Well-Defined Thermo-Responsive Copolymers Based on Oligo(Ethylene Glycol) Methacrylate and Pentafluorostyrene for the Removal of Organic Dyes from Water
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
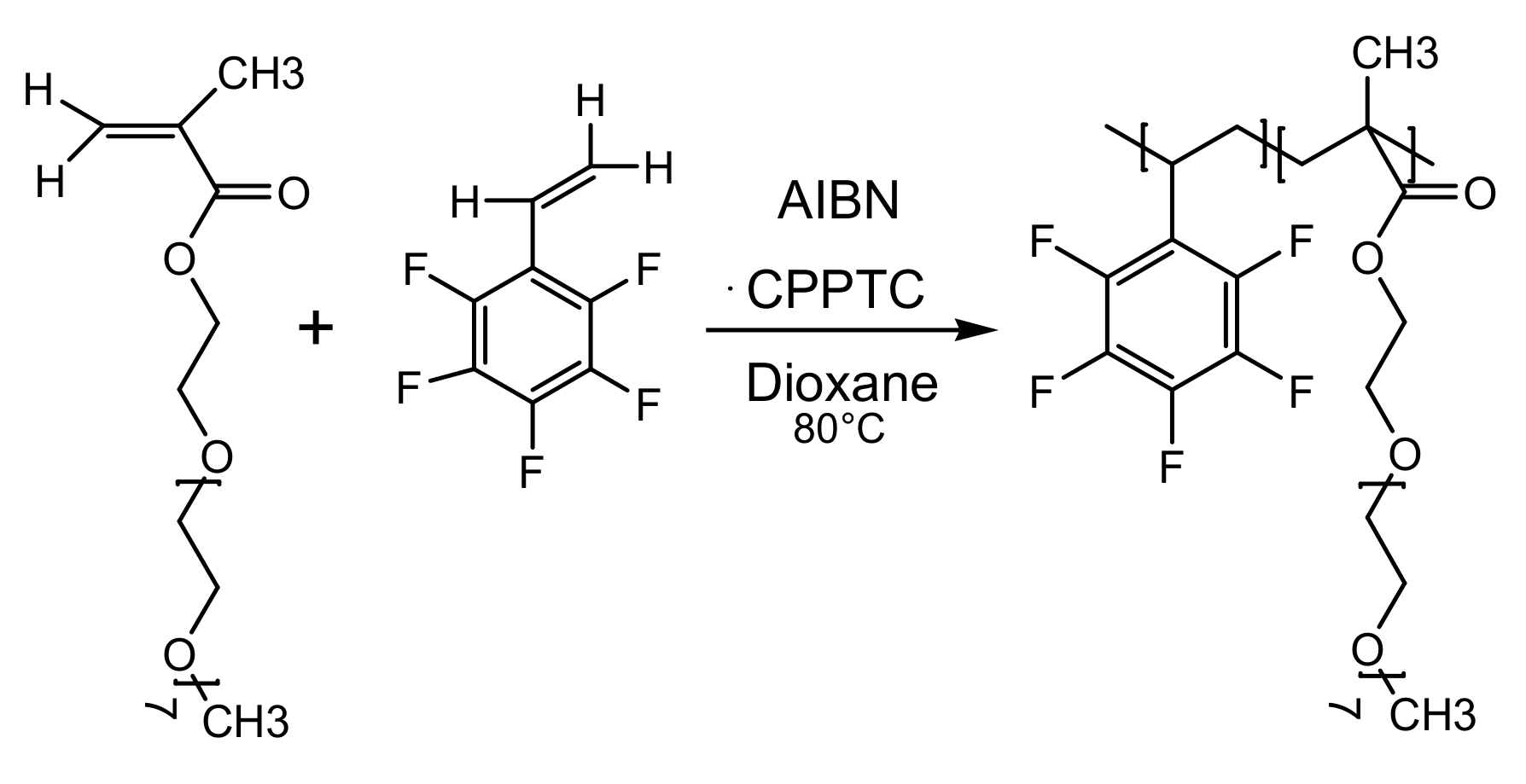
2.2. Synthesis of Copolymers of OEGMA and PFS (PFG) via RAFT
2.3. Polymer Characterization
2.4. Characterization of the Self-Assembly Properties
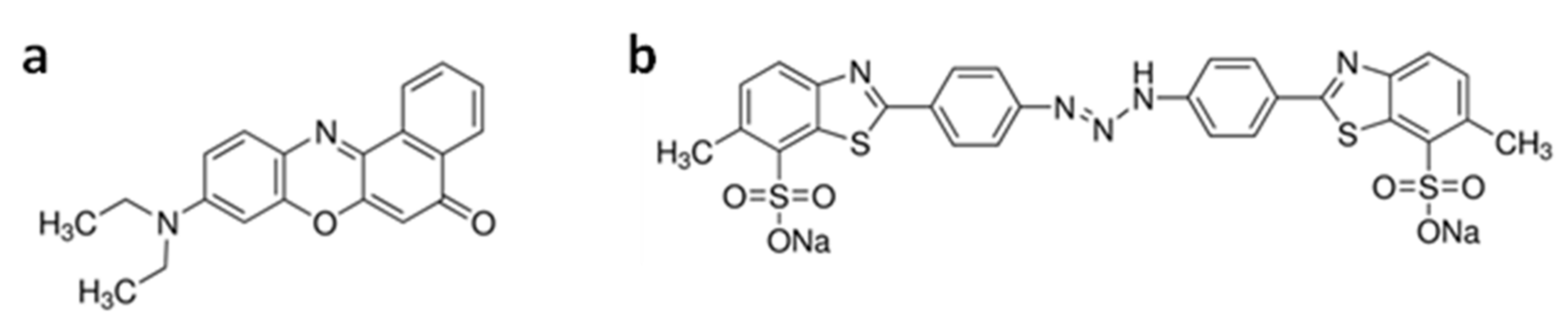
2.5. Dye Removal Analyses
3. Results and Discussion
3.1. Syntheses and Chemical Characterizations of Copolymers of OEGMA and PFS
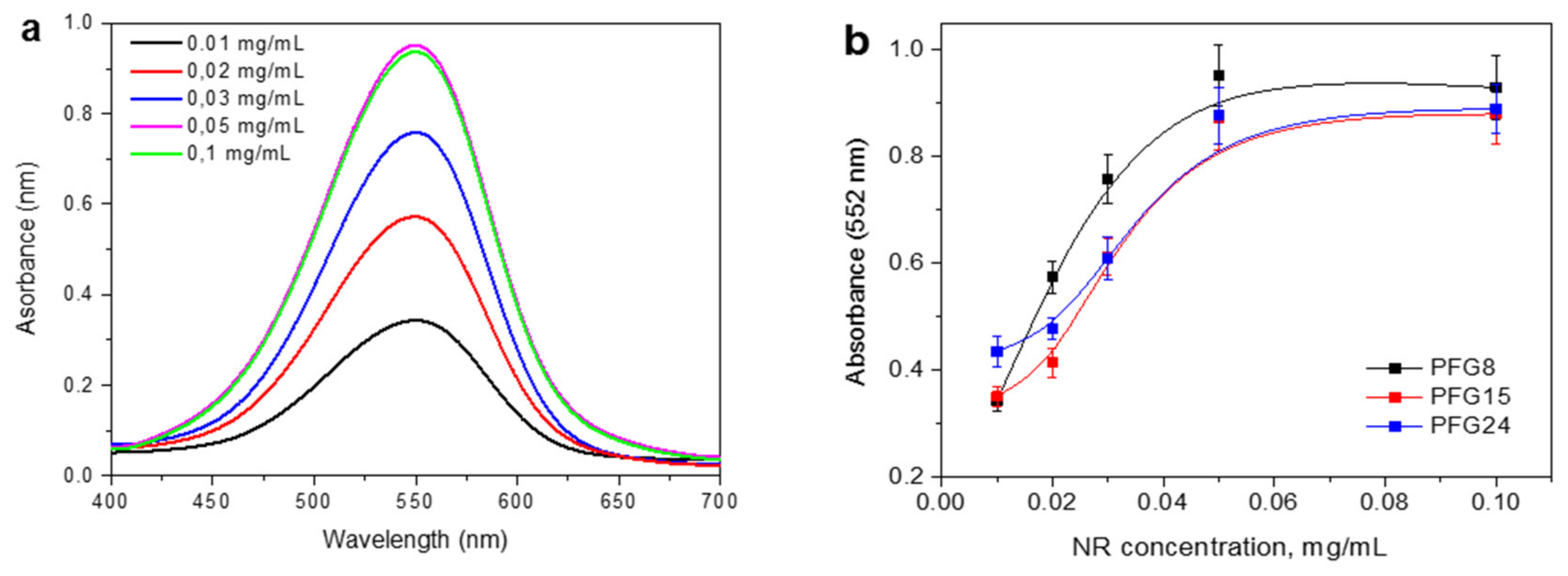
3.2. Self-Assembly of PFG Copolymers in Aqueous Solution
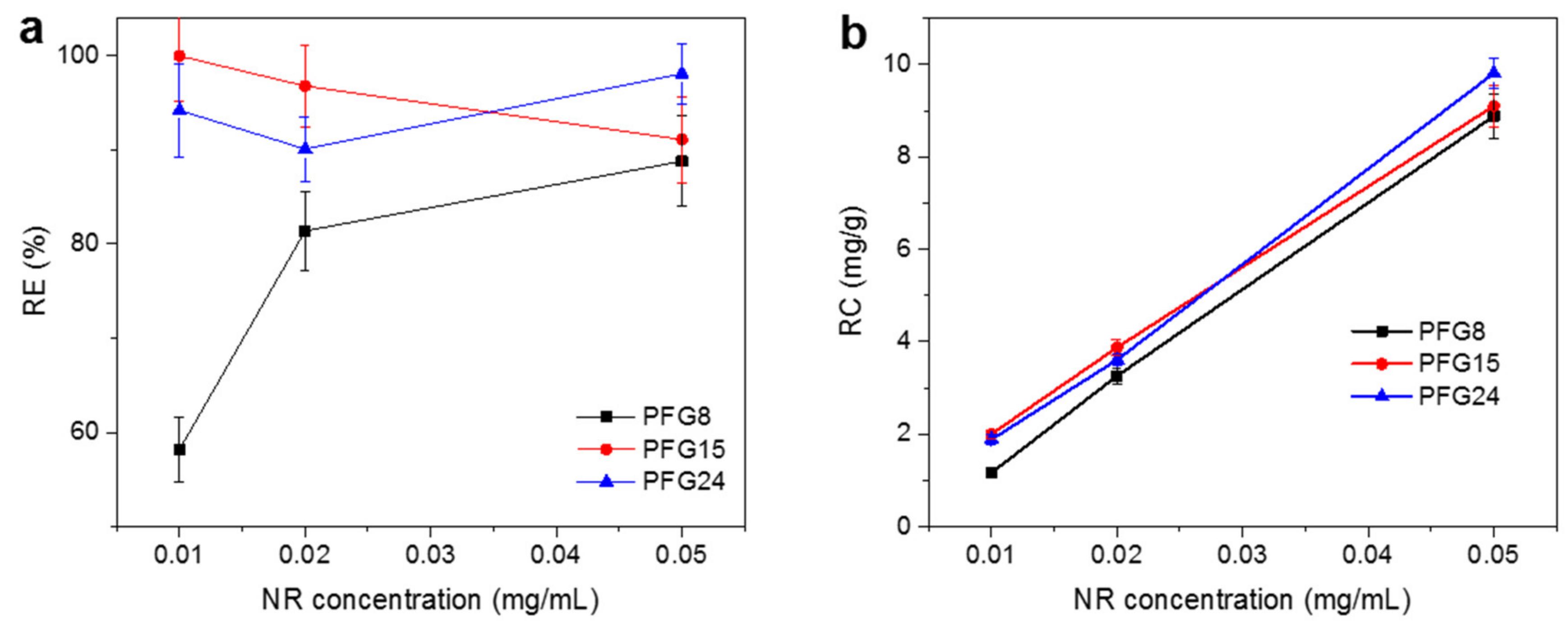
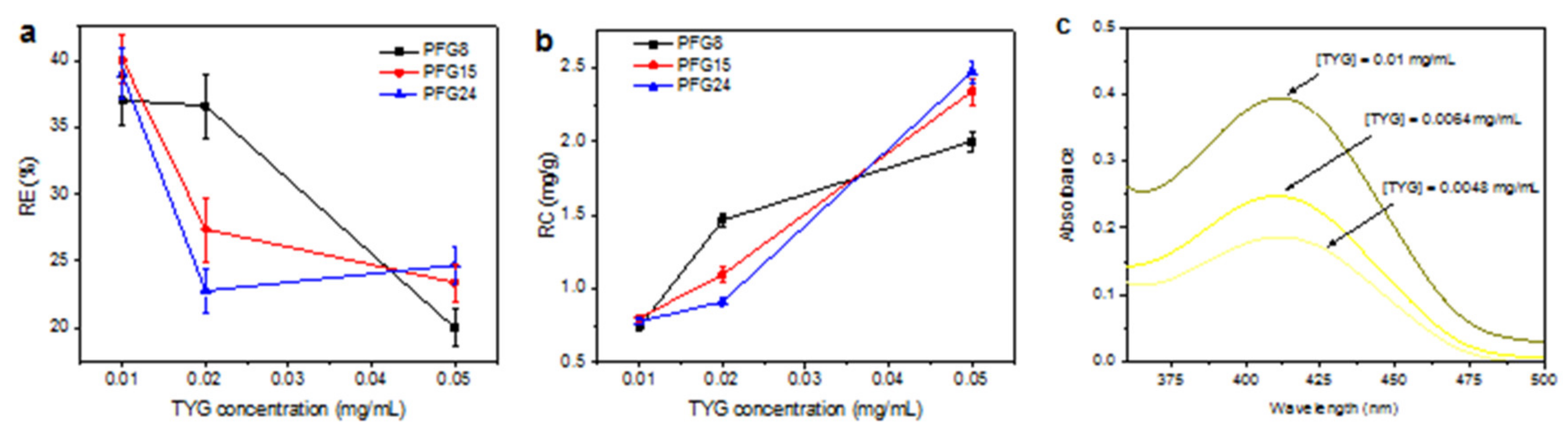
3.3. Evaluation of PFG Copolymers for Water Purification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Badi, N. Non-linear PEG-based thermo-responsive polymer systems. Prog. Polym. Sci. 2017, 66, 54–79. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P. Temperature and pH stimuli-responsive polymers and their applications in controlled and self-regulated drug delivery. J. Appl. Pharm. 2010, 2, 1–10. [Google Scholar]
- Aguilar, M.R.; Román, J.S. Smart Polymers and Their Applications; Woodhead Publishing: Cambridge, UK, 2014; pp. 1–11. [Google Scholar]
- Kim, Y.J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Sponchioni, M.; Palmiero, U.C.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Schmidt, S.; Motschmann, H.; Hellweg, T.; von Klitzing, R. Thermo-responsive surfaces by spin-coating of PNIPAM-co-PAA microgels: A combined AFM and ellipsometry study. Polymer 2008, 49, 749–756. [Google Scholar] [CrossRef]
- Huang, X.; Witte, K.L.; Bergbreiter, D.E.; Wong, C.H. Homogenous enzymatic synthesis using a thermo-responsive water-soluble polymer support. Adv. Synth. Catal. 2001, 343, 675–681. [Google Scholar] [CrossRef]
