Papain Covalently Immobilized on Chitosan–Clay Nanocomposite Films: Application in Synthetic and Real White Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzyme, Chemicals and Wines
2.2. Preparation of CS–Clay Nanocomposite Films by Solvent Casting
2.3. Physical Characterization of CS–Clay Nanocomposite Films
2.4. Papain Immobilization on CS–Clay Nanocomposite Films
2.5. Proteolytic Activity Assay
2.6. Kinetic Characterization of Immobilized Papain
2.7. Wine Stabilization Treatment in the Batch-Scale Stirred Reactor
2.8. Wine Protein Content Determination
2.9. Heat Test
2.10. Statistical Analysis
3. Results and Discussion
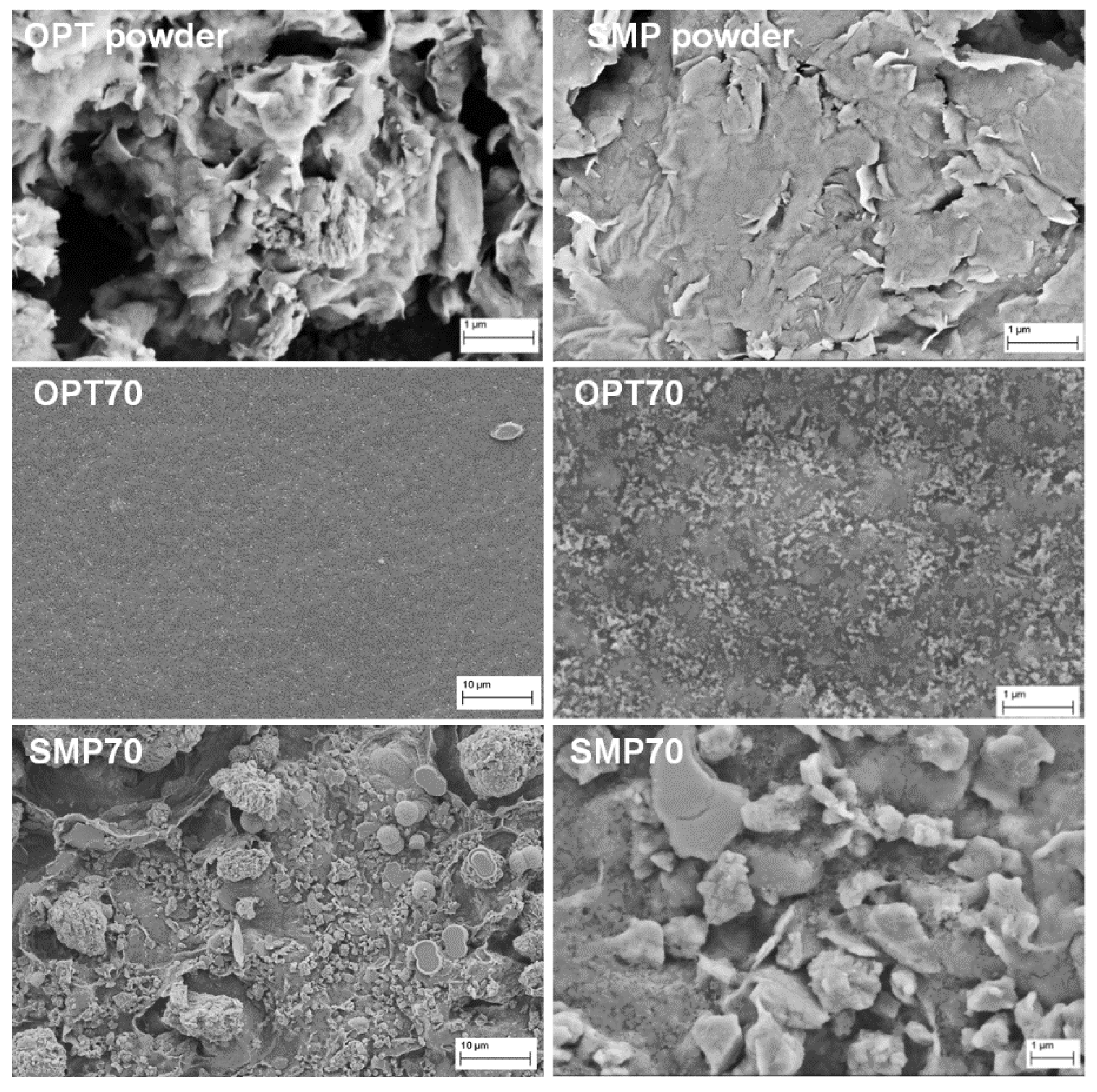
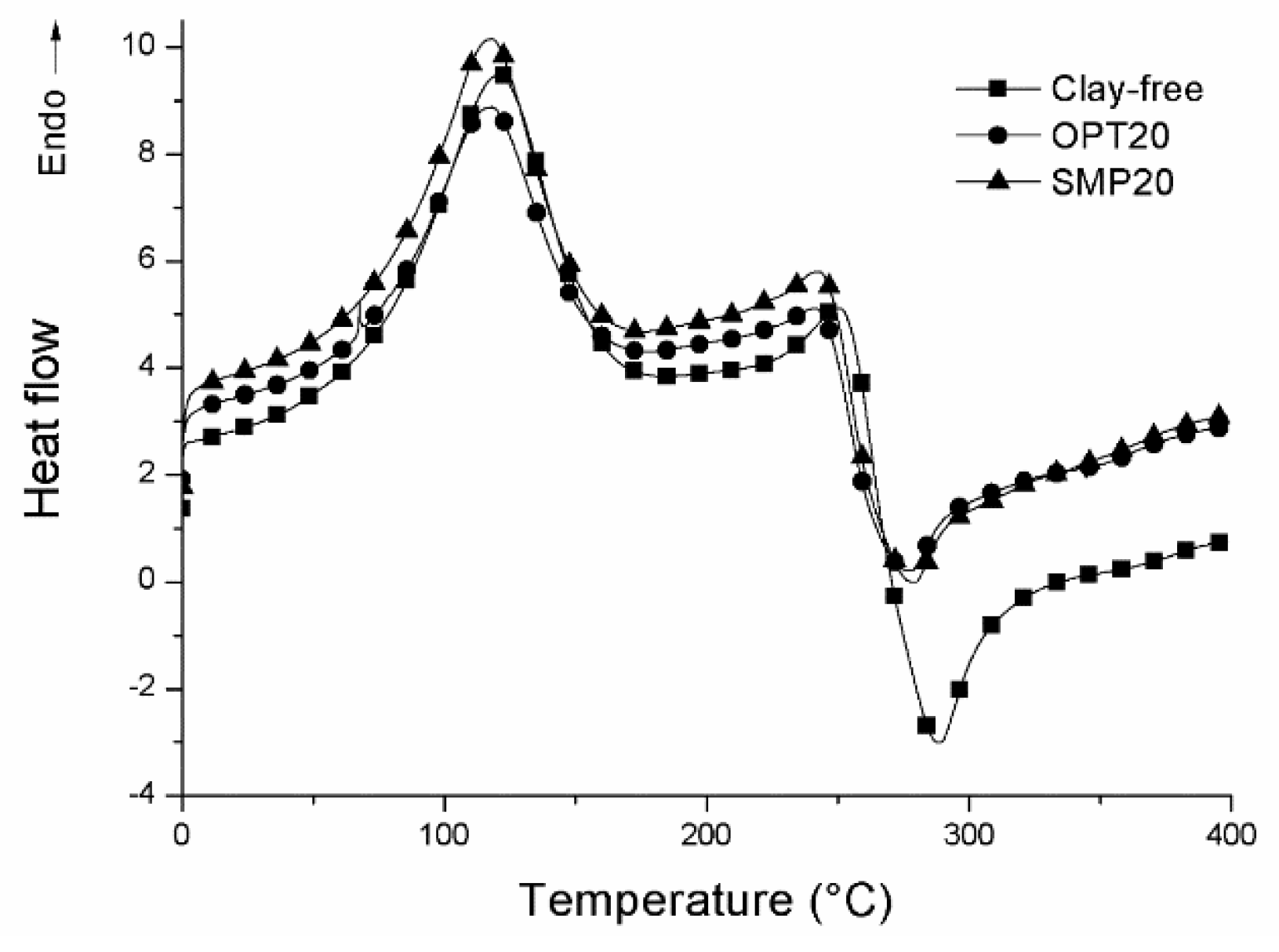
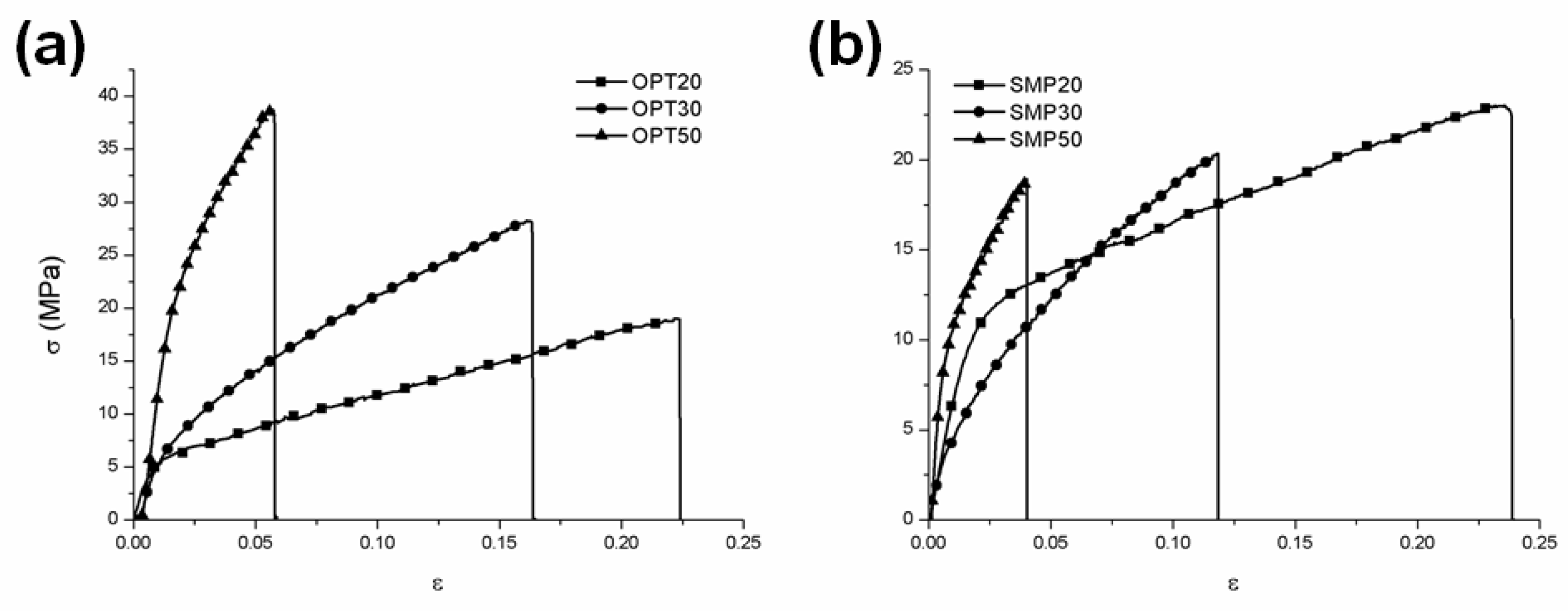
3.1. Physical Properties of CS–Clay Nanocomposite Films
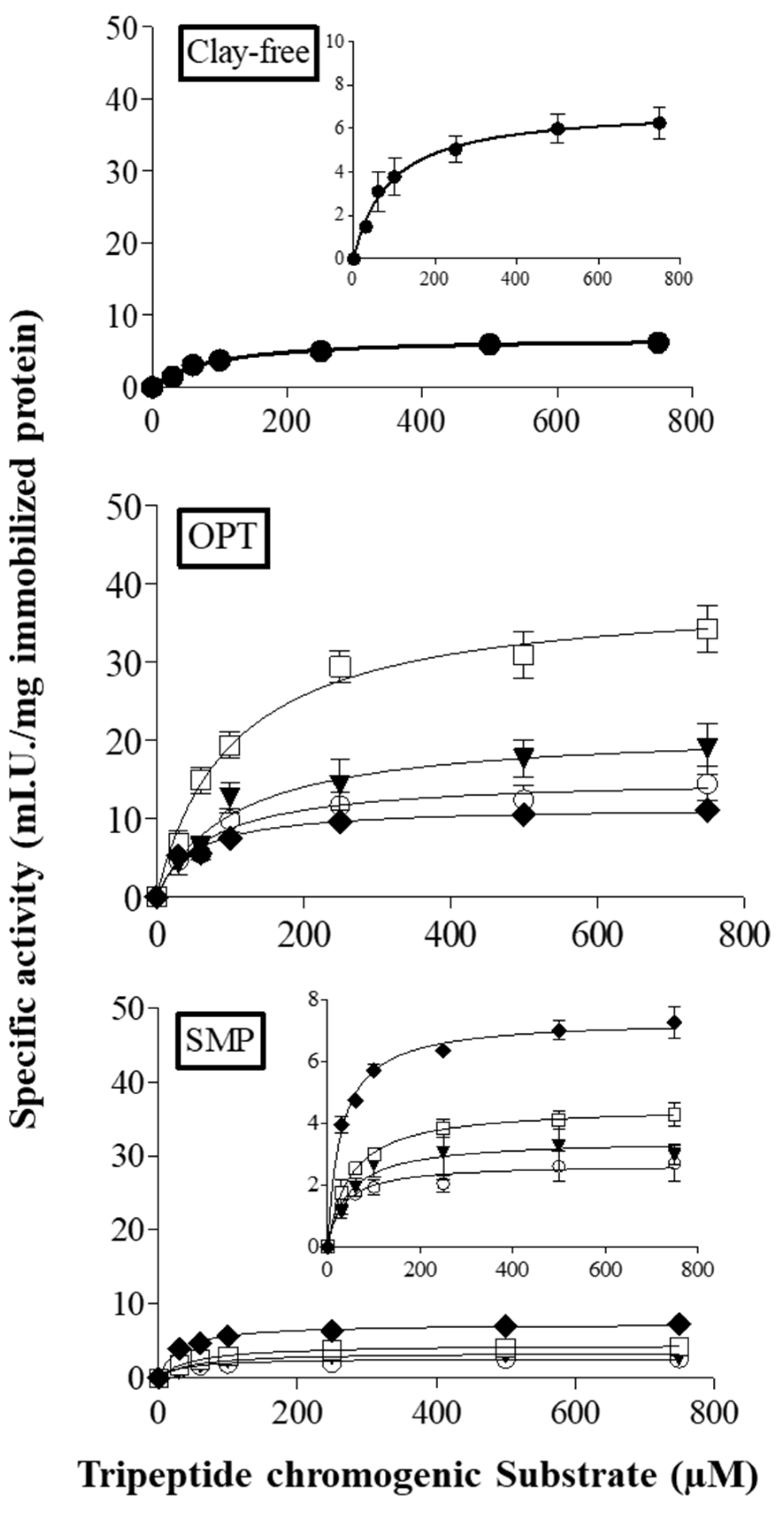
3.2. Kinetic Properties of Papain Immobilized on CS–Clay Nanocomposite Films
3.3. Wine Stabilization Treatment in Batch-Scale Stirred Reactor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ottone, C.; Romero, O.; Aburto, C.; Illanes, A.; Wilson, L. Biocatalysis in the winemaking industry: Challenges and opportunities for immobilized enzymes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 595–621. [Google Scholar] [CrossRef]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ. Chem. Lett. 2020, 18, 315–323. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Liburdi, K.; Straniero, R.; Benucci, I.; Garzillo, A.M.V.; Esti, M. Lysozyme immobilized on micro-sized magnetic particles: Kinetic parameters at wine pH. Appl. Biochem. Biotechnol. 2012, 166, 1736–1746. [Google Scholar] [CrossRef]
