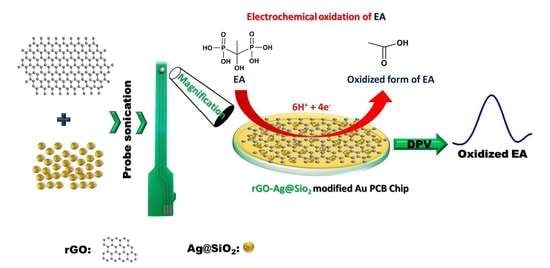
Differential Pulse Voltammetric Electrochemical Sensor for the Detection of Etidronic Acid in Pharmaceutical Samples by Using rGO-Ag@SiO2/Au PCB
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Apparatus
2.2. Synthesis of Ag@SiO2
2.3. Preparation of rGO
2.4. Construction of the rGO-Ag@SiO2 PCB Sensing Platform
2.4.1. Self-Assembly of rGO-Ag@SiO2
2.4.2. Surface Modification of the Au PCB
2.5. Real Sample Preparation
3. Results and Discussion
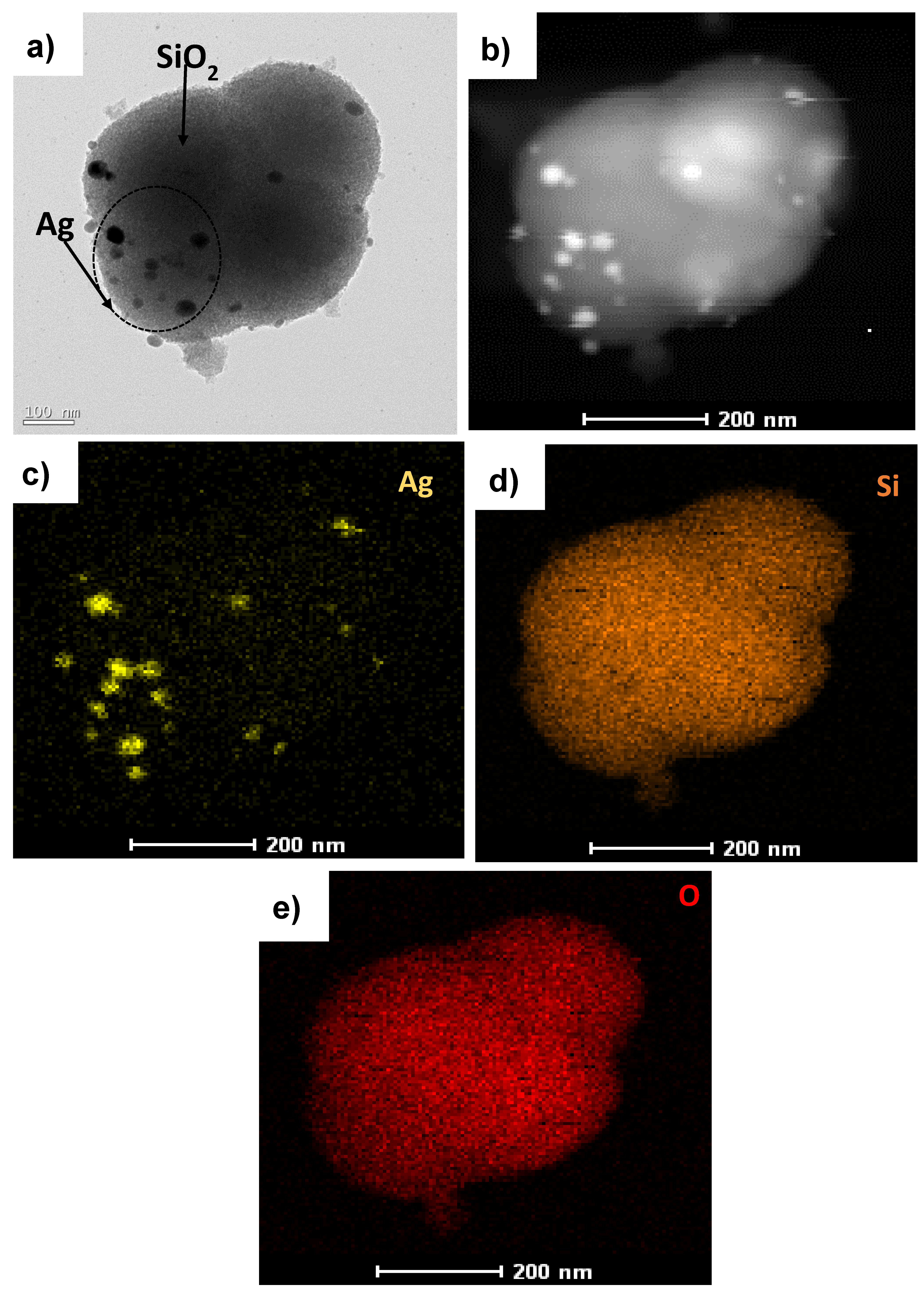
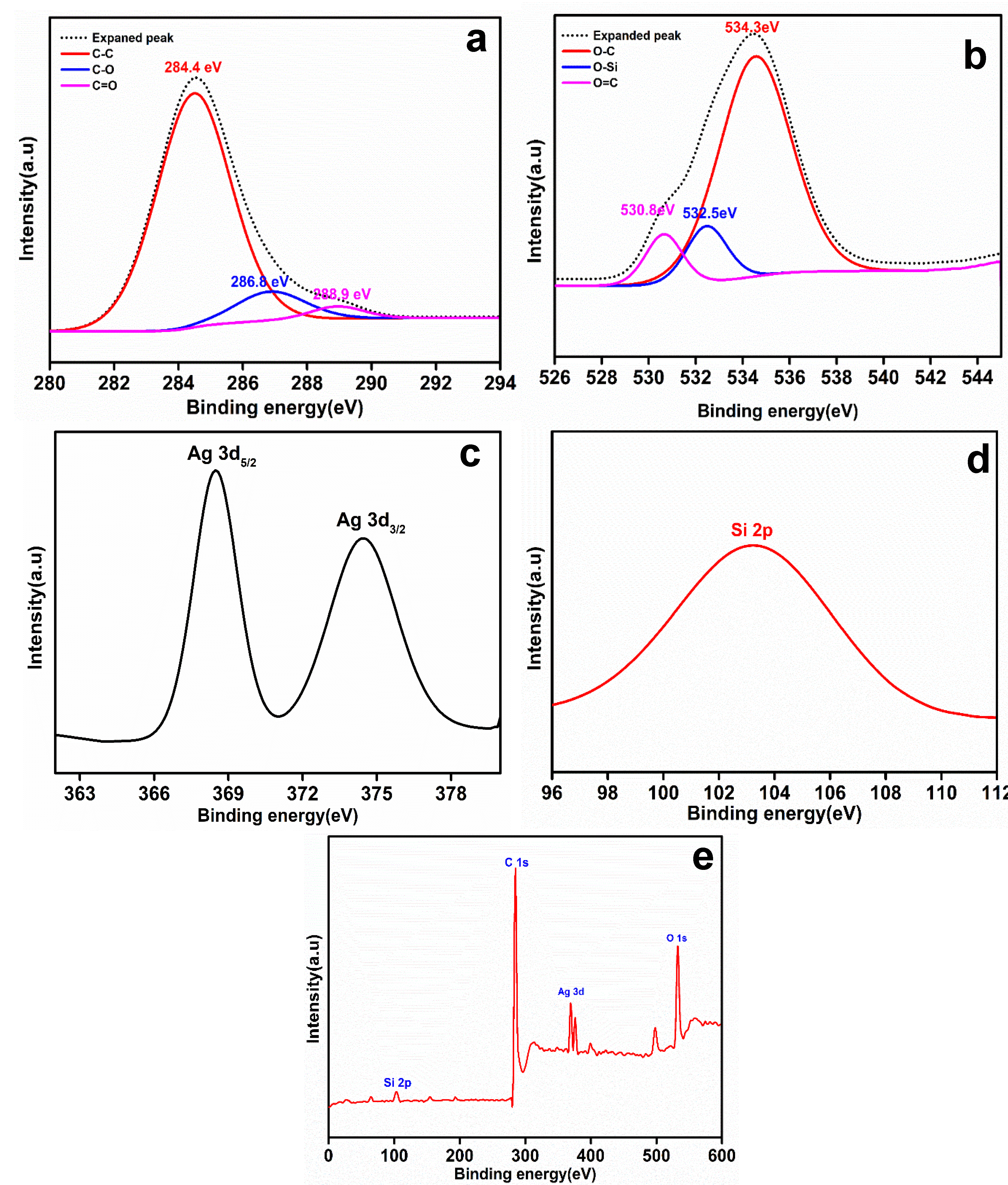
3.1. Characterization
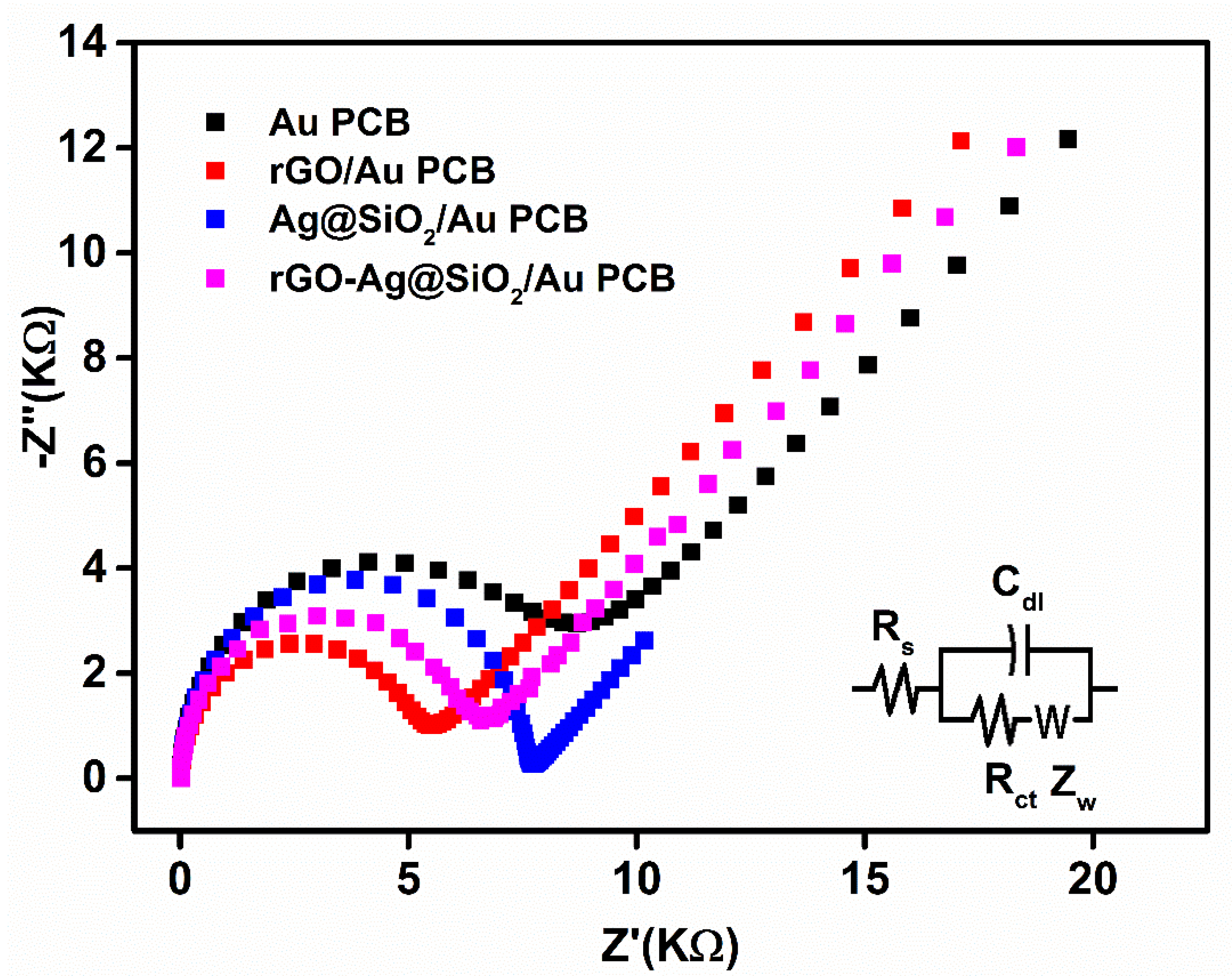
3.2. Electrochemical Characterization of rGO-Ag@SiO2/Au PCB
3.3. Electrochemical Oxidation of EA in the rGO-Ag@SiO2/Au PCB
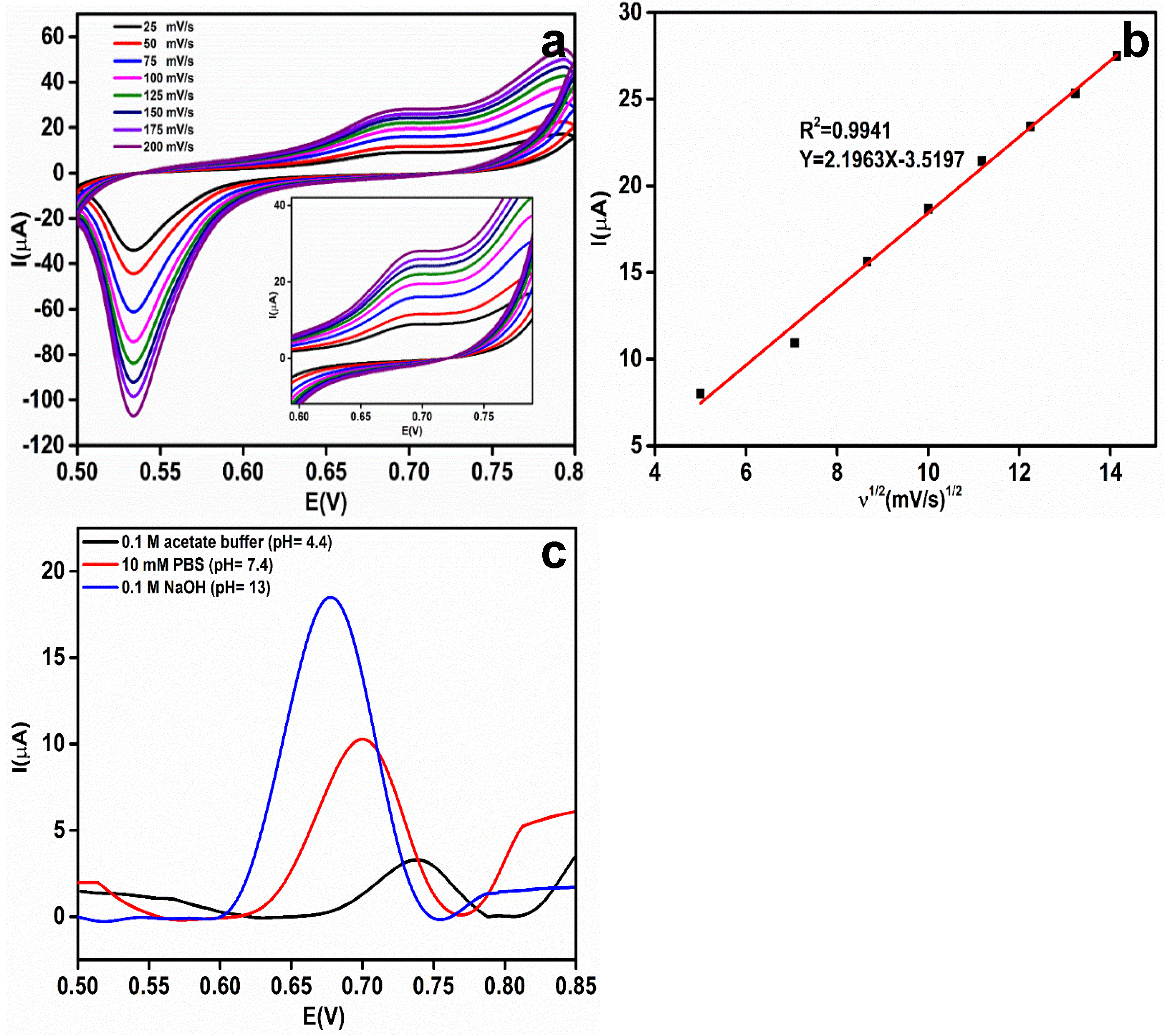
3.4. Effect of Scan Rate and pH
3.5. Mechanism of the Detection of EA
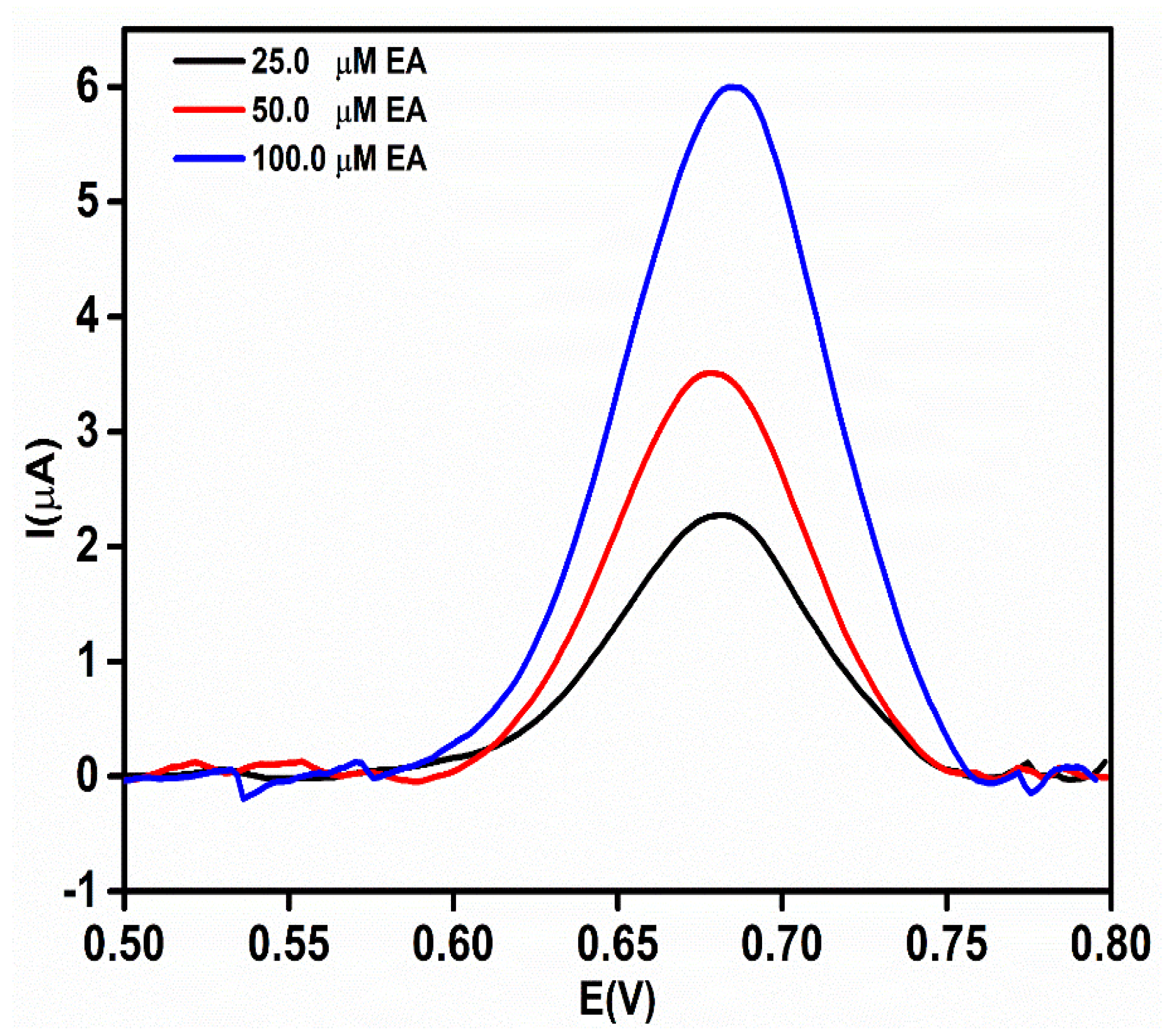
3.6. DPV Study
3.7. Stability, Reproducibility, and Reusability
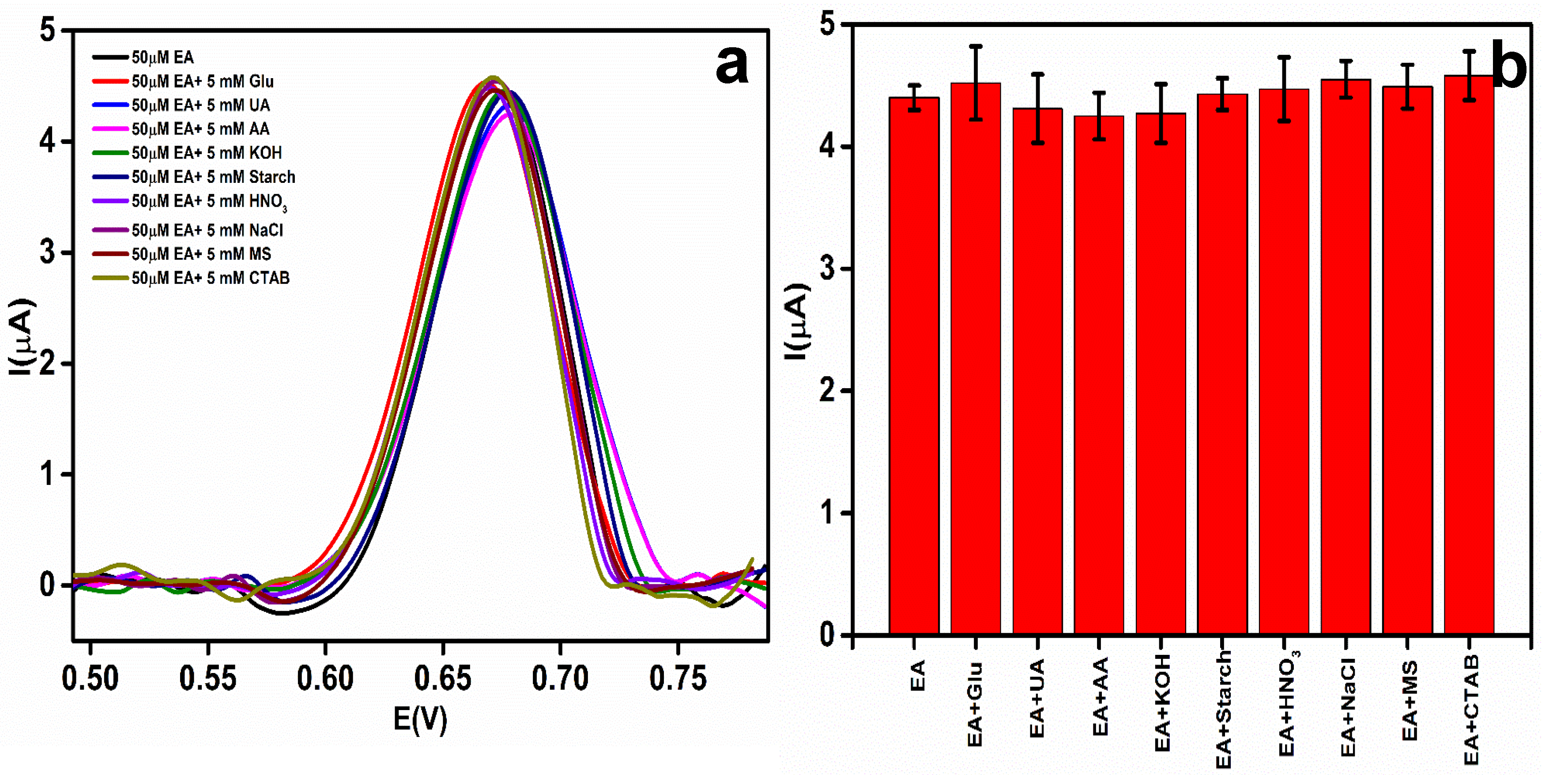
3.8. Interference Study
3.9. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Ioachimescu, A.; Licata, A. Etidronate: What is its place in treatment of primary osteoporosis and other demineralizing diseases today? Curr. Osteoporos. Rep. 2007, 5, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Reszka, A.A.; Rodan, G.A. Mechanism of action of bisphosphonates. Curr. Osteoporos. Rep. 2003, 1, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, Y.; Guo, C.; Chen, D. Current Topics in Osteoporosis; World Scientific Publishing, Co.: Singapore, 2005; pp. 1–551. [Google Scholar] [CrossRef]