- Parasuraman, D.; Serpe, M.J. Poly (N-Isopropylacrylamide) Microgel-Based Assemblies for Organic Dye Removal from Water. ACS Appl. Mater. Interfaces 2011, 3, 4714–4721. [Google Scholar] [CrossRef]
- Parasuraman, D.; Leung, E.; Serpe, M.J. Poly (N-isopropylacrylamide) microgel based assemblies for organic dye removal from water: Microgel diameter effects. Colloid Polym. Sci. 2012, 290, 1053–1064. [Google Scholar] [CrossRef]
- Truong, N.P.; Whittaker, M.R.; Anastasaki, A.; Haddleton, D.M.; Quinn, J.F.; Davis, T.P. Facile production of nanoaggregates with tuneable morphologies from thermo-responsive P(DEGMA-co-HPMA). Polym. Chem. 2016, 7, 430–440. [Google Scholar] [CrossRef]
- Zuppardi, F.; Chiacchio, F.R.; Sammarco, R.; Malinconico, M.; D’Ayala, G.G.; Cerruti, P. Fluorinated oligo(ethylene glycol) methacrylate-based copolymers: Tuning of self assembly properties and relationship with rheological behavior. Polymer 2017, 112, 169–179. [Google Scholar] [CrossRef]
- Thivaios, I.; Bokias, G. Adsorption of Nile Red by poly(N-isopropylacrylamide) gels in binary water/tetrahydrofuran mixtures. J. Appl. Polym. Sci. 2010, 116, 1509–1514. [Google Scholar] [CrossRef]
- Özkahraman, B.; Acar, I.; Emik, S. Removal of cationic dyes from aqueous solutions with poly (N-isopropylacrylamide-co-itaconic acid) hydrogels. Polym. Bull. 2011, 66, 551–570. [Google Scholar] [CrossRef]
- Saitoh, T.; Yoshida, Y.; Matsudo, T.; Fujiwara, S.; Dobashi, A.; Iwaki, K.; Suzuki, Y.; Matsubara, C. Concentration of Hydrophobic Organic Compounds by Polymer-Mediated Extraction. Anal. Chem. 1999, 71, 4506–4512. [Google Scholar] [CrossRef]
- Tian, Y.; Ju, B.; Zhang, S.; Hou, L. Thermo-responsive cellulose ether and its flocculation behavior for organic dye removal. Carbohydr. Polym. 2016, 136, 1209–1217. [Google Scholar] [CrossRef]
