Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review
Abstract
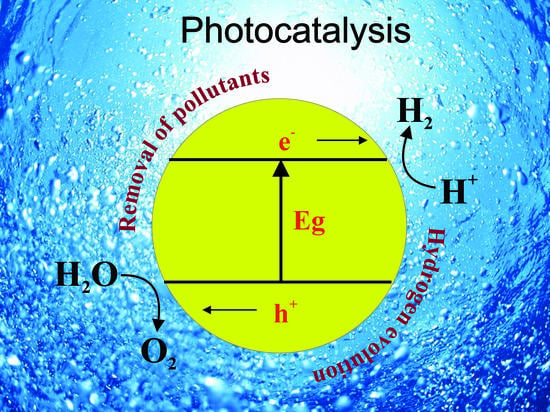
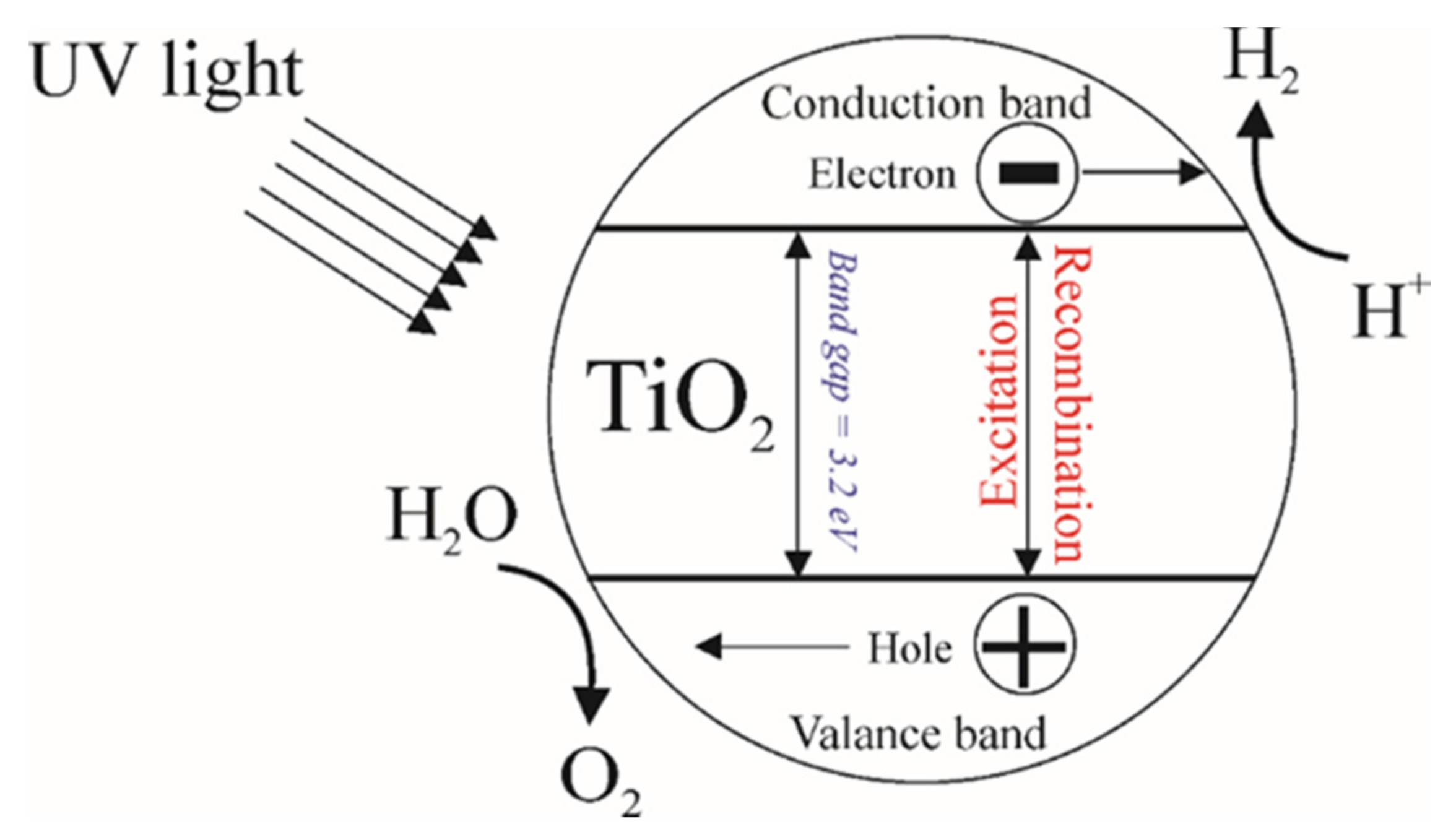
1. Introduction
2. Factors Affecting the Efficiency of Photocatalysis and Techniques Used to Improve the Efficiency of TiO2-Based Photocatalysts
2.1. Lifetime of Photogenerated Charge Carriers
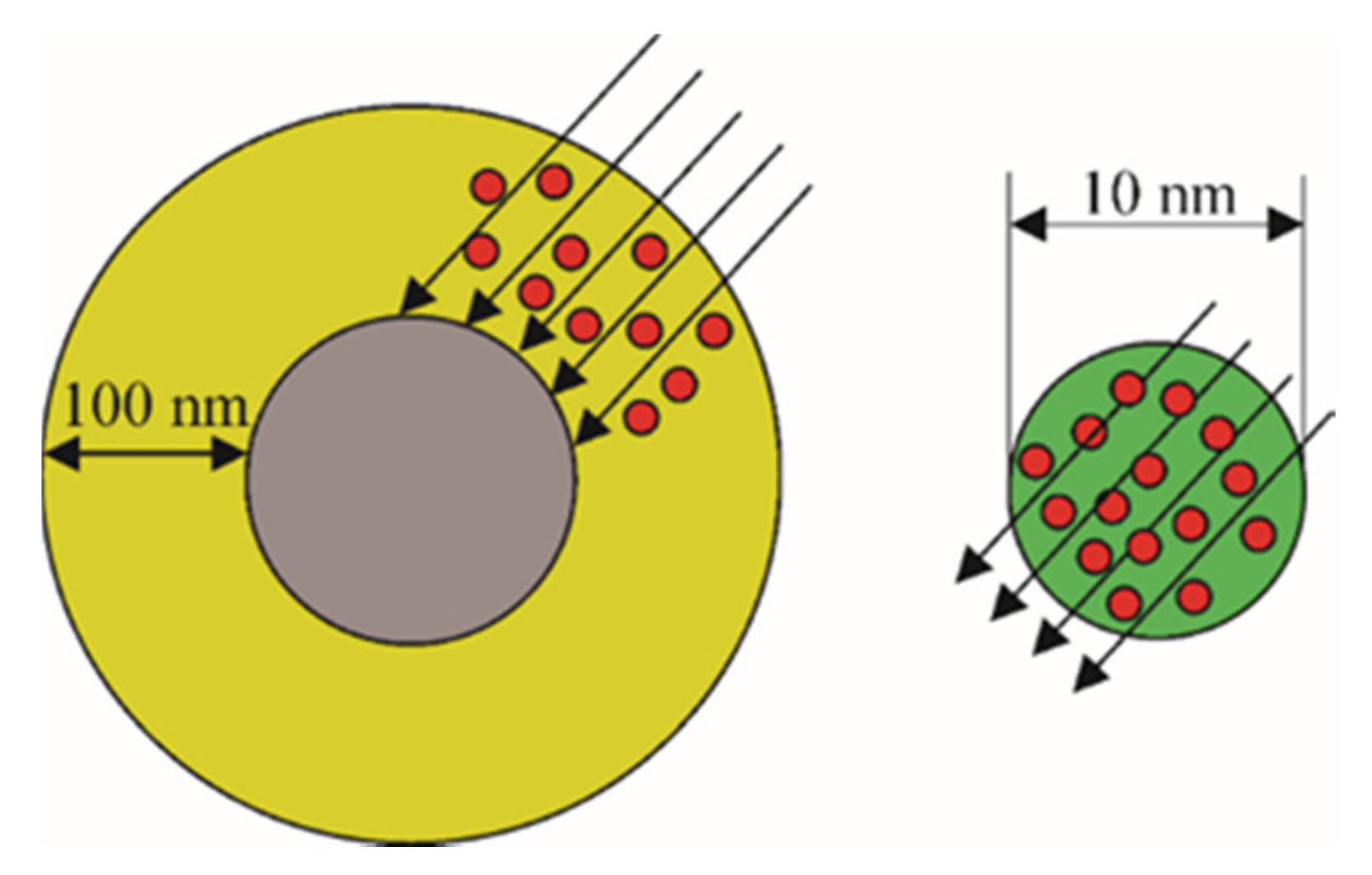
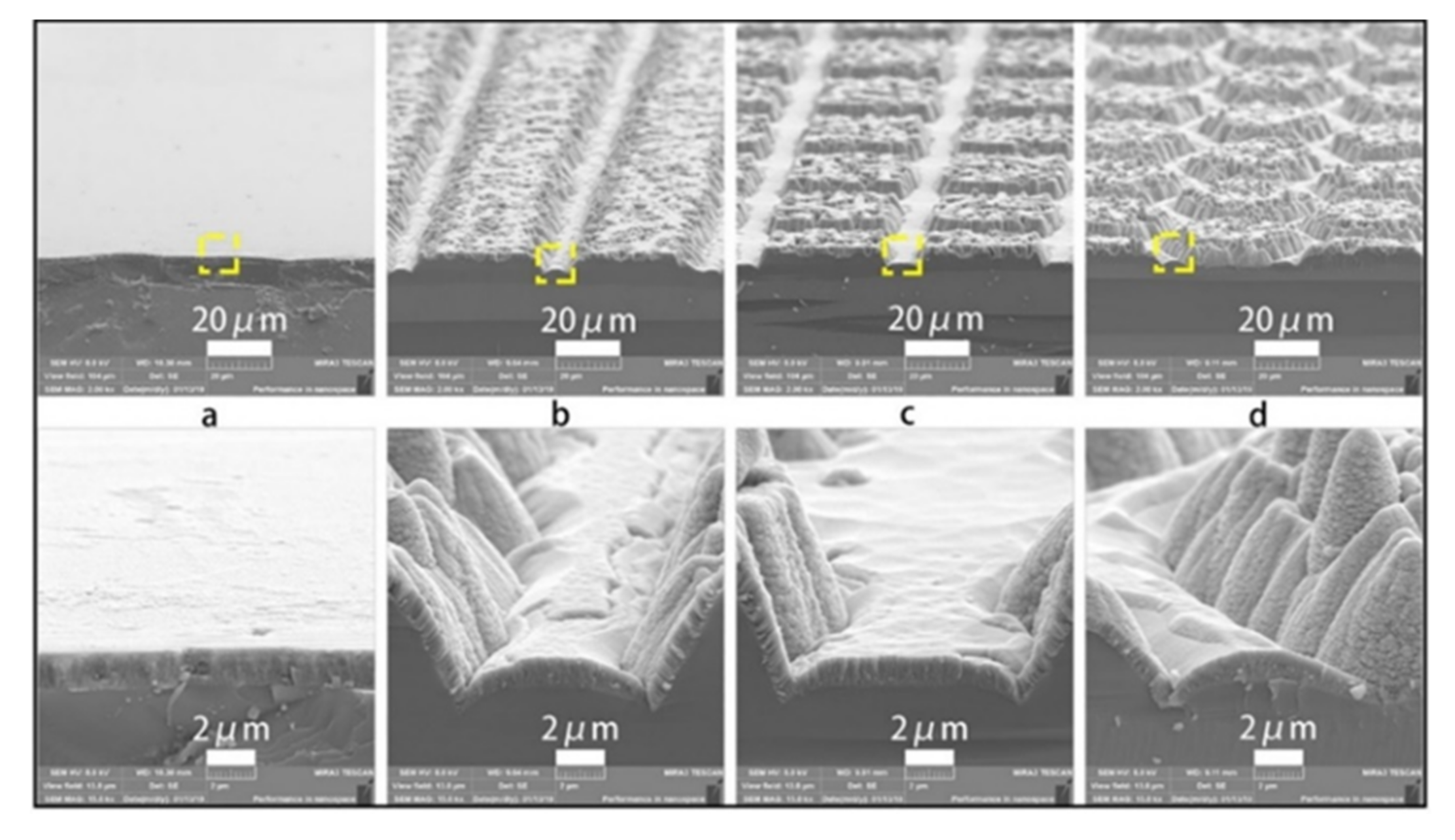
2.2. The Particle Size of Photocatalyst
2.3. Doping with Cations
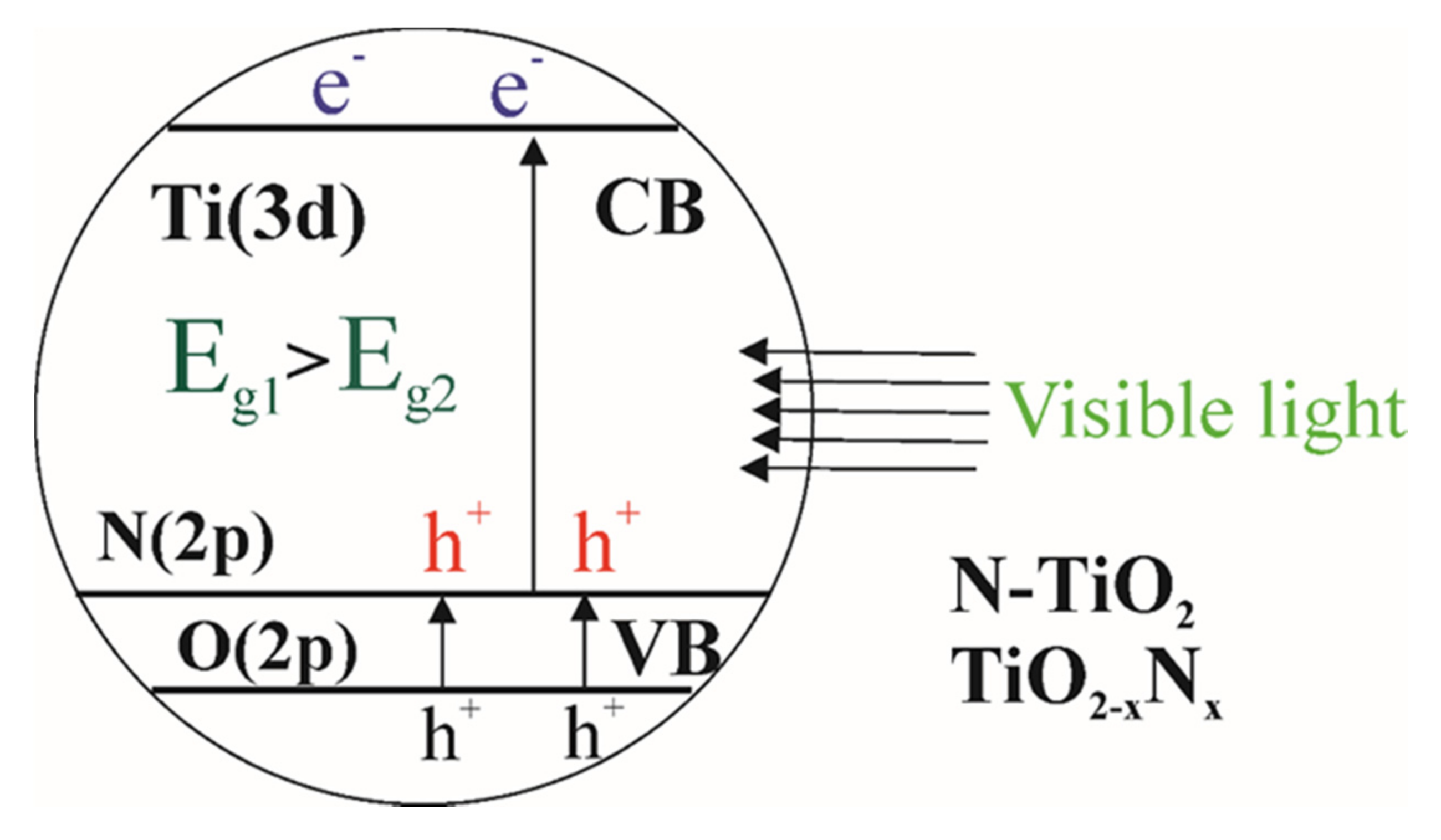
2.4. Doping with Anionic Elements
2.5. Doping/Loading with Metal Nanoparticles
3. The Utilization of Photocatalysts Based on TiO2
3.1. Hydrogen Evolution
3.2. Photodegradation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samokhvalov, A. Hydrogen by photocatalysis with nitrogen codoped titanium dioxide. Renew. Sustain. Energy Rev. 2017, 72, 981–1000. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Juan, J.C.; Show, P.-L.; Pan, G.-T. Recent developments of strontium titanate for photocatalytic water splitting application. Int. J. Hydrogen Energy 2019, 44, 14316–14340. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Li, S.; Li, X.; Ge, L.; Gao, Y.; Li, N. The roles and mechanism of cocatalysts in photocatalytic water splitting to produce hydrogen. Chin. J. Catal. 2020, 41, 642–671. [Google Scholar] [CrossRef]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.; Jiangyong, H. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Bingham, M.; Mills, A. Photonic efficiency and selectivity study of M (M = Pt, Pd, Au and Ag)/TiO2 photocatalysts for methanol reforming in the gas phase. J. Photochem. Photobiol. A Chem. 2020, 389, 112257. [Google Scholar] [CrossRef]
- Paz, Y. Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl. Catal. B Environ. 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Kou, J.; Lu, C.; Wang, J.; Chen, Y.; Xu, Z.; Varma, R.S. Selectivity Enhancement in Heterogeneous Photocatalytic Transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef]
- Parrino, F.; Bellardita, M.; García-López, E.I.; Marcì, G.; Loddo, V.; Palmisano, L. Heterogeneous Photocatalysis for Selective Formation of High-Value-Added Molecules: Some Chemical and Engineering Aspects. ACS Catal. 2018, 8, 11191–11225. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, L.; Su, R.; Hu, B.; Wang, Z.; Jin, Y.; Wang, Y.; Besenbacher, F.; Dong, M. Nanostructured heterogeneous photo-catalysts for hydrogen production and water splitting: A comprehensive insight. Appl. Mater. Today 2019, 17, 159–182. [Google Scholar] [CrossRef]
- Vajda, K.; Saszet, K.; Kedves, E.Z.; Kása, Z.; Danciu, V.; Baia, L.; Magyari, K.; Hernádi, K.; Kovács, G.; Pap, Z. Shape-controlled agglomeration of TiO2 nanoparticles. New insights on polycrystallinity vs. single crystals in photocatalysis. Ceram. Int. 2016, 42, 3077–3087. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, G.; Irudayaraj, A.; Josephine, R.L. Tuning the optical band Gap of pure TiO2 via photon induced method. Optik 2019, 179, 889–894. [Google Scholar]
- Allen, N.S.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J.; Mahdjoub, N.; Hill, C. Characterisation and photocatalytic assessment of TiO2 nano-polymorphs: Influence of crystallite size and influence of thermal treatment on paint coatings and dye fading kinetics. J. Phys. Chem. Solids 2019, 126, 131–142. [Google Scholar] [CrossRef]
