Carbon Quantum Dots Modified (002) Oriented Bi2O2CO3 Composites with Enhanced Photocatalytic Removal of Toluene in Air
Abstract
1. Introduction
2. Materials and Methods
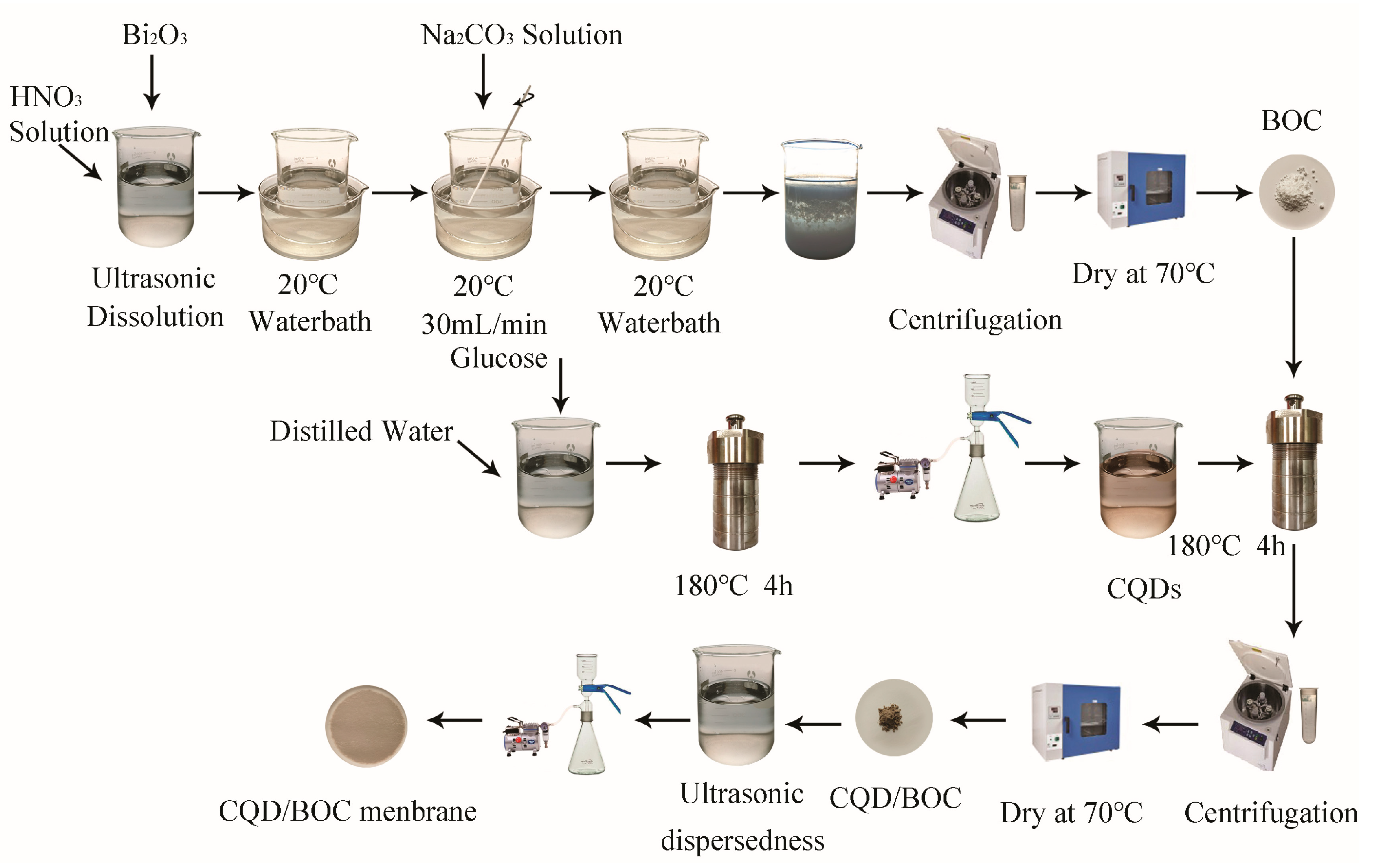
2.1. Fabrication of CQD/BOC Membranes
2.1.1. Preparation of Bi2O2CO3 Composites
2.1.2. Preparation of CQDs
2.1.3. Preparation of CQDs/BOC Composites
2.1.4. Fabrication of CQD/BOC Membranes
2.2. Characterization
2.3. Photocatalytictest
3. Results and Discussion
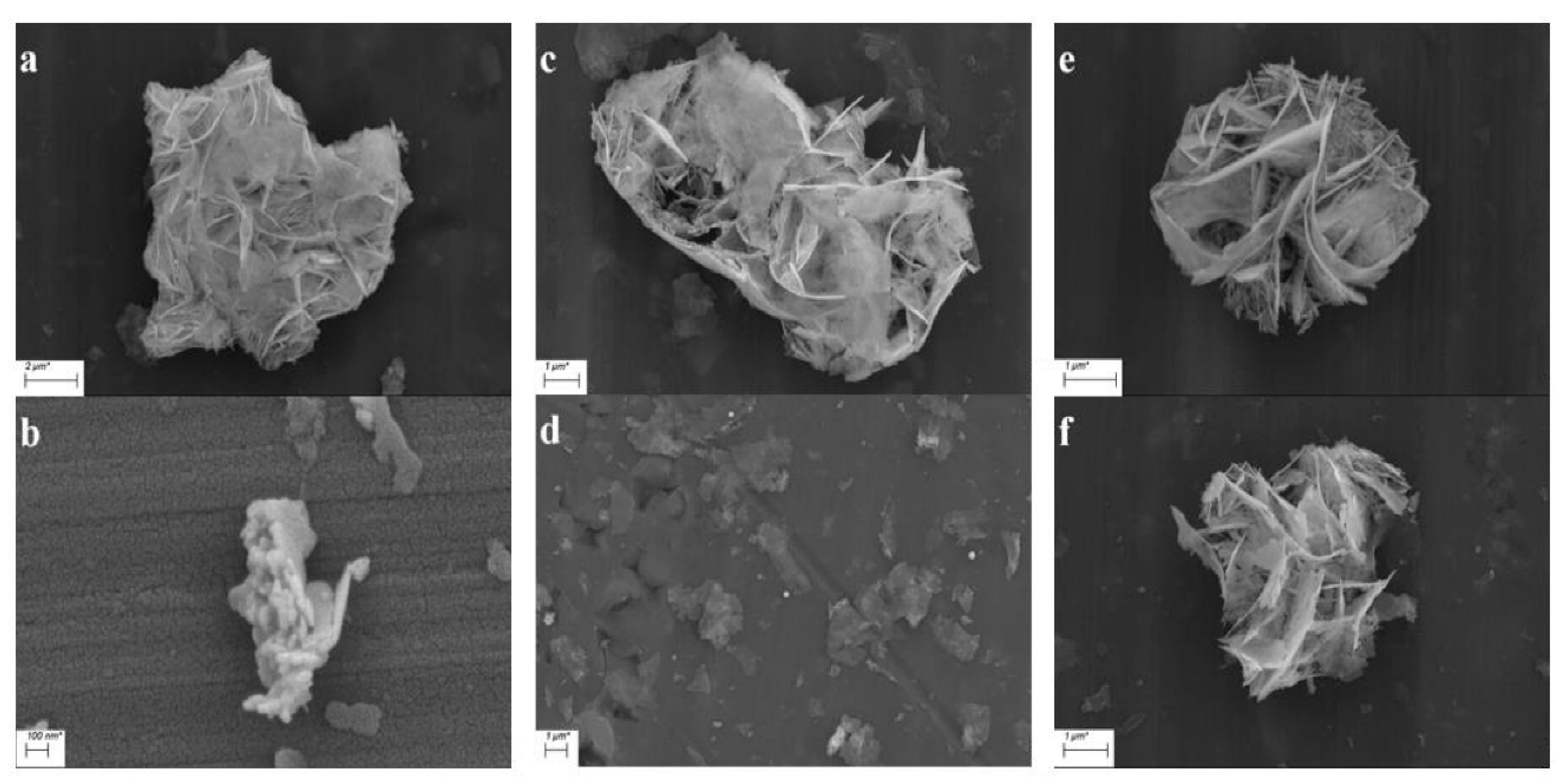
3.1. Morphology Analysis
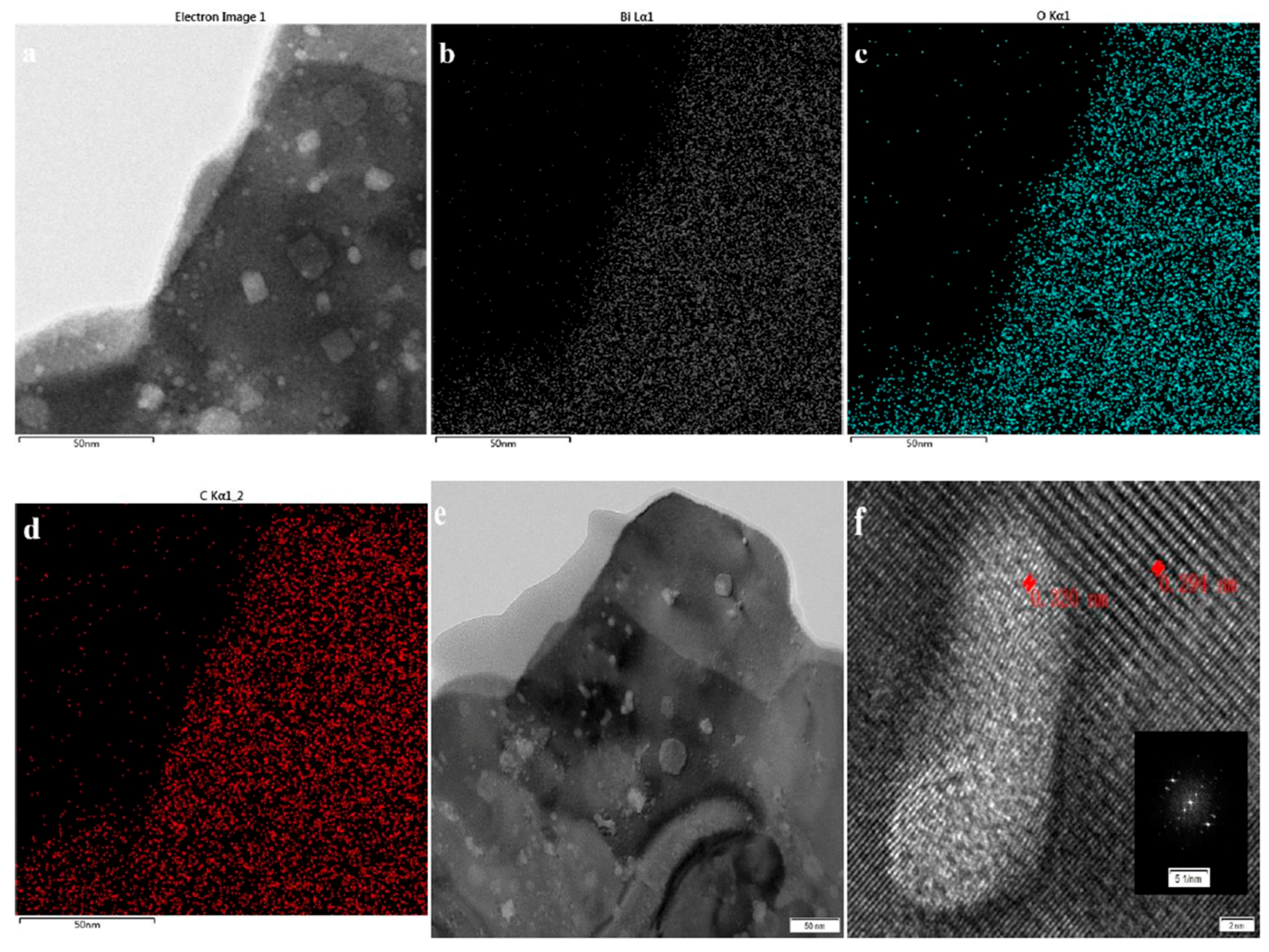
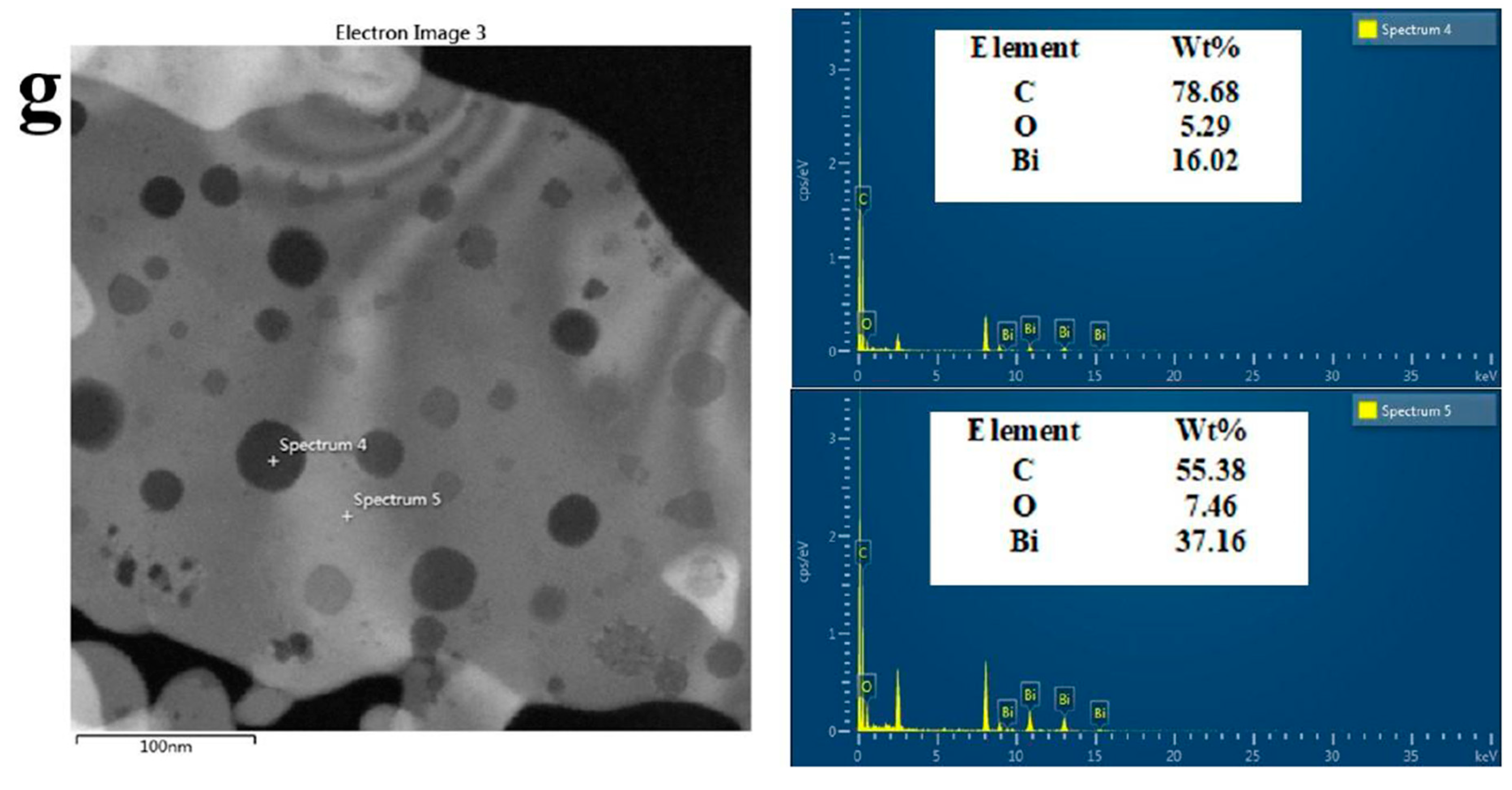
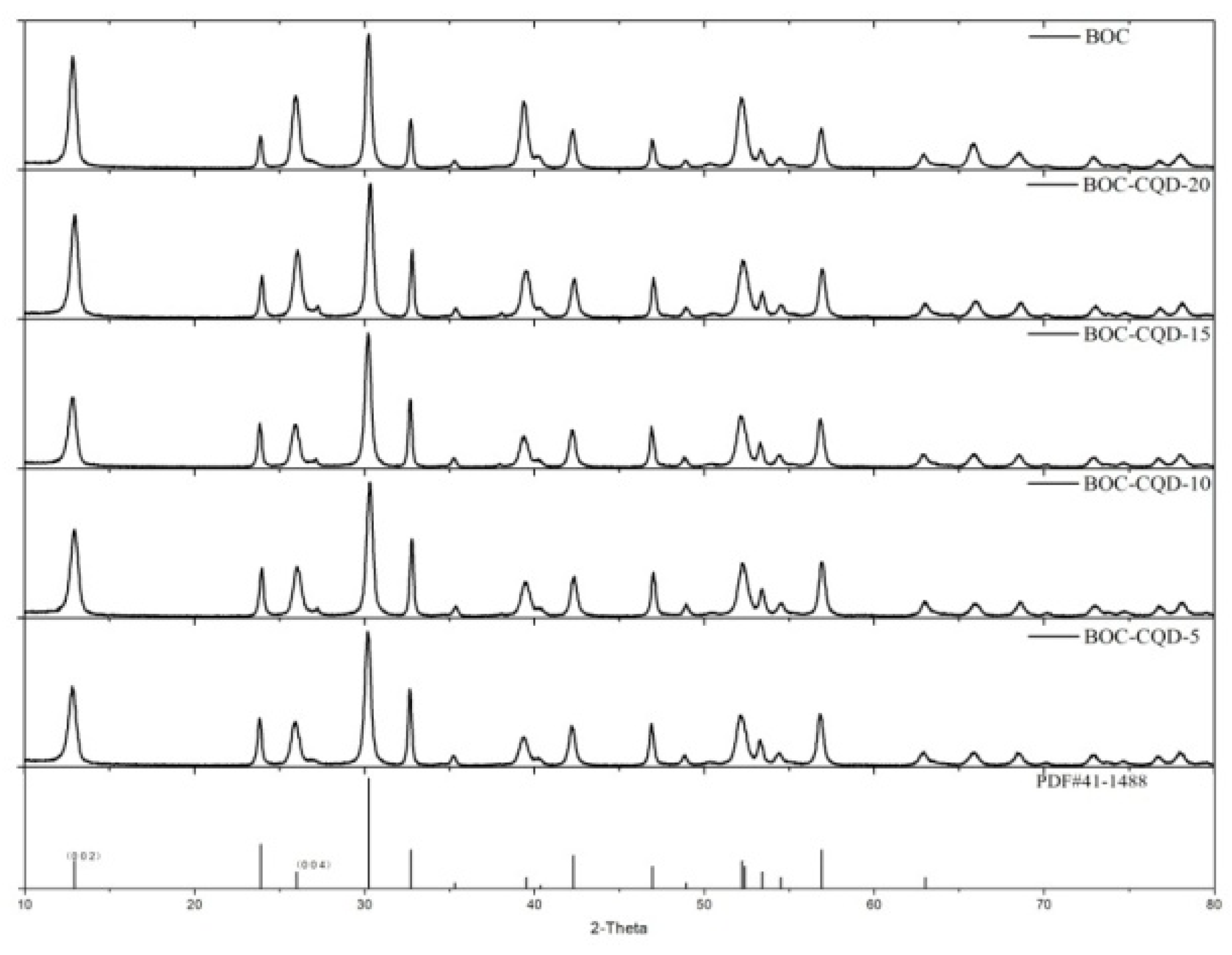
3.2. Structure and Composition Analysis
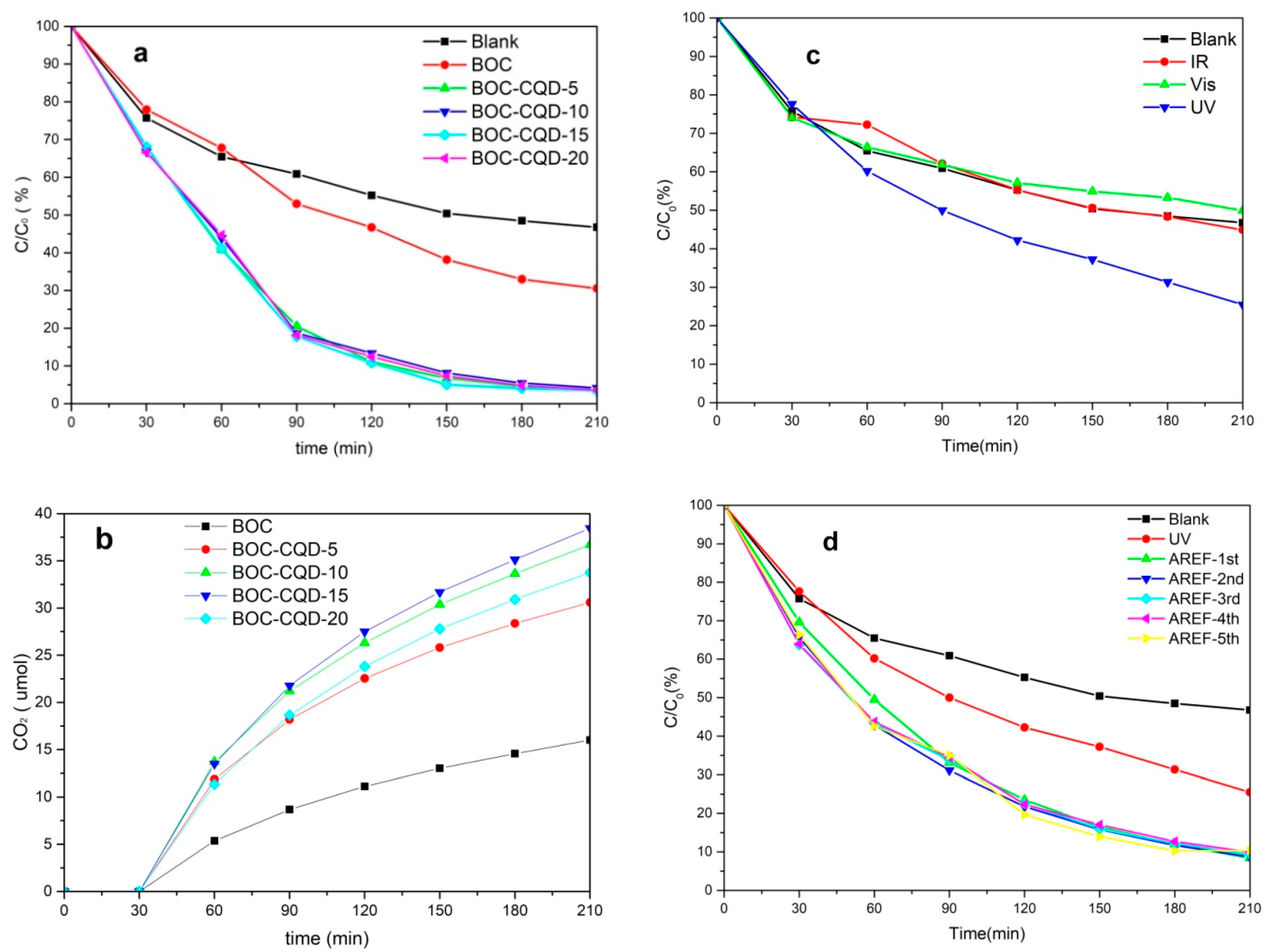
3.3. Photocatalytic Properties
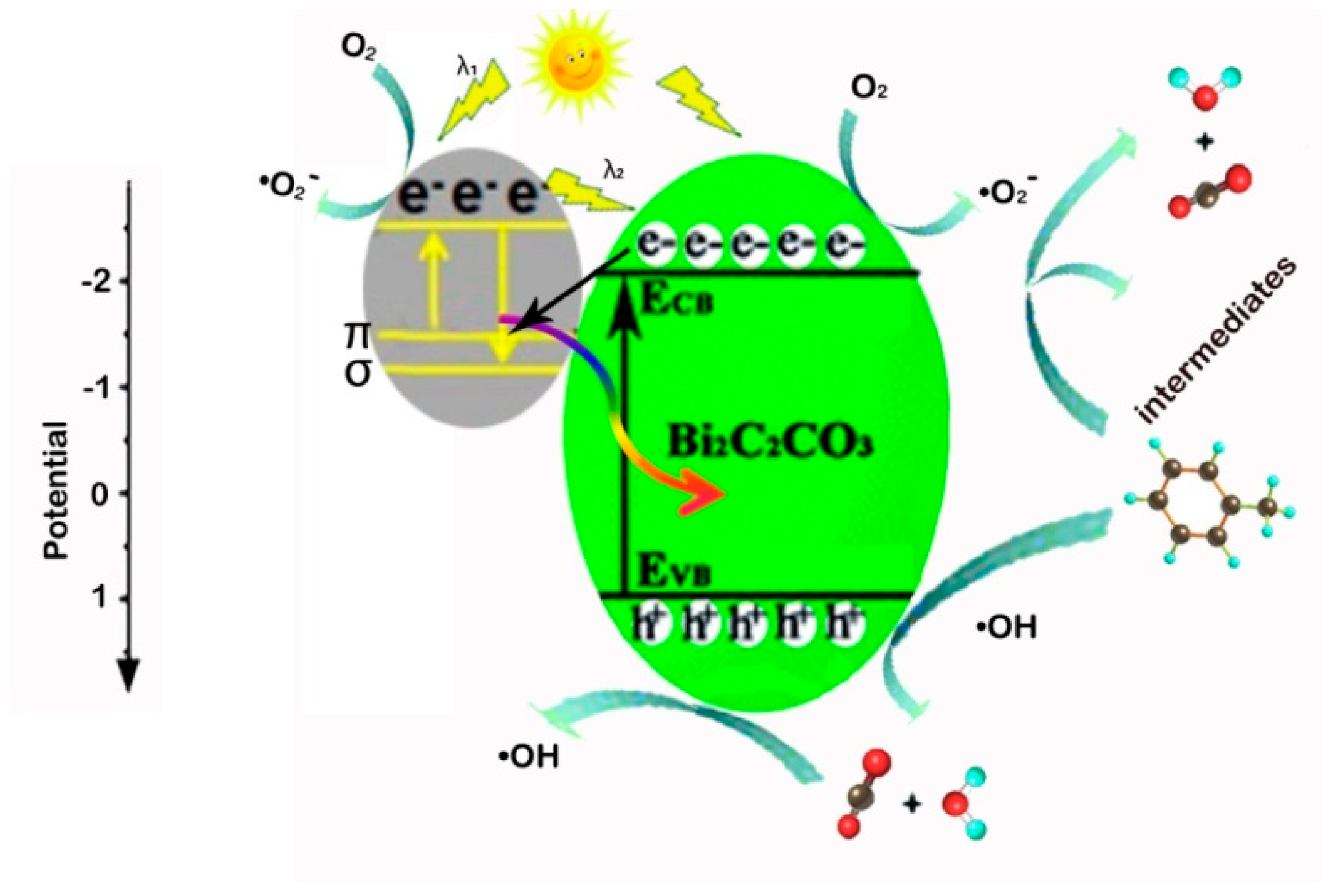
3.4. Photocatalytic Degradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brown, S.; Sim, M.R.; Abramson, M.J.; Gray, C.N. Concentrations of volatile organic compounds in indoor air—A review. Indoor Air 1994, 4, 123–134. [Google Scholar] [CrossRef]
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Wolkoff, P. Trends in Europe to reduce the indoor air pollution of VOCs. Indoor Air 2003, 13, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, F.; Lee, C.-S.; Pant, B.; Bolourani, G.; Lakdawala, N.; Bastani, A. Evaluation of various activated carbons for air cleaning—Towards design of immune and sustainable buildings. Atmos. Environ. 2008, 42, 8176–8184. [Google Scholar] [CrossRef]
