3.1. Investigation of Early Stage of CNT Growth on Inconel 600 Surface
In order to address the effect of pretreatments on physical or chemical change of the substrate surface, we first performed a detailed investigation of Inconel surface after the pretreatment. The condition of thermal annealing and plasma treatment was employed from our previous works as mentioned in
Section 2.1.
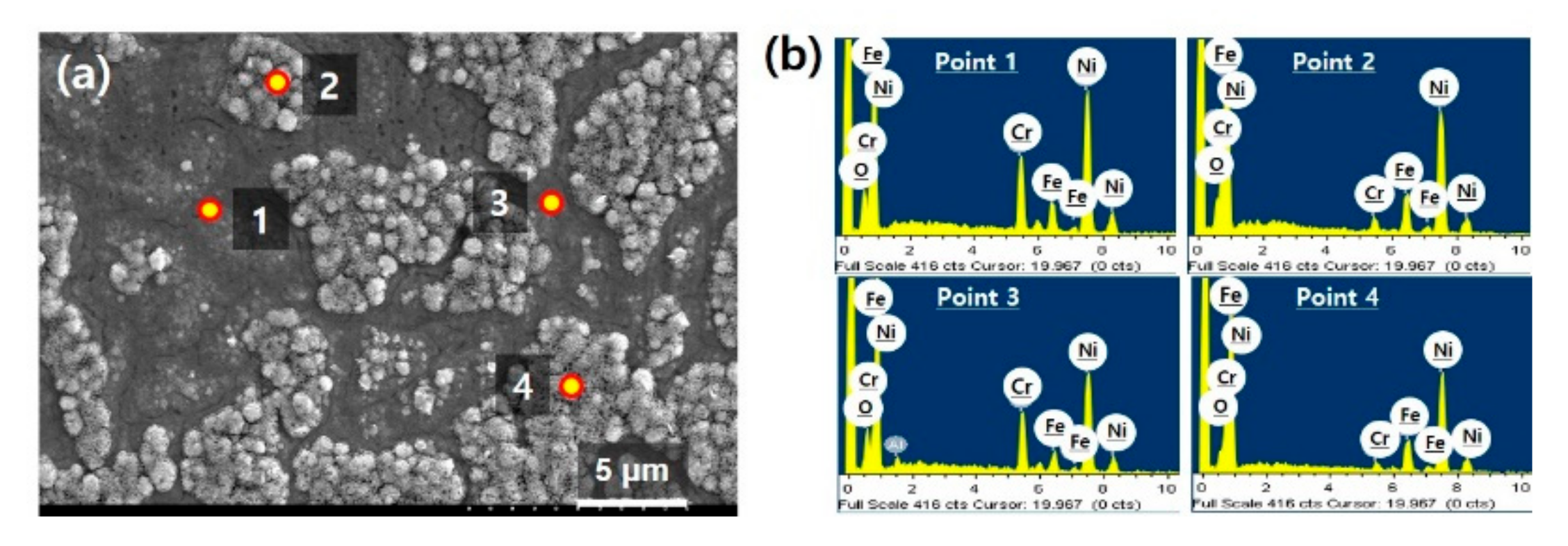
Figure 1a is a SEM image of Inconel 600 surface taken after the pretreatment. It was found the substrate was divided into two regions having different morphologies on the surface. While the regions designated points 1 and 3 look flattened, points 2 and 4 indicate roughened regions on the substrate. In addition to the morphologic difference, to identify the difference in chemical composition of two regions, we further performed EDS analyses. The spectra and quantitative result are presented in
Figure 1b and
Table 2, respectively. It was found the flattened regions (points 1 and 3) had similar chemical composition to that of Inconel 600. However, it was very interesting that the roughened regions (points 2 and 4) show increased Ni and Fe content and significantly reduced Cr content compared to the Inconel and flattened regions. It could be expected that not only the physical change in morphology, but also the chemical change such as compositional segregation occurred during the pretreatments.
Next, to explore the morphologic difference in a nanoscale between two regions, we adopted high resolution SEM observation and the results are shown in
Figure 2.
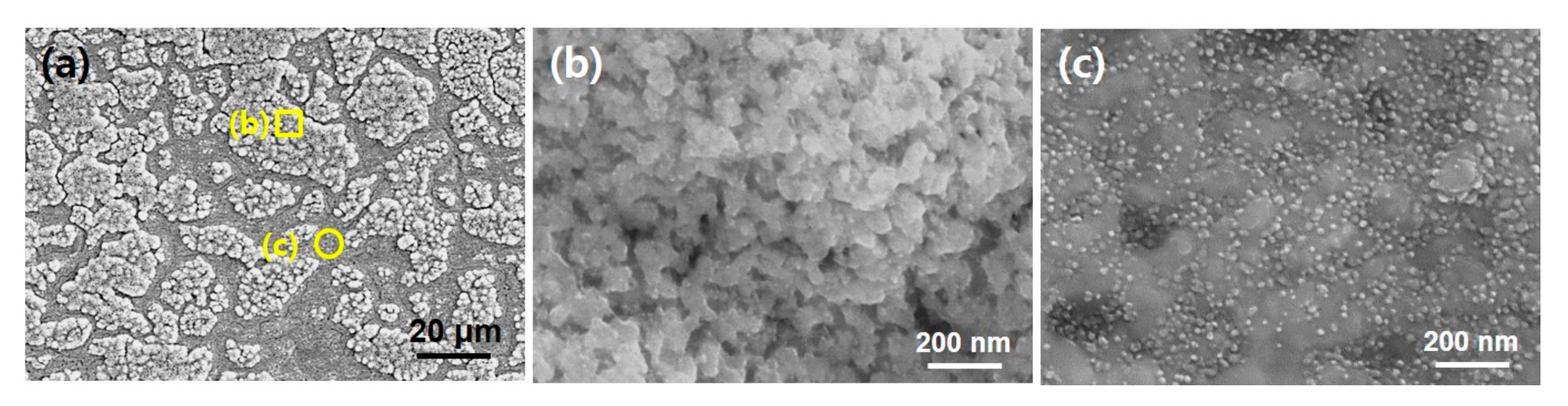
Figure 2a is a large area SEM image and very similar to
Figure 1a. High magnification SEM observation was performed as indicated with yellow square and circle in
Figure 2a.
