Effect of Nanostructured Scaffold on Human Adipose-Derived Stem Cells: Outcome of In Vitro Experiments
Abstract
1. Introduction
2. Materials and Methods
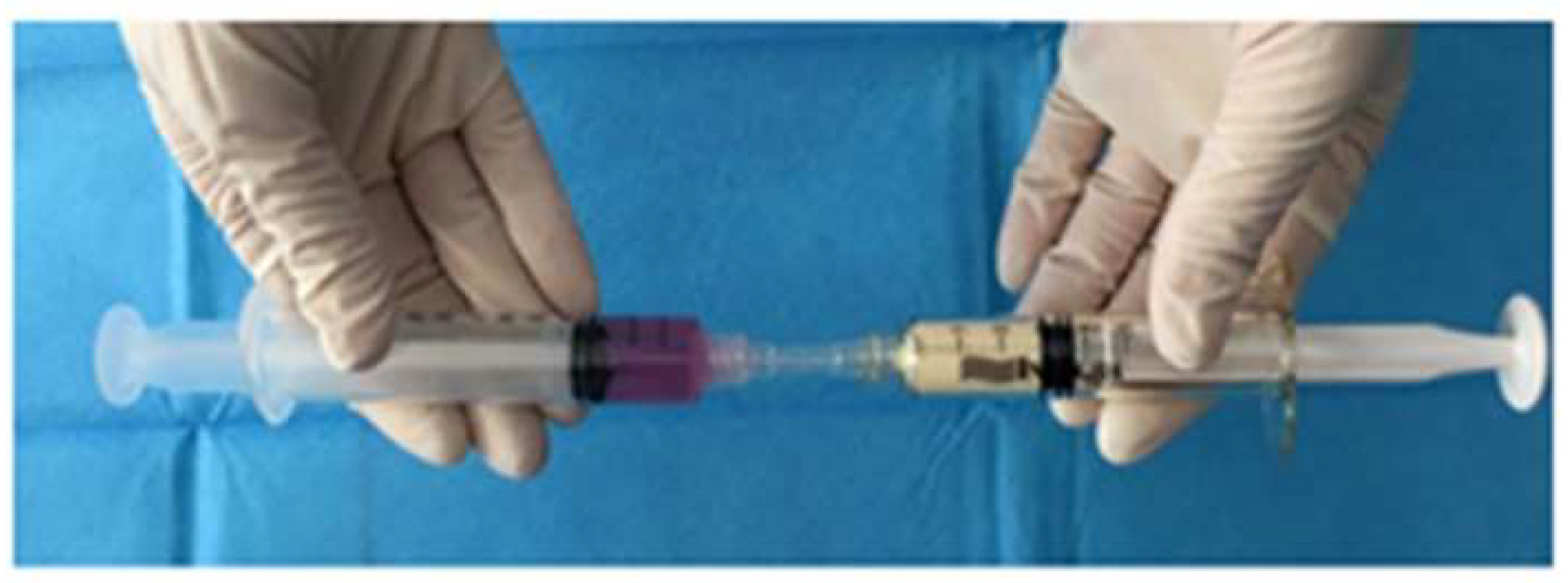
2.1. Flowable Wound Matrix
2.2. Human Adipose-Derived Stem Cell (hASC) Isolation and Culture
2.3. hASC Characterization
2.4. Flowable Wound Matrix (FWM)-hASC Mixture Preparation
2.5. Cell Viability/Proliferation Assay
2.6. Optical and Electron Microscopy
2.7. Gene Expression
2.8. Enzyme-Linked Immunosorbent Assay (ELISA) on hASC Culture Medium
2.9. Statistical Analysis
3. Results
3.1. Cell Viability
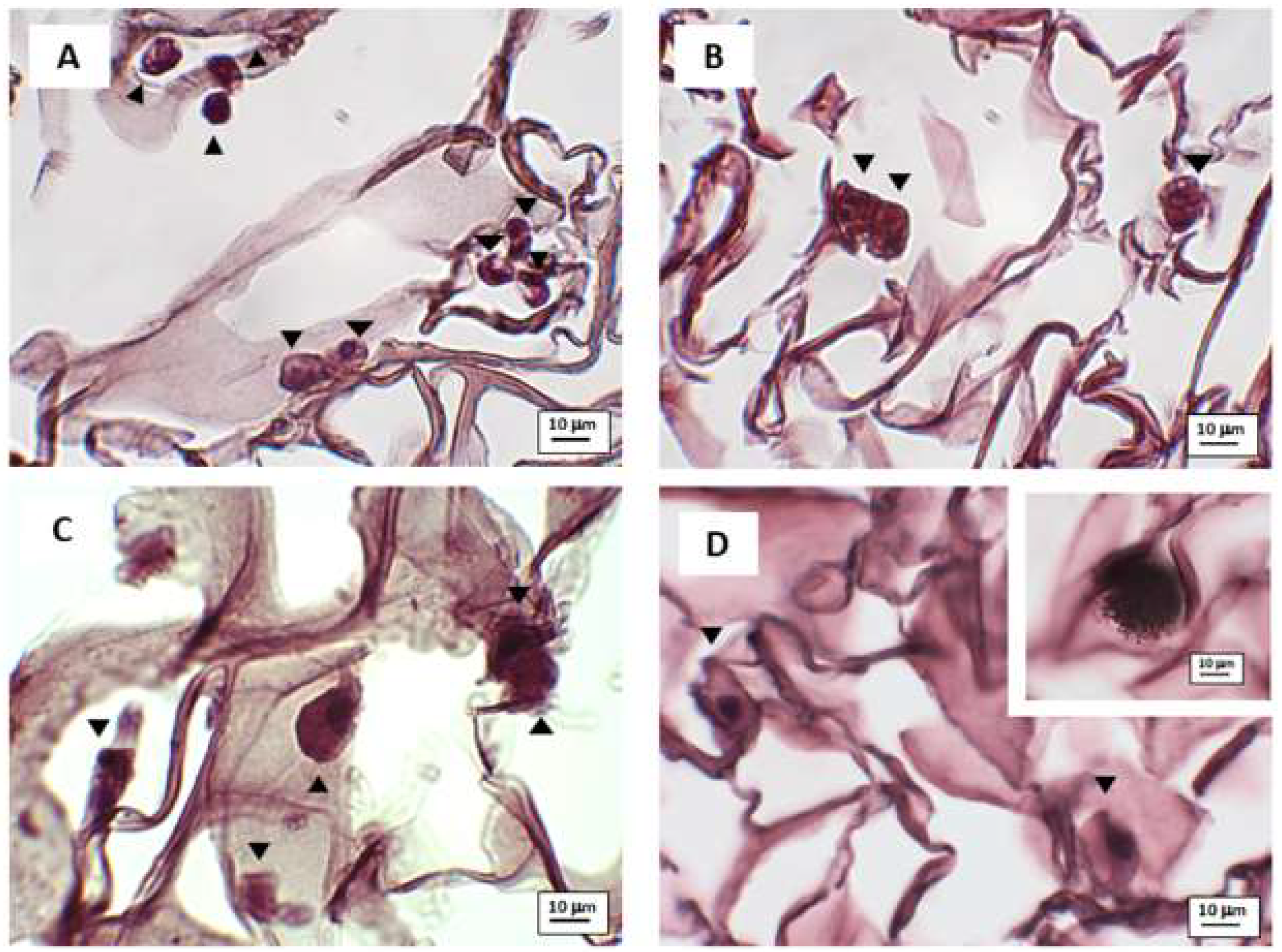
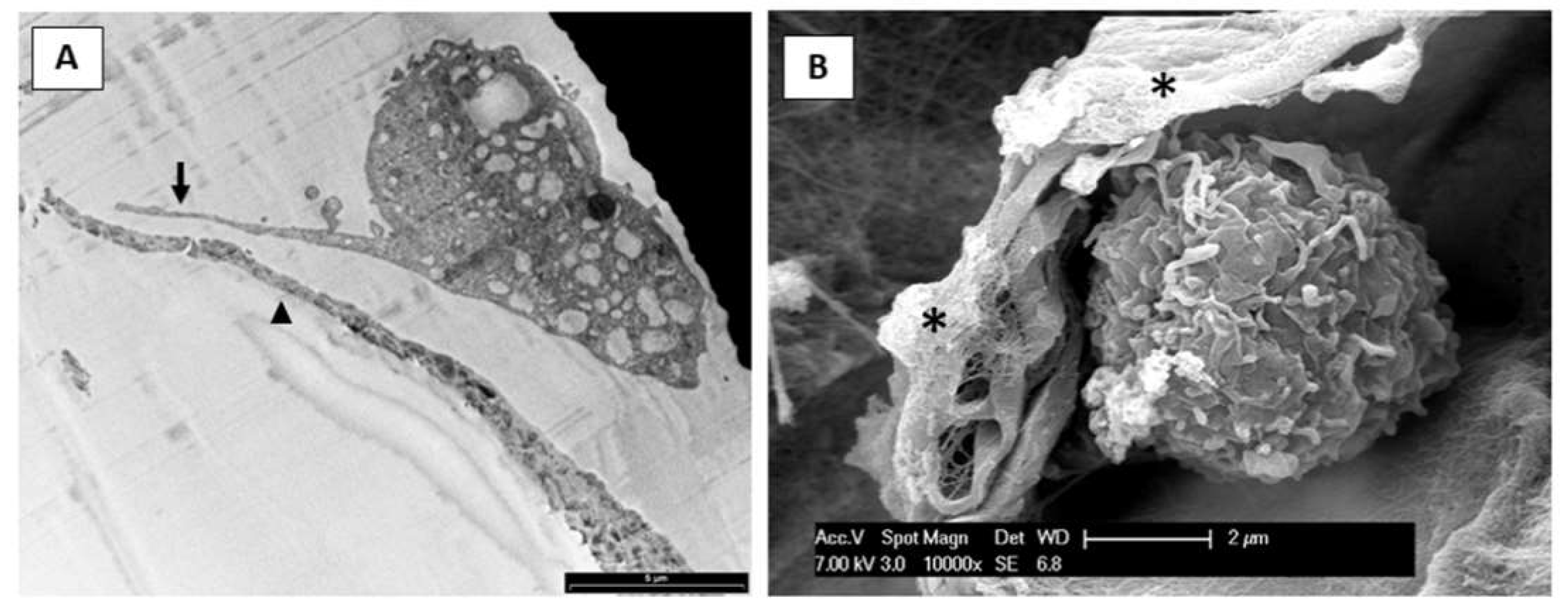
3.2. Microscopy Observation
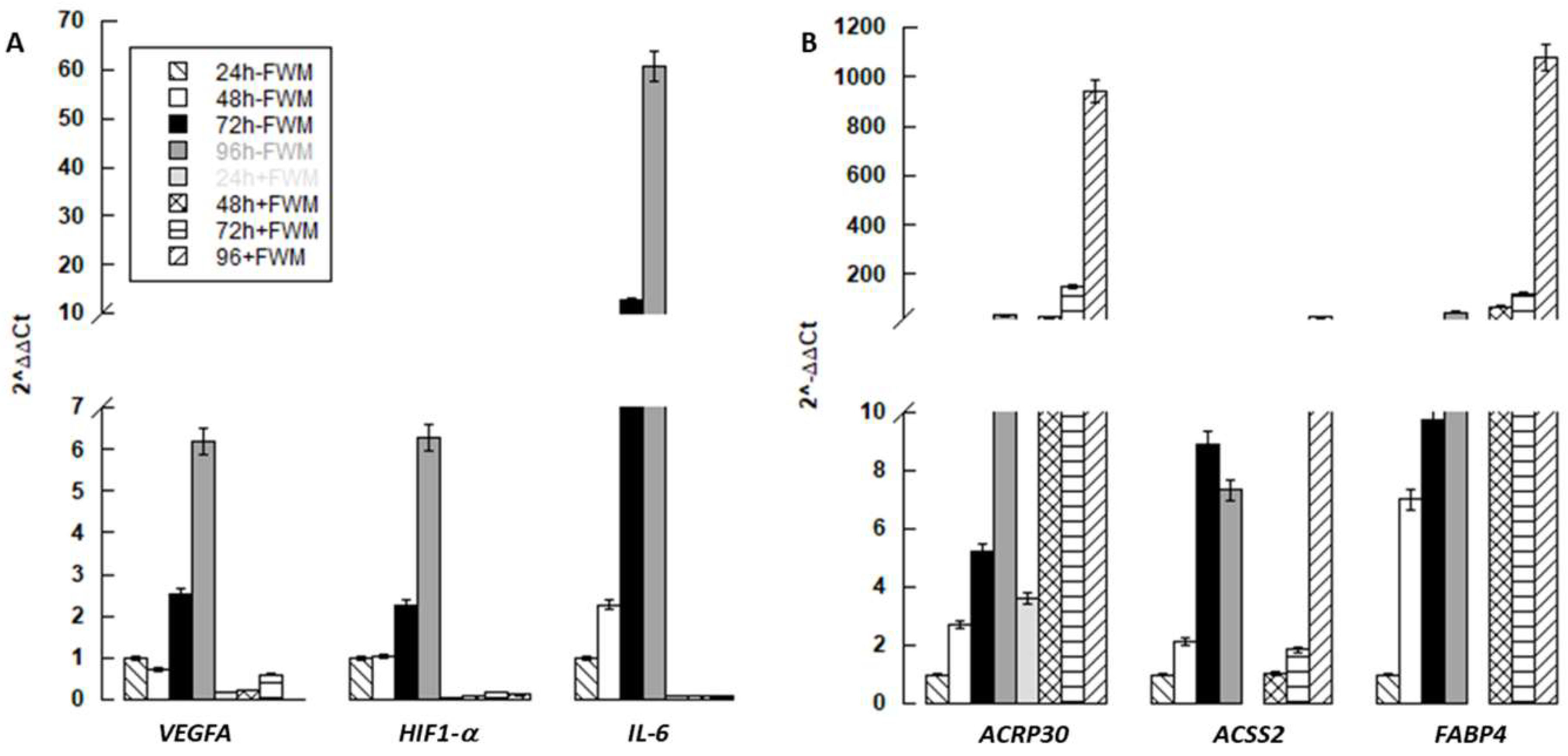
3.3. Gene Expression
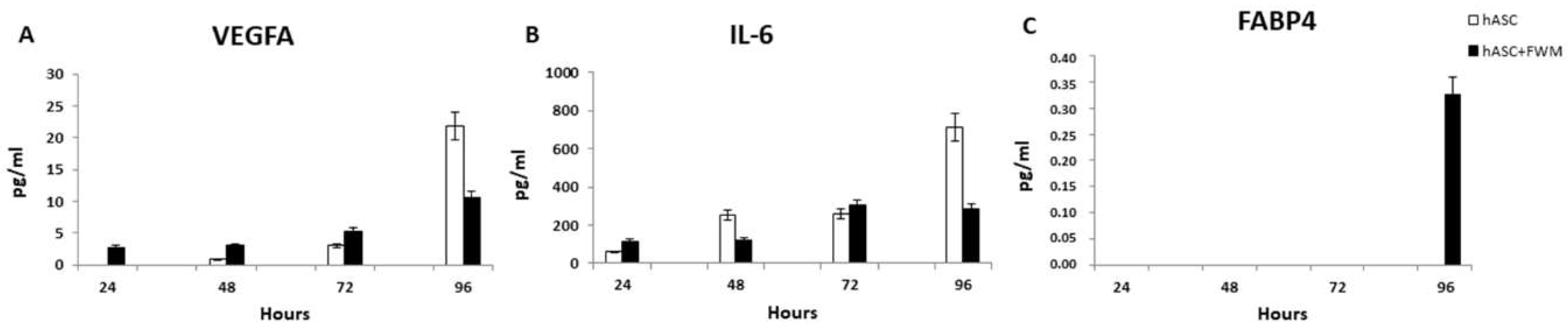
3.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Schwartz, M.A.; DeSimone, D.W. Cell adhesion receptors in mechanotransduction. Curr. Opin. Cell Biol. 2008, 20, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.K.K.; Wong, S.T.; Lim, C.K.; Kung, T.Y.S.; Yap, C.H.; Ramagopal, Y.; Romer, L.H.; Yim, E.K.F. Nanotopography Modulates Mechanotransduction of Stem Cells and Induces Differentiation through Focal Adhesion Kinase. ACS Nano 2013, 7, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, A.M.; Di Pompeo, F.S.; De Mezerville, R. Nanotechnology, nanosurfaces and silicone gel breast implants: Current aspects. Case Rep. Plast. Surg. Hand Surg. 2017, 4, 99–113. [Google Scholar] [CrossRef]