- Zappino, M.; Cacciotti, I.; Benucci, I.; Nanni, F.; Liburdi, K.; Valentini, F.; Esti, M. Bromelain immobilization on microbial and animal source chitosan films, plasticized with glycerol, for application in wine-like medium: Microstructural, mechanical and catalytic characterizations. Food Hydrocoll. 2015, 45, 41–47. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Liburdi, K.; Nanni, F.; Esti, M. Chitosan beads from microbial and animal sources as enzyme supports for wine application. Food Hydrocoll. 2016, 61, 191–200. [Google Scholar] [CrossRef]
- Benucci, I.; Mazzocchi, C.; Lombardelli, C.; Cacciotti, I.; Esti, M. Multi-enzymatic systems immobilized on chitosan beads for pomegranate juice treatment in fluidized bed reactor: Effect on haze-active molecules and chromatic properties. Food Bioprocess Tech. 2019, 12, 1559–1572. [Google Scholar] [CrossRef]
- Cacciotti, I.; Pallotto, F.; Scognamiglio, V.; Moscone, D.; Arduini, F. Reusable optical multi-plate sensing system for pesticide detection by using electrospun membranes as smart support for acetylcholinesterase immobilisation. Mater. Sci. Eng. C 2020, 111, 110744. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, H.; Yang, M.; Shen, G.; Yu, R. Amperometric glucose biosensor based on a surface treated nanoporous ZrO2/chitosan composite film as immobilization matrix. Anal. Chim. Acta 2004, 525, 213–220. [Google Scholar] [CrossRef]