- Siegel, J. Primary and secondary consequences of indoor air cleaners. Indoor Air 2016, 26, 88–96. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef]
- Hamza, M.A.; El-Shazly, A.N.; Tolba, S.A.; Allam, N.K. Novel Bi-based photocatalysts with unprecedented visible light-driven hydrogen production rate: Experimental and DFT insights. Chem. Eng. J. 2019, 384, 123351. [Google Scholar] [CrossRef]
- Li, M.; Huang, H.; Yu, S.; Tian, N.; Zhang, Y. Facet, junction and electric field engineering of bismuth-based materials for photocatalysis. ChemCatChem 2018, 10, 4477–4496. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Zhao, X.; Xiao, X.; Sun, F.; Zuo, X.; Nan, J. Small-molecule surface modified bismuth-based semiconductors as a new class of visible-light-driven photocatalytic materials: Structure-dependent photocatalytic properties and photosensitization mechanism. Chem. Eng. J. 2020, 380, 122546. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Li, G.; Zhang, L. Conversion of a Bi nanowire array to an array of Bi-Bi2O3 core-shell nanowires and Bi2O3 nanotubes. Small 2006, 2, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Shi, Q.; Zhang, M.; Xie, L.; Chen, J.; Yang, X.; Chen, M.; Zhao, W. Enhanced photocatalytic oxidation of toluene with a coral-like direct Z-scheme BiVO4/g-C3N4 photocatalyst. J. Alloy. Compd. 2017, 714, 619–626. [Google Scholar] [CrossRef]