Figure 2b,c is highly magnified views of rough and flat regions, respectively. It is interesting the morphologic difference was evident between two regions. In detail, while the aggregated surface morphology was observed in roughened region (
Figure 2b), the nanoparticles with 10–20 nm in diameter were uniformly dispersed over the flattened region (
Figure 2c).
In general, it has been known the surface morphology is closely related with the final structures of CNT and growth yield as well. Thus, we investigated the behavior of early stage of CNT growth with respect to the different morphology of the pretreated substrate surface as shown in
Figure 2. We introduced the feedstock gas for 5 s and instantly evacuated the chamber.
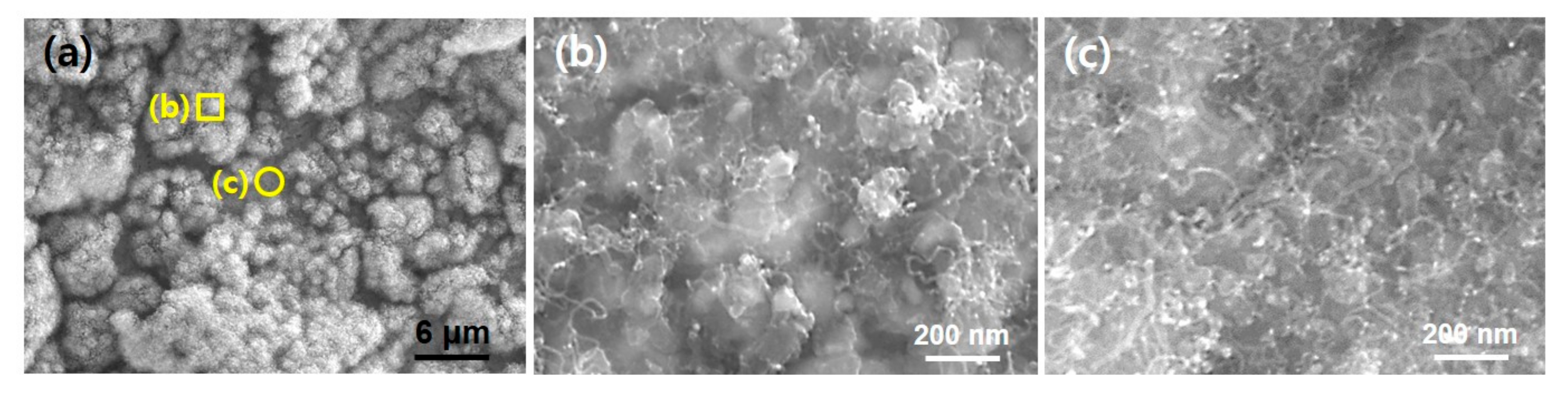
Figure 3a shows a large area SEM image of Inconel 600 substrate after the CNT growth for 5 s. As shown in
Figure 1a and
Figure 2a, the substrate showed both particle like morphology (indicated by yellow square (b)) and flattened surface (indicated by yellow circle (c)). After the 5 s growth, it is found that while the crumbled and very short tubular structures are formed in the roughened region, more dense tubular structures are produced at the flat surface region as shown in
Figure 3b,c, respectively. From the SEM images, the tube length appeared below 1 μm and diameter ranges of 10–20 nm which is very similar to that of nanoparticles in
Figure 2c. It can be supposed that the tubular structures in
Figure 3 would be CNTs grown from the catalytic nanoparticles in
Figure 2c.
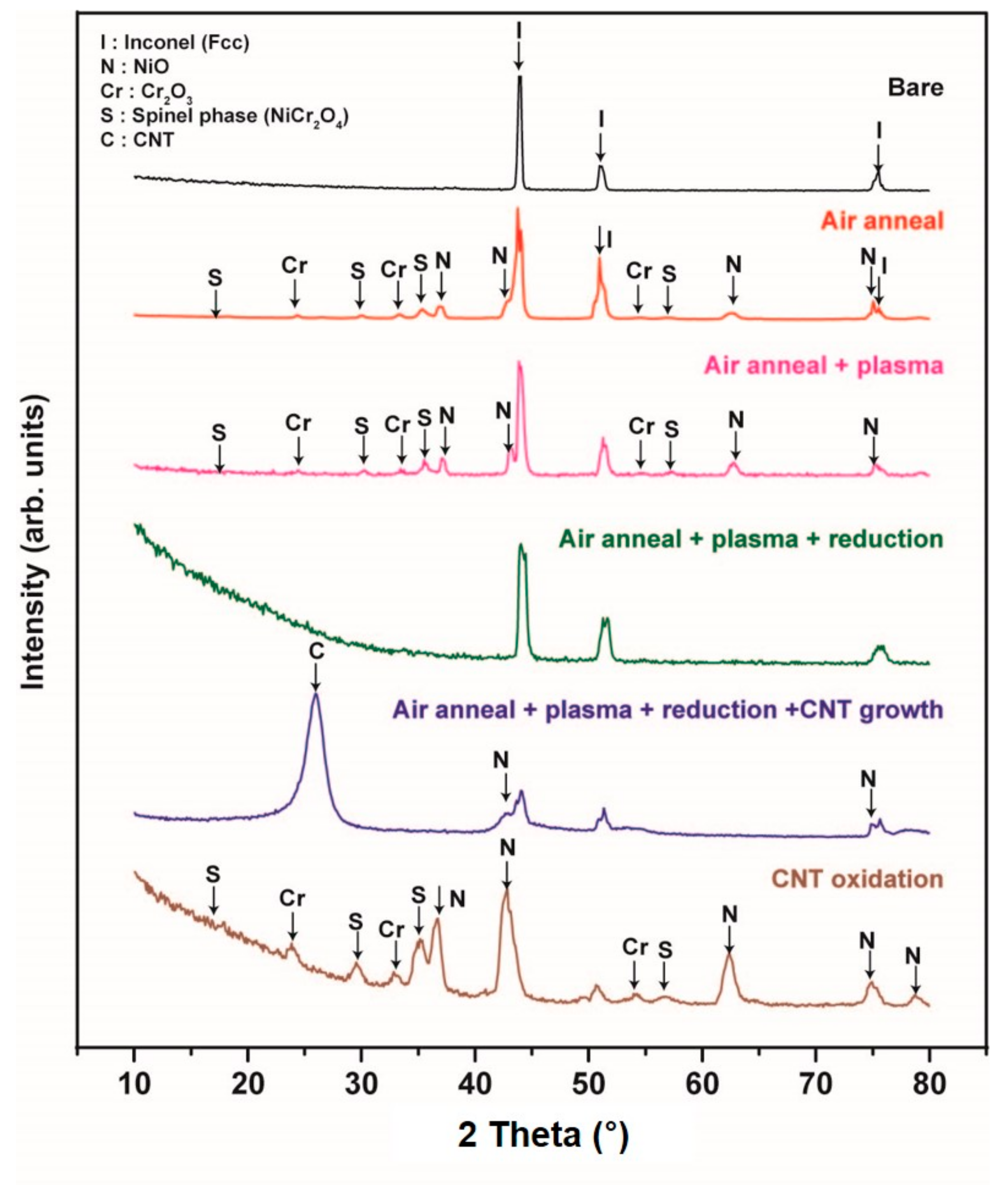
Figure 4 shows a summary of XRD profiles performed in order to study the compositional change on the substrate at each step. Those are as-purchased, air annealing, plasma treatment, reduction before growth, CNT growth and CNT oxidation after growth. As-purchased bare substrate, all peaks came from Inconel 600. Then, some oxide phases, such as NiCr
3O
4, Cr
2O
3 and NiO, were detected after thermal annealing at 725 °C for 10 min under air. The detected phases were not changed after the successive Ar plasma treatment for 30 min at 500 °C. The plasma power was 10.5 W.
