Antimicrobial and Biocompatible Polycaprolactone and Copper Oxide Nanoparticle Wound Dressings against Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Bacterial Strains
2.2. Effect of CuONPs on MRSA Growth
2.3. Preparation of Active Films
2.4. Antimicrobial Activity of Active PCL Films
2.5. Characterization of the Active Films
2.6. Preparation of Active Extracts for Biological Activity
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Genotoxicity Assay
2.10. Copper Content Release from the Active Films to the Extracts
2.11. Hemocompatibility
2.12. Statistical Analysis
3. Results and Discussion
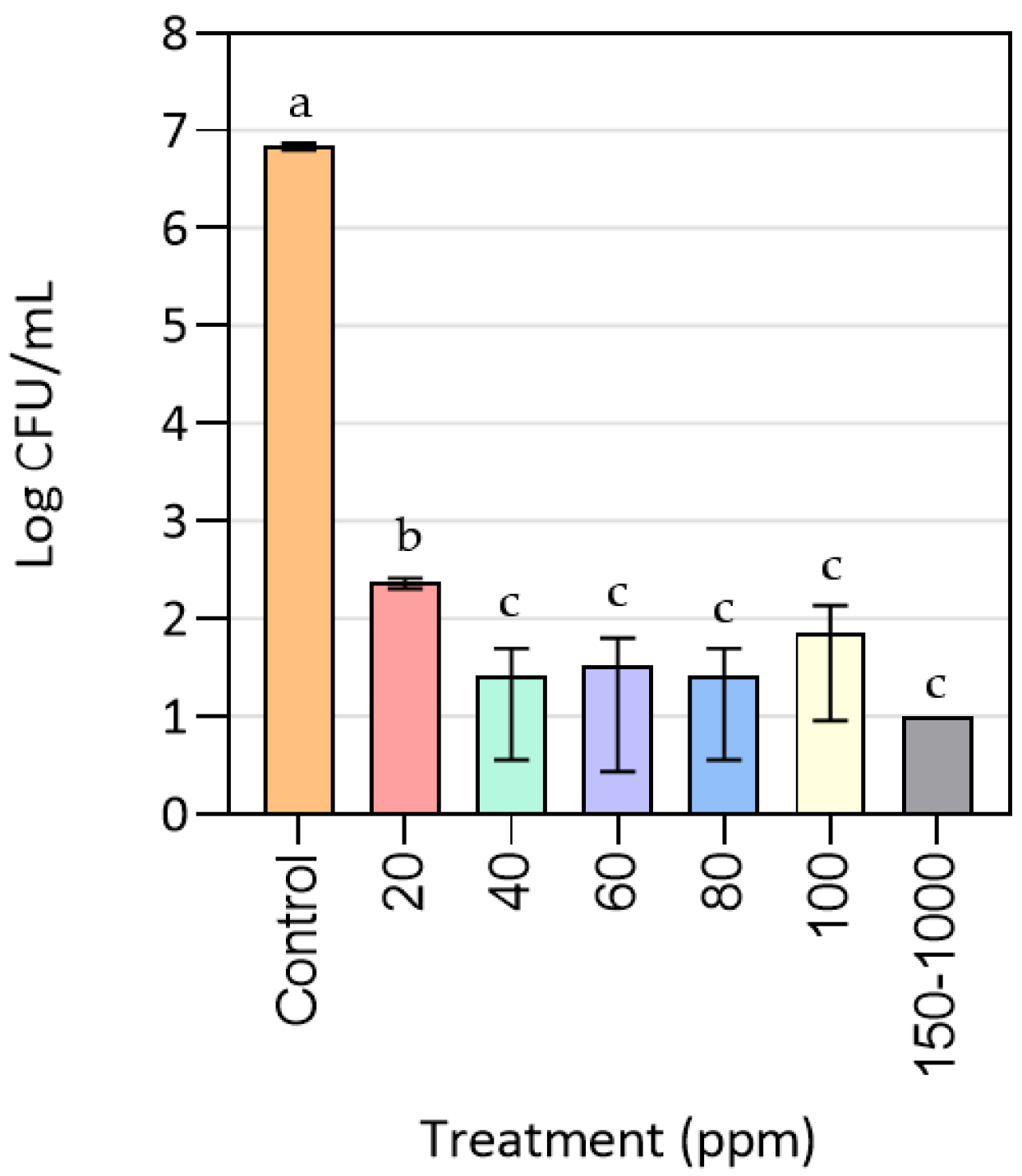
3.1. Effect of CuONPs on MRSA Growth
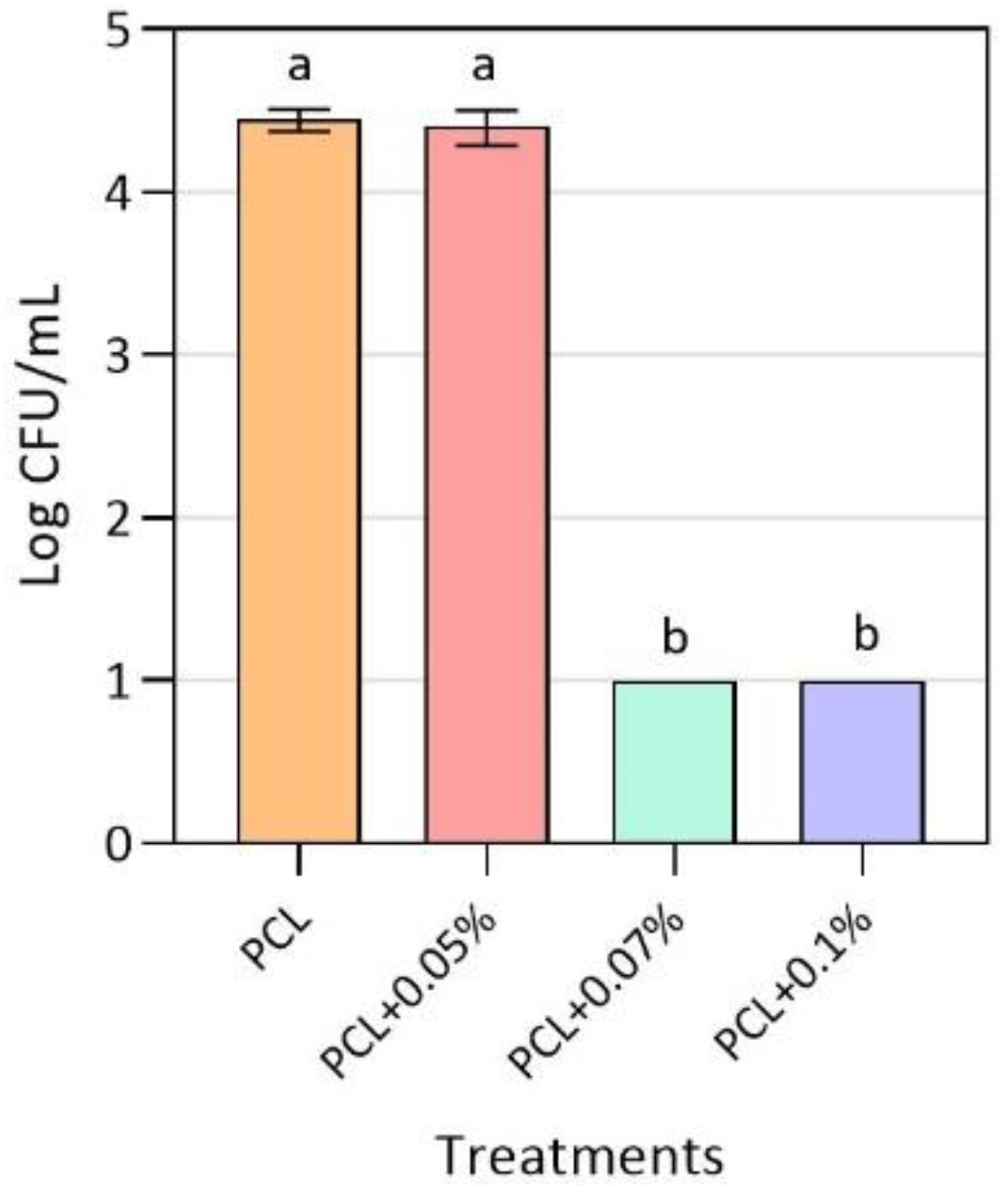
3.2. Antimicrobial Activity of Active PCL Films
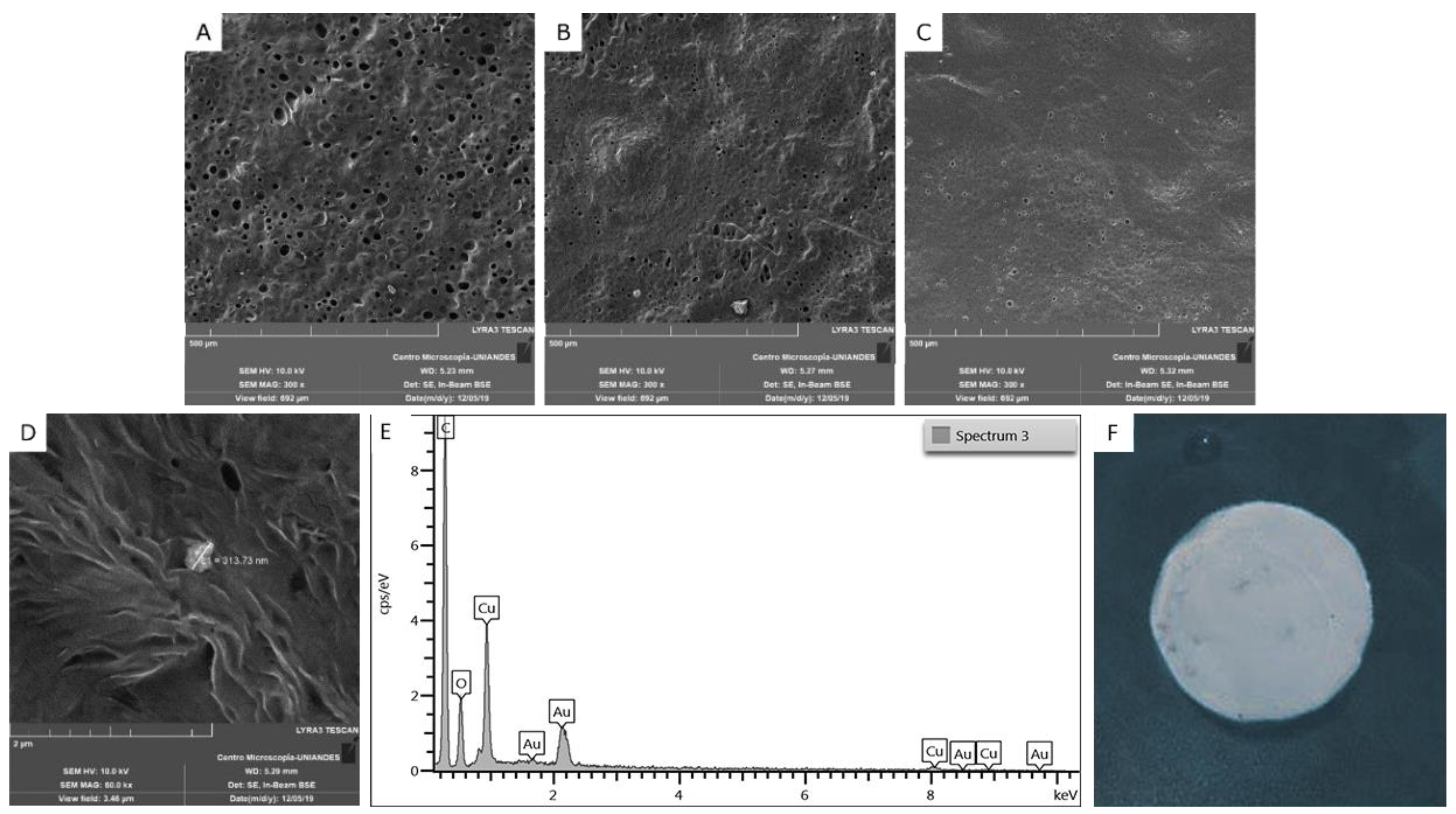
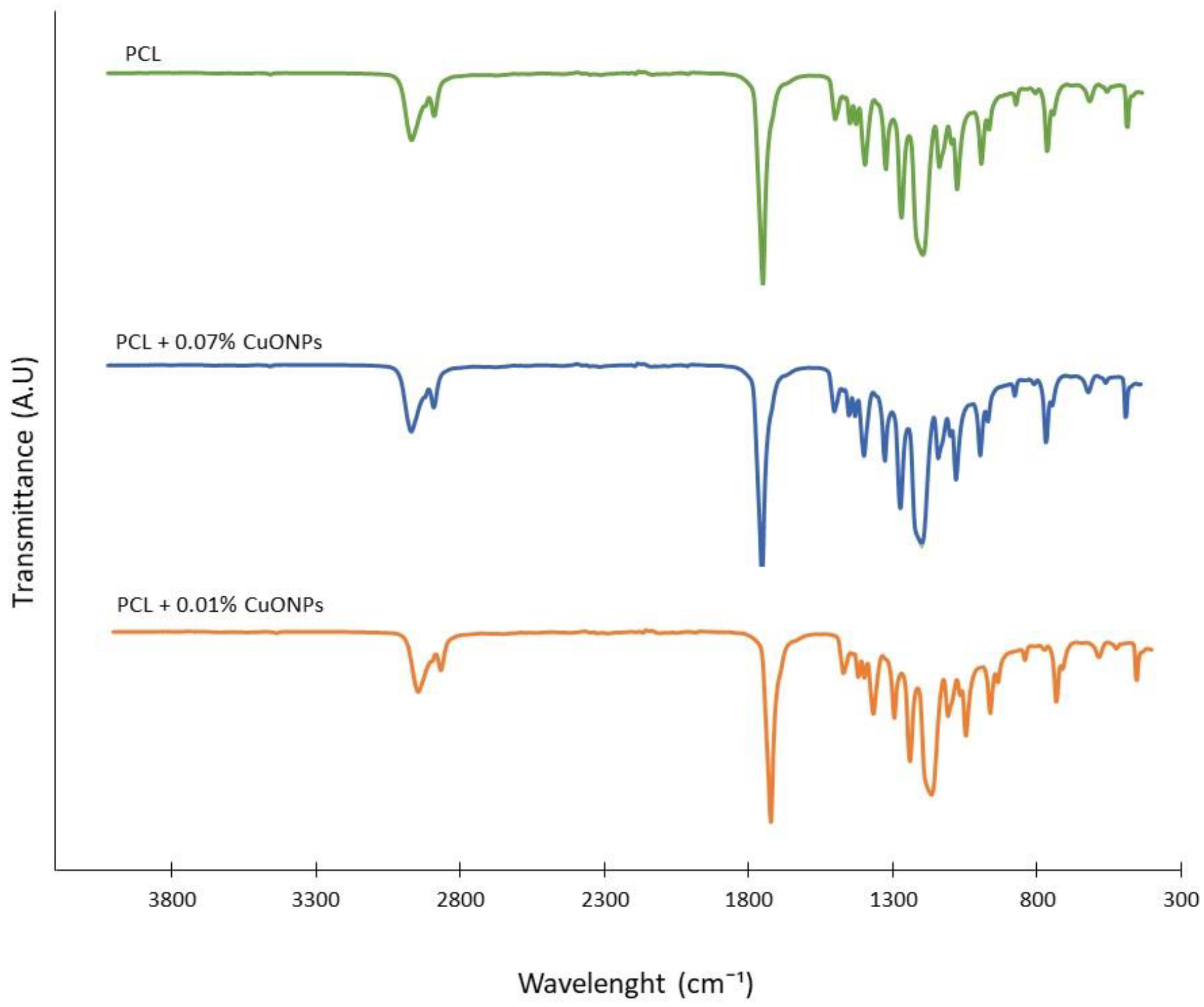
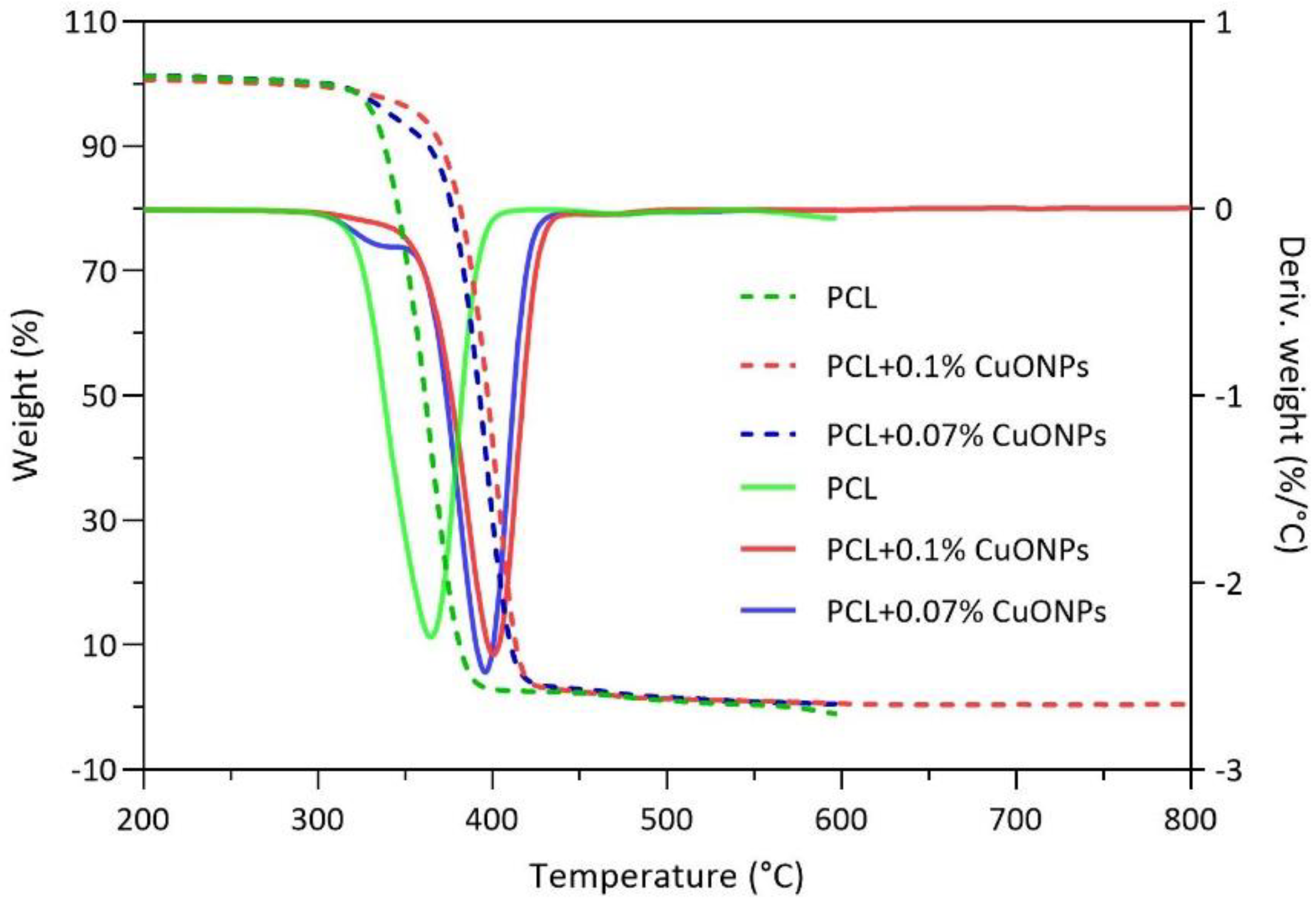
3.3. Films Characterization
3.4. Cell Viability Assays
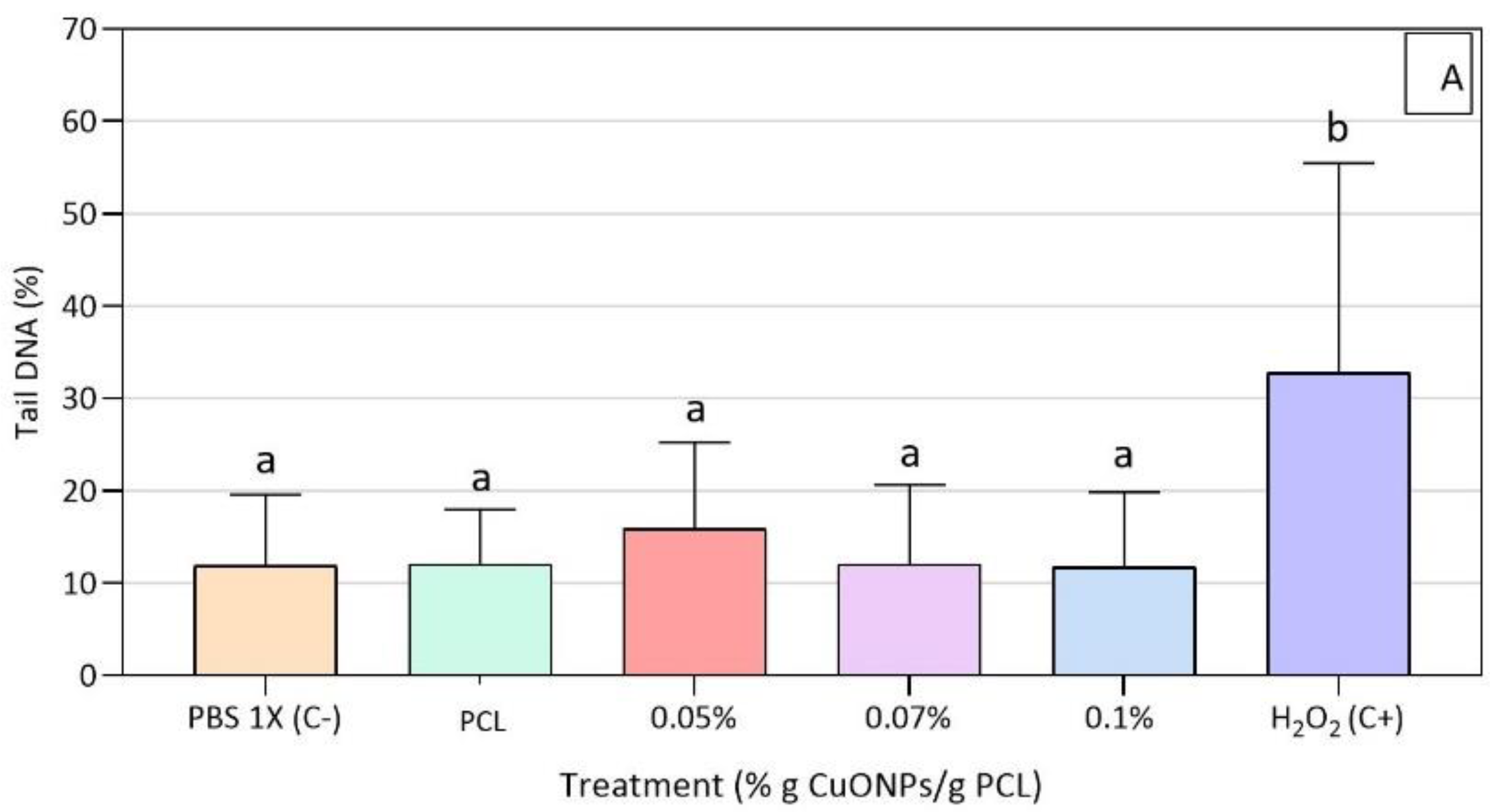
3.5. Genotoxic Effect of Active Extracts on HFF-1 Cells
3.6. CuONPs Content in the Active Extracts
3.7. Hemocompatibility Assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velázquez-Meza, M.E. Surgimiento y diseminación de Staphylococcus aureus meticilinorresistente. Salud Publ. Mex. 2005, 47, 381–387. [Google Scholar] [CrossRef]
- Ravichandran, P.; Chitti, S.P. Antimicrobial Dressing for Diabetic Foot Ulcer Colonized with MRSA. Online J. Biol. Sci. 2015, 15, 282–291. [Google Scholar] [CrossRef][Green Version]
- Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial Activity of Silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, S.; Elmi, A.; Hallaj Nezhadi, S. Copper Nanoparticles as Antibacterial Agents. J. Mol. Pharm. Org. Process Res. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Jain, P.; Pradeep, T. Potential of Silver Nanoparticle-Coated Polyurethane Foam As an Antibacterial Water Filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Park, J.; Osaka, T.; Park, S. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-zacheo, T.; Alessio, M.D.; Zambonin, P.G.; Traversa, E.; et al. Copper Nanoparticle/Polymer Composites with Antifungal and Bacteriostatic Properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Palza, H.; Quijada, R.; Delgado, K. Antimicrobial polymer composites with copper micro- and nanoparticles: Effect of particle size and polymer matrix. J. Bioact. Compat. Polym. 2015, 30, 366–380. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, J.; Borkow, G.; Mishal, J.; Magen, E.; Zatcoff, R.; Shemer-Avni, Y. Copper oxide impregnated textiles with potent biocidal activities. J. Ind. Text. 2006, 35, 323–335. [Google Scholar] [CrossRef]
- Borkow, G.; Zatcoff, R.C.; Gabbay, J. Reducing the risk of skin pathologies in diabetics by using copper impregnated socks. Med. Hypotheses 2009, 73, 883–886. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, K.K.; Shin, S.; Park, S.M.; Hah, S.S. Comparative toxicity studies of ultra-pure Ag, Au, Co, and Cu nanoparticles generated by laser ablation in biocompatible aqueous solution. Bull. Korean Chem. Soc. 2012, 33, 3265–3268. [Google Scholar] [CrossRef][Green Version]
- Castro-Mayorga, J.L.; Fabra, M.J.; Pourrahimi, A.M.; Olsson, R.T.; Lagaron, J.M. The impact of zinc oxide particle morphology as an antimicrobial and when incorporated in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging and food contact surfaces applications. Food Bioprod. Process. 2017, 101, 32–44. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: Synthesis, Properties, and Applications. Encycl. Polym. Sci. Technol. 2002, 1–36. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Downes, S. Physicochemical characterisation of degrading polycaprolactone scaffolds. Polym. Degrad. Stab. 2010, 95, 2269–2276. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Macosko, C.W.; Kweon, H.Y.; Yoo, M.K.; Park, I.K.; Kim, T.H.; Lee, H.C.S.; Oh, J.S.; Akaike, T.; Cho, C.S. A novel degradable polycarbonate networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar]