- Barfidokht, A.; Mishra, R.K.; Seenivasan, R.; Liu, S.; Hubble, L.J.; Wang, J.; Hall, D.A. Wearable electrochemical glove-based sensor for rapid and on-site detection of fentanyl. Sens. Actuators B Chem. 2019, 296, 126422. [Google Scholar] [CrossRef]
- Mabrouk, M.; Hammad, S.F.; Abdelaziz, M.A.; Mansour, F.R. Determination of Etidronate in Pharmaceutical Formulations by RP-HPLC Method with Indirect UV Detection. Arab. J. Med. Sci. 2018, 1, 1–4. [Google Scholar] [CrossRef]
- Zacharis, C.K.; Tzanavaras, P.D. Determination of bisphosphonate active pharmaceutical ingredients in pharmaceuticals and biological material: A review of analytical methods. J. Pharm. Biomed. Anal. 2008, 48, 483–496. [Google Scholar] [CrossRef]
- Panneer Selvam, S.; Yun, K. A self-assembled silver chalcogenide electrochemical sensor based on rGO-Ag2Se for highly selective detection of serotonin. Sens. Actuators B Chem. 2020, 302. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, Y.; Xu, S.; Feng, M.; Yu, Y.; Yang, W.; Li, H. A highly sensitive sensor based on ordered mesoporous ZnFe2O4 for electrochemical detection of dopamine. Anal. Chim. Acta 2020, 1096, 26–33. [Google Scholar] [CrossRef]
- Xu, H.; Shao, M.; Chen, T.; Zhuo, S.; Wen, C.; Peng, M. Magnetism-assisted assembled porous Fe3O4 nanoparticles and their electrochemistry for dopamine sensing. Microporous Mesoporous Mater. 2012, 153, 35–40. [Google Scholar] [CrossRef]