Before the growth of CNT, the oxide phases disappeared because we changed the inlet gases of the CVD chamber to make reduction environment. From this result, we can expect the important factor to yield CNT would be a surface morphology in a nanoscale. After the growth of CNT for 30 min at 525 °C, we can clearly observe the diffraction peaks at 26° and 43° corresponding to (002) and (100) of the hexagonal graphite structure, respectively. In addition, we intentionally oxidized the CNT sample at 725 °C for 10 min to remove and investigate the surface state. The obtained XRD profile showedthe same pattern as that of annealed or plasma pretreated sample.
According to the investigation about the initial CNT growth stage, we can briefly conclude that the growth of CNT can be facilitated by the pretreatment consisting of thermal annealing and plasma ion bombardment due to the modified surface morphology having catalytic nanoparticles.
3.2. Growth Optimization of CNT on Inconel 600 Substrate
In this section, we demonstrate the results of growth optimization of CNT by varying the growth temperature and plasma power. First, we changed the growth temperature from 375 °C to 725 °C while plasma power was kept at 10.5 W.
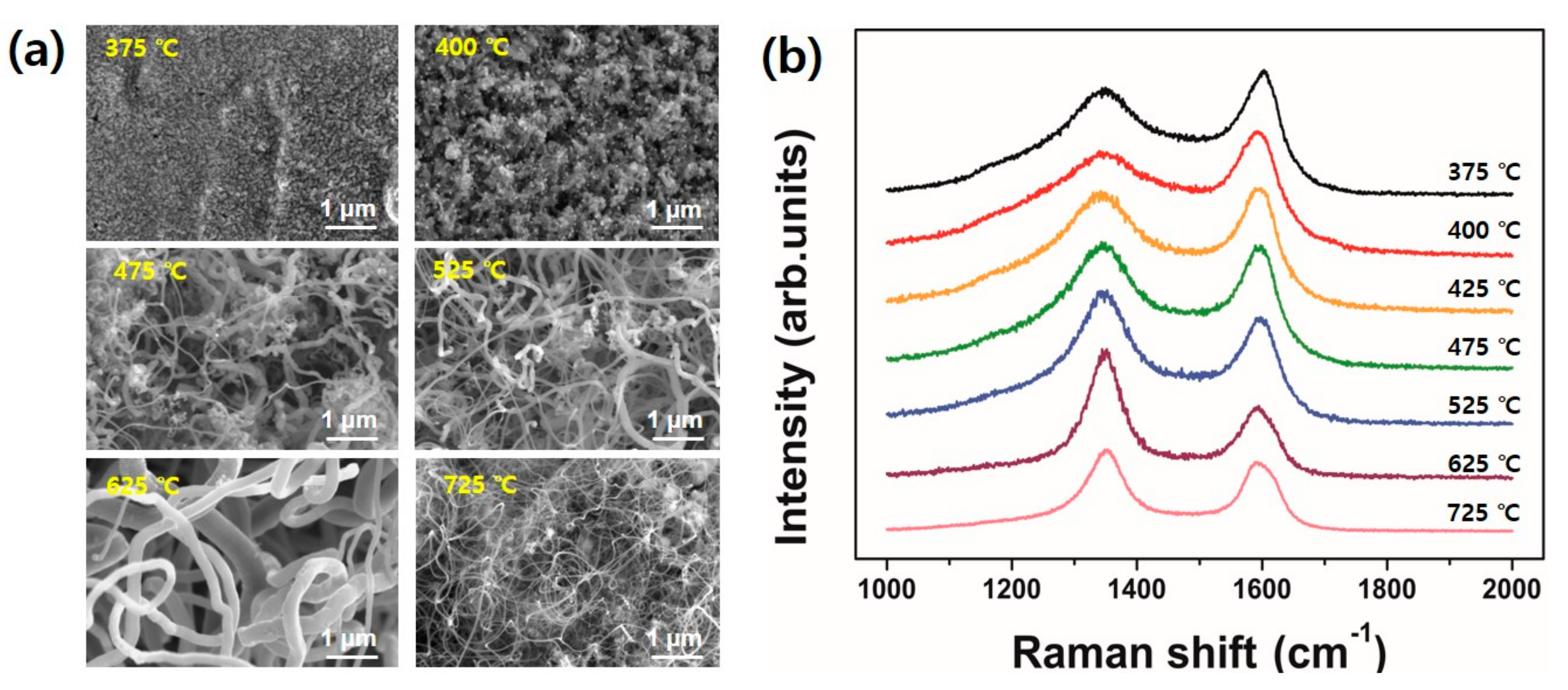
Figure 5a is SEM images showing the different morphologies of CNT grown at specified temperature and their corresponding Raman profiles are displayed in
Figure 5b. At the growth temperature of 375 °C and 400 °C, it is hard to observe tubular structures in the SEM images although the samples produce a typical Raman spectra of multi-walled CNTs. As the growth temperature increases, for instance 475 °C and 525 °C, relatively long CNTs are observed. The value of I
D/I
G ranges from 0.86 to 1.23. Although higher value of I
D/I
G implies lower crystallinity of the nanotube structure, the value ranges obtained in this study is not critical range to reflect the structural degree of the CNTs as we confirmed from our previous works [
5]. It is interesting that the CNT sample grown at 625 °C is a mixture of very thick and thin tubes. On the other hand, the CNTs grown at 725 °C looks thinner than other tubes obtained in this study. In addition, while the value of I
D/I
G from CNTs grown at 625 °C reaches 1.85, but CNTs grown at 725 °C shows a relatively lower I
D/I
G value of 1.15. At the present, it is expected the difference may be related to the morphology of catalytic nanoparticles on the substrate surface induced by the pretreatments. In addition, enhanced surface diffusion at higher growth temperature would produce smaller nanoparticles with the aid of plasma ion bombardment and finally nucleate the thinner CNTs. However, further investigation is required for extensive understanding.