- Cruz-Acuña, R.; García, A.J. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Boil. 2016, 57, 324–333. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.S.; Fu, J. Forcing stem cells to behave: A biophysical perspective of the cellular microenvironment. Annu. Rev. Biophys. 2012, 41, 519–542. [Google Scholar] [CrossRef]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef]
- Warburton, D.; Perin, L.; Defilippo, R.; Bellusci, S.; Shi, W.; Driscoll, B. Stem/Progenitor Cells in Lung Development, Injury Repair, and Regeneration. Proc. Am. Thorac. Soc. 2008, 5, 703–706. [Google Scholar] [CrossRef]
- Coraux, C.; Roux, J.; Jolly, T.; Birembaut, P. Epithelial Cell-Extracellular Matrix Interactions and Stem Cells in Airway Epithelial Regeneration. Proc. Am. Thorac. Soc. 2008, 5, 689–694. [Google Scholar] [CrossRef]
- Wang, N. Review of cellular mechanotransduction. J. Phys. D: Appl. Phys. 2017, 50, 233002. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, J.; Mei, X.; Chi, G.; Li, L.; Song, Y.; He, X.; Li, Y.-L. Regulatory effects of dermal papillary pluripotent stem cells on polarization of macrophages from M1 to M2 phenotype in vitro. Transpl. Immunol. 2018, 52, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.K.; Mishra, V.K.; Dubey, R.; Deng, Y.-H.; Tsai, F.-C.; Deng, W.-P. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 2200. [Google Scholar] [CrossRef] [PubMed]
- Niada, S.; Giannasi, C.; Gualerzi, A.; Banfi, G.; Brini, A.T. Differential Proteomic Analysis Predicts Appropriate Applications for the Secretome of Adipose-Derived Mesenchymal Stem/Stromal Cells and Dermal Fibroblasts. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D. Vascularization Strategies for Tissue Engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef]