- Wang, Y.; Jianga, S.; Liu, F.; Zhao, C.; Zhao, D.; Li, X. Study on preparation and toluene removal of BiOI/Bi2WO6/ACF photocatalyst. Appl. Surf. Sci. 2019, 488, 161–169. [Google Scholar] [CrossRef]
- Fan, W.; Li, H.; Zhao, F.; Xiao, X.; Huang, Y.; Ji, H.; Tong, Y. Boosting photocatalytic performance of (001) BiOI: Enhance donor density and separation efficiency of the photogenerated electrons and holes. Chem. Commun. 2016, 52, 5316–5319. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, P.; Yuan, X.; Zhang, Y.; Huang, H.; Wang, L.; Dong, F. Pivotal roles of artificial oxygen vacancies in enhancing photocatalytic activity and selectivity on Bi2O2CO3 nanosheets. Chin. J. Catal. 2019, 40, 620–630. [Google Scholar] [CrossRef]
- Chen, L.; Huang, R.; Yin, S.-F.; Luo, S.-L.; Au, C.-T. Flower-like Bi2O2CO3: Facile synthesis and their photocatalytic application in treatment of dye-containing wastewater. Chem. Eng. J. 2012, 123, 193–194. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Yang, K.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. Facile template-free synthesis of Bi2O2CO3 hierarchical microflowers and their associated photocatalytic activity. Chemphyschem 2010, 11, 2167–2173. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Z.; Zhang, K.; Dong, F.; Zhou, Y. Topochemical transformation of low energy crystal facets to high energy facets: A case from Bi2O2CO3 {001} facets to β-Bi2O3 {001} facets with improved photocatalytic oxidation of NO. Cryst. Eng. Comm. 2015, 17, 6098–6102. [Google Scholar] [CrossRef]