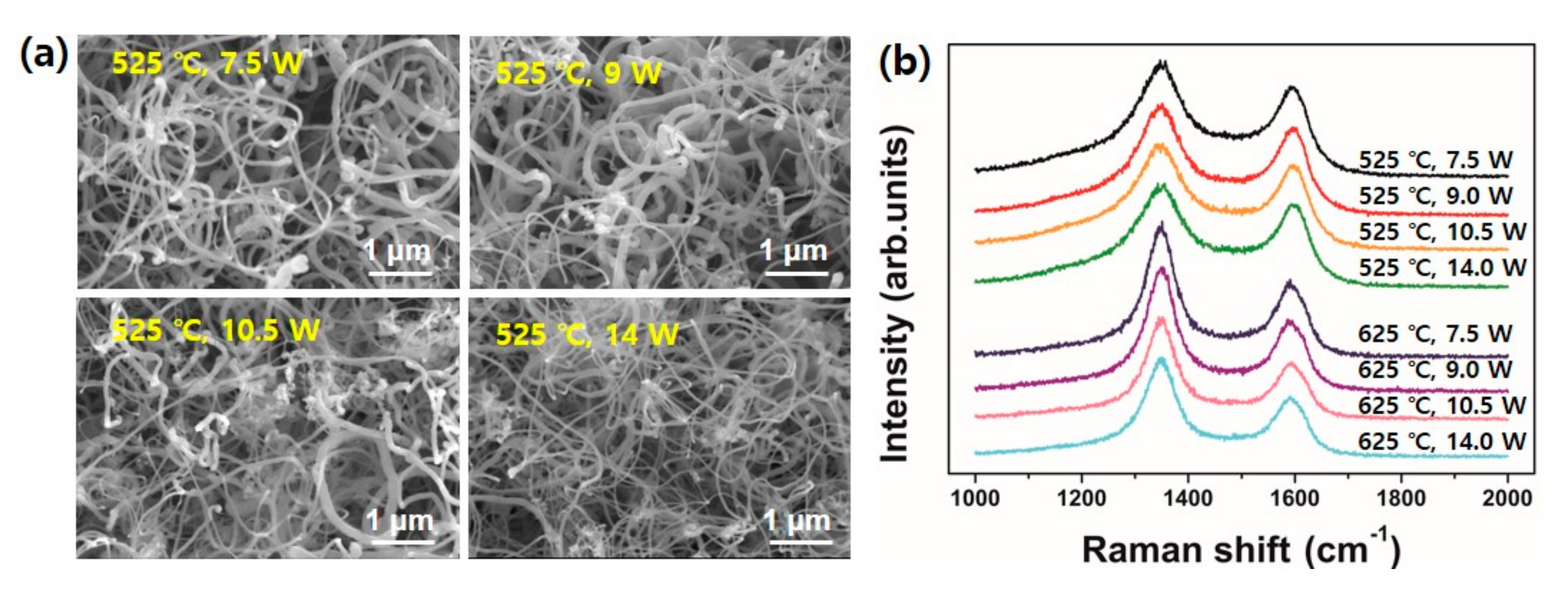
The growth temperature was fixed at 525 °C when we investigated the effect of plasma power on CNT growth. The results are shown in
Figure 6a. As the plasma power get increased, the tube diameters appeared thinner. It can be clearly observed when we compare the CNT samples between 10.5 W and 14 W of applied plasma power. Here, it is expected the plasma ion bombardment with high power results in smaller catalytic nanoparticles as we confirmed in
Figure 2c. It is plausible because a higher powered plasma generally possesses higher plasma density and ion bombardment energy as well.
Figure 6b shows Raman spectra obtained from the samples grown at 525 °C and 625 °C with different plasma powers. It is interesting the value of I
D/I
G at the same growth temperature shows almost the same value regardless of the applied plasma power. It implies, from a CNT structural point of view, the effect of plasma power applied at the pretreatment stage is relatively small compared to the growth temperature. However, it is noteworthy the plasma pretreatment is a crucial to form a catalytic nanoparticles and thus facilitate the nucleation of CNTs at an initial growth stage.
Finally, we can summarize the optimized pretreatment condition is a mixed process of thermal annealing at 725 °C for 10 min and successive plasma ion bombardment with Ar gas at 500 °C with 10.5 W for 30 min.