- Cherubino, M.; Valdatta, L.; Balzaretti, R.; Pellegatta, I.; Rossi, F.; Protasoni, M.; Tedeschi, A.; Accolla, R.S.; Bernardini, G.; Gornati, R. Human adipose-derived stem cells promote vascularization of collagen-based scaffolds transplanted into nude mice. Regen. Med. 2016, 11, 261–271. [Google Scholar] [CrossRef]
- Borgese, M.; Rossi, F.; Bonfanti, P.; Colombo, A.; Mantecca, P.; Valdatta, L.; Bernardini, G.; Gornati, R. Recovery ability of human adipose stem cells exposed to cobalt nanoparticles: Outcome of dissolution. Nanomedecine 2020, 15, 453–465. [Google Scholar] [CrossRef]
- Palombella, S.; Pirrone, C.; Cherubino, M.; Valdatta, L.; Bernardini, G.; Gornati, R. Identification of reference genes for qPCR analysis during hASC long culture maintenance. PLoS ONE 2017, 12, e0170918. [Google Scholar] [CrossRef]
- Pirrone, C.; Rossi, F.; Cappello, S.; Borgese, M.; Mancini, G.; Bernardini, G.; Gornati, R. Evaluation of biomarkers in Mytilus galloprovincialis as an integrated measure of biofilm-membrane bioreactor (BF-MBR) system efficiency in mitigating the impact of oily wastewater discharge to marine environment: A microcosm approach. Aquat. Toxicol. 2018, 198, 49–62. [Google Scholar] [CrossRef]
- Bava, A.; Cappellini, F.; Pedretti, E.; Rossi, F.; Caruso, E.; Vismara, E.; Chiriva-Internati, M.; Bernardini, G.; Gornati, R. Heparin and Carboxymethylchitosan Metal Nanoparticles: An Evaluation of Their Cytotoxicity. BioMed Res. Int. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Rossi, F.; Bernardini, G.; Bonfanti, P.; Colombo, A.; Prati, M.; Gornati, R. Effects of TCDD on Spermatogenesis Related Factor-2 (SRF-2): Gene expression in Xenopus. Toxicol. Lett. 2009, 191, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and Mechanical Properties Significantly Influence Cell Attachment, Proliferation, and Migration within Collagen Glycosaminoglycan Scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef]
- Traore, M.A.; George, S.C. Tissue Engineering the Vascular Tree. Tissue Eng. Part B Rev. 2017, 23, 505–514. [Google Scholar] [CrossRef]
- Li, Y.Y.; Choy, T.H.; Ho, F.C.; Chan, B.P. Scaffold composition affects cytoskeleton organization, cell-matrix interaction and the cellular fate of human mesenchymal stem cells upon chondrogenic differentiation. Biomaterials 2015, 52, 208–220. [Google Scholar] [CrossRef]
- Vandrovcová, M.; Bačáková, L. Adhesion, Growth and Differentiation of Osteoblasts on Surface-Modified Materials Developed for Bone Implants. Physiol. Res. 2011, 60, 403–417. [Google Scholar] [CrossRef]
- Krishna, L.; Dhamodaran, K.; Jayadev, C.; Chatterjee, K.; Shetty, R.; Khora, S.S.; Das, D. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res. Ther. 2016, 7, 188. [Google Scholar] [CrossRef]
- Kundu, A.K.; Khatiwala, C.B.; Putnam, A.J. Extracellular Matrix Remodeling, Integrin Expression, and Downstream Signaling Pathways Influence the Osteogenic Differentiation of Mesenchymal Stem Cells on Poly(Lactide-Co-Glycolide) Substrates. Tissue Eng. Part A 2009, 15, 273–283. [Google Scholar] [CrossRef]