- Yusoff, N.; Pandikumar, A.; Ramaraj, R.; Lim, H.N.; Huang, N.M. Gold nanoparticle based optical and electrochemical sensing of dopamine. Microchim. Acta 2015, 182, 2091–2114. [Google Scholar] [CrossRef]
- Roy, S.; Soin, N.; Bajpai, R.; Misra, D.S.; McLaughlin, J.A.; Roy, S.S. Graphene oxide for electrochemical sensing applications. J. Mater. Chem. 2011, 21, 14725–14731. [Google Scholar] [CrossRef]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M. A graphene oxide-based electrochemical sensor for sensitive determination of 4-nitrophenol. J. Hazard. Mater. 2012, 201–202, 250–259. [Google Scholar] [CrossRef]
- Zhu, N.; Han, S.; Gan, S.; Ulstrup, J.; Chi, Q. Graphene paper doped with chemically compatible prussian blue nanoparticles as nanohybrid electrocatalyst. Adv. Funct. Mater. 2013, 23, 5297–5306. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Liu, Y.; Li, J. Graphene and its derivatives for the development of solar cells, photoelectrochemical, and photocatalytic applications. Energy Environ. Sci. 2013, 6, 1362–1387. [Google Scholar] [CrossRef]
- Joung, D.; Singh, V.; Park, S.; Schulte, A.; Seal, S.; Khondaker, S.I. Anchoring ceria nanoparticles on reduced graphene oxide and their electronic transport properties. J. Phys. Chem. C 2011, 115, 24494–24500. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Ebenso, E.E. Electrochemical determination of serotonin in urine samples based on metal oxide nanoparticles/MWCNT on modified glassy carbon electrode. Sens. Bio-Sens. Res. 2017, 13, 17–27. [Google Scholar] [CrossRef]
