Preparation of PBS/PLLA/HAP Composites by the Solution Casting Method: Mechanical Properties and Biocompatibility
Abstract
1. Introduction
2. Experimental Method
2.1. Chemicals and Materials
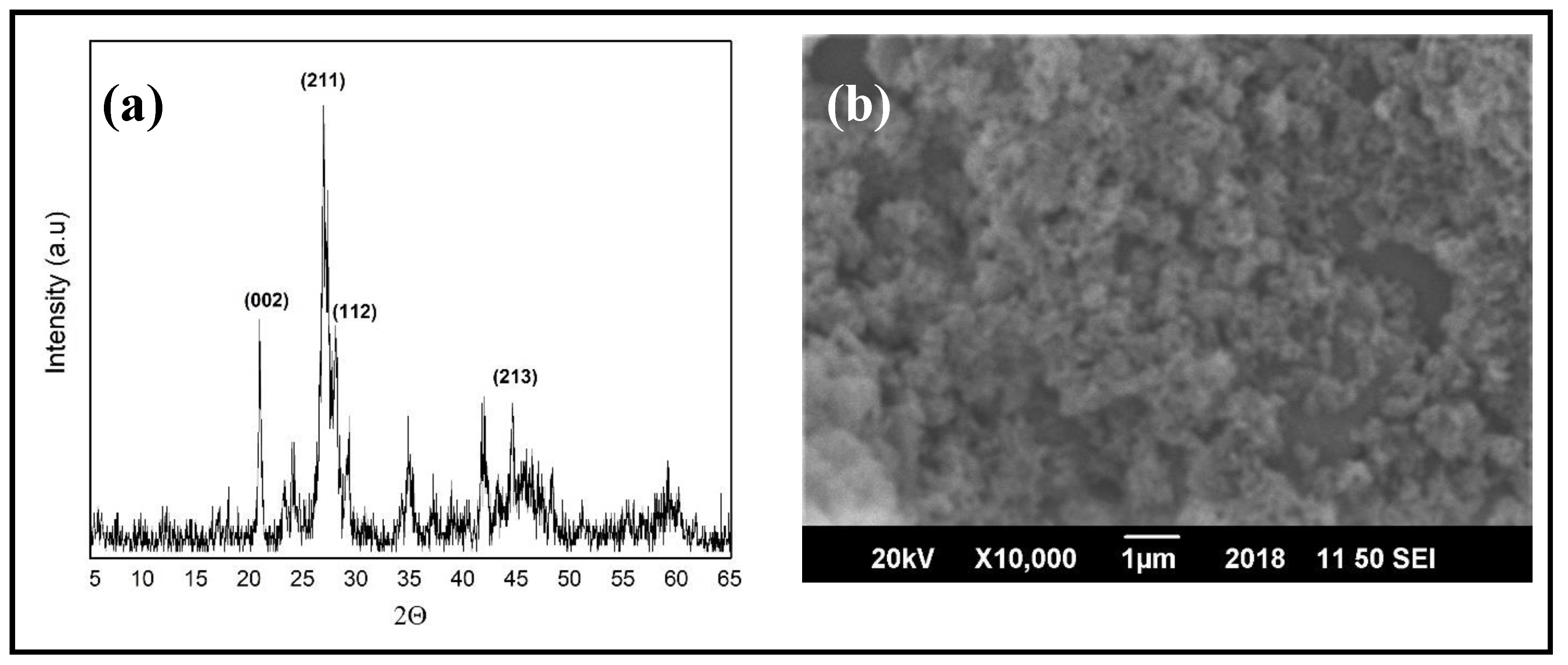
2.2. Synthesis of Hydroxyapatite Nanoparticles (HAPs)
- A total of 130 g of calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) was dissolved in 800 mL distilled water, followed by continuous stirring, while maintaining the temperature at 80 °C and pH at 12 by adding ammonia solution;
- Separately, 38 g of di-ammonium hydrogen phosphate ((NH4)2HPO4) was dissolved in 550 mL distilled water under similar conditions;
- Ca(NO3)2·4H2O solution was added drop wise to (NH4)2HPO4 solution and the obtained solution was stirred for 2 h at 80 °C;
- For the precipitation of HAPs, the obtained solution was left for 24 h at room temperature and afterwards, was repeatedly washed with distilled water;
- After washing, the solution was filtered and the obtained powder (HAPs) was dried at 80 °C for 24 h. The dried powder was calcined at 550 °C for 3 h.