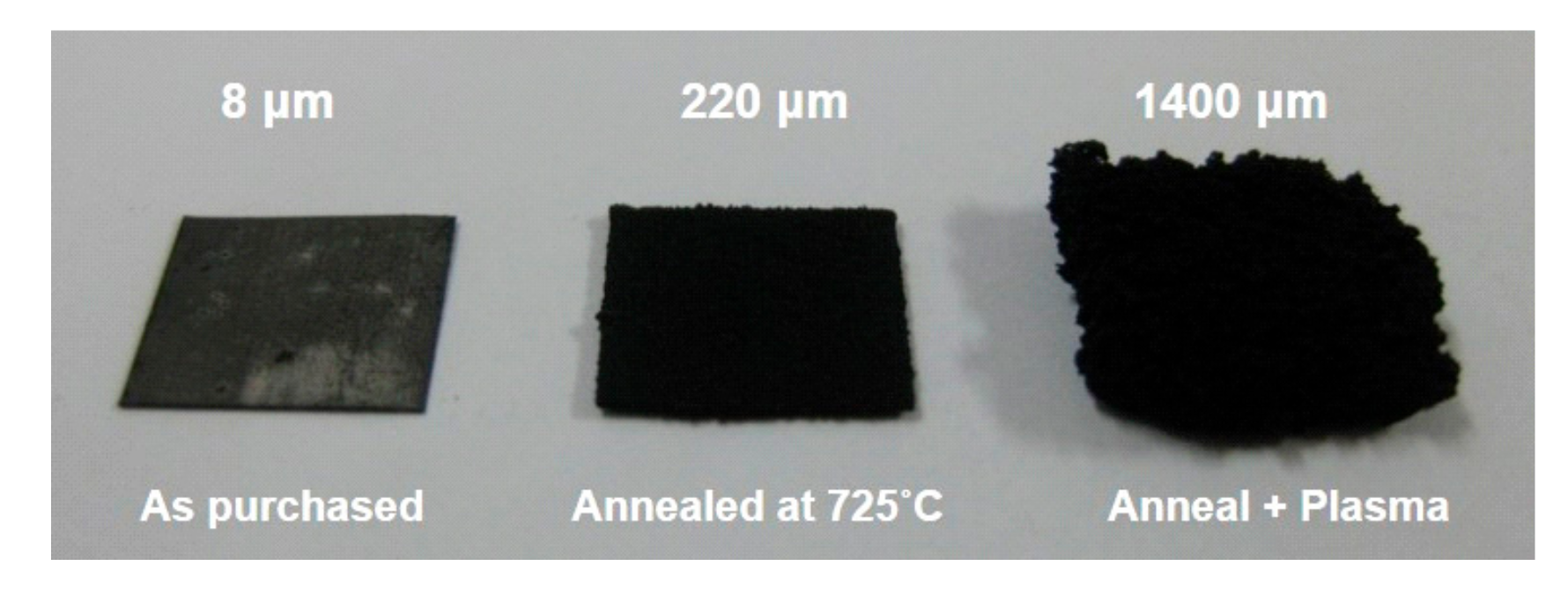
Figure 7 clearly shows the effect of substrate pretreatment of Inconel 600 on CNT growth. Again, the growth was performed at 525 °C for 30 min.
In order to demonstrate the advantage of direct growth of CNTs on metallic substrate, we performed a field emission (FE) measurement using the CNTs grown on conventional SiO
2 wafer and Inconel 600. Briefly, the FE measurement was performed using a simple diode configuration and the distance between CNT top surface and the anode was kept at 500 μm. The chamber was evacuated below 5 × 10
−7 Torr.
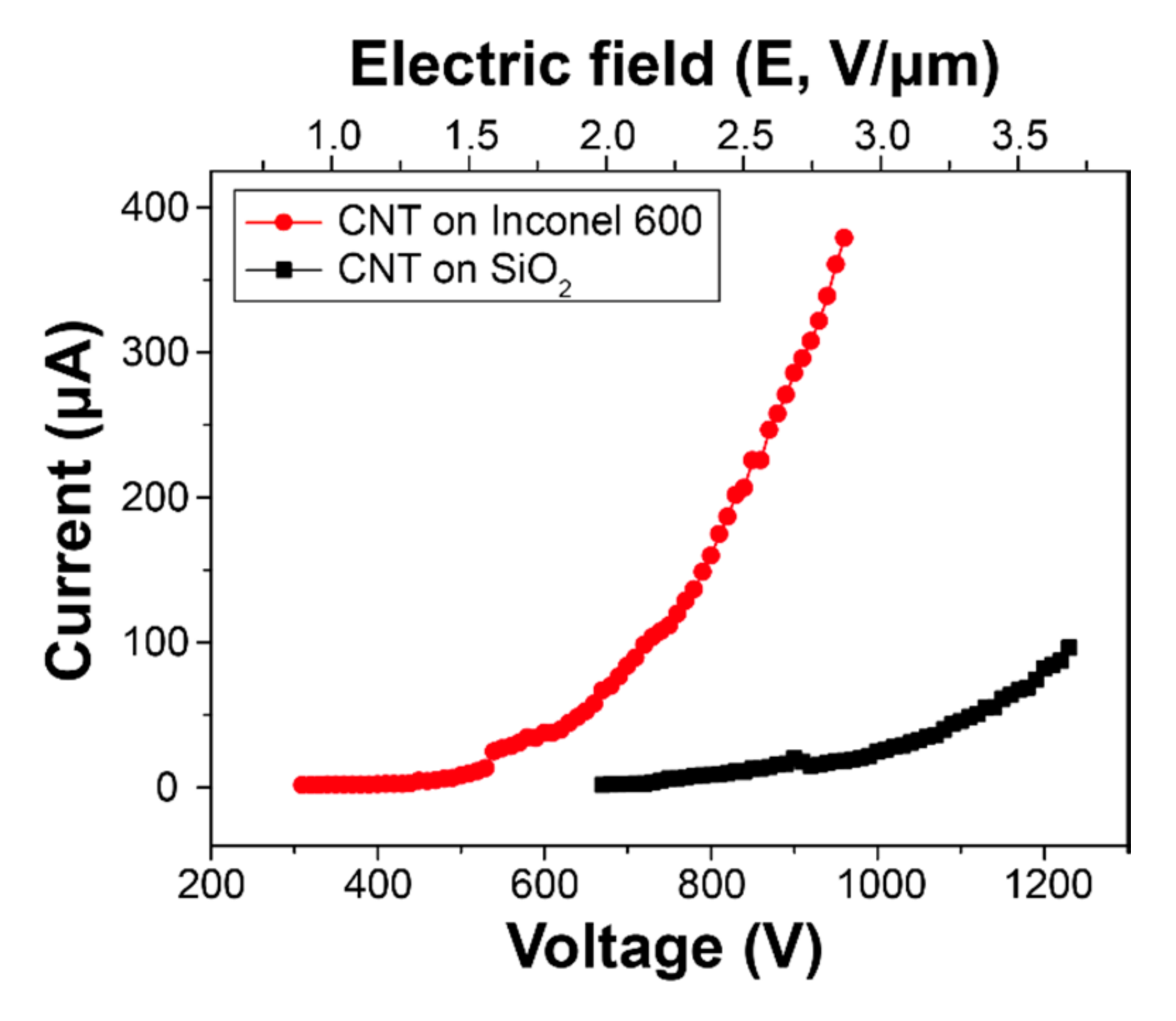
Figure 8 shows current–voltage curves of the CNTs grown on SiO
2 wafer and Inconel 600. Because FE phenomenon is based on electron tunneling, we estimated field enhancement factor (
β) using the Fowler-Nordheim (FN) plot from
Figure 8. The
β can be calculated as following relation [
17,
18].
where,
B is the FN constant (6.83 × 10
3 V eV
−3/2 μm
−1),
φ is a work function of CNTs (5 eV) [
19],
D is distance between CNTs and anode (500 μm) and slope of the F–N plot. In this study, while the
β from CNTs grown on SiO
2 wafer was estimated 4957, the
β from CNTs on Inconel 600 shows 8177. The significant improvement in the FE performance would be a result of direct growth on metallic substrate.