- Hao, W.; Teng, F.; Gu, W.; Liu, Z.; Zhang, A.; Liu, Z. Investigation on the effect of an anion layer on photocatalytic activity: Carbonate vs. oxalate. New J. Chem. 2017, 41, 7073–7080. [Google Scholar] [CrossRef]
- Madhusudan, P.; Ran, J.; Zhang, J.; Yu, J.; Liu, G. Novel urea assisted hydrothermal synthesis of hierarchical BiVO4/Bi2O2CO3 nanocomposites with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2011, 110, 286–295. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Zai, J.T.; Miao, X.; Zou, Q.; Su, Y.Z.; Wang, K.X.; Qian, X.F. Hierarchical Bi2O2CO3 Microspheres with Improved Visible-Light-Driven Photocatalytic Activity. Cryst. Eng. Comm. 2011, 13, 4010–4017. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, J.; Shen, X.; Wang, J.; Wang, B.; Gao, D. Br-doped Bi2O2CO3 nanosheets with improved electronic structure and accelerated charge migration for outstanding photocatalytic behavior. Appl. Surf. Sci. 2019, 470, 63–73. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhou, Y.; Liu, S.; Liang, Y.; Luo, T.; Dai, G. Enhanced visible-light photocatalytic activity of Bi2O2CO3 nanoplates by Fe-doping in the degradation of rhodamine B. Mater. Res. Bull. 2018, 107, 438–445. [Google Scholar] [CrossRef]
- Lan, Y.; Li, Z.; Xie, W.; Li, D.; Yan, G.; Guo, S.; Pan, C.; Wu, J. In situ fabrication of I-doped Bi2O2CO3/g-C3N4 heterojunctions for enhanced photodegradation activity under visible light. J. Hazard. Mater. 2020, 385, 121622. [Google Scholar] [CrossRef]