- Muñoz-Escobar, A.; Ruíz-Baltazar, Á.d.J.; Reyes-López, S.Y. Novel Route of Synthesis of PCL-CuONPs Composites With Antimicrobial Properties. Dose Response 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Escobar, N.M.O.; Quiroga, J.A.; Trujillo, T.G. Using Positive Deviance in the prevention and control of MRSA infections in a Colombian hospital: A time-series analysis. Epidemiol. Infect. 2019, 145, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Gianino, E.; Miller, C.; Gilmore, J. bioengineering Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Bharti, J.; Mathur, A. Study of the antimicrobial effect of the silver nanoparticles against biofilm producing Staphylococcus aureus strains. Int. J. Sci. Res. Publ. 2017, 7, 153–163. [Google Scholar]
- Standard, I. ISO 10993-4: Biological Evaluation of Medical Devices Part 4—Selection of Tests for Interactions with Blood; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Escobar, J.J.; Nino, V.A.T.; Bonilla, L.L.C.; Quintero, M.P.; Diaz, J.P.O.; Camargo, C.M.; Briceno, J.C. In vitro Biological Evaluation of Small Intestinal Submucosa—Chitosan/Hyaluronic Acid Tridimensional Scaffolds as Deep Wound Healing Dressings. Int. Semin. Biomed. Eng. 2018, 1–9. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Feichtmeier, N.S.; Ruchter, N.; Zimmermann, S.; Sures, B.; Leopold, K. A direct solid sampling analysis method for the detection of silver nanoparticles in biological matrices. Anal. Bioanal. Chem. 2016, 408, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Du, L.; Zhao, Y.T.; Tian, W.Q. In vitro hemocompatibility and cytotoxicity evaluation of halloysite nanotubes for biomedical application. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. 2013, 73, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Padalhin, A.R.; Ba Linh, N.T.; Ki Min, Y.; Lee, B.T. Evaluation of the cytocompatibility hemocompatibility in vivo bone tissue regenerating capability of different PCL blends. J. Biomater. Sci. Polym. Ed. 2014, 25, 487–503. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Kaur, A.; Fanourakis, G. Influence of Phosphate Dispersing Agents on Particle Size Distribution of Soil Fines; University of Johannesburg: Johannesburg, South Africa, 2016. [Google Scholar]