2.3. Preparation of PBS/PLLA Polymer Blends
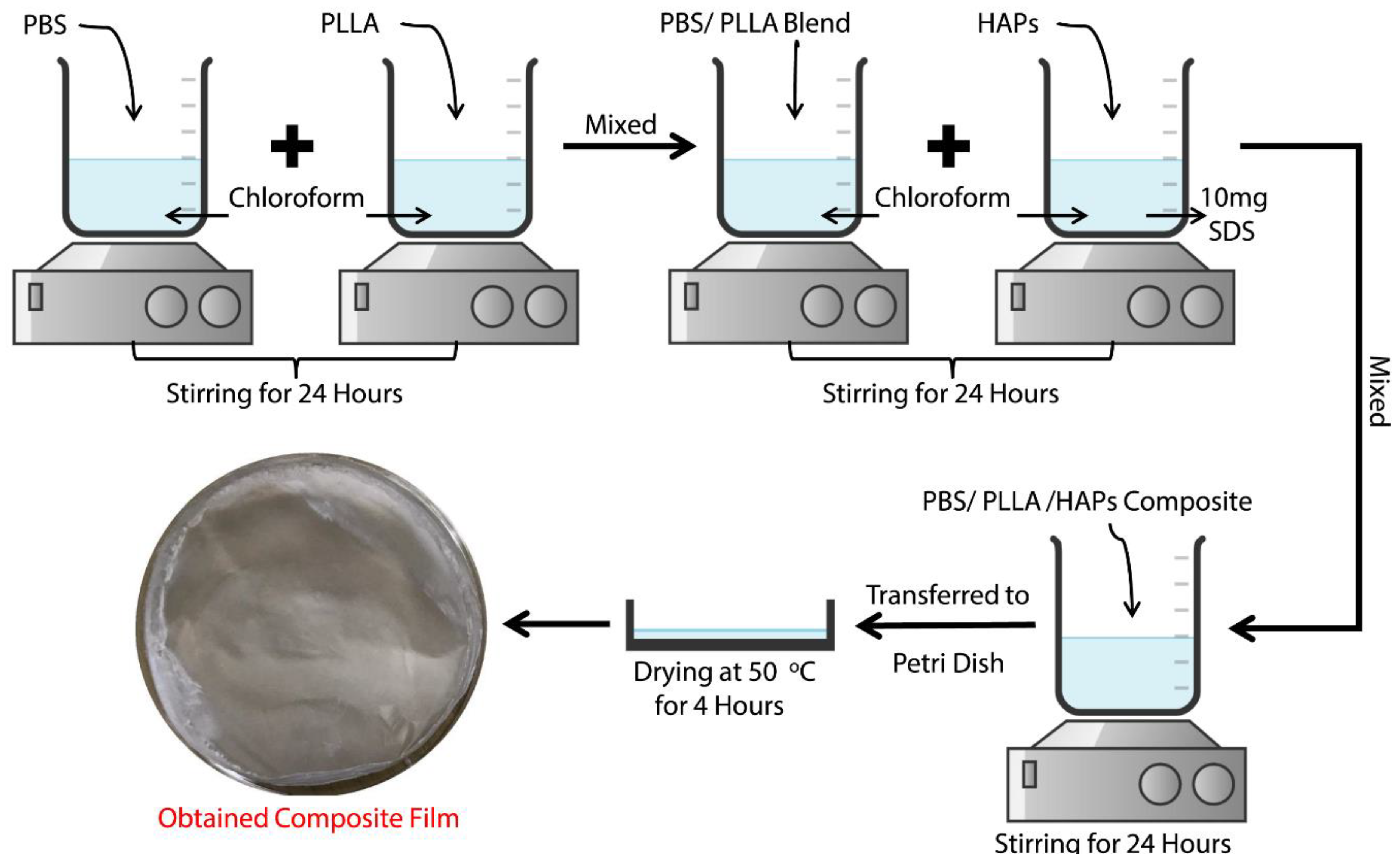
2.4. Preparation of the PBS/PLLA/HAP Composite
2.5. Characterization
3. Results and Discussion
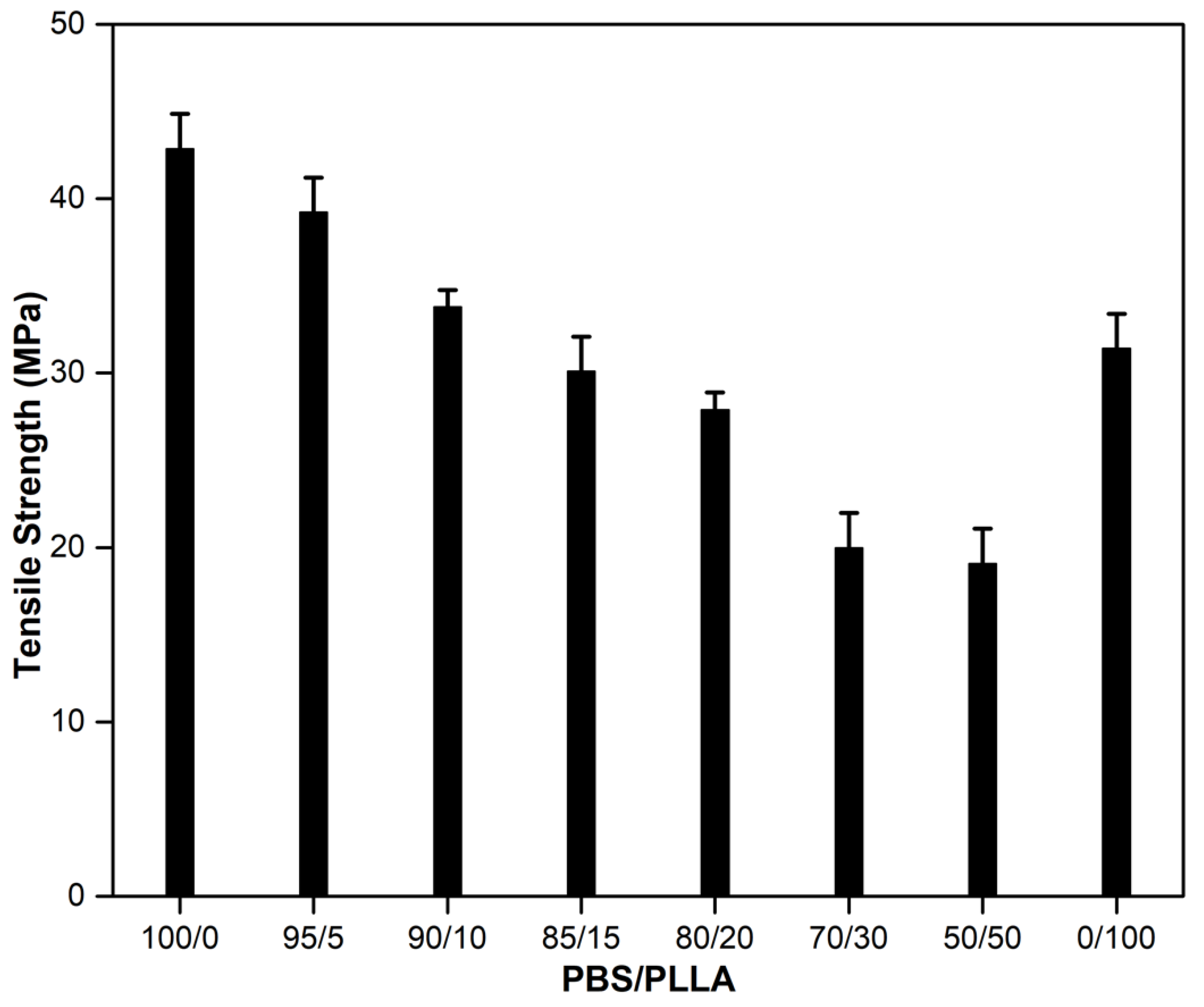
3.1. Mechanical Properties of PBS/PLLA Polymer Blends and PBS/PLLA/HAP Composites
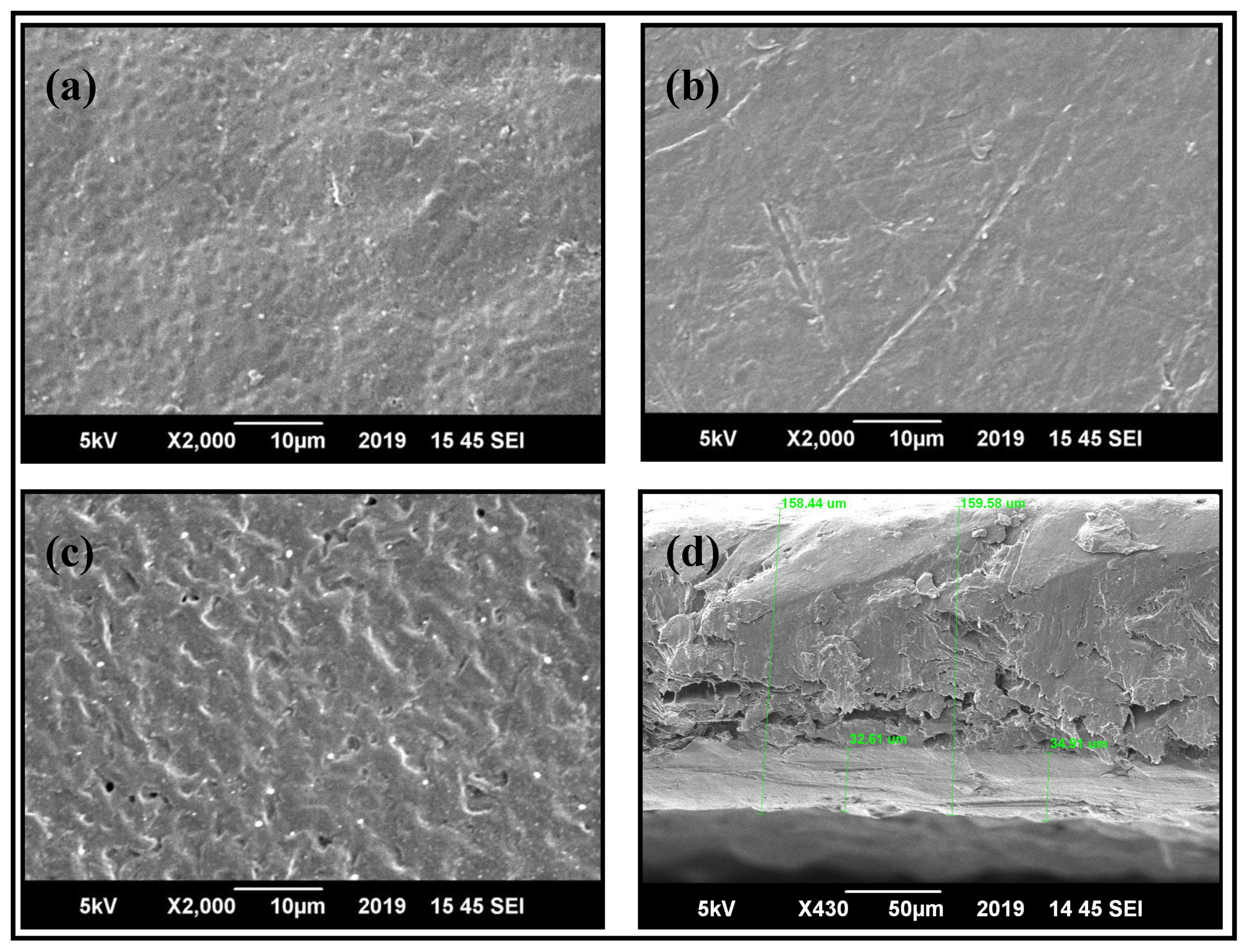
3.2. Morphological Studies of PBS/PLLA Polymer Blends and PBS/PLLA/HAP Composites
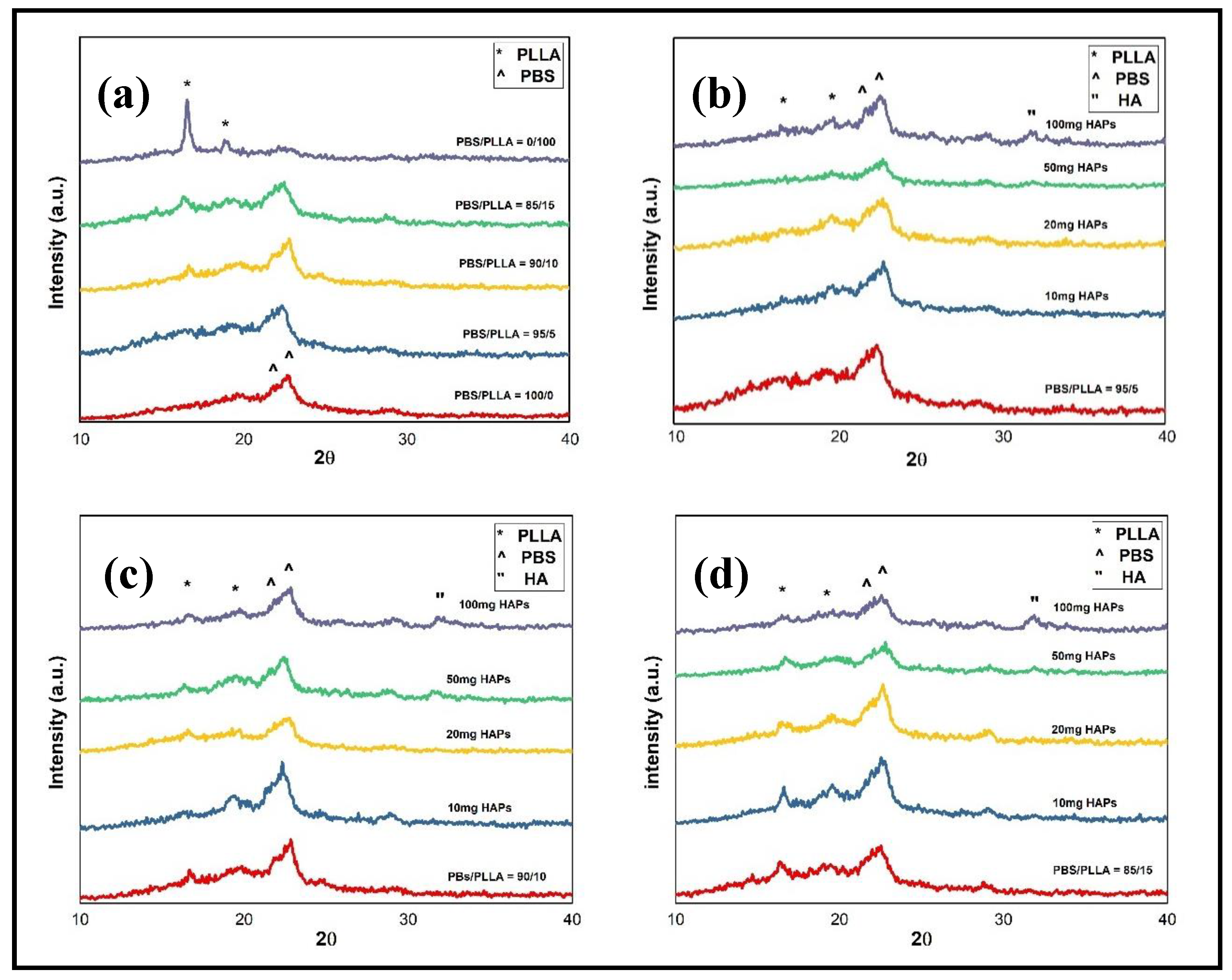
3.3. Structural Studies of PBS/PLLA Polymer Blends and PBS/PLLA/HAP Composites
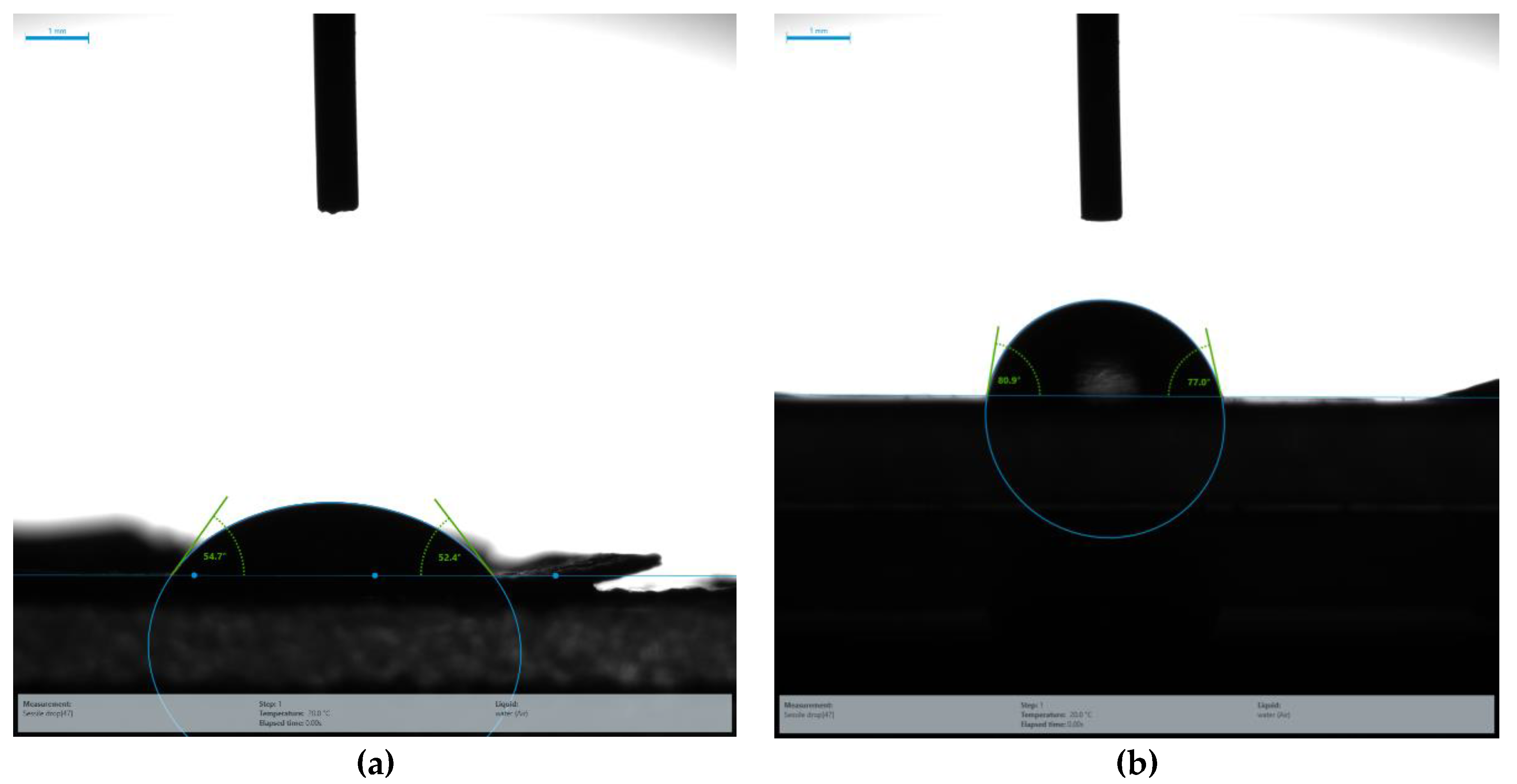
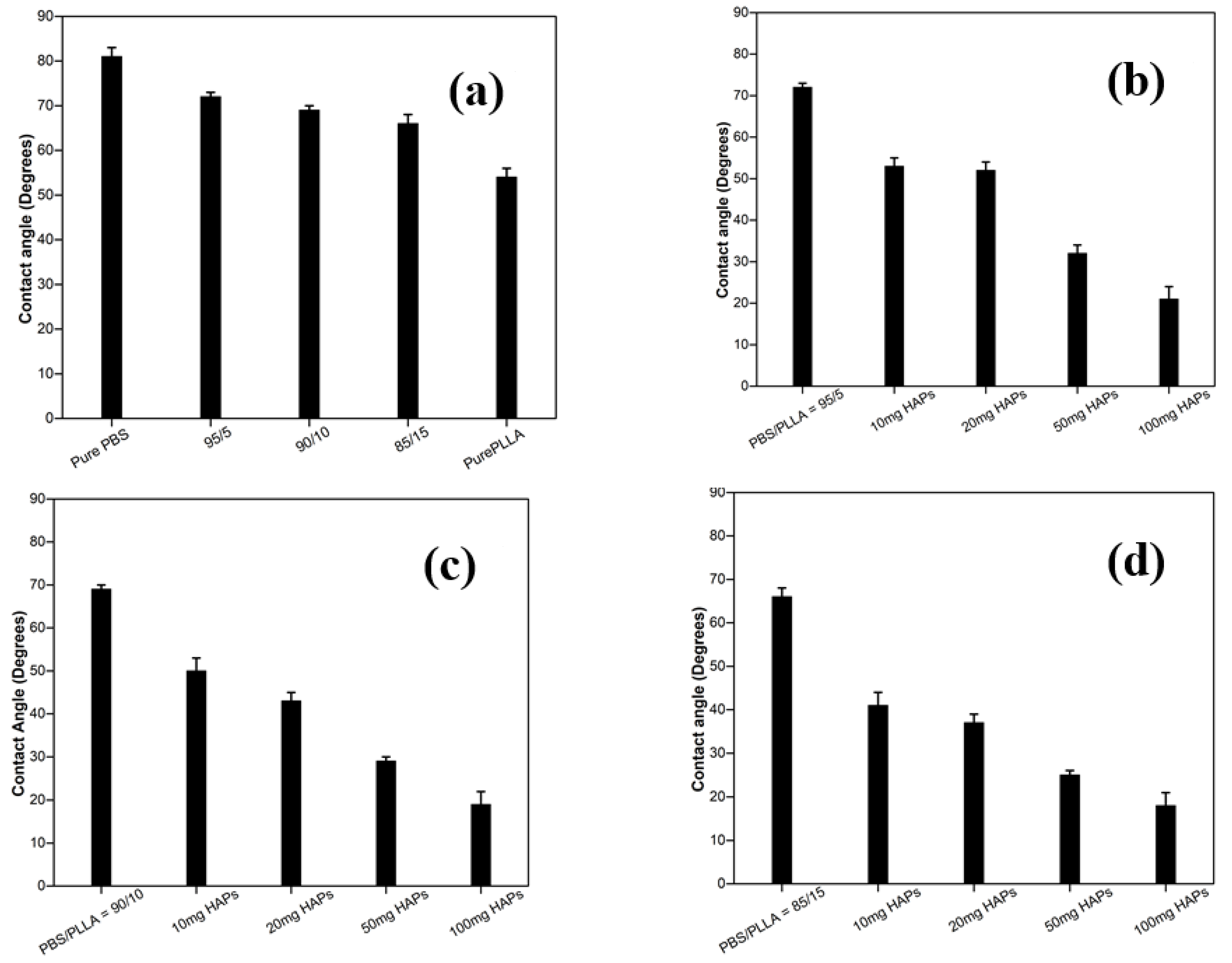
3.4. Contact Angle Analysis of PBS/PLLA Polymer Blends and PBS/PLLA/HAP Composites
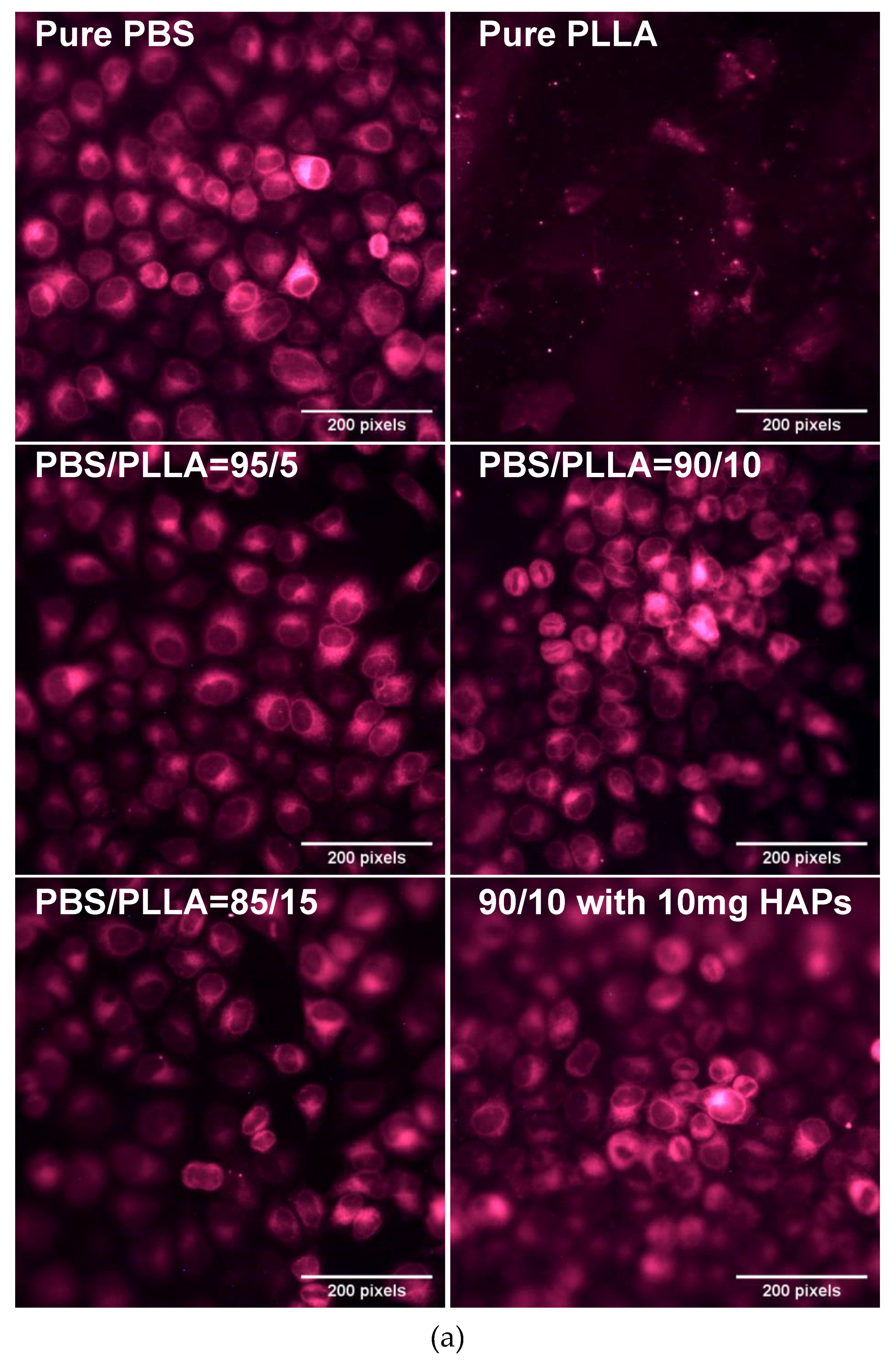
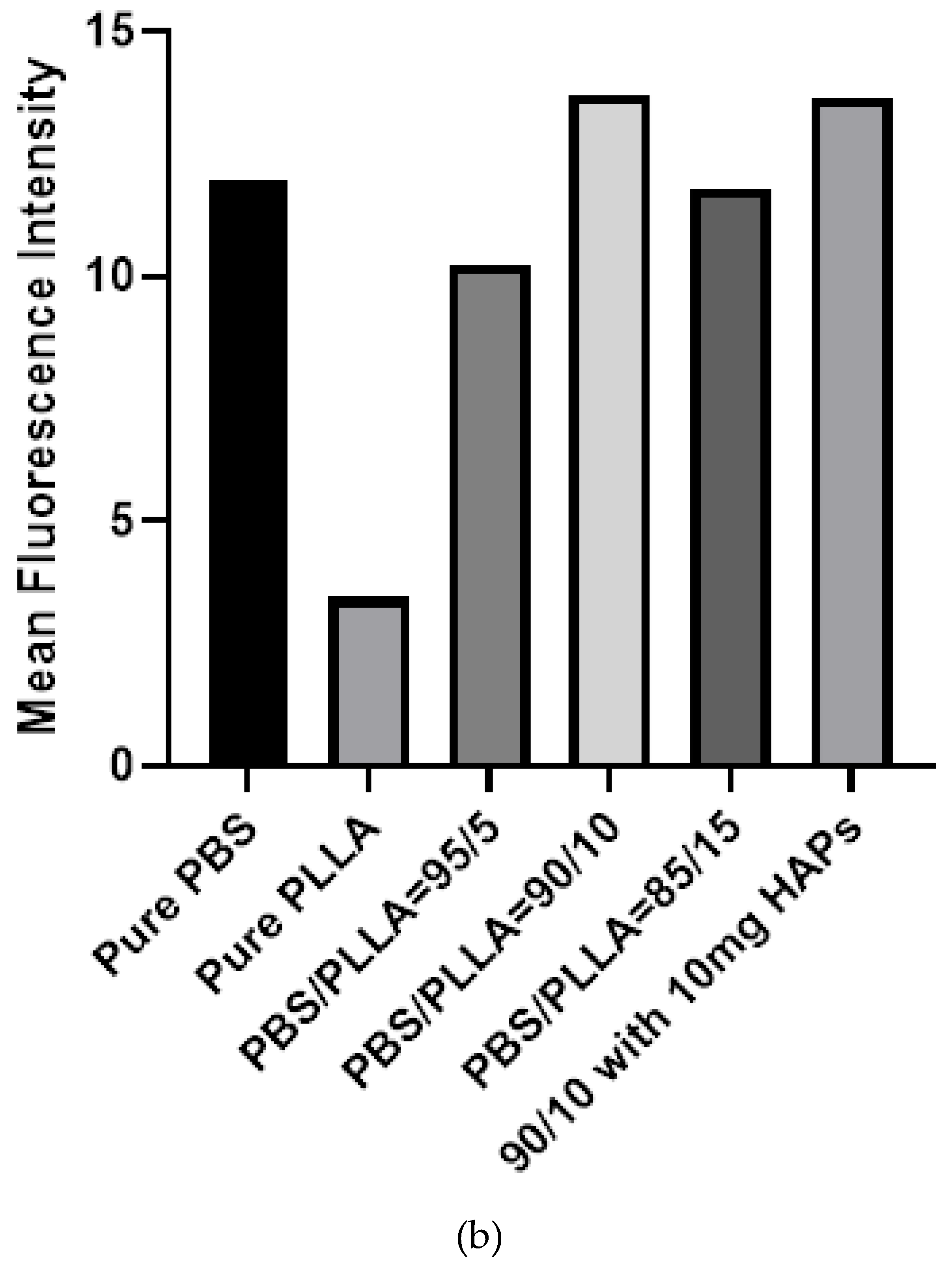
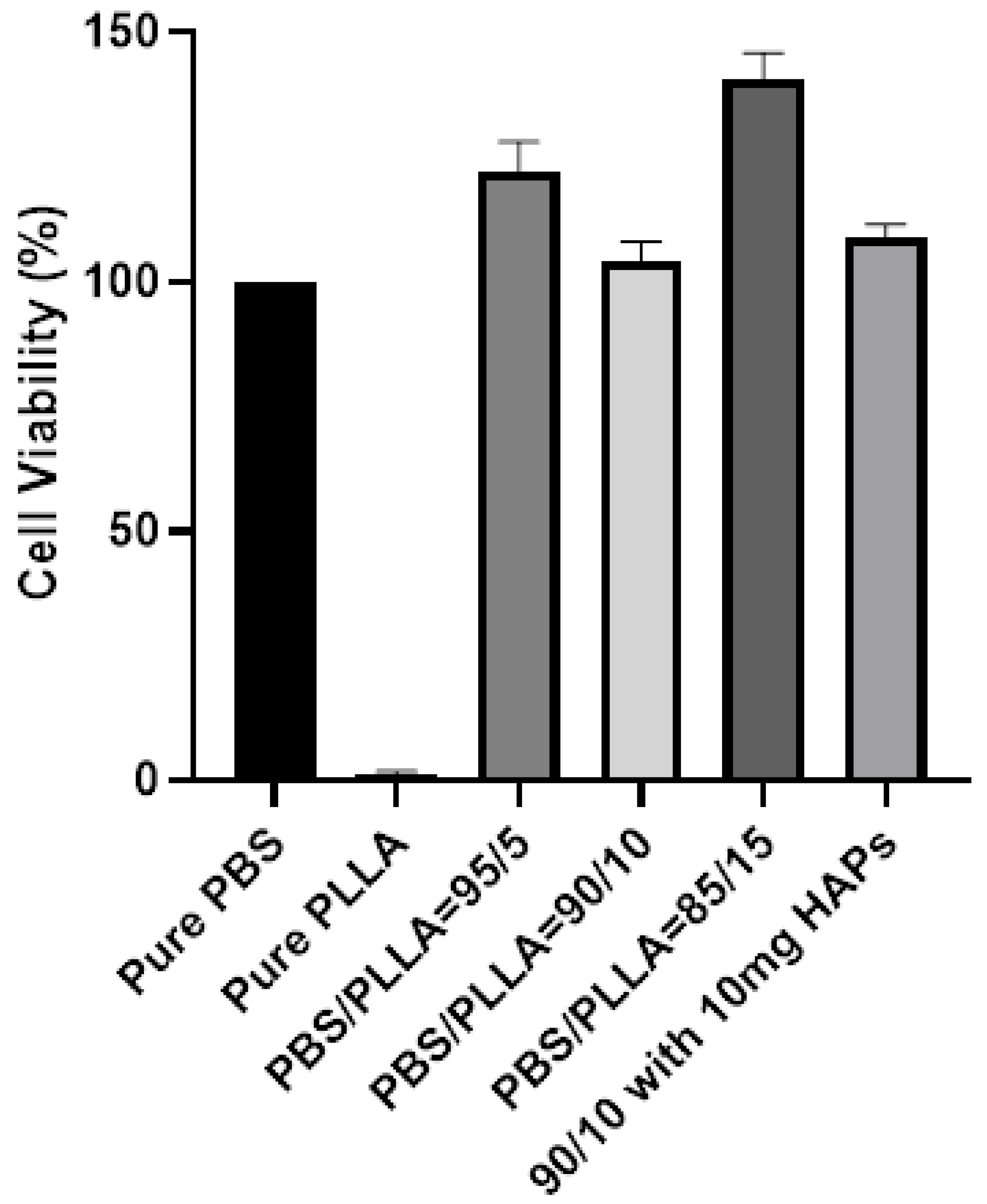
3.5. Biological Response of PBS/PLLA Polymer Blends and PBS/PLLA/HAP Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pneumaticos, S.G.; Triantafyllopoulos, G.K.; Basdra, E.K.; Papavassiliou, A.G. Segmental bone defects: From cellular and molecular pathways to the development of novel biological treatments. J. Cell. Mol. Med. 2010, 14, 2561–2569. [Google Scholar] [CrossRef]