- Yang, H.C.; Hou, J.; Chen, V.; Xu, Z.K. Surface and interface engineering for organic—Inorganic composite membranes. J. Mater. Chem. A 2016, 4, 9716–9729. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan nanocomposite coatings for food, paints, and water treatment applications. Appl. Sci. 2019, 9, 2409. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Li, Y.C.; Xiao, H.; Wang, X. Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym. 2018, 199, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The effect of nanofillers on the functional properties of biopolymer-based films: A review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Lombardelli, C.; Benucci, I.; Esti, M. Clay/chitosan biocomposite systems as novel green carriers for covalent immobilization of food enzymes. J. Mater. Res. Technol. 2019, 8, 3644–3652. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Inno. 2020, 101067. [Google Scholar] [CrossRef]
- Islam, M.A.; Tan, Y.L.; Islam, M.A.; Romić, M.; Hameed, B.H. Chitosan–bleaching earth clay composite as an efficient adsorbent for carbon dioxide adsorption: Process optimization. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 9–15. [Google Scholar] [CrossRef]
- Mastanjević, K.; Krstanović, V.; Lukinac, J.; Jukić, M.; Vulin, Z.; Mastanjević, K. Beer—The importance of colloidal stability (non-biological haze). Fermentation 2018, 4, 91. [Google Scholar] [CrossRef]
- Cerreti, M.; Liburdi, K.; Benucci, I.; Spinelli, S.E.; Lombardelli, C.; Esti, M. Optimization of pectinase and protease clarification treatment of pomegranate juice. LWT Food Sci. Technol. 2017, 82, 58–65. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Liburdi, K.; Acciaro, G.; Zappino, M.; Esti, M. Immobilised native plant cysteine proteases: Packed-bed reactor for white wine protein stabilisation. J. Food Sci. Tech. 2016, 53, 1130–1139. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binging. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gaspar, L.M.; Machado, A.; Coutinho, R.; Sousa, S.; Santos, R.; Xavier, A.; Simões, J.S. Development of potential yeast protein extracts for red wine clarification and stabilization. Front. Microbial. 2019, 10, 2310. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.Y.; Young, T.H.; Yao, C.H.; Chiu, W.Y. Properties of the poly (vinyl alcohol)/chitosan blend and its effect on the culture of fibroblast in vitro. Biomaterials 1999, 20, 1479–1487. [Google Scholar] [CrossRef]
- Kittur, F.S.; Prashanth, K.H.; Sankar, K.U.; Tharanathan, R.N. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Sakurai, K.; Maegawa, T.; Takahashi, T. Glass transition temperature of chitosan and miscibility of chitosan/poly (N-vinyl pyrrolidone) blends. Polymer 2000, 41, 7051–7056. [Google Scholar] [CrossRef]