- Crespi, R.; Capparé, P.; Gherlone, E. Dental Implants Placed in Extraction Sites Grafted With Different Bone Substitutes: Radiographic Evaluation at 24 Months. J. Periodontol. 2009, 80, 1616–1621. [Google Scholar] [CrossRef]
- Crespi, R.; Capparé, P.; Gherlone, E. Osteotome Sinus Floor Elevation and Simultaneous Implant Placement in Grafted Biomaterial Sockets: 3 Years of Follow-Up. J. Periodontol. 2010, 81, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Tetè, G.; Capparé, P.; Gherlone, E.F. New Application of Osteogenic Differentiation from HiPS Stem Cells for Evaluating the Osteogenic Potential of Nanomaterials in Dentistry. Int. J. Environ. Res. Public Heal. 2020, 17, 1947. [Google Scholar] [CrossRef]
- Crespi, R.; Mariani, E.; Benasciutti, E.; Capparé, P.; Cenci, S.; Gherlone, E. Magnesium-Enriched Hydroxyapatite versus Autologous Bone in Maxillary Sinus Grafting: Combining Histomorphometry with Osteoblast Gene Expression Profiles Ex Vivo. J. Periodontol. 2009, 80, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, H.; Liu, Y.; Liu, L.; Zhang, W.; Luo, E.; Luo, E. Effect of adiponectin secreted from adipose-derived stem cells on bone-fat balance and bone defect healing. J. Tissue Eng. Regen. Med. 2019, 13, 2055–2066. [Google Scholar] [CrossRef]
- Dimascio, L.; Voermans, C.; Uqoezwa, M.; Duncan, A.; Lu, D.; Wu, J.; Sankar, U.; Reya, T. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J. Immunol. 2007, 178, 3511–3520. [Google Scholar] [CrossRef]