- Allo, B.A.; Costa, D.O.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactive and biodegradable nanocomposites and hybrid biomaterials for bone regeneration. J. Funct. Biomater. 2012, 3, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds based bone tissue engineering: The role of chitosan. Tissue Eng. Part B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Boroujeni, N.M.; Zhou, H.; Luchini, T.J.; Bhaduri, S.B. Development of monetite/phosphorylated chitosan composite bone cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Hesaraki, S.; Nezafati, N. In vitro biocompatibility of chitosan/hyaluronic acid-containing calcium phosphate bone cements. Bioprocess Biosyst. Eng. 2014, 37, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Dong, Y.; Chen, X.; Lin, R. Ectopic osteogenesis and scaffold biodegradation of tissue engineering bone composed of chitosan and osteo-induced bone marrow mesenchymal stem cellsin vivo. Chin. Med. J. 2014, 127, 322–328. [Google Scholar]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Mikos, A.G. Mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007, 13, 927–938. [Google Scholar] [CrossRef]
- di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef]
- Gautam, S.; Chou, C.-F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater. Sci. Eng. C 2014, 34, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A. Advent and maturation of regenerative medicine. Tissue Eng. Regen. Med. 2013, 10, 155–159. [Google Scholar] [CrossRef]
- Lickorish, D.; Guan, L.; Davies, J. A three-phase, fully resorbable, polyester/calcium phosphate scaffold for bone tissue engineering: Evolution of scaffold design. Biomaterials 2007, 28, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.; Blaker, J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef]
- Liaqat, U.; Ko, H.; Suh, H.; Lee, M.; Pyun, J.-C. Surface modification of parylene-N films for the culture of osteoblast-like cells (MG-63). Appl. Surf. Sci. 2016, 378, 277–285. [Google Scholar] [CrossRef]
- Liaqat, U.; Ko, H.; Suh, H.; Lee, M.; Pyun, J.-C. UV-irradiated parylene surfaces for proliferation and differentiation of PC-12 cells. Enzym. Microb. Technol. 2017, 97, 1–10. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Hua, K.; Zhang, W.; Ji, J.; Yang, X. Enhanced mechanical properties and degradability of poly (butylene succinate) and poly (lactic acid) blends. Iran. Polym. J. 2013, 22, 267–275. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Mikos, A.G. From material to tissue: Biomaterial development, scaffold fabrication, and tissue engineering. AIChE J. 2008, 54, 3048–3067. [Google Scholar] [CrossRef] [PubMed]