- Vielkind, M.; Kampen, I.; Kwade, A. Zinc Oxide Nanoparticles in Bacterial Growth Medium: Optimized Dispersion and Growth Inhibition of Pseudomonas putida. Adv. Nanopart. 2013, 2, 287–293. [Google Scholar] [CrossRef]
- Kaweeteerawat, C.; Chang, C.H.; Roy, K.R.; Liu, R.; Li, R.; Toso, D.; Fischer, H.; Ivask, A.; Ji, Z.; Zink, J.I.; et al. Cu Nanoparticles Have Different Impacts in Escherichia coli and Lactobacillus brevis than Their Microsized and Ionic Analogues. ACS Nano 2015, 9, 7215–7225. [Google Scholar] [CrossRef] [PubMed]
- Namburu, P.K.; Kulkarni, D.P.; Misra, D.; Das, D.K. Viscosity of copper oxide nanoparticles dispersed in ethylene glycol and water mixture. Exp. Therm. Fluid Sci. 2007, 32, 397–402. [Google Scholar] [CrossRef]
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.P.; Warszyński, P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Coll. Surf. B Biointerfaces 2015, 128, 17–22. [Google Scholar] [CrossRef]
- Ladomersky, E.; Petris, M.J. Copper tolerance and virulence in bacteria. Metallomics 2015, 7, 957–964. [Google Scholar] [CrossRef]
- Castro Mayorga, J.L.; Fabra Rovira, M.J.; Cabedo Mas, L.; Sánchez Moragas, G.; Lagarón Cabello, J.M. Antimicrobial nanocomposites and electrospun coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J. Appl. Polym. Sci. 2018, 135, 1–11. [Google Scholar] [CrossRef]
- Zou, P.; Liu, H.; Li, Y.; Huang, J.; Dai, Y. Surface dextran modified electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) fibrous scaffold promotes the proliferation of bone marrow-derived mesenchymal stem cells. Mater. Lett. 2016, 179, 109–113. [Google Scholar] [CrossRef]
