Štefanić, G.; Pucić, I. Gamma-irradiation synthesis of iron oxide nanoparticles in the presence of PEO, PVP or CTAB. Radiat. Phys. Chem. 2016, 124, 75–83. [Google Scholar] [CrossRef]
- Marić, I.; Štefanić, G.; Gotić, M.; Jurkin, T. The impact of dextran sulfate on the radiolytic synthesis of magnetic iron oxide nanoparticles. J. Mol. Struct. 2019, 1183, 126–136. [Google Scholar] [CrossRef]
- Sutherland, T.I.; Sparks, C.J.; Joseph, J.M.; Wang, Z.; Whitaker, G.; Sham, T.K.; Wren, J.C. Effect of ferrous ion concentration on the kinetics of radiation-induced iron-oxide nanoparticle formation and growth. Phys. Chem. Chem. Phys. 2017, 19, 695–708. [Google Scholar] [CrossRef]
- Wang, S.; Xin, H.; Qian, Y. Preparation of nanocrystalline Fe3O4 by γ-ray radiation. Mater. Lett. 1997, 33, 113–116. [Google Scholar] [CrossRef]
- Yakabuskie, P.A.; Joseph, J.M.; Keech, P.; Botton, G.A.; Guzonas, D.; Wren, J.C. Iron oxyhydroxide colloid formation by gamma-radiolysis. Phys. Chem. Chem. Phys. 2011, 13, 7198–7206. [Google Scholar] [CrossRef]
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J.-L.; Delcourt, M.-O. Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids. New. J. Chem. 1998, 22, 1239–1255. [Google Scholar] [CrossRef]
- Jurkin, T.; Pucić, I. Poly(ethylene oxide) irradiated in the solid state, melt and aqueous solution—A DSC and WAXD study. Radiat. Phys. Chem. 2012, 81, 1303–1308. [Google Scholar] [CrossRef]
- Savaş, H.; Güven, O. Gelation, swelling and water vapor permeability behavior of radiation synthesized poly(ethylene oxide) hydrogels. Radiat. Phys. Chem. 2002, 64, 35–40. [Google Scholar] [CrossRef]
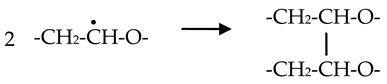
- Ulanski, P.; Rosiak, J.M.; Zainuddin, A. Pulse radiolysis of poly(ethylene oxide) in aqueous solutions. I. Formation of macroradicals. Radiat. Phys. Chem. 1995, 46, 913–916. [Google Scholar] [CrossRef]
- Ulanski, P.; Rosiak, J.M.; Zainuddin, A. Pulse radiolysis of poly(ethylene oxide) in aqueous solutions. II. Decay of macroradicals. Radiat. Phys. Chem. 1995, 46, 917–920. [Google Scholar] [CrossRef]
- Jiang, C.Z.; Yang, S.Y.; Gan, N.; Pan, H.C.; Liu, H. A method for determination of [Fe3+]/[Fe2+] ratio in superparamagnetic iron oxide. J. Magn. Magn. Mater. 2017, 439, 126–134. [Google Scholar] [CrossRef]
- Marić, I.; Gotić, M.; Štefanić, G.; Pustak, A.; Jurkin, T. γ-irradiation generated ferrous ions affect the formation of magnetite and feroxyhyte. Radiat. Phys. Chem. 2020, 170, 108648. [Google Scholar] [CrossRef]
- Marić, I.; Dražić, G.; Štefanić, G.; Zadro, K.; Gotić, M.; Jurkin, T. Characterization of radiolytically synthesized feroxyhyte and magnetite nanoparticles. Mater. Charact. 2020, 159, 110038. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Tugarova, A.V. Sample treatment in Mössbauer spectroscopy for protein-related analyses: Nondestructive possibilities to look inside metal-containing biosystems. Talanta 2017, 174, 819–837. [Google Scholar] [CrossRef]