- Wang, F.; Hu, S. Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim. Acta 2009, 165, 1–22. [Google Scholar] [CrossRef]
- Chazalviel, J.-N.; Allongue, P. On the Origin of the Efficient Nanoparticle Mediated Electron Transfer across a Self-Assembled Monolayer. J. Am. Chem. Soc. 2011, 133, 762–764. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Wu, M.; Ge, S.L.; Zhu, Y.; Wang, Q.J.; He, P.G.; Fang, Y.Z. Simultaneous electrochemical determination of sulphite and nitrite by a gold nanoparticle/graphene-chitosan modified electrode. Fenxi Huaxue Chin. J. Anal. Chem. 2013, 41, 1232–1237. [Google Scholar] [CrossRef]
- Keerthi, M.; Boopathy, G.; Chen, S.M.; Chen, T.W.; Rwei, S.P.; Liu, X. An efficient electrochemical sensor based on Ag nanoparticle decorated MnO2 /reduced graphene oxide ternary nanocomposite for detection of acetaminophen in human urine sample. Int. J. Electrochem. Sci. 2019, 14, 346–358. [Google Scholar] [CrossRef]
- Nantaphol, S.; Chailapakul, O.; Siangproh, W. Sensitive and selective electrochemical sensor using silver nanoparticles modified glassy carbon electrode for determination of cholesterol in bovine serum. Sens. Actuators B Chem. 2015, 207, 193–198. [Google Scholar] [CrossRef]
- Veerapandian, M.; Yun, K. Methylene blue dye coated silver-silica nanoparticles with dual functionality fabricated by injection pump and ultrasonochemistry. Mater. Res. Bull. 2013, 48, 1817–1823. [Google Scholar] [CrossRef]