- Saitoh, T.; Asano, K.; Hiraide, M. Removal of phenols in water using chitosan-conjugated thermo-responsive polymers. J. Hazard. Mater. 2011, 185, 1369–1373. [Google Scholar] [CrossRef]
- Tokuyama, H.; Hisaeda, J.; Nii, S.; Sakohara, S. Removal of heavy metal ions and humic acid from aqueous solutions by co-adsorption onto thermosensitive polymers. Sep. Purif. Technol. 2010, 71, 83–88. [Google Scholar] [CrossRef]
- Lutz, J.F. Thermo-Switchable Materials Prepared Using the OEGMA-Platform. Adv. Mater. 2011, 23, 2237–2243. [Google Scholar] [CrossRef]
- Gawlitza, K.; Radulescu, A.; von Klitzing, R.; Wellert, S. On the structure of biocompatible, thermo-responsive poly(ethylene glycol) microgels. Polymer 2014, 55, 6717–6724. [Google Scholar] [CrossRef]
- Cappelli, A.; Paolino, M.; Grisci, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Boccia, A.C.; Samperi, F.; Battiato, S.; Paccagnini, E.; et al. A click chemistry-based “grafting through” approach to the synthesis of a biorelevant polymer brush. Polym. Chem. 2011, 2, 2518–2527. [Google Scholar] [CrossRef]
- Lutz, J.F.; Hoth, A. Preparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)ethyl Methacrylate and Oligo(ethylene glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Chenal, M.; Mura, S.; Marchal, C.; Gigmes, D.; Charleux, B.; Fattal, E.; Couvreur, P.; Nicolas, J. Facile Synthesis of Innocuous Comb-Shaped Polymethacrylates with PEG Side Chains by Nitroxide-Mediated Radical Polymerization in Hydroalcoholic Solutions. Macromolecules 2010, 43, 9291–9303. [Google Scholar] [CrossRef]
- Rinaldi, D.; Hamaide, T.; Graillat, C.; D’Agosto, F.; Spitz, R.; Georges, S.; Mosquet, M.; Maitrasse, P. RAFT copolymerization of methacrylic acid and poly(ethylene glycol) methyl ether methacrylate in the presence of a hydrophobic chain transfer agent in organic solution and in water. J. Polym. Sci. Part A: Polym. Chem. 2009, 47, 3045–3055. [Google Scholar] [CrossRef]