- Román, J.S.; García, P.G. Partially biodegradable polyacrylic-polyester composites for internal bone fracture fixation. Biomaterials 1991, 12, 236–241. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Yoon, S.-D.; Kwon, Y.-S.; Lee, K.-S. Biodegradation and biocompatibility of poly L-lactic acid implantable mesh. Int. Neurourol. J. 2017, 21, S48. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Costa-Pinto, A.; Correlo, V.; Sol, P.; Bhattacharya, M.; Srouji, S.; Livne, E.; Reis, R.; Neves, N. Chitosan-poly (butylene succinate) scaffolds and human bone marrow stromal cells induce bone repair in a mouse calvaria model. J. Tissue Eng. Regen. Med. 2012, 6, 21–28. [Google Scholar] [CrossRef]
- Wang, H.; Ji, J.; Zhang, W.; Zhang, Y.; Jiang, J.; Wu, Z.; Pu, S.; Chu, P.K. Biocompatibility and bioactivity of plasma-treated biodegradable poly (butylene succinate). Acta Biomater. 2009, 5, 279–287. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.; Bikiaris, D. Thermal degradation mechanism of poly (ethylene succinate) and poly (butylene succinate): Comparative study. Thermochim. Acta 2005, 435, 142–150. [Google Scholar] [CrossRef]