- Veerapandian, M.; Neethirajan, S. chitosan nanoparticles: Fabrication, electrode. RSC Adv. 2015, 75015–75024. [Google Scholar] [CrossRef]
- Yang, S.; Yue, W.; Huang, D.; Chen, C.; Lin, H.; Yang, X. A facile green strategy for rapid reduction of graphene oxide by metallic zinc. RSC Adv. 2012, 2, 8827–8832. [Google Scholar] [CrossRef]
- Sakthisabarimoorthi, A.; Martin Britto Dhas, S.A.; Jose, M. Nonlinear optical properties of Ag@SiO2 core-shell nanoparticles investigated by continuous wave He-Ne laser. Mater. Chem. Phys. 2018, 212, 224–229. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Wang, Z.; Wang, Y.; Dai, J.; Gao, L.; Wei, M.; Yan, Y.; Li, C. A polydopamine-based molecularly imprinted polymer on nanoparticles of type SiO2@rGO@Ag for the detection of λ-cyhalothrin via SERS. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishna, S.; Sureshkumar, K.; Varley, T.S.; Nagaraju, D.H.; Ramakrishnappa, T. In situ reduction and functionalization of graphene oxide with l-cysteine for simultaneous electrochemical determination of cadmium(ii), lead(ii), copper(ii), and mercury(ii) ions. Anal. Methods 2014, 6, 8698–8705. [Google Scholar] [CrossRef]
- Sprenger, D.; Bach, H.; Meisel, W.; Gütlich, P. XPS study of leached glass surfaces. J. Non. Cryst. Solids 1990, 126, 111–129. [Google Scholar] [CrossRef]