- Huang, H.; Wang, J.; Dong, F.; Guo, Y.; Tian, N.; Zhang, Y.; Zhang, T. Highly Efficient Bi2O2CO3 Single-Crystal Lamellas with Dominantly Exposed {001} Facets. Cryst. Growth Des. 2015, 15, 534–537. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Y.; Wang, F.; Zhang, K.; Yu, S.; Cao, K. Polyaniline-decorated {001} facets of Bi2O2CO3 nanosheets: In situ oxygen vacancy formation and enhanced visible light photocatalytic activity. ACS Appl. Mater. Interfaces 2015, 7, 730–737. [Google Scholar] [CrossRef]
- Yang, C.; Xue, Z.; Qin, J.; Sawangphruk, M.; Rajendran, S.; Zhang, X.; Liu, R. Visible Light-Driven Photocatalytic H-2 Generation and Mechanism Insights into Bi2O2CO3/G-C3N4 Z-Scheme Photocatalyst. J. Phys. Chem. C 2019, 123, 4795–4804. [Google Scholar] [CrossRef]
- Fan, H.; Zhou, H.; Li, H.; Liu, X.; Ren, C.; Wang, F.; Li, W. Novel Ag2CrO4/Bi2O2CO3 heterojunction: Simple preparation, wide visible light absorption band and excellent photocatalytic activity. Chem. Phys. 2019, 517, 60–66. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Li, Y.; Shang, X.; Jia, D.; Li, C. Direct Z-scheme Bi2O2CO3/porous g-C3N4 heterojunction for improved photocatalytic degradation performance. J. Taiwan Inst. Chem. Eng. 2020, 106, 74–85. [Google Scholar] [CrossRef]
- Tian, J.; Leng, Y.; Zhao, Z.; Xia, Y.; Sang, Y.; Hao, P.; Zhan, J.; Li, M.; Liu, H. Carbon quantum dots/hydrogenated TiO2 nanobelt heterostructures and their broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Nano Energy 2015, 11, 419–427. [Google Scholar] [CrossRef]