- Serrano, M.C.; Pagani, R.; Vallet-Regí, M.; Peña, J.; Rámila, A.; Izquierdo, I.; Portolés, M.T. In vitro biocompatibility assessment of poly(ε-caprolactone) films using L929 mouse fibroblasts. Biomaterials 2004, 25, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, D.; Niu, Y.; He, T.; Chen, K.C.; Xu, K. Alternating block polyurethanes based on PCL and PEG as potential nerve regeneration materials. J. Biomed. Mater. Res. Part A 2014, 102, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Delgado, K.; Quijada, R.; Palma, R.; Palza, H. Polypropylene with embedded copper metal or copper oxide nanoparticles as a novel plastic antimicrobial agent. Lett. Appl. Microbiol. 2011, 53, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.A.; Zapata, P.A.; Rabagliati, F.M.; Azócar, M.I.; Muñoz, L.A.; Zhou, X.; Thompson, G.E.; Páez, M.A. Antibacterial and non-cytotoxic effect of nanocomposites based in polyethylene and copper nanoparticles. J. Mater. Sci. Mater. Med. 2015, 26, 129. [Google Scholar] [CrossRef]
- Usha, R.; Prabu, E.; Palaniswamy, M.; Venil, C.K.; Rajendran, R. Synthesis of Metal Oxide Nano Particles by Streptomyces Sp for Development of Antimicrobial Textiles. Glob. J. Biotechnol. Biochem. 2010, 5, 153–160. [Google Scholar]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Wehrschuetz-Sigl, E.; Hasmann, A.; Guebitz, G.; Gedanken, A. CuO-cotton nanocomposite: Formation, morphology, and antibacterial activity. Surf. Coat. Technol. 2009, 204, 54–57. [Google Scholar] [CrossRef]
- Wu, C.S. Physical properties and biodegradability of maleated-polycaprolactone/starch composite. Polym. Degrad. Stab. 2003, 80, 127–134. [Google Scholar] [CrossRef]
- Persenaire, O.; Alexandre, M.; Degée, P.; Dubois, P. Mechanisms and kinetics of thermal degradation of poly(ε-caprolactone). Biomacromolecules 2001, 2, 288–294. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Jimenez, A.; Kenny, J.M.; Garrigós, M.C. Characterization and disintegrability under composting conditions of PLA-based nanocomposite films with thymol and silver nanoparticles. Polym. Degrad. Stab. 2016, 132, 2–10. [Google Scholar] [CrossRef]