- Feng, J.; Fan, D.; Wang, Q.; Ma, L.; Wei, W.; Xie, J.; Zhu, J. Facile synthesis silver nanoparticles on different xerogel supports as highly efficient catalysts for the reduction of p-nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 743–756. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Liu, H.; Suen, N.T.; Yu, X.; Feng, L. Electrochemical Hydrogen Evolution Reaction Efficiently Catalyzed by Ru2P Nanoparticles. ChemSusChem 2018, 11, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Rong, K.; Huang, L.; Zhang, H.; Zhai, J.; Fang, Y.; Dong, S. Electrochemical fabrication of nanoporous gold electrodes in a deep eutectic solvent for electrochemical detections. Chem. Commun. 2018, 54, 8853–8856. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.G.M.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the electrochemical area of screen-printed electrochemical sensing platforms. Biosensors 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Veera Manohara Reddy, Y.; Sravani, B.; Agarwal, S.; Gupta, V.K.; Madhavi, G. Electrochemical sensor for detection of uric acid in the presence of ascorbic acid and dopamine using the poly(DPA)/SiO2@Fe3O4 modified carbon paste electrode. J. Electroanal. Chem. 2018, 820, 168–175. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Rawool, C.R.; Srivastava, A.K. Voltammetric determination of pyrazinamide at graphene-zinc oxide nanocomposite modified carbon paste electrode employing differential pulse voltammetry. Sens. Actuators B Chem. 2016, 237, 196–205. [Google Scholar] [CrossRef]