- Li, H.; Liu, R.; Liu, Y.; Huang, H.; Yu, H.; Ming, H.; Lian, S.; Lee, S.-T.; Kang, Z. Carbon quantum dots/Cu2O composites with protruding nanostructures and their highly efficient (near) infrared photocatalytic behavior. J. Mater. Chem. 2012, 22, 17470–17475. [Google Scholar] [CrossRef]
- Zhang, H.; Ming, H.; Lian, S.; Huang, H.; Li, H.; Zhang, L.; Liu, Y.; Kang, Z.; Lee, S.-T. Fe2O3/carbon quantum dots complex photocatalysts and their enhanced photocatalytic activity under visible light. Dalton Trans. 2011, 40, 10822–10825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, H.; Ming, H.; Li, H.; Zhang, L.; Liu, Y.; Kang, Z. Carbon quantum dots/Ag3PO4 complex photocatalysts with enhanced photocatalytic activity and stability under visible light. J. Mater. Chem. 2012, 22, 10501–10506. [Google Scholar] [CrossRef]
- Bai, P.; Tong, X.; Wan, J.; Gao, Y.; Xue, S. Flower-like Bi2O2CO3-mediated selective oxidative coupling processes of amines under visible light irradiation. J. Catal. 2019, 374, 257–265. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Xu, H.; Qiao, L.; Luo, Y.; Lin, Y.; Nan, C. Synthesis and broadband spectra photocatalytic properties of Bi2O2(CO3)1−xSx. Materials 2018, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, H.; Luo, Y.; Xu, Y.; Liu, J.; Lin, Y. (002) Oriented Bi2O2CO3 nanosheets with enhanced photocatalytic performance for toluene removal in air. Catalysts 2020, 10, 389. [Google Scholar] [CrossRef]