- van Blitterswijk, C.; Hesseling, S.; Grote, J.; Koerten, H.; de Groot, K. The biocompatibility of hydroxyapatite ceramic: A study of retrieved human middle ear implants. J. Biomed. Mater. Res. 1990, 24, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Vagaská, B.; Bačáková, L.; Filovaá, E.; Balík, K. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol. Res. 2010, 59. [Google Scholar]
- Guo, W.; Zhang, Y.; Zhang, W. Mechanical properties and crystallization behavior of hydroxyapatite/poly (butylenes succinate) composites. J. Biomed. Mater. Res. Part A 2013, 101, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, S.; el Sawi, I.; Bagheri, Z.S.; Turcotte, G.; Bougherara, H. Fabrication and characterization of novel biomimetic PLLA/cellulose/hydroxyapatite nanocomposite for bone repair applications. Mater. Sci. Eng. C 2014, 39, 120–125. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Liu, J.; Zhu, W.; Wang, D. Investigation of the in vitro degradation of a novel polylactide/nanohydroxyapatite composite for artificial bone. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Li, G.; Qin, S.; Liu, X.; Zhang, D.; He, M. Structure and properties of nano-hydroxyapatite/poly (butylene succinate) porous scaffold for bone tissue engineering prepared by using ethanol as porogen. J. Biomater. Appl. 2019, 33, 776–791. [Google Scholar] [CrossRef]