- Lopez-Herrera, M.E.; Greneche, J.M.; Varret, F. Analysis of the Mössbauer quadrupole spectra of some amorphous fluorides. Phys. Rev. B 1983, 28, 4944. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; De Grave, E. Application of Mössbauer spectroscopy in Earth Sciences. In Mössbauer Spectroscopy Tutorial Book, 1st ed.; Yoshida, Y., Langouche, G., Eds.; Springer: Berlin, Germany, 2013; pp. 91–186. [Google Scholar]
- Gracien, E.B.; Ruimin, Z.; Kanza, L.K.; Lopaka, I. Effects of pH on the morphology of iron oxides synthesized under gamma-irradiation. J. Radioanal. Nucl. Chem. 2006, 270, 473–478. [Google Scholar] [CrossRef]
- Spinks, J.W.T.; Woods, R.J. Introduction to Radiation Chemistry, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1990; p. 262. [Google Scholar]
- Spotheim-Maurizot, M.; Mostafavi, M.; Douki, T.; Belloni, J. Radiation Chemistry. From Basics to Application in Material and Life Sciences; EDP Sciences: Les Ulis, France, 2008; pp. 120–121. [Google Scholar]
- Swiatla-Wojcik, D. Computation of the effect of pH on spur chemistry in water radiolysis at elevated temperatures. Nukleonika 2008, 53, S31–S37. [Google Scholar]
- Matheson, M.S.; Mamou, A.; Silverman, J.; Rabani, J. Reaction of hydroxyl radicals with polyethylene oxide in aqueous solution. J. Phys. Chem. 1973, 77, 2420–2424. [Google Scholar] [CrossRef]
- Dey, G.R.; Remita, H.; Mostafavi, M. Radiolytic reduction of Fe(II) in 2-propanol. Chem. Phys. Lett. 2006, 431, 83–87. [Google Scholar] [CrossRef]
- Le Caër, S.; Rotureau, P.; Brunet, F.; Charpentier, T.; Blain, G.; Renault, J.P.; Mialocq, J.-C. Radiolysis of confined water: Hydrogen production at a high dose. Chem. Phys. Chem. 2005, 6, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Minkova, L.; Stamenova, R.; Tsvetanov, C.; Nedkov, E. Structural studies of radiation-crosslinked poly(ethylene oxide). J. Polym. Sci. Part. B 1989, 27, 621–642. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Fillers, 2nd ed.; ChemTec Publishing: Toronto, ON, Canada, 2000; pp. 485–495, 501–505. [Google Scholar]
- Karaoğlu, E.; Kavas, H.; Baykal, A.; Toprak, M.S.; Sözeri, H. Effect of hydrolyzing agents on the properties of poly(ethylene glycol)-Fe3O4 nanocomposite. Nano Micro Lett. 2011, 3, 79–85. [Google Scholar] [CrossRef]
- Mathur, S.; Moudgil, B.M. Adsorption Mechanism of PEO on oxide surfaces. J. Colloid Interface Sci. 1997, 196, 92–98. [Google Scholar] [CrossRef]
- Weeber, R.; Kantorovich, S.; Holm, C. Ferrogels crosslinked by magnetic particles: Field-driven deformation and elasticity studied using computer simulations. J. Chem. Phys. 2015, 143, 154901. [Google Scholar] [CrossRef]