- Xian, T.; Sun, X.; Di, L.; Zhou, Y.; Ma, J.; Li, H.; Yang, H. Carbon Quantum Dots (CQDs) decorated Bi2O3-x hybrid photocatalysts with promising nir-light-driven photodegradation activity for AO7. Catalysts 2019, 9, 1031. [Google Scholar] [CrossRef]
- Liang, N.; Wang, M.; Jin, L.; Huang, S.; Chen, W.; Xu, M.; He, Q.; Zai, J.; Fang, N.; Qian, X. Highly efficient Ag2O/Bi2O2CO3 p-n heterojunction photocatalysts with improved visible-light responsive activity. ACS Appl. Mater. Interfaces 2014, 6, 11698–11705. [Google Scholar] [CrossRef]
- Wang, C.; Shao, C.; Liu, Y.; Zhang, L. Photocatalytic properties BiOCl and Bi2O3 nanofibers prepared by electrospinning. Scr. Mater. 2008, 59, 332–335. [Google Scholar] [CrossRef]
- Houc, W.; Hu, T.; Du, N.; Zhang, R.; Liu, J.-Q.; Hou, W. Wavelength-dependent differences in photocatalytic performance between BiOBr nanosheets with dominant exposed (001) and (010) facets. Appl. Catal. B Environ. 2016, 187, 342–349. [Google Scholar]
- Li, H.T.; Kang, Z.H.; Liu, Y.; Lee, S.T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, H.; Yi, Z.; Wang, X.X. Enhanced photocatalytic performance by hybridization of Bi2WO6 nanoparticles with honeycomb-like porous carbon skeleton. J. Environ. Manag. 2019, 248, 109341. [Google Scholar] [CrossRef] [PubMed]
- Hennezel, O.; Pichat, P.; Ollis, D.F. Benzene and toluene gas-phase photocatalytic degradation over H2O and HCL pretreated TiO2: By-products and mechanisms. J. Photochem. Photobiol. A Chem. 1998, 118, 197–204. [Google Scholar] [CrossRef]