- Sivalingam, G.; Madras, G. Thermal degradation of binary physical mixtures and copolymers of poly(ε-caprolactone), poly(D, L-lactide), poly(glycolide). Polym. Degrad. Stab. 2004, 84, 393–398. [Google Scholar] [CrossRef]
- Chrissafis, K.; Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part I: An overview on thermal decomposition of addition polymers. Thermochim. Acta 2011, 523, 1–24. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.D.R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Verma, A.; Alakhtani, S.; Ali, B.A. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int. J. Toxicol. 2013, 32, 296–307. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.B.; Jandhyam, S.; Dukhande, V.V.; Bhushan, A.; Daniels, C.K.; Leung, S.W.; Lai, J.C.K. Differential cytotoxicity of metallic oxide nanoparticles in mammalian cells. In NSTI-Nanotech, Nanotechnology, Proceedings of the 2008 Nanotechnology Conference and Trade Show, National Harbor, MD, USA, 29 June–1 July 2008; Taylor & Francis Inc.: Abingdon, UK, 2008; Volume 2, pp. 130–133. [Google Scholar]
- Javed, R.; Ahmed, M.; Haq, I.; Nisa, S.; Zia, M. PVP and PEG doped CuO nanoparticles are more biologically active: Antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mater. Sci. Eng. C 2017, 79, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Hutmacher, D.W.; Schantz, J.T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. A 2009, 90, 906–919. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Teoh, S.H.; Hutmacher, D.W. Comparison of the degradation of polycaprolactone and polycaprolactone-(β-tricalcium phosphate) scaffolds in alkaline medium. Polym. Int. 2007, 56, 718–728. [Google Scholar] [CrossRef]
- Hartmann, A.; Plappert, U.; Poetter, F.; Suter, W. Comparative study with the alkaline Comet assay and the chromosome aberration test. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 536, 27–38. [Google Scholar] [CrossRef]