- Sinhmar, A.; Setia, H.; Kumar, V.; Sobti, A.; Toor, A.P. Enhanced photocatalytic activity of nickel and nitrogen codoped TiO2 under sunlight. Environ. Technol. Innov. 2020, 18, 100658. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Wei, Z.; Shi, L. A Fundamental DFT Study of Anatase (TiO2) Doped with 3d Transition Metals for High Photocatalytic Activities. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2018, 33, 403–408. [Google Scholar] [CrossRef]
- Benjwal, P.; De, B.; Kar, K.K. 1-D and 2-D morphology of metal cation co-doped (Zn, Mn) TiO2 and investigation of their photocatalytic activity. Appl. Surf. Sci. 2018, 427, 262–272. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.; Xue, J.; Gao, G.; Liu, X.; Jia, H.; Li, Q.; Xu, B. The dual effects of RGO films in TiO2/CdSe heterojunction: Enhancing photocatalytic activity and improving photocorrosion resistance. Appl. Surf. Sci. 2019, 481, 1515–1523. [Google Scholar] [CrossRef]
- Kernazhitsky, L.; Shymanovska, V.; Gavrilko, T.; Naumov, V.; Kshnyakin, V.; Khalyavka, T. A comparative study of optical absorption and photocatalytic properties of nanocrystalline single-phase anatase and rutile TiO2 doped with transition metal cations. J. Solid State Chem. 2013, 198, 511–519. [Google Scholar] [CrossRef]
- Song, E.; Kim, Y.-T.; Choi, J. Anion additives in rapid breakdown anodization for nonmetal-doped TiO2 nanotube powders. Electrochem. Commun. 2019, 109, 106610. [Google Scholar] [CrossRef]
- Fadlallah, M.M. Magnetic, electronic, optical, and photocatalytic properties of nonmetal- and halogen-doped anatase TiO2 nanotubes. Phys. E Low Dimens. Syst. Nanostruct. 2017, 89, 50–56. [Google Scholar] [CrossRef]
- Varnagiris, S.; Medvids, A.; Lelis, M.; Milcius, D.; Antuzevics, A. Black carbon-doped TiO2 films: Synthesis, characterization and photocatalysis. J. Photochem. Photobiol. A Chem. 2019, 382, 111941. [Google Scholar] [CrossRef]
- Li, G.; Zou, B.; Feng, S.; Shi, H.; Liao, K.; Wang, Y.; Wang, W.; Zhang, G. Synthesis of N-Doped TiO2 with good photocatalytic property. Phys. B Condens. Matter 2020, 588, 412184. [Google Scholar] [CrossRef]
- Li, H.; Qiu, L.; Bharti, B.; Dai, F.; Zhu, M.; Ouyang, F.; Lin, L. Efficient photocatalytic degradation of acrylonitrile by Sulfur-Bismuth co-doped F-TiO2/SiO2 nanopowder. Chemosphere 2020, 249, 126135. [Google Scholar] [CrossRef]
- He, D.; Li, Y.; Wu, J.; Yang, Y.; An, Q. Carbon wrapped and doped TiO2 mesoporous nanostructure with efficient visible-light photocatalysis for NO removal. Appl. Surf. Sci. 2017, 391, 318–325. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel one-step synthesis of nitrogen-doped TiO2 by flame aerosol technique for visible-light photocatalysis: Effect of synthesis parameters and secondary nitrogen (N) source. Chem. Eng. J. 2018, 350, 324–334. [Google Scholar] [CrossRef]
- Jiang, J.X.; Zhang, Q.Q.; Li, Y.H.; Li, L. Three-dimensional network graphene aerogel for enhancing adsorption and visible light photocatalysis of nitrogen-doped TiO2. Mater. Lett. 2019, 234, 298–301. [Google Scholar] [CrossRef]