According to the investigation about the initial CNT growth stage, we can briefly conclude that the growth of CNT can be facilitated by the pretreatment consisting of thermal annealing and plasma ion bombardment due to the modified surface morphology having catalytic nanoparticles.
3.3. Comparison of CNT Growth Behavior with Other Ni-Based Superalloys
Based on the results of growth behavior of CNT on Inconel 600, we compare the effect of pretreatments with other metallic substrates, Ni-based superalloys. Ni plate was also used for a comparison. As shown in
Table 1, Invar 42 is a representative low thermal expansion alloy composed of mainly Ni and Fe. Hastelloy C276 is a Ni–Mo–Cr alloy with excellent corrosion resistance.
Because Ni is a common element in the alloys, it is possible to directly grow CNTs on the alloy surface. Thus, it is important to explore the change of surface state by changing the pretreatments in order to achieve efficient growth of CNTs as we investigated in Inconel 600.
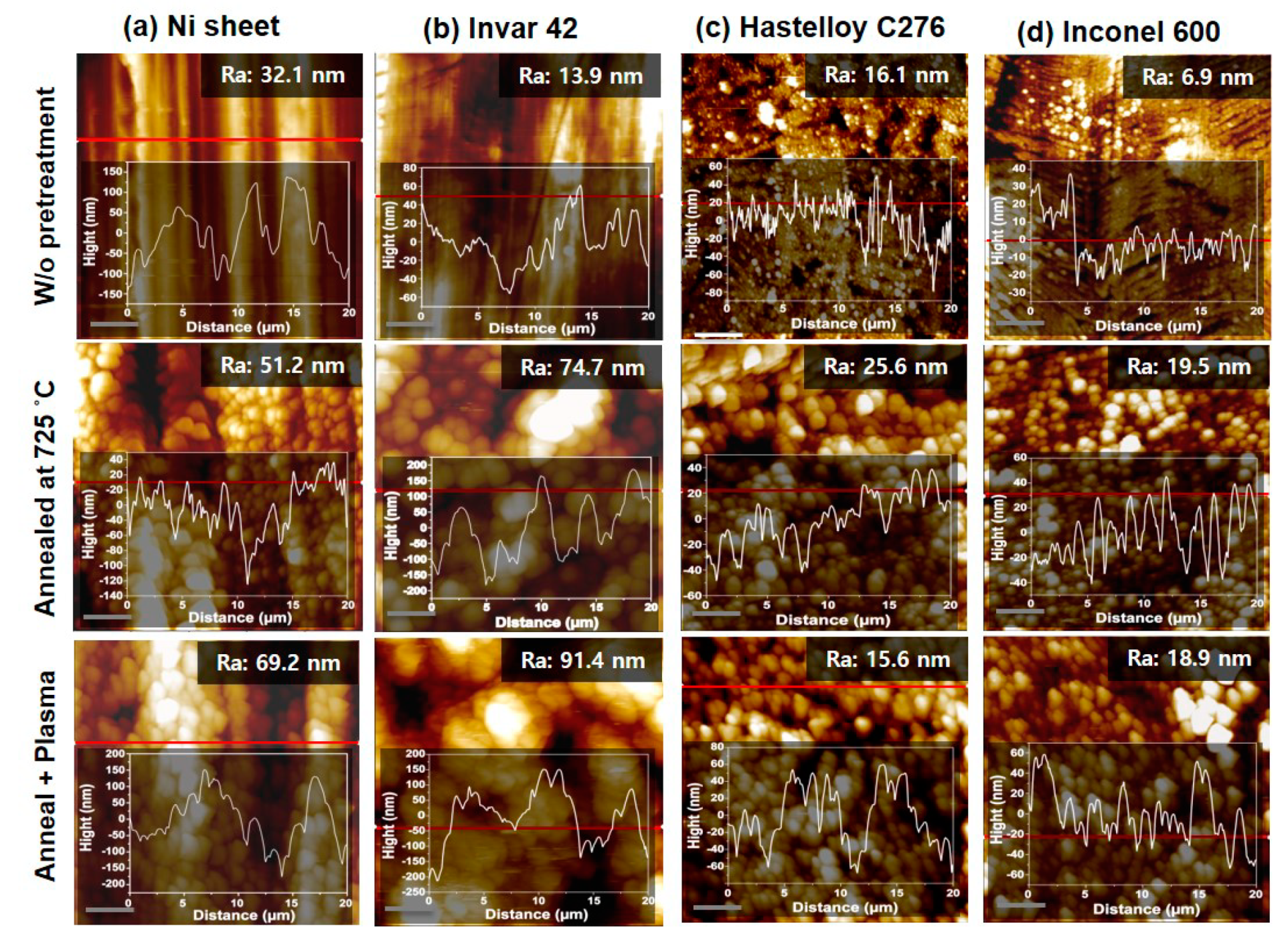
Figure 9 shows AFM topographic images of Ni sheet, Invar 42, Hastelloy C276 and Inconel 600 with respect to before pretreatment, thermal annealing at 725 °C and additional Ar plasma treatment with 10.5 W at 500 °C. It can be found from AFM images that the surface structure shows nanoparticle morphology and the quantitative surface roughness presented by arithmetical mean height (Ra) value slightly increased by the pretreatments. This well agrees with the height profiles inserted in each image. Using these substrates, we performed the CNT growth with different growth temperatures.