- Khalid, M.; Mujahid, M.; Amin, S.; Rawat, R.; Nusair, A.; Deen, G. Effect of surfactant and heat treatment on morphology, surface area and crystallinity in hydroxyapatite nanocrystals. Ceram. Int. 2013, 39, 39–50. [Google Scholar] [CrossRef]
- Chen, G.-X.; Kim, H.-S.; Kim, E.-S.; Yoon, J.-S. Compatibilization-like effect of reactive organoclay on the poly (L-lactide)/poly (butylene succinate) blends. Polymer 2005, 46, 11829–11836. [Google Scholar] [CrossRef]
- Zamiri, A.; De, S. Mechanical properties of hydroxyapatite single crystals from nanoindentation data. J. Mech. Behav. Biomed. Mater. 2011, 4, 146–152. [Google Scholar] [CrossRef]
- Aronov, D.; Rosen, R.; Ron, E.; Rosenman, G. Tunable hydroxyapatite wettability: Effect on adhesion of biological molecules. Process Biochem. 2006, 41, 2367–2372. [Google Scholar] [CrossRef]
- Tihan, T.G.; Ionita, M.D.; Popescu, R.G.; Iordachescu, D. Effect of hydrophilic–hydrophobic balance on biocompatibility of poly (methyl methacrylate) (PMMA)–hydroxyapatite (HA) composites. Mater. Chem. Phys. 2009, 118, 265–269. [Google Scholar] [CrossRef]

| PBS/PLLA | HAPs (mg) | Tensile Strength (MPa) (Mean ± SD) |
|---|---|---|
| 95/5 | 0 | 39.2 ± 2.0 |
| 10 | 39.9 ± 2.8 | |
| 20 | 39.0 ± 1.7 | |
| 50 | 51.2 ± 1.3 | |
| 100 | 43.1 ± 0.8 | |
| 90/10 | 0 | 33.8 ± 1.0 |
| 10 | 56.9 ± 1.4 | |