- Marques, J.; Gomes, T.D.; Forte, M.A.; Silva, R.F.; Tavares, C.J. A new route for the synthesis of highly-active N-doped TiO2 nanoparticles for visible light photocatalysis using urea as nitrogen precursor. Catal. Today 2019, 326, 36–45. [Google Scholar] [CrossRef]
- Du, M.; Qiu, B.; Zhu, Q.; Xing, M.; Zhang, J. Fluorine doped TiO2/mesocellular foams with an efficient photocatalytic activity. Catal. Today 2019, 327, 340–346. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Ma, R.; Xue, Y.; Zheng, S. Fluorine doped anatase TiO2 with exposed reactive (001) facets supported on porous diatomite for enhanced visible-light photocatalytic activity. Microporous Mesoporous Mater. 2017, 243, 281–290. [Google Scholar] [CrossRef]
- Wei, J.Q.; Chen, X.J.; Wang, P.F.; Han, Y.B.; Xu, J.C.; Hong, B.; Jin, H.X.; Jin, D.F.; Peng, X.L.; Li, J.; et al. High surface area TiO2/SBA-15 nanocomposites: Synthesis, microstructure and adsorption-enhanced photocatalysis. Chem. Phys. 2018, 510, 47–53. [Google Scholar] [CrossRef]
- Hafez, H.S. Synthesis of highly-active single-crystalline TiO2 nanorods and its application in environmental photocatalysis. Mater. Lett. 2009, 63, 1471–1474. [Google Scholar] [CrossRef]
- Vargas Hernández, J.; Coste, S.; García Murillo, A.; Carrillo Romo, F.; Kassiba, A. Effects of metal doping (Cu, Ag, Eu) on the electronic and optical behavior of nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Belošević-Čavor, J.; Koteski, V.; Umićević, A.; Ivanovski, V. Effect of 5d transition metals doping on the photocatalytic properties of rutile TiO2. Comput. Mater. Sci. 2018, 151, 328–337. [Google Scholar] [CrossRef]
- Unal, F.A.; Ok, S.; Unal, M.; Topal, S.; Cellat, K.; Şen, F. Synthesis, characterization, and application of transition metals (Ni, Zr, and Fe) doped TiO2 photoelectrodes for dye-sensitized solar cells. J. Mol. Liq. 2020, 299, 112177. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Yang, L.; Ren, L.; Wang, D.; Ye, J. Metal nanoparticles induced photocatalysis. Natl. Sci. Rev. 2017, 4, 761–780. [Google Scholar] [CrossRef]
- Yao, G.Y.; Zhao, Z.Y.; Liu, Q.L.; Dong, X.D.; Zhao, Q.M. Theoretical calculations for localized surface plasmon resonance effects of Cu/TiO2 nanosphere: Generation, modulation, and application in photocatalysis. Sol. Energy Mater. Sol. Cells 2020, 208, 110385. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Xiu, Z.; Guo, M.; Zhao, T.; Pan, K.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in Ti3+ self-doped nanostructured TiO2 visible light photocatalysts for environmental and energy applications. Chem. Eng. J. 2020, 382, 123011. [Google Scholar] [CrossRef]
- Pulido Melián, E.; Nereida Suárez, M.; Jardiel, T.; Calatayud, D.G.; Del Campo, A.; Doña-Rodríguez, J.M.; Araña, J.; González Díaz, O.M. Highly photoactive TiO2 microspheres for photocatalytic production of hydrogen. Int. J. Hydrogen Energy 2019, 44, 24653–24666. [Google Scholar] [CrossRef]