- Popescu, E.D.; Zaharescu, T.; Gavrilǎ, G. Insulation properties of degrading LDPE modified with metallic oxides. Optoelectr. Adv. Mater. Rapid Commun. 2011, 5, 1056–1061. [Google Scholar]
- Davenas, J.; Stevenson, I.; Celette, N.; Cambon, S.; Gardette, J.L.; Rivaton, A.; Vignoud, L. Stability of polymers under ionizing radiation. The many faces of radiation interaction with polymers. Nucl. Instrum. Methods Phys. Res. Sect. B 2002, 191, 653–661. [Google Scholar] [CrossRef]
- Agrawal, S.K.; Sanabria-DeLong, N.; Tew, G.N.; Bhatia, S.R. Nanoparticle-reinforced associative network hydrogels. Langmuir 2008, 24, 13148–13154. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Corbella, L.; Senger, B.; Boulmedais, F.; Hernández, R. Photoresponsive nanometer-scale iron alginated hydrogels: A study of gel-sol transition using quartz crystal microbalance. Langmuir 2019, 35, 11397–11405. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Li, G.; Liu, X.; Wu, S.; Tong, Z. Redox-responsive gel-sol/sol-gel transition in poly(acrylic acid) aqueous solution containing Fe(III) ions swiched by light. J. Am. Chem. Soc. 2008, 130, 16166–16167. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, Z.; Chen, X. Thermal and rheological properties of poly(vinyl alcohol) and water-soluble chitosan hydrogels prepared by combination of γ-ray irradiation and freeze thawing. J. Appl. Polym. Sci. 2008, 109, 3825–3830. [Google Scholar] [CrossRef]
- Van Brederode, R.A.; Rodriguez, F.; Cocks, G.C. Crosslinking poly(ethylene oxide) in dilute solutions by gamma rays. J. Appl. Polym. Sci. 1968, 12, 2097–2104. [Google Scholar] [CrossRef]
- Fechine, G.J.M.; Barros, J.A.G.; Alcântara, M.R.; Catalani, L.H. Fluorescence polarization and rheological studies of the poly(N-vinyl-2-pyrrolidone) hydrogels produced by UV radiation. Polymer 2006, 47, 2629–2633. [Google Scholar] [CrossRef]





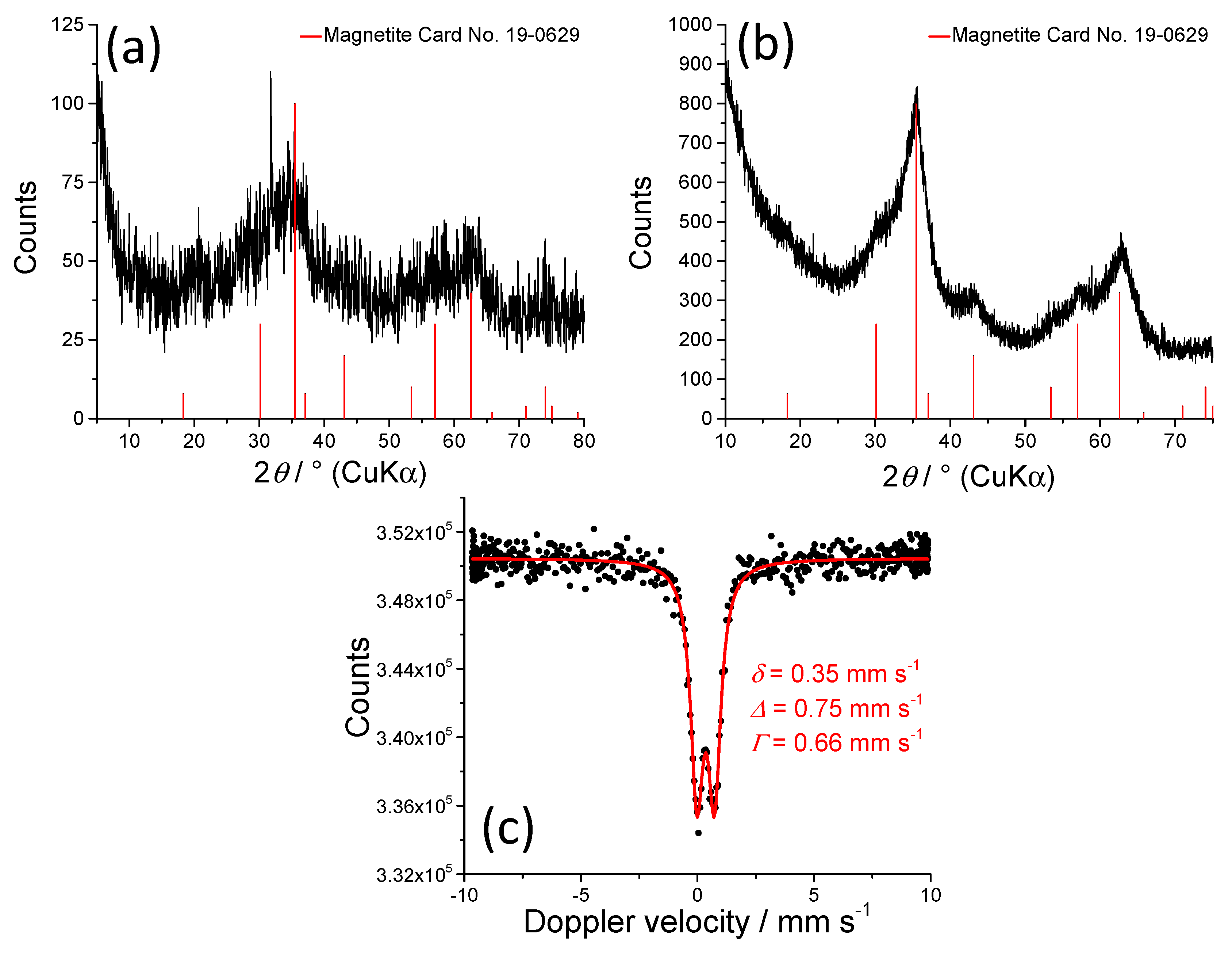

| Sample | Phase | hkl | 2θ/° | FWHM/° | Dhkl/nm |
|---|---|---|---|---|---|
| unirradiated precursor (5 wt% Fe3+, 0.2 M 2-propanol) | ferrihydrite | 110 | ~35 | 5.1 | 1.7 |
| PEO + halite (impurity) | |||||
| gel - 50 kGy (5 wt% Fe3+, 0.2 M 2-propanol) | ferrihydrite | 110 | 35.8 | 1.9 | 4.5 |
| PEO + halite (impurity) | |||||
| gel - 130 kGy (5 wt% Fe3+, 0.2 M 2-propanol) | magnetite | 311 | 35.5 | 3.6 | 2.3 |
| PEO + halite (impurity) | |||||
| gel - 300 kGy (5 wt% Fe3+, 0.2 M 2-propanol) | magnetite | - | 35.3 | 3.5 | 2.4 |
| halite + unidentified impurity | |||||
| gel - 130 kGy (20 wt% Fe3+, 0.2 M 2-propanol) | magnetite | 311 | 35.5 | 2.6 | 3.3 |
| PEO + halite (impurity) | |||||
| gel - 300 kGy (20 wt% Fe3+, 0.2 M 2-propanol) | magnetite | 311 | 35.4 | 0.6 | 13.9 |
| PEO + halite (impurity) | |||||
| powder - 50 kGy (20 wt% Fe3+, 0.8 M 2-propanol) | magnetite | 311 | 35.5 | 3.6 | 2.3 |