- Chen, T.; Wu, Y.-W.; Lu, H.; Guo, Y.; Tang, Z.-H. Adiponectin enhances osteogenic differentiation in human adipose-derived stem cells by activating the APPL1-AMPK signaling pathway. Biochem. Biophys. Res. Commun. 2015, 461, 237–242. [Google Scholar] [CrossRef]
- Berner, H.S.; Lyngstadaas, S.P.; Spahr, A.; Monjo, M.; Thommesen, L.; Drevon, C.A.; Syversen, U.; Reseland, J.E. Adiponectin and its receptors are expressed in bone-forming cells. Bone 2004, 35, 842–849. [Google Scholar] [CrossRef]
- Kanazawa, I.; Yamaguchi, T.; Yano, S.; Yamauchi, M.; Yamamoto, M.; Sugimoto, T. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Boil. 2007, 8, 51. [Google Scholar] [CrossRef]
- Karan, D.; David, J.R.; Capy, P. Molecular evolution of the AMP-forming Acetyl-CoA synthetase. Gene 2001, 265, 95–101. [Google Scholar] [CrossRef]
- Starai, V.; Escalante-Semerena, J.C. Acetyl-coenzyme A synthetase (AMP forming). Cell. Mol. Life Sci. 2004, 61, 2020–2030. [Google Scholar] [CrossRef]
- Gao, X.; Lin, S.-H.; Ren, F.; Li, J.-T.; Chen, J.-J.; Yao, C.-B.; Yang, H.-B.; Jiang, S.-X.; Yan, G.-Q.; Wang, D.; et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun. 2016, 7, 11960. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Furukawa, T.; Yoshii, H.; Mori, T.; Kiyono, Y.; Waki, A.; Kobayashi, M.; Tsujikawa, T.; Kudo, T.; Okazawa, H.; et al. Cytosolic acetyl-CoA synthetase affected tumor cell survival under hypoxia: The possible function in tumor acetyl-CoA/acetate metabolism. Cancer Sci. 2009, 100, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, S.Y.; Park, S.Y.; Kim, Y.M.; Lee, M.H.; Ryu, H.M.; Kim, J.M. Mesenchymal progenitor cells in the human umbilical cord. Ann. Hematol. 2004, 83, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Liu, W.; Kuang, S. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. FASEB J. 2012, 27, 277–287. [Google Scholar] [CrossRef]