- Huang, G.; Liu, X.; Shi, S.; Li, S.; Xiao, Z.; Zhen, W.; Liu, S.; Wong, P.K. Hydrogen producing water treatment through mesoporous TiO2 nanofibers with oriented nanocrystals. Chin. J. Catal. 2020, 41, 50–61. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Liu, S.; Gao, L.; Mao, L.; Fan, Z.; Shangguan, W.; Jiang, Z. Non-noble metal Cu as a cocatalyst on TiO2 nanorod for highly efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2018, 445, 527–534. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, P.; Zhao, J.; Zhang, Y.; Hu, J.; Shao, G. Plasmon enhancement on photocatalytic hydrogen production over the Z-scheme photosynthetic heterojunction system. Appl. Catal. B Environ. 2017, 210, 297–305. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Matsushita, Y.; Abdel-moneim, A. Fabrication of efficient TiO2-RGO heterojunction composites for hydrogen generation via water-splitting: Comparison between RGO, Au and Pt reduction sites. Appl. Surf. Sci. 2017, 423, 185–196. [Google Scholar] [CrossRef]
- Wu, L.; Shi, S.; Li, Q.; Zhang, X.; Cui, X. TiO2 nanoparticles modified with 2D MoSe2 for enhanced photocatalytic activity on hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 720–728. [Google Scholar] [CrossRef]
- Xing, X.; Zhu, H.; Zhang, M.; Xiao, L.; Li, Q.; Yang, J. Effect of heterojunctions and phase-junctions on visible-light photocatalytic hydrogen evolution in BCN-TiO2 photocatalysts. Chem. Phys. Lett. 2019, 727, 11–18. [Google Scholar] [CrossRef]
- Li, F.; Xiao, X.; Zhao, C.; Liu, J.; Li, Q.; Guo, C.; Tian, C.; Zhang, L.; Hu, J.; Jiang, B. TiO2-on-C3N4 double-shell microtubes: In-situ fabricated heterostructures toward enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2020, 572, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Y.; Bao, J.; Fang, J.; Zhao, S.; Zhang, Y.; Sheng, X.; Chen, W. Structure regulation of ZnS@g-C3N4/TiO2 nanospheres for efficient photocatalytic H2 production under visible-light irradiation. Chem. Eng. J. 2018, 346, 226–237. [Google Scholar] [CrossRef]
- Melián, E.P.; López, C.R.; Méndez, A.O.; Díaz, O.G.; Suárez, M.N.; Doña Rodríguez, J.M.; Navío, J.A.; Fernández Hevia, D. Hydrogen production using Pt-loaded TiO2 photocatalysts. Int. J. Hydrogen Energy 2013, 38, 11737–11748. [Google Scholar] [CrossRef]
- Vaiano, V.; Lara, M.A.; Iervolino, G.; Matarangolo, M.; Navio, J.A.; Hidalgo, M.C. Photocatalytic H2 production from glycerol aqueous solutions over fluorinated Pt-TiO2 with high {001} facet exposure. J. Photochem. Photobiol. A Chem. 2018, 365, 52–59. [Google Scholar] [CrossRef]
- Yasuda, M.; Matsumoto, T.; Yamashita, T. Sacrificial hydrogen production over TiO2-based photocatalysts: Polyols, carboxylic acids, and saccharides. Renew. Sustain. Energy Rev. 2018, 81, 1627–1635. [Google Scholar] [CrossRef]
- López, C.R.; Melián, E.P.; Ortega Méndez, J.A.; Santiago, D.E.; Doña Rodríguez, J.M.; González Díaz, O. Comparative study of alcohols as sacrificial agents in H2 production by heterogeneous photocatalysis using Pt/TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2015, 312, 45–54. [Google Scholar] [CrossRef]
- Velázquez, J.J.; Fernández-González, R.; Díaz, L.; Pulido Melián, E.; Rodríguez, V.D.; Núñez, P. Effect of reaction temperature and sacrificial agent on the photocatalytic H2-production of Pt-TiO2. J. Alloys Compd. 2017, 721, 405–410. [Google Scholar] [CrossRef]