- Zeng, M.; Fang, Z.; Xu, C. Effect of compatibility on the structure of the microporous membrane prepared by selective dissolution of chitosan/synthetic polymer blend membrane. J. Membr. Sci. 2004, 230, 175–181. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Basak, E.; Aydemir, T.; Dinçer, A.; Becerik, S.Ç. Comperative study of catalase immobilization on chitosan, magnetic chitosan and chitosan-clay composite beads. Artif. Cells Nanomed. Biotechnol. 2013, 41, 408–413. [Google Scholar] [CrossRef]
- Chang, M.Y.; Juang, R.S. Activities, stabilities, and reaction kinetics of three free and chitosan–clay composite immobilized enzymes. Enzyme Microb. Technol. 2005, 36, 75–82. [Google Scholar] [CrossRef]
- Jiang, D.S.; Long, S.Y.; Huang, J.; Xiao, H.Y.; Zhou, J.Y. Immobilization of pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem. Eng. J. 2005, 25, 15–23. [Google Scholar] [CrossRef]
| Manzoni | Sauvignon Blanc | |
|---|---|---|
| pH | 3.37 ± 0.01 | 3.34 ± 0.01 |
| Total acidity (g/L tartaric acid) | 6.00 ± 0.05 | 6.00 ± 0.05 |
| Alcohol level (% v/v) | 11.2 ± 0.3 | 13.2 ± 0.2 |
| Free SO2 (mg/L) | 6 ± 1 | 10 ± 1 |
| Total SO2 (mg/L) | 30 ± 2 | 48 ± 3 |
| Total phenols (mg/L catechin) | 217 ± 1 | 219 ± 4 |
| Total protein (mg/L) | 465 ± 43 | 120 ± 7 |
| ΔNTU Index0 | 600 ± 11 | 116 ± 8 |
| Clay-Free | OPT | SMP | |||||
|---|---|---|---|---|---|---|---|
| 20 | 30 | 50 | 20 | 30 | 50 | ||