- Yamamoto, T.; Furuhashi, M.; Sugaya, T.; Oikawa, T.; Matsumoto, M.; Funahashi, Y.; Matsukawa, Y.; Gotoh, M.; Miura, T. Transcriptome and Metabolome Analyses in Exogenous FABP4- and FABP5-Treated Adipose-Derived Stem Cells. PLoS ONE 2016, 11, e0167825. [Google Scholar] [CrossRef]
- De Luca, A.; Lamura, L.; Gallo, M.; Maffia, V.; Normanno, N. Mesenchymal stem cell-derived interleukin-6 and vascular endothelial growth factor promote breast cancer cell migration. J. Cell. Biochem. 2012, 113, 3363–3370. [Google Scholar] [CrossRef]
- Soleymaninejadian, E.; Pramanik, K.; Samadian, E. Immunomodulatory Properties of Mesenchymal Stem Cells: Cytokines and Factors. Am. J. Reprod. Immunol. 2011, 67, 1–8. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Salgado, A.J. Mesenchymal stem cells secretome: Current trends and future challenges. Neural Regen. Res. 2019, 15, 75–77. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]

| Gene Name | Primer Sequence 5′-3′ | Melting Temperature (°C) | Accession Number |
|---|---|---|---|
| Hs GAPDH | FW CCCTTCATTGACCTCAACTAC | 61.5 | M17851.1 |
| Rev CATTGATCACAAGCTTCCCG | 61.6 | ||
| Hs β2m | FW TTCTGGCCTGGAGGCTATC | 60.0 | AB021288.1 |
| Rev TCAGGAAATTTGACTTTCCATTC | 59.0 | ||
| Hs VEGFA | FW GGAGTCCAACATCACCAT | 60.5 | AY047581.1 |
| Rev GCTGTAGGAAGCTCATCT | 59.9 | ||
| Hs IL-6 | FW ACTCACCTCTTCAGAACG | 60.0 | M14584.1 |
| Rev CCTCTTTGCTGCTTTCAC | 60.4 | ||