- Kampouri, S.; Stylianou, K.C. Dual-Functional Photocatalysis for Simultaneous Hydrogen Production and Oxidation of Organic Substances. ACS Catal. 2019, 9, 4247–4270. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liu, C.; Luo, J.; Crittenden, J.; Liu, X.; Cai, T.; Yuan, J.; Pei, Y.; Liu, Y. Photocatalytic wastewater purification with simultaneous hydrogen production using MoS2 QD-decorated hierarchical assembly of ZnIn2S4 on reduced graphene oxide photocatalyst. Water Res. 2017, 121, 11–19. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Nasillo, G.; Palmisano, L. Photocatalytic Solar Light H2 Production by Aqueous Glucose Reforming: Photocatalytic Solar Light H2 Production by Aqueous Glucose Reforming. Eur. J. Inorg. Chem. 2018, 2018, 4522–4532. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Li, Z.; Meng, X. Photocatalytic Reforming for Hydrogen Evolution: A Review. Catalysts 2020, 10, 335. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, B.J.; Jeong, S.; Jeon, K.J.; Chung, K.H.; Jung, S.C. Characteristics of hydrogen production by photocatalytic water splitting using liquid phase plasma over Ag-doped TiO2 photocatalysts. Environ. Res. 2020, 188, 109630. [Google Scholar] [CrossRef]
- Wu, G.; Chen, T.; Su, W.; Zhou, G.; Zong, X.; Lei, Z.; Li, C. H2 production with ultra-low CO selectivity via photocatalytic reforming of methanol on Au/TiO2 catalyst. Int. J. Hydrogen Energy 2008, 33, 1243–1251. [Google Scholar] [CrossRef]
- Udayabhanu; Reddy, N.L.; Shankar, M.V.; Sharma, S.C.; Nagaraju, G. One-pot synthesis of Cu-TiO2/CuO nanocomposite: Application to photocatalysis for enhanced H2 production, dye degradation & detoxification of Cr (VI). Int. J. Hydrogen Energy 2020, 45, 7813–7828. [Google Scholar]
- Xing, X.; Tang, S.; Hong, H.; Jin, H. Concentrated solar photocatalysis for hydrogen generation from water by titania-containing gold nanoparticles. Int. J. Hydrogen Energy 2020, 45, 9612–9623. [Google Scholar] [CrossRef]
- Su, E.C.; Huang, B.S.; Lee, J.T.; Wey, M.Y. Excellent dispersion and charge separation of SrTiO3-TiO2 nanotube derived from a two-step hydrothermal process for facilitating hydrogen evolution under sunlight irradiation. Sol. Energy 2018, 159, 751–759. [Google Scholar] [CrossRef]
- Sultanov, F.; Daulbayev, C.; Bakbolat, B.; Daulbayev, O.; Bigaj, M.; Mansurov, Z.; Kuterbekov, K.; Bekmyrza, K. Aligned composite SrTiO3/PAN fibers as 1D photocatalyst obtained by electrospinning method. Chem. Phys. Lett. 2019, 737, 136821. [Google Scholar] [CrossRef]
- Sultanov, F.; Bakbolat, B.; Mansurov, Z.; Pei, S.-S.; Ebrahim, R.; Daulbayev, C.; Urazgaliyeva, A.; Tulepov, M. Spongy Structures Coated with Carbon Nanomaterialsfor Efficient Oil/Water Separation. Eurasian Chem. Technol. J. 2017, 19, 127. [Google Scholar] [CrossRef]
- Sultanov, F.; Bakbolat, B.; Daulbaev, C.; Urazgalieva, A.; Azizov, Z.; Mansurov, Z.; Tulepov, M.; Pei, S.S. Sorptive Activity and Hydrophobic Behavior of Aerogels Based on Reduced Graphene Oxide and Carbon Nanotubes. J. Eng. Phys. Thermophys. 2017, 90, 826–830. [Google Scholar] [CrossRef]
- Luo, L.; Li, J.; Dai, J.; Xia, L.; Barrow, C.J.; Wang, H.; Jegatheesan, J.; Yang, M. Bisphenol A removal on TiO2–MoS2–reduced graphene oxide composite by adsorption and photocatalysis. Process. Saf. Environ. Prot. 2017, 112, 274–279. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Tran, M.L.; Tran, T.T.V.; Juang, R.S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar] [CrossRef]
- Kumar, K.Y.; Saini, H.; Pandiarajan, D.; Prashanth, M.K.; Parashuram, L.; Raghu, M.S. Controllable synthesis of TiO2 chemically bonded graphene for photocatalytic hydrogen evolution and dye degradation. Catal. Today 2020, 340, 170–177. [Google Scholar] [CrossRef]