| TIHB (°C) | 121 ± 0.5 | 118 ± 0.4 | 124 ± 0.7 | 111 ± 0.6 | 118 ± 0.4 | 115 ± 0.4 | 105 ± 0.3 |
| Td (°C) | 287 ± 1.2 | 274 ± 1.3 | 272 ± 0.9 | 260 ± 1.0 | 279 ± 1.1 | 280 ± 1.4 | 284 ± 1.5 |
| ΔHmI (J/g) | 259 ± 1.5 | 259 ± 1.4 | 238 ± 1.2 | 142 ± 0.9 | 274 ± 1.1 | 296 ± 1.3 | 335 ± 2.0 |
| E (MPa) | σmax (MPa) | εmax | |
|---|---|---|---|
| Clay-Free | 579 ± 23 | 16 ± 1 | 0.30 ± 0.01 |
| OPT-20 | 738 ± 20 | 21± 6 | 0.22 ± 0.08 |
| OPT-30 | 653 ± 71 | 23 ± 4 | 0.13 ± 0.05 |
| OPT-50 | 1670 ± 202 | 36 ± 7 | 0.06 ± 0.03 |
| SMP-20 | 580 ± 55 | 24 ± 4 | 0.28 ± 0.05 |
| SMP-30 | 528 ± 4 | 22 ± 3 | 0.12 ± 0.03 |
| SMP-50 | 1582 ± 210 | 19 ± 4 | 0.04 ± 0.01 |
| Sample | IY (%) | Vmax (mIU mg−1PI) | KM (μM) | kcat (min−1) | Ka (min−1 μM−1) | R2 |
|---|---|---|---|---|---|---|
| Clay-free | 45 ± 6 b,c | 6.9 ± 0.2 e | 88 ± 9 a | 3183 ± 0 e | 36 ± 1 d | 0.99 |
| OPT-20 | 41 ± 4 c,d | 15.3 ± 0.9 c | 75 ± 16 a,b | 6535 ± 1 c | 87 ± 4 c | 0.97 |
| OPT-30 | 26 ± 5 e | 21.4 ± 1.0 b | 106 ± 23 a | 12507 ± 1 b | 118 ± 6 b | 0.98 |
| OPT-50 | 29 ± 2 de | 38.9 ± 2.0 a | 102 ± 14 a | 20417 ± 2 a | 199 ± 3 a | 0.99 |
| OPT-70 | 55 ± 3 b | 11.6 ± 0.5 d | 50 ± 8 b,c | 4225 ± 0 d | 85 ± 3 c | 0.98 |
| SMP-20 | 54 ± 7 a,b,c | 2.6 ± 0.1 f | 34 ± 8 c | 1017 ± 0 h | 30 ± 2 d,e | 0.97 |
| SMP-30 | 41 ± 5 c,d | 3.5 ± 0.2 f | 46 ± 11 b,c | 642 ± 0 i | 14 ± 1 f | 0.97 |
| SMP-50 | 56 ± 2 b | 4.5 ± 0.1 f | 49 ± 2 b,c | 1221 ± 0 g | 25 ± 0 e | 1.00 |
| SMP-70 | 60 ± 4 a | 7.3 ±0.1 e | 29 ± 3 c | 2503 ± 0 f | 86 ± 1 c | 1.00 |