- Guazzelli, E.; Martinelli, E.; Galli, G.; Cupellini, L.; Jurinovich, S.; Mennucci, B. Single-chain self-folding in an amphiphilic copolymer: An integrated experimental and computational study. Polymer 2019, 161, 33–40. [Google Scholar] [CrossRef]
- Han, S.; Hagiwara, M.; Ishizone, T. Synthesis of Thermally Sensitive Water-Soluble Polymethacrylates by Living Anionic Polymerizations of Oligo(ethylene glycol) Methyl Ether Methacrylates. Macromolecules 2003, 36, 8312–8319. [Google Scholar] [CrossRef]
- Ishizone, T.; Seki, A.; Hagiwara, M.; Han, S.; Yokoyama, H.; Oyane, A.; Deffieux, A.; Carlotti, S. Anionic Polymerizations of Oligo(ethylene glycol) Alkyl Ether Methacrylates: Effect of Side Chain Length and Alkyl Group of Side Chain on Cloud Point in Water. Macromolecules 2008, 41, 2963–2967. [Google Scholar] [CrossRef]
- Delplace, V.; Tardy, A.; Harrisson, S.; Mura, S.; Gigmes, D.; Guillaneuf, Y.; Nicolas, J. Degradable and Comb-Like PEG-Based Copolymers by Nitroxide-Mediated Radical Ring-Opening Polymerization. Biomacromolecules 2013, 14, 3769–3779. [Google Scholar] [CrossRef]
- Kwan, S.; Maric, M. Thermo-responsive polymers with tunable cloud point temperatures grafted from chitosan via nitroxide mediated polymerization. Polymer 2016, 86, 69–82. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, H.; Puttick, S.; Reid, J.; Bernardi, S.; Searles, D.J.; Whittaker, A.K. Conformation of Hydrophobically Modified Thermo-responsive Poly(OEGMA-co-TFEA) across the LCST Revealed by NMR and Molecular Dynamics Studies. Macromolecules 2015, 48, 3310–3317. [Google Scholar] [CrossRef]
- Xu, S.; Liu, W. Synthesis and surface characterization of an amphiphilic fluorinated copolymer via emulsifier-free emulsion polymerization of RAFT. J. Fluor. Chem. 2008, 129, 125–130. [Google Scholar] [CrossRef]