- Rajender, G.; Kumar, J.; Giri, P.K. Interfacial charge transfer in oxygen deficient TiO2-graphene quantum dot hybrid and its influence on the enhanced visible light photocatalysis. Appl. Catal. B Environ. 2018, 224, 960–972. [Google Scholar] [CrossRef]
- Ali, M.H.H.; Al-Afify, A.D.; Goher, M.E. Preparation and characterization of graphene—TiO2 nanocomposite for enhanced photodegradation of Rhodamine-B dye. Egypt. J. Aquat. Res. 2018, 44, 263–270. [Google Scholar] [CrossRef]
- Ton, N.N.T.; Dao, A.T.N.; Kato, K.; Ikenaga, T.; Trinh, D.X.; Taniike, T. One-pot synthesis of TiO2/graphene nanocomposites for excellent visible light photocatalysis based on chemical exfoliation method. Carbon 2018, 133, 109–117. [Google Scholar] [CrossRef]
- Lettieri, S.; Gargiulo, V.; Pallotti, D.K.; Vitiello, G.; Maddalena, P.; Alfè, M.; Marotta, R. Evidencing opposite charge-transfer processes at TiO2/graphene-related materials interface through a combined EPR, photoluminescence and photocatalysis assessment. Catal. Today 2018, 315, 19–30. [Google Scholar] [CrossRef]
- Shahbazi, R.; Payan, A.; Fattahi, M. Preparation, evaluations and operating conditions optimization of nano TiO2 over graphene based materials as the photocatalyst for degradation of phenol. J. Photochem. Photobiol. A Chem. 2018, 364, 564–576. [Google Scholar] [CrossRef]
- Khan, H.; Jiang, Z.; Berk, D. Molybdenum doped graphene/TiO2 hybrid photocatalyst for UV/visible photocatalytic applications. Sol. Energy 2018, 162, 420–430. [Google Scholar] [CrossRef]
- Sun, X.; Ji, S.; Wang, M.; Dou, J.; Yang, Z.; Qiu, H.; Kou, S.; Ji, Y.; Wang, H. Fabrication of porous TiO2-RGO hybrid aerogel for high-efficiency, visible-light photodegradation of dyes. J. Alloys Compd. 2020, 819, 153033. [Google Scholar] [CrossRef]
- Sultanov, F.R.; Mansurov, Z.A.; Daulbayev, C.; Urazgaliyeva, A.A.; Bakbolat, B.; Pei, S.-S. Study of Sorption Capacity and Surface Morphology of Carbon Nanomaterials/Chitosan Based Aerogels. Eur. Chem. Technol. J. 2016, 18, 19. [Google Scholar] [CrossRef]
- Sultanov, F.R.; Pei, S.S.S.; Auyelkhankyzy, M.; Smagulova, G.; Lesbayev, B.T.; Mansurov, Z.A. Aerogels Based on Graphene Oxide with Addition of Carbon Nanotubes: Synthesis and Properties. Eur. Chem. Technol. J. 2014, 16, 265–269. [Google Scholar] [CrossRef]
- Ali, M.H.H.; Al-Qahtani, K.M.; El-Sayed, S.M. Enhancing photodegradation of 2,4,6 trichlorophenol and organic pollutants in industrial effluents using nanocomposite of TiO2 doped with reduced graphene oxide. Egypt. J. Aquat. Res. 2019, 45, 321–328. [Google Scholar] [CrossRef]
- Nadimi, M.; Ziarati Saravani, A.; Aroon, M.A.; Ebrahimian Pirbazari, A. Photodegradation of methylene blue by a ternary magnetic TiO2/Fe3O4/graphene oxide nanocomposite under visible light. Mater. Chem. Phys. 2019, 225, 464–474. [Google Scholar] [CrossRef]