- Heli, H.; Faramarzi, F.; Sattarahmady, N. Voltammetric investigation and amperometric detection of the bisphosphonate drug sodium alendronate using a copper nanoparticles-modified electrode. J. Solid State Electrochem. 2010, 14, 2275–2283. [Google Scholar] [CrossRef]
- Heli, H.; Faramarzi, F.; Jabbari, A.; Parsaei, A.; Moosavi-Movahedi, A.A. Electrooxidation and determination of etidronate using copper nanoparticles and microparticles-modified carbon paste electrodes. J. Braz. Chem. Soc. 2010, 21, 16–24. [Google Scholar] [CrossRef]
- Goodarzi, Z.; Maghrebi, M.; Zavareh, A.F.; Mokhtari-Hosseini, Z.-B.; Ebrahimi-hoseinzadeh, B.; Zarmi, A.H.; Barshan-tashnizi, M. Evaluation of nicotine sensor based on copper nanoparticles and carbon nanotubes. J. Nanostruct. Chem. 2015, 5, 237–242. [Google Scholar] [CrossRef]
- Benedito Da Silva, O.; MacHado, S.A.S. Evaluation of the detection and quantification limits in electroanalysis using two popular methods: Application in the case study of paraquat determination. Anal. Methods 2012, 4, 2348–2354. [Google Scholar] [CrossRef]
- Ma, X.; Chao, M.; Wang, Z. Electrochemical detection of dopamine in the presence of epinephrine, uric acid, and ascorbic acid were administered using a graphene-modified electrode. Anal. Methods 2012, 4, 1687–1692. [Google Scholar] [CrossRef]





| Sensor Parameters | EA |
|---|---|
| Oxidation potential (V) | 0.67 |
| Linearity range (µM) | 2.0–200.0 |
| Slope of calibration curve | 0.0497 |
| Standard deviation of blanks | 1.12 × 10−8 |
| LOD (µM) | 0.68 |
| Operational stability (Retained peak current after 20 cycles) | 92.7% |