| powder - 130 kGy (20 wt% Fe3+, 0.8 M 2-propanol) | magnetite | 311 | 35.5 | 2.6 | 3.3 |
| Initial Mass Percentage of Fe3+ */% | Mass Percentage of PEO Aqueous Solution/% | D/kGy | G′ (Max)/Pa | Yield Point/Pa | Flow Point/Pa | Loss Factor (tan δ = G″/G′) |
|---|---|---|---|---|---|---|
| 0 | 1.85 | 50 | 816 | 56.0 | 166.3 | 0.01 |
| 5 | 1.85 | 50 | 1115 | 117.4 | 192 | 0.11 |
| 20 | 1.85 | 50 | 1907 | 192.8 | 375 | 0.08 |
| 0 | 1.85 | 130 | 2336 | 98.5 | 377.3 | 0.04 |
| 5 | 1.85 | 130 | 5519 | 201.9 | 439.2 | 0.05 |
| 20 | 1.85 | 130 | 7300 | 455.3 | 788.3 | 0.04 |
| 0 | 1.85 | 300 | 2784 | 124.2 | 559.7 | 0.04 |
| 5 | 1.85 | 300 | 10,325 | 386.3 | 736.3 | 0.03 |
| 20 | 1.85 | 300 | 7220 | 355.0 | 1086 | 0.03 |
| 0 | 4 | 130 | 6507 | 298.6 | 1261 | 0.03 |
| 2.3 ** | 4 | 130 | 1800 | 82.4 | 517.4 | 0.05 |
| 9.3 ** | 4 | 130 | 1200 | 72.8 | 450.2 | 0.09 |
| wt% Fe3+ | 0 | 5 | 20 | 0 | 5 | 20 | 0 | 5 | 20 | 0 | 2.3 | 9.3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% PEO | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 | 4.0 | 4.0 | 4.0 |
| Dose (kGy) | 50 | 50 | 50 | 130 | 130 | 130 | 300 | 300 | 300 | 130 | 130 | 130 |
| Recovery 3ITT test (%) t = 60 s | 96.1 | 90.7 | 84.7 | 70.8 | 59.7 | 60.5 | 87.0 | 57.6 | 57.1 | 74.6 | 90.4 | 92.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marić, I.; Šijaković Vujičić, N.; Pustak, A.; Gotić, M.; Štefanić, G.; Grenèche, J.-M.; Dražić, G.; Jurkin, T. Rheological, Microstructural and Thermal Properties of Magnetic Poly(Ethylene Oxide)/Iron Oxide Nanocomposite Hydrogels Synthesized Using a One-Step Gamma-Irradiation Method. Nanomaterials 2020, 10, 1823. https://doi.org/10.3390/nano10091823
Marić I, Šijaković Vujičić N, Pustak A, Gotić M, Štefanić G, Grenèche J-M, Dražić G, Jurkin T. Rheological, Microstructural and Thermal Properties of Magnetic Poly(Ethylene Oxide)/Iron Oxide Nanocomposite Hydrogels Synthesized Using a One-Step Gamma-Irradiation Method. Nanomaterials. 2020; 10(9):1823. https://doi.org/10.3390/nano10091823
Chicago/Turabian StyleMarić, Ivan, Nataša Šijaković Vujičić, Anđela Pustak, Marijan Gotić, Goran Štefanić, Jean-Marc Grenèche, Goran Dražić, and Tanja Jurkin. 2020. "Rheological, Microstructural and Thermal Properties of Magnetic Poly(Ethylene Oxide)/Iron Oxide Nanocomposite Hydrogels Synthesized Using a One-Step Gamma-Irradiation Method" Nanomaterials 10, no. 9: 1823. https://doi.org/10.3390/nano10091823
APA StyleMarić, I., Šijaković Vujičić, N., Pustak, A., Gotić, M., Štefanić, G., Grenèche, J.-M., Dražić, G., & Jurkin, T. (2020). Rheological, Microstructural and Thermal Properties of Magnetic Poly(Ethylene Oxide)/Iron Oxide Nanocomposite Hydrogels Synthesized Using a One-Step Gamma-Irradiation Method. Nanomaterials, 10(9), 1823. https://doi.org/10.3390/nano10091823