- Park, K.; Ali, I.; Kim, J.O. Photodegradation of perfluorooctanoic acid by graphene oxide-deposited TiO2 nanotube arrays in aqueous phase. J. Environ. Manag. 2018, 218, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Al Kausor, M.; Chakrabortty, D. Facile fabrication of N-TiO2/Ag3PO4@GO nanocomposite toward photodegradation of organic dye under visible light. Inorg. Chem. Commun. 2020, 116, 107907. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, M.; Tong, Y.; Wang, H.; Zhou, X.; Sheng, X.; Sun, Y.; Chen, C. Improved photoelectrocatalytic degradation of tetrabromobisphenol A with silver and reduced graphene oxide-modified TiO2 nanotube arrays under simulated sunlight. Ecotoxicol. Environ. Saf. 2019, 182, 109472. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, G.; Li, L.; Dong, X.; Zhang, X. Enhanced activation of peroxymonosulfate by nitrogen-doped graphene/TiO2 under photo-assistance for organic pollutants degradation: Insight into N doping mechanism. Chemosphere 2020, 244, 125526. [Google Scholar] [CrossRef]
- Chen, F.; An, W.; Li, Y.; Liang, Y.; Cui, W. Fabricating 3D porous PANI/TiO2-graphene hydrogel for the enhanced UV-light photocatalytic degradation of BPA. Appl. Surf. Sci. 2018, 427, 123–132. [Google Scholar] [CrossRef]
- Ghasemi, A.; Azzouz, R.; Laipple, G.; Kabak, K.E.; Heavey, C. Optimizing capacity allocation in semiconductor manufacturing photolithography area—Case study: Robert Bosch. J. Manuf. Syst. 2020, 54, 123–137. [Google Scholar] [CrossRef]
- Daulbaev, C.B.; Dmitriev, T.P.; Sultanov, F.R.; Mansurov, Z.A.; Aliev, E.T. Obtaining Three-Dimensional Nanosize Objects on a “3D Printer + Electrospinning” Machine. J. Eng. Phys. Thermophys. 2017, 90, 1115–1118. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Zuo, X.; Wen, F.; Jiang, H.; Cao, H.; Pei, Y. Micro-patterned TiO2 films for photocatalysis. Mater. Lett. 2019, 254, 448–451. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, R.; Zhang, M.; Gao, S. Solvothermal preparation of Fe-doped TiO2 nanotube arrays for enhancement in visible light induced photoelectrochemical performance. J. Alloys Compd. 2017, 690, 139–144. [Google Scholar] [CrossRef]
- Zhang, J.J.; Qi, P.; Li, J.; Zheng, X.C.; Liu, P.; Guan, X.X.; Zheng, G.P. Three-dimensional Fe2O3-TiO2-graphene aerogel nanocomposites with enhanced adsorption and visible light-driven photocatalytic performance in the removal of RhB dyes. J. Ind. Eng. Chem. 2018, 61, 407–415. [Google Scholar] [CrossRef]
- Lesbayev, A.B.; Smagulova, G.T.; Kim, S.; Prikhod’ko, N.G.; Manakov, S.M.; Guseinov, N.; Mansurov, Z.A. Solution-Combustion Synthesis and Characterization of Fe3O4 Nanoparticles. Int. J. Self Propag. High Temp. Synth. 2018, 27, 195–197. [Google Scholar] [CrossRef]
- Soltani-nezhad, F.; Saljooqi, A.; Shamspur, T.; Mostafavi, A. Photocatalytic degradation of imidacloprid using GO/Fe3O4/TiO2-NiO under visible radiation: Optimization by response level method. Polyhedron 2019, 165, 188–196. [Google Scholar] [CrossRef]
- Rajoriya, S.; Bargole, S.; George, S.; Saharan, V.K.; Gogate, P.R.; Pandit, A.B. Synthesis and characterization of samarium and nitrogen doped TiO2 photocatalysts for photo-degradation of 4-acetamidophenol in combination with hydrodynamic and acoustic cavitation. Sep. Purif. Technol. 2019, 209, 254–269. [Google Scholar] [CrossRef]