- Shemper, B.S.; Mathias, L.J. Syntheses and characterization of statistical and block fluorinated copolymers with linear and star-like architectures via ATRP. Eur. Polym. J. 2004, 40, 651–665. [Google Scholar] [CrossRef]
- Erol, F.E.; Sinirlioglu, D.; Cosgun, S.; Muftuoglu, A.E. Synthesis of Fluorinated Amphiphilic Block Copolymers Based on PEGMA, HEMA, and MMA via ATRP and CuAAC Click Chemistry. Int. J. Polym. Sci. 2014, 2014, Article ID 464806. [Google Scholar] [CrossRef]
- Koiry, B.P.; Chakrabarty, A.; Singha, N.K. Fluorinated amphiphilic block copolymers via RAFT polymerization and their application as surf-RAFT agent in miniemulsion polymerization. RSC Adv. 2015, 5, 15461–15468. [Google Scholar] [CrossRef]
- Hansen, N.M.L.; Haddleton, D.M.; Hvilsted, S. Fluorinated bio-acceptable polymers via an ATRP macroinitiator approach. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 5770–5780. [Google Scholar] [CrossRef]
- Koda, Y.; Terashima, T.; Takenaka, M.; Sawamoto, M. Star Polymer Gels with Fluorinated Microgels via Star–Star Coupling and Cross-Linking for Water Purification. ACS Macro Lett. 2015, 4, 377–380. [Google Scholar] [CrossRef]
- Tan, J.; Liu, W.; Wang, Z. Preparation and self-assembly of pH-sensitive amphiphilic comb-shaped copolymer containing long fluorinated side chains. J. Macromol. Sci. A 2016, 53, 716–724. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, H.; Whittaker, A.K. NMR investigation of effect of dissolved salts on the thermoresponsive behavior of oligo(ethylene glycol)-methacrylate-based polymers. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2375–2385. [Google Scholar] [CrossRef]
- Topuzoğullari, M.; Bulmus, V.; Dalgakiran, E.; Dinçer, S. pH- and temperature-responsive amphiphilic diblock copolymers of 4-vinylpyridine and oligoethyleneglycol methacrylate synthesized by RAFT polymerization. Polym. 2014, 55, 525–534. [Google Scholar] [CrossRef]