| 20 | 45.0 ± 2.0 | |
| 50 | 23.9 ± 2.8 | |
| 100 | 29.6 ± 1.5 | |
| 85/15 | 0 | 30.1 ± 2.1 |
| 10 | 26.2 ± 1.9 | |
| 20 | 27.9 ± 0.2 | |
| 50 | 27.5 ± 1.1 | |
| 100 | 17.8 ± 2.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.; Hussain, Z.; Liaqat, U.; Liaqat, M.A.; Zahoor, M. Preparation of PBS/PLLA/HAP Composites by the Solution Casting Method: Mechanical Properties and Biocompatibility. Nanomaterials 2020, 10, 1778. https://doi.org/10.3390/nano10091778
Khan MA, Hussain Z, Liaqat U, Liaqat MA, Zahoor M. Preparation of PBS/PLLA/HAP Composites by the Solution Casting Method: Mechanical Properties and Biocompatibility. Nanomaterials. 2020; 10(9):1778. https://doi.org/10.3390/nano10091778
Chicago/Turabian StyleKhan, Muzamil Ahmad, Zakir Hussain, Usman Liaqat, Muhammad Arman Liaqat, and Muhammad Zahoor. 2020. "Preparation of PBS/PLLA/HAP Composites by the Solution Casting Method: Mechanical Properties and Biocompatibility" Nanomaterials 10, no. 9: 1778. https://doi.org/10.3390/nano10091778
APA StyleKhan, M. A., Hussain, Z., Liaqat, U., Liaqat, M. A., & Zahoor, M. (2020). Preparation of PBS/PLLA/HAP Composites by the Solution Casting Method: Mechanical Properties and Biocompatibility. Nanomaterials, 10(9), 1778. https://doi.org/10.3390/nano10091778