- Isari, A.A.; Hayati, F.; Kakavandi, B.; Rostami, M.; Motevassel, M.; Dehghanifard, E. N, Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: An effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem. Eng. J. 2020, 392, 123685. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Wang, H.; Wang, F.; Wang, D.; Gao, Z.; Wang, X.; Xu, F.; Jiang, K. Ultrasonic-assisted synthesis of two dimensional BiOCl/MoS2 with tunable band gap and fast charge separation for enhanced photocatalytic performance under visible light. J. Colloid Interface Sci. 2019, 533, 539–547. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Q.; Zhang, Z.; Liu, X.; Zhao, J.; Cheng, S.; Zong, B.; Dai, W.L. Carbon nitride nanosheets decorated with WO3 nanorods: Ultrasonic-assisted facile synthesis and catalytic application in the green manufacture of dialdehydes. Appl. Catal. B Environ. 2015, 165, 511–518. [Google Scholar] [CrossRef]
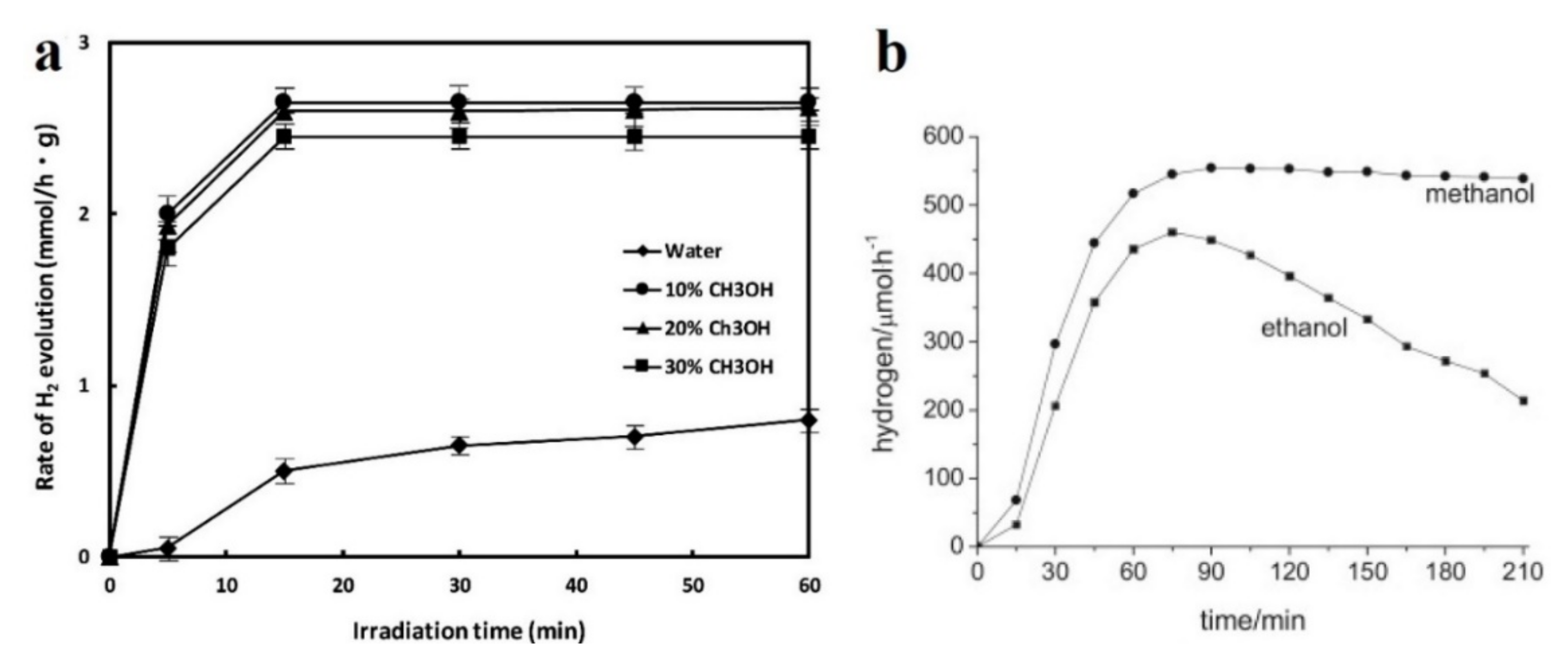
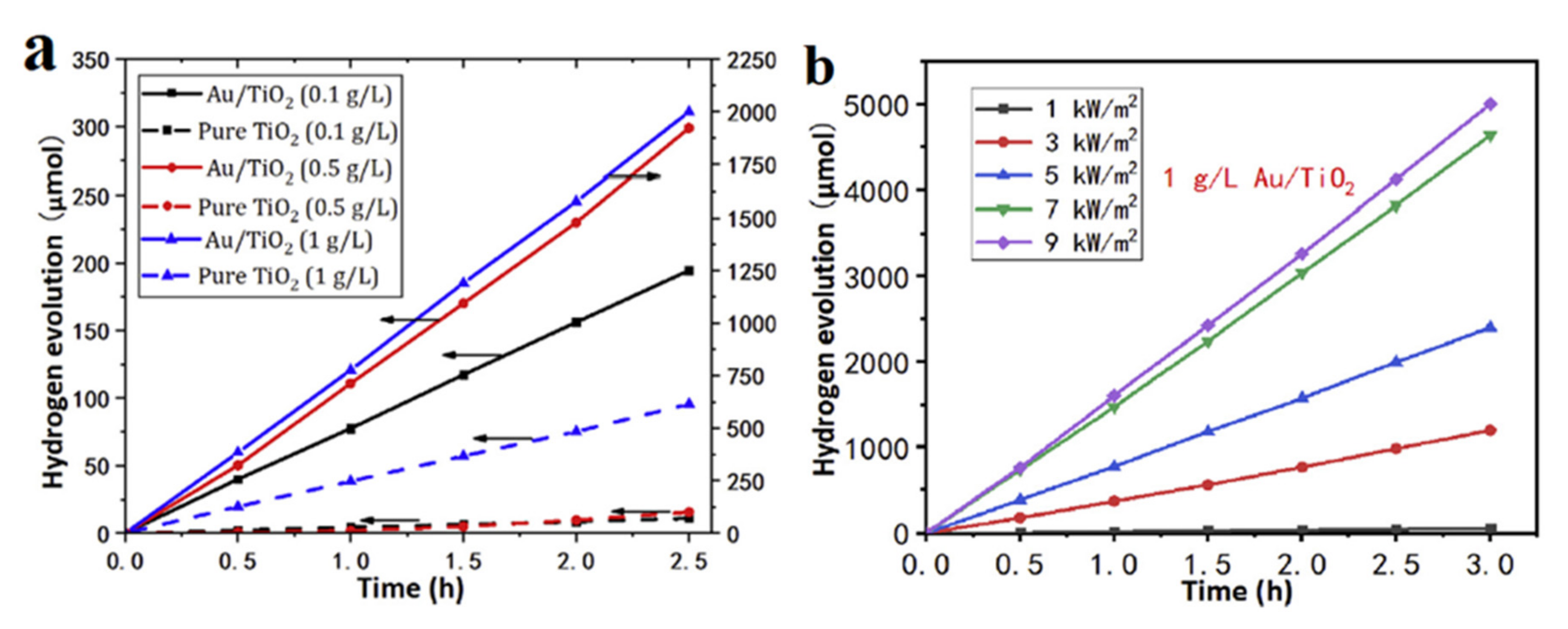
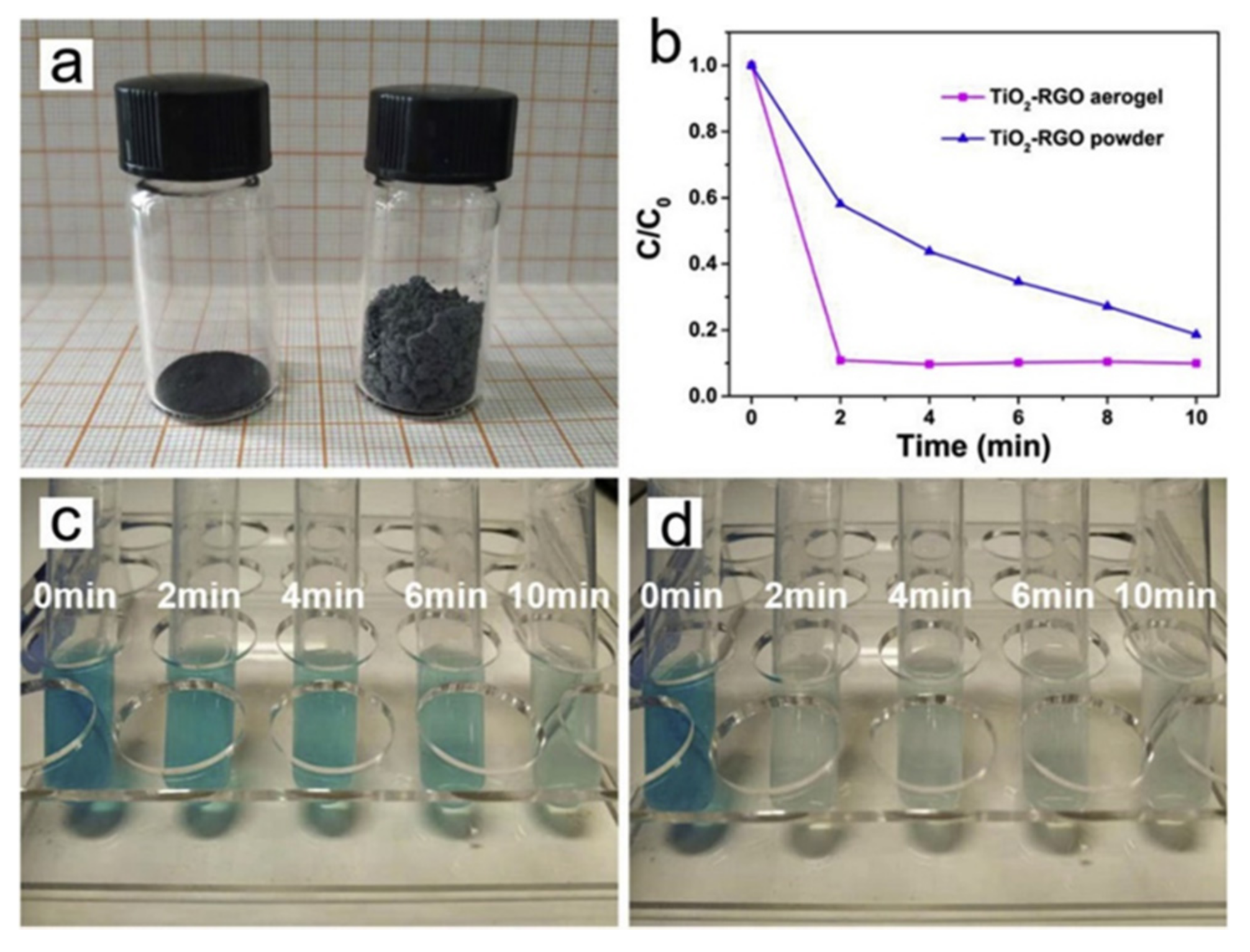


| Photocatalyst | Structure | Light Source | Sacrificial Agents | Evolution H2 | Ref. |
|---|---|---|---|---|---|
| Au/TiO2 Pt/TiO2 | microspheres | 15 W fluorescent tubes (λmax = 365 nm) | 25% vol. methanol | 1118 µmol h−1 2125 µmol h−1 | [42] |
| TiO2 | nanofibers | 300 W Xenon lamp | 10% vol. methanol | 3200 μmol h−1 g−1 | [43] |
| Cu/TiO2 | nanorods | 300 W Xe lamp (λ > 300 nm) | 20% vol. methanol | 1023.8 μmol h−1 | [44] |
| TiO2/WO3/Au | nanofibers | 300 W Xe arc lamp | 35% vol. methanol | 269.63 µmol h−1 | [45] |
| M/TiO2/rGO M = Au or Pt | nanoparticles | 300 W Xenon lamp (λ > 300 nm) | 20% vol. methanol | 670 µmol h−1 | [46] |
| MoSe2/TiO2 | nanoparticles | Xe arc lamp (PLS-SXE300) | 10% vol. methanol | 4.9 μmol h−1 | [47] |
| BCN-TiO2 | nanosheets& nanoparticles | 300 W xenon lamp with a UV-cutoff filter (λ ≥ 420 nm) | 20% vol. triethanolamine | 68.54 μmol h−1 g−1 | [48] |
| TiO2/C3N4 | double-shell microtubes | 300 W xenon lamp | 20% vol. methanol | 10.1 mmol h−1 g−1 | [49] |
| ZnS@g-C3N4/TiO2 | nanospheres | 300 W Xenon lamp (λ > 400 nm) | 10% vol. triethanolamine | 422 μmol h−1 g−1 | [50] |
| Photocatalyst | Organic Pollutant | Light Source | Irradiation Time | Efficiency | Ref. |
|---|---|---|---|---|---|
| TiO2@rGO | 2,4,6 trichlorophenol | Mercury lamp (11 W) | 180 min | 90% | [80] |
| TiO2/Fe3O4/GO | Methylene blue | Halogen lamp (500 W) | 90 min | 76% | [81] |
| GO/TiO2 nanotubes | Perfluorooctanoic acid | UV lamp (8 W) | 240 min | 97% | [82] |
| N-TiO2/Ag3PO4@GO | Acid Blue 25 | Halogen bulb (250 W) | 20 min | 98% | [83] |
| Ag and rGO modified TiO2 | Tetrabromobisphenol A | Xenon light (500 W) | 80 min | 99.6% | [84] |
| N-doped graphene/TiO2 | Bisphenol A | Mercury lamp (300 W) | 60 min | 100% | [85] |
| 3D polyaniline/TiO2/rGO hydrogel | BPA | Mercury lamp (500 W) | 40 min | 100% | [86] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. https://doi.org/10.3390/nano10091790
Bakbolat B, Daulbayev C, Sultanov F, Beissenov R, Umirzakov A, Mereke A, Bekbaev A, Chuprakov I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials. 2020; 10(9):1790. https://doi.org/10.3390/nano10091790
Chicago/Turabian StyleBakbolat, Baglan, Chingis Daulbayev, Fail Sultanov, Renat Beissenov, Arman Umirzakov, Almaz Mereke, Askhat Bekbaev, and Igor Chuprakov. 2020. "Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review" Nanomaterials 10, no. 9: 1790. https://doi.org/10.3390/nano10091790
APA StyleBakbolat, B., Daulbayev, C., Sultanov, F., Beissenov, R., Umirzakov, A., Mereke, A., Bekbaev, A., & Chuprakov, I. (2020). Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials, 10(9), 1790. https://doi.org/10.3390/nano10091790