- Ahamed, M.; Siddiqui, M.A.; Akhtar, M.J.; Ahmad, I.; Pant, A.B.; Alhadlaq, H.A. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem. Biophys. Res. Commun. 2010, 396, 578–583. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Yang, L.; Zheng, Y.; Long, J.; Jia, J.; Xiao, S.; Liu, J. Activation of ERK and P53 regulates copper oxide nanoparticle-induced cytotoxicity in keratinocytes and fibroblasts. Int. J. Nanomed. 2014, 9, 4763–4772. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, C.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Cytotoxic origin of copper(II) oxide nanoparticles: Comparative studies with micron-sized particles, leachate, and metal salts. ACS Nano 2011, 5, 7214–7225. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.M.; Limbach, L.K.; Van Duc, L.; Krumeich, F.; Athanassiou, E.K.; Gerber, L.C.; Moch, H.; Stark, W.J. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010, 197, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J.; Dardik, R.; Eidelman, A.I.; Lavie, Y.; Grunfeld, Y.; Ikher, S.; Huszar, M.; Zatcoff, R.C.; Marikovsky, M. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen. 2010, 18, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, S.; Richard, M.; Ann, O.G.; Chris, D.; Jonas, W. Microbial Cellulose Wound Dressing for Treating Chronic Wounds. U.S. Patent 10,864,804, 27 January 2005. [Google Scholar]
- Stricker-Krongrad, A.-H.; Alikhassy, Z.; Matsangos, N.; Sebastian, R.; Marti, G.; Lay, F.; Harmon, J.W. Efficacy of Chitosan-Based Dressing for Control of Bleeding in Excisional Wounds. Eplasty 2018, 18, e14. [Google Scholar]
- Arul, V.; Kartha, R.; Jayakumar, R. A therapeutic approach for diabetic wound healing using biotinylated GHK incorporated collagen matrices. Life Sci. 2007, 80, 275–284. [Google Scholar] [CrossRef]
- Dominiak, M.; Łysiak-Drwal, K.; Solski, L.; Zywicka, B.; Rybak, Z.; Gedrange, T. Evaluation of healing processes of intraosseous defects with and without guided bone regeneration and platelet rich plasma: An animal study. Ann. Anat. 2012, 194, 549–555. [Google Scholar] [CrossRef]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef]