| Hs HIF1α | FW CAAGTCCTCAAAGGACAG | 59.7 | AF304431.1 |
| Rev TGGTAGTGGTGGCATTAG | 60.1 | ||
| Hs FABP4 | FW AAGTCAAGAGCACCATAACCT | 63.3 | NM_001442.2 |
| Rev GCATTCCACCACCAGTTTATC | 63.4 | ||
| Hs ACRP30 | FW GGAAGGAGAGCGTAATGGA | 62.7 | NM_004797.3 |
| Rev AGTTGGTGTCATGGTAGAGAA | 62.7 | ||
| Hs ACSS2 | FW ATACAAGGTGACCAAGTTCTACA | 63.3 | NM_018677.3 |
| Rev GTGACAGGCTCATCTCCAA | 63.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgese, M.; Barone, L.; Rossi, F.; Raspanti, M.; Papait, R.; Valdatta, L.; Bernardini, G.; Gornati, R. Effect of Nanostructured Scaffold on Human Adipose-Derived Stem Cells: Outcome of In Vitro Experiments. Nanomaterials 2020, 10, 1822. https://doi.org/10.3390/nano10091822
Borgese M, Barone L, Rossi F, Raspanti M, Papait R, Valdatta L, Bernardini G, Gornati R. Effect of Nanostructured Scaffold on Human Adipose-Derived Stem Cells: Outcome of In Vitro Experiments. Nanomaterials. 2020; 10(9):1822. https://doi.org/10.3390/nano10091822
Chicago/Turabian StyleBorgese, Marina, Ludovica Barone, Federica Rossi, Mario Raspanti, Roberto Papait, Luigi Valdatta, Giovanni Bernardini, and Rosalba Gornati. 2020. "Effect of Nanostructured Scaffold on Human Adipose-Derived Stem Cells: Outcome of In Vitro Experiments" Nanomaterials 10, no. 9: 1822. https://doi.org/10.3390/nano10091822
APA StyleBorgese, M., Barone, L., Rossi, F., Raspanti, M., Papait, R., Valdatta, L., Bernardini, G., & Gornati, R. (2020). Effect of Nanostructured Scaffold on Human Adipose-Derived Stem Cells: Outcome of In Vitro Experiments. Nanomaterials, 10(9), 1822. https://doi.org/10.3390/nano10091822