- Chaduc, I.; Crepet, A.; Boyron, O.; Charleux, B.; D’Agosto, F.; Lansalot, M. Effect of the pH on the RAFT Polymerization of Acrylic Acid in Water. Application to the Synthesis of Poly(acrylic acid)-Stabilized Polystyrene Particles by RAFT Emulsion Polymerization. Macromolecules 2013, 46, 6013–6023. [Google Scholar] [CrossRef]
- Chaduc, I.; Girod, M.; Antoine, R.; Charleux, B.; D’Agosto, F.; Lansalot, M. Batch Emulsion Polymerization Mediated by Poly(methacrylic acid) MacroRAFT Agents: One-Pot Synthesis of Self-Stabilized Particles. Macromolecules 2013, 45, 5881–5893. [Google Scholar] [CrossRef]
- Lansalot, M.; Farcet, C.; Charleux, B.; Vairon, J.-P.; Pirri, R. Controlled Free-Radical Miniemulsion Polymerization of Styrene Using Degenerative Transfer. Macromolecules 1999, 32, 7354–7360. [Google Scholar] [CrossRef]
- Chong, Y.K.; Krstina, J.; Le, T.P.T.; Moad, G.; Postma, A.; Rizzardo, E.; Thang, S.H. Thiocarbonylthio Compounds [SC(Ph)S−R] in Free Radical Polymerization with Reversible Addition-Fragmentation Chain Transfer (RAFT Polymerization). Role of the Free-Radical Leaving Group (R). Macromolecules 2003, 36, 2256–2272. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Ma, J.; Guo, C.; Tang, Y.; Wang, J.; Zheng, L.; Liang, X.; Chen, S.; Liu, H. Microenvironmental and conformational structure of triblock copolymers in aqueous solution by 1H and 13C NMR spectroscopy. J. Colloid Interface Sci. 2006, 299, 953–961. [Google Scholar] [CrossRef]
- Porsch, C.; Hansson, S.; Nordgren, N.; Malmström, E.E. Thermo-responsive cellulose-based architectures: Tailoring LCST using poly(ethylene glycol) methacrylates. Polym. Chem. 2011, 2, 1114. [Google Scholar] [CrossRef]