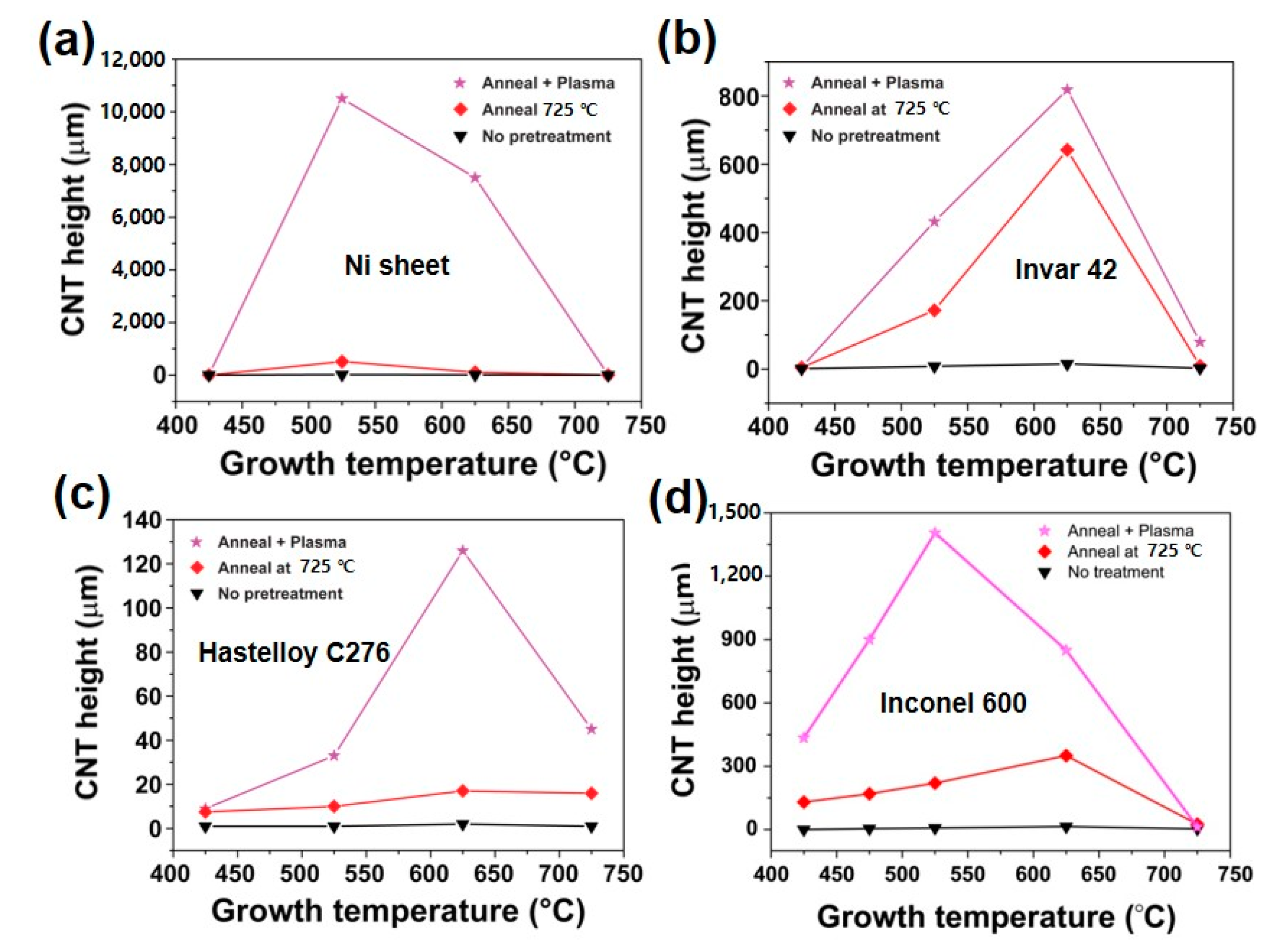
Figure 10 is a summary of CNT growth results. All substrates produced the highest CNT film thickness are undergone thermal annealing and additional Ar plasma treatment as we obtained in Inconel 600. The only difference is an optimal growth temperature, which produces the highest CNT forest thickness. In detail, the optimized growth temperature was determined at 525 °C for Ni and Inconel 600. On the other hand, the temperature is found to be 625 °C for Invar 42 and Hastelloy C276 substrates. The difference may be attributed to the existence of high temperature elements such as Mo and Cr in Invar 42 and Hastelloy C276. In particular, the poor growth of CNTs on Hastelloy C276 would be caused by a large content of Mo, which is inactive element in CNT growth.
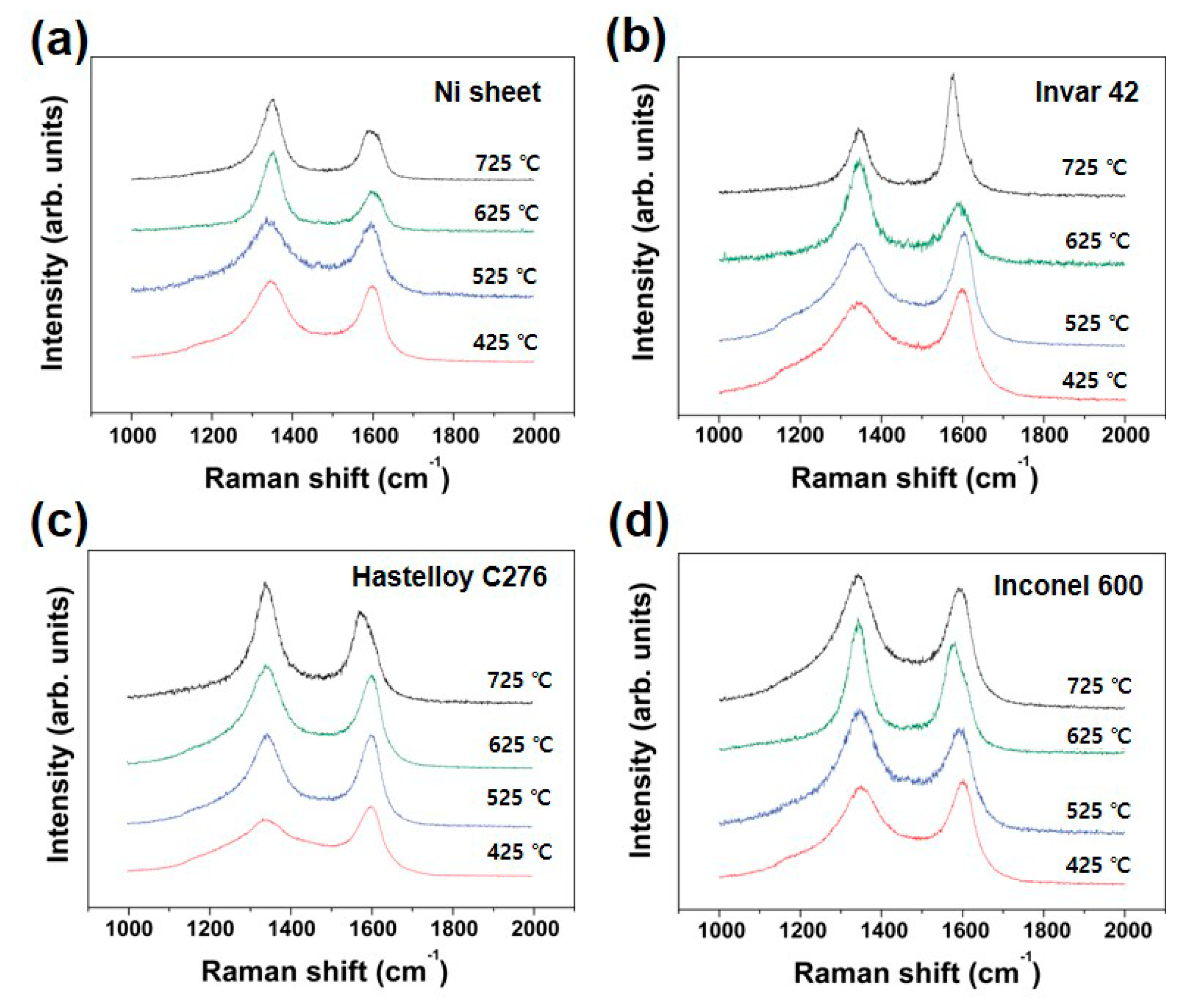
Figure 11 summarizes Raman spectra from CNTs grown using different substrates and growth temperature. All substrates were also treated with thermal annealing and successive Ar plasma treatment. Then, CNTs were grown at the optimized growth temperature. Although the CNTs grown at higher temperature show lower I