| Treatment | Net Haze after Heat Test | Residual Protein Content | ||
|---|---|---|---|---|
| ΔNTU | Reduction (TRY, %) | mg BSAeq/L | Reduction (%) | |
| Manzoni | ||||
| Untreated wine | 601 ± 11 a | - | 465 ± 63 a | - |
| OPT-50 | 102 ± 41 b | 83 | 124 ± 35 b | 73 |
| Sauvignon Blanc | ||||
| Untreated wine | 116 ± 8 a | - | 124 ± 1 a | - |
| OPT-50 | 80 ± 1 b | 31 | 109 ± 1 b | 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benucci, I.; Lombardelli, C.; Cacciotti, I.; Esti, M. Papain Covalently Immobilized on Chitosan–Clay Nanocomposite Films: Application in Synthetic and Real White Wine. Nanomaterials 2020, 10, 1622. https://doi.org/10.3390/nano10091622
Benucci I, Lombardelli C, Cacciotti I, Esti M. Papain Covalently Immobilized on Chitosan–Clay Nanocomposite Films: Application in Synthetic and Real White Wine. Nanomaterials. 2020; 10(9):1622. https://doi.org/10.3390/nano10091622
Chicago/Turabian StyleBenucci, Ilaria, Claudio Lombardelli, Ilaria Cacciotti, and Marco Esti. 2020. "Papain Covalently Immobilized on Chitosan–Clay Nanocomposite Films: Application in Synthetic and Real White Wine" Nanomaterials 10, no. 9: 1622. https://doi.org/10.3390/nano10091622
APA StyleBenucci, I., Lombardelli, C., Cacciotti, I., & Esti, M. (2020). Papain Covalently Immobilized on Chitosan–Clay Nanocomposite Films: Application in Synthetic and Real White Wine. Nanomaterials, 10(9), 1622. https://doi.org/10.3390/nano10091622