- Schild, H.G.; Tirrell, D.A. Microcalorimetric detection of lower critical solution temperatures in aqueous polymer solutions. J. Phys. Chem. 1990, 94, 4352–4356. [Google Scholar] [CrossRef]
- Ray, A.; Das, S.; Chattopadhyay, N. Aggregation of Nile Red in Water: Prevention through Encapsulation in β-Cyclodextrin. ACS Omega 2019, 4, 15–24. [Google Scholar] [CrossRef]
- Zhu, C.; Xia, Y.; Zai, Y.; Dai, Y.; Liu, X.; Bian, J.; Liu, Y.; Liu, J.; Li, G. Adsorption and desorption behaviors of HPEI and thermo-responsive HPEI based gels on anionic and cationic dyes. Chem. Eng. J. 2019, 369, 863–873. [Google Scholar] [CrossRef]
- Zhou, J.; Hao, B.; Wang, L.; Ma, J.; Cheng, W. Preparation and characterization of nano-TiO2/chitosan/poly(N-isopropylacrylamide) composite hydrogel and its application for removal of ionic dyes. Sep. Purif. Technol. 2017, 176, 193–199. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, H.W.; Ma, W. Spectrophotometric Determination of Cationic Surfactant with Titan Yellow. Asian J. Chem. 2013, 25, 2736–2738. [Google Scholar] [CrossRef]
- Salem, M.A.; Salem, I.A.; Hanfy, M.G.; Zaki, A.B. Removal of Titan Yellow Dye From Aqueous Solution By Polyaniline/Fe3O4 Nanocomposite. Eur. Chem. Bull. 2016, 5, 113–118. [Google Scholar]
- Shi, Q.X.; Li, Y.; Wang, L.; Wang, J.; Cao, Y.L. Preparation of supported chitosan adsorbent with high adsorption capacity for Titan Yellow removal. Int. J. Biol. Macromol. 2020, 152, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, X.; Qin, D.; Da, W.; Hou, B.; Hao, C.; Wu, J.; Jingbo, W. Construction of magnetic lignin-based adsorbent and its adsorption properties for dyes. J. Hazard. Mater. 2019, 369, 50–61. [Google Scholar] [CrossRef]
- Lutz, J.F.; Akdemir, Ö.; Hoth, A. Point by Point Comparison of Two Thermosensitive Polymers Exhibiting a Similar LCST: Is the Age of Poly(NIPAM) Over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef] [PubMed]
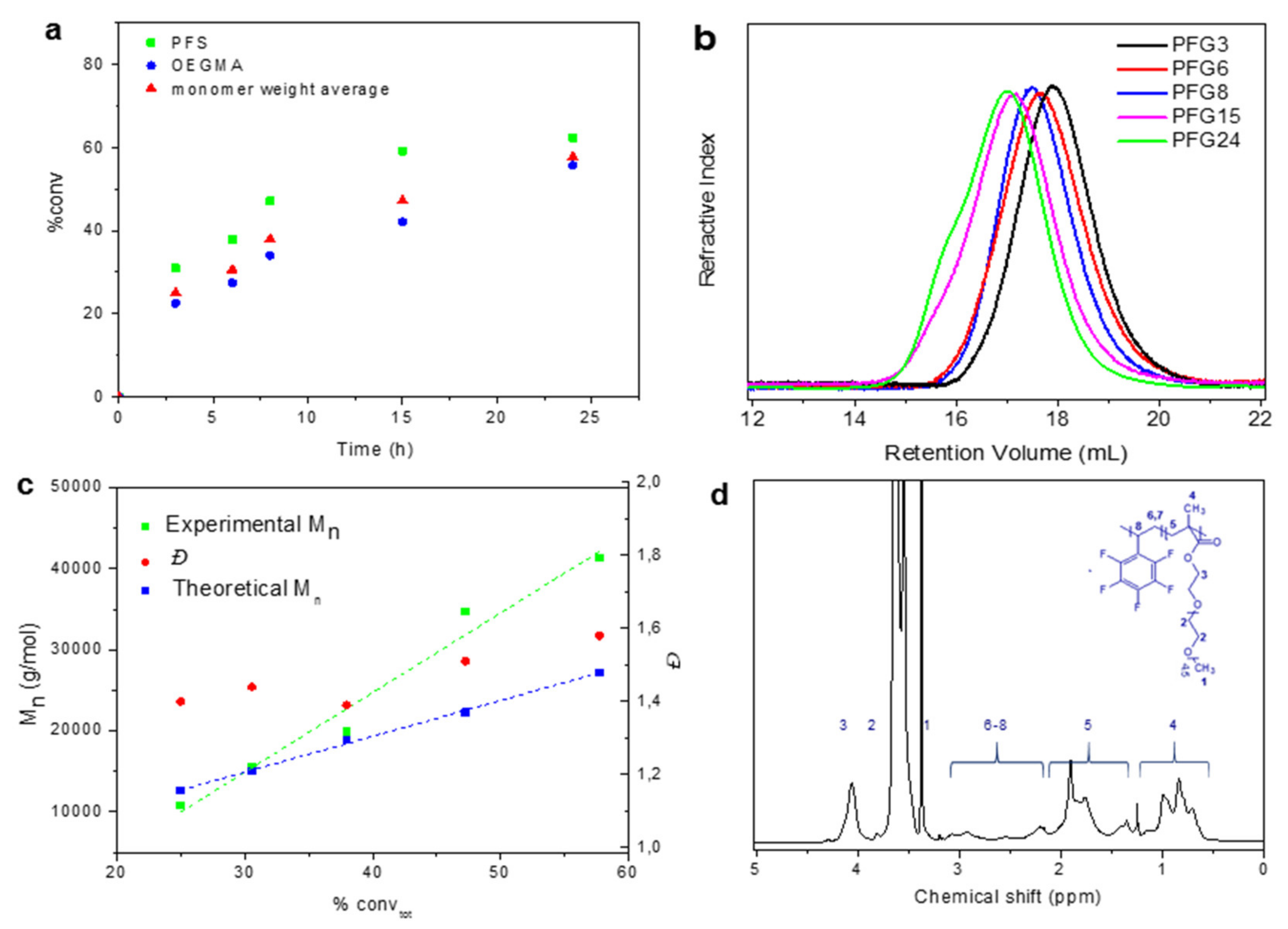