| BOC | BOC-CQD-5 | BOC-CQD-10 | BOC-CQD-15 | BOC-CQD-20 | |
|---|---|---|---|---|---|
| Surface area (m2/g) | 12.293 | 17.189 | 18.842 | 17.699 | 17.266 |
| Total pore volume for pores with Diameter less than 194.68 nm at P/Po = 0.990027 (cm3/g) | 0.0952 | 0.1441 | 0.1254 | 0.1248 | 0.0934 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Wang, H.; Luo, Y.; Xu, Y.; Liu, J.; Lin, R.; Gao, Y.; Lin, Y. Carbon Quantum Dots Modified (002) Oriented Bi2O2CO3 Composites with Enhanced Photocatalytic Removal of Toluene in Air. Nanomaterials 2020, 10, 1795. https://doi.org/10.3390/nano10091795
Ding J, Wang H, Luo Y, Xu Y, Liu J, Lin R, Gao Y, Lin Y. Carbon Quantum Dots Modified (002) Oriented Bi2O2CO3 Composites with Enhanced Photocatalytic Removal of Toluene in Air. Nanomaterials. 2020; 10(9):1795. https://doi.org/10.3390/nano10091795
Chicago/Turabian StyleDing, Junping, Huanchun Wang, Yidong Luo, Yushuai Xu, Jinsheng Liu, Ruichu Lin, Yuchen Gao, and Yuanhua Lin. 2020. "Carbon Quantum Dots Modified (002) Oriented Bi2O2CO3 Composites with Enhanced Photocatalytic Removal of Toluene in Air" Nanomaterials 10, no. 9: 1795. https://doi.org/10.3390/nano10091795
APA StyleDing, J., Wang, H., Luo, Y., Xu, Y., Liu, J., Lin, R., Gao, Y., & Lin, Y. (2020). Carbon Quantum Dots Modified (002) Oriented Bi2O2CO3 Composites with Enhanced Photocatalytic Removal of Toluene in Air. Nanomaterials, 10(9), 1795. https://doi.org/10.3390/nano10091795