| Step | Temperature (°C) | Heating Rate (°C/s) | Holding Time (s) |
|---|---|---|---|
| Drying I | 90 | 3 | 20 |
| Drying II | 110 | 5 | 10 |
| Pyrolysis I | 350 | 50 | 20 |
| Pyrolysis II | 1100 | 300 | 10 |
| Gas adaptation | 1100 | 0 | 5 |
| Atomization | 2000 | 1500 | 4 |
| Cleaning | 2450 | 500 | 4 |
| Samples | Cu+ (ppb) |
|---|---|
| PCL + 0.1% CuONPs | 7.84 ± 2.79 abd |
| PCL + 0.07% CuONPs | 5.99 ± 1.37 bde |
| PCL + 0.05% CuONPs | 1.36 ± 0.47 cef |
| PCL + 0.1% CuONPs + HFF-1 cells | 4.80 ± 0.20 de |
| PCL + 0.07% CuONPs + HFF-1 cells | 2.93 ± 0.14 ef |
| PCL + 0.05% CuONPs + HFF-1 cells | 1.22 ± 0.19 f |
| Samples | H (%) |
|---|---|
| PCL | 0.254 ± 0.096 a |
| PCL + 0.07% CuONPs | 0.263 ± 0.101 a |
| Saline solution 0.9% (w/v) | 0.247 ± 0.095 a |
| Distilled water | 8.487 ± 1.244 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcucho, J.; Narváez, D.M.; Castro-Mayorga, J.L. Antimicrobial and Biocompatible Polycaprolactone and Copper Oxide Nanoparticle Wound Dressings against Methicillin-Resistant Staphylococcus aureus. Nanomaterials 2020, 10, 1692. https://doi.org/10.3390/nano10091692
Balcucho J, Narváez DM, Castro-Mayorga JL. Antimicrobial and Biocompatible Polycaprolactone and Copper Oxide Nanoparticle Wound Dressings against Methicillin-Resistant Staphylococcus aureus. Nanomaterials. 2020; 10(9):1692. https://doi.org/10.3390/nano10091692
Chicago/Turabian StyleBalcucho, Jennifer, Diana M. Narváez, and Jinneth Lorena Castro-Mayorga. 2020. "Antimicrobial and Biocompatible Polycaprolactone and Copper Oxide Nanoparticle Wound Dressings against Methicillin-Resistant Staphylococcus aureus" Nanomaterials 10, no. 9: 1692. https://doi.org/10.3390/nano10091692
APA StyleBalcucho, J., Narváez, D. M., & Castro-Mayorga, J. L. (2020). Antimicrobial and Biocompatible Polycaprolactone and Copper Oxide Nanoparticle Wound Dressings against Methicillin-Resistant Staphylococcus aureus. Nanomaterials, 10(9), 1692. https://doi.org/10.3390/nano10091692