| Sample | Time (h) | PFS/OEGMAtheo a | PFS/OEGMA b | Mn theo c (Da) | Mnd (Da) | Ð | LCSTe (°C) | Dhf (nm) |
|---|---|---|---|---|---|---|---|---|
| PFG3 | 3 | 0.59 | 0.50 | 12,613 | 10,840 | 1.40 | 26.1 ± 0.6 | 750 ± 25 |
| PFG6 | 6 | 0.59 | 0.57 | 15,022 | 15,500 | 1.44 | 26.0 ± 0.5 | 1415 ± 46 |
| PFG8 | 8 | 0.59 | 0.62 | 18,931 | 19,900 | 1.39 | 26.8 ± 0.8 | 1363 ± 38 |
| PFG15 | 15 | 0.60 | 0.60 | 22,300 | 34,660 | 1.51 | 27.5 ± 0.7 | 1290 ± 68 |
| PFG24 | 24 | 0.50 | 0.56 | 27,176 | 41,350 | 1.58 | 30.2 ± 0.4 | 565 ± 37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuppardi, F.; Malinconico, M.; D’Agosto, F.; D’Ayala, G.G.; Cerruti, P. Well-Defined Thermo-Responsive Copolymers Based on Oligo(Ethylene Glycol) Methacrylate and Pentafluorostyrene for the Removal of Organic Dyes from Water. Nanomaterials 2020, 10, 1779. https://doi.org/10.3390/nano10091779
Zuppardi F, Malinconico M, D’Agosto F, D’Ayala GG, Cerruti P. Well-Defined Thermo-Responsive Copolymers Based on Oligo(Ethylene Glycol) Methacrylate and Pentafluorostyrene for the Removal of Organic Dyes from Water. Nanomaterials. 2020; 10(9):1779. https://doi.org/10.3390/nano10091779
Chicago/Turabian StyleZuppardi, Federica, Mario Malinconico, Franck D’Agosto, Giovanna Gomez D’Ayala, and Pierfrancesco Cerruti. 2020. "Well-Defined Thermo-Responsive Copolymers Based on Oligo(Ethylene Glycol) Methacrylate and Pentafluorostyrene for the Removal of Organic Dyes from Water" Nanomaterials 10, no. 9: 1779. https://doi.org/10.3390/nano10091779
APA StyleZuppardi, F., Malinconico, M., D’Agosto, F., D’Ayala, G. G., & Cerruti, P. (2020). Well-Defined Thermo-Responsive Copolymers Based on Oligo(Ethylene Glycol) Methacrylate and Pentafluorostyrene for the Removal of Organic Dyes from Water. Nanomaterials, 10(9), 1779. https://doi.org/10.3390/nano10091779