D/I
G value, the obtained value is not sensitive to exactly reflect the structural difference of the CNTs. The situation is very similar to that of Inconel 600 as we confirmed in
Figure 5 and
Figure 6.
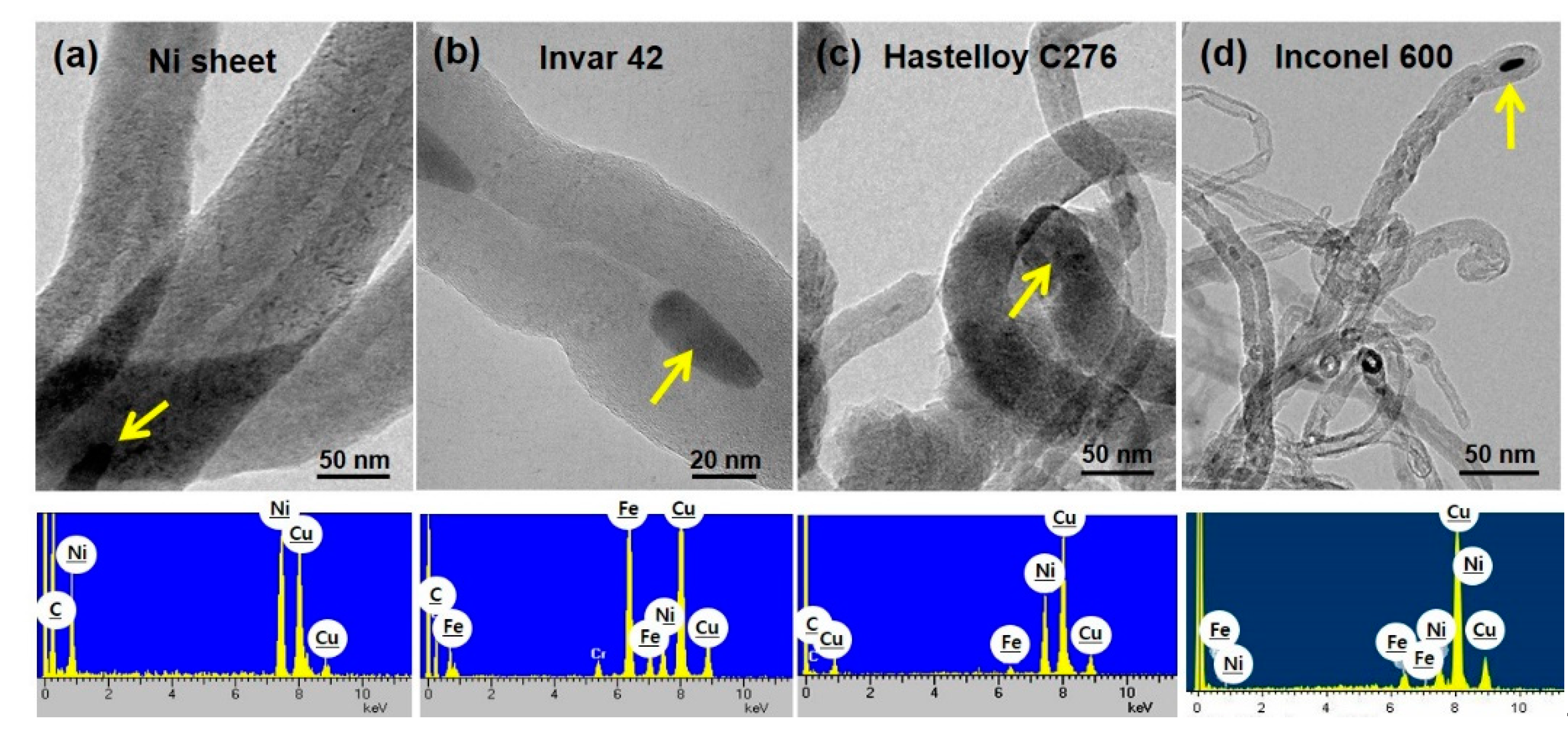
In order to investigate the detailed nanostructures, we performed TEM observation using the representative CNT samples from each substrate and the results are shown in
Figure 12. High resolution TEM images of the CNTs reveal the graphitic layers along the tube axis. The main elements of the catalysts designated by yellow arrows are confirmed as Ni and Fe as presented in EDS results. This well agrees with the chemical composition of the alloys. Cu peak is attributed to the TEM grid.
Finally, we believe that the pretreatments yields highly efficient growth of CNTs on Ni-based alloys by the formation of catalytic nanoparticles with appropriate roughened surface.