| Repeatability (%RSD) | 3.51% |
| Reproducibility (%RSD) | 2.88% |
| Storage stability (Retained peak current after four weeks) | 93.1% |
| Interferent Added | Concentration of Interferent Added (mM) | Concentration of EA Added (µM) | Ep of EA (V) | Peak Current of EA (µA) |
|---|---|---|---|---|
| - | 0 | 50 | 0.675 | 4.43 |
| Glucose | 5 | 50 | 0.668 | 4.52 |
| UA | 5 | 50 | 0.679 | 4.31 |
| AA | 5 | 50 | 0.679 | 4.26 |
| KOH | 5 | 50 | 0.676 | 4.27 |
| Starch | 5 | 50 | 0.678 | 4.43 |
| HNO3 | 5 | 50 | 0.670 | 4.47 |
| NaCl | 5 | 50 | 0.673 | 4.55 |
| MS | 5 | 50 | 0.673 | 4.49 |
| CTAB | 5 | 50 | 0.671 | 4.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panneer Selvam, S.; Chinnadayyala, S.R.; Cho, S.; Yun, K. Differential Pulse Voltammetric Electrochemical Sensor for the Detection of Etidronic Acid in Pharmaceutical Samples by Using rGO-Ag@SiO2/Au PCB. Nanomaterials 2020, 10, 1368. https://doi.org/10.3390/nano10071368
Panneer Selvam S, Chinnadayyala SR, Cho S, Yun K. Differential Pulse Voltammetric Electrochemical Sensor for the Detection of Etidronic Acid in Pharmaceutical Samples by Using rGO-Ag@SiO2/Au PCB. Nanomaterials. 2020; 10(7):1368. https://doi.org/10.3390/nano10071368
Chicago/Turabian StylePanneer Selvam, Sathish, Somasekhar R. Chinnadayyala, Sungbo Cho, and Kyusik Yun. 2020. "Differential Pulse Voltammetric Electrochemical Sensor for the Detection of Etidronic Acid in Pharmaceutical Samples by Using rGO-Ag@SiO2/Au PCB" Nanomaterials 10, no. 7: 1368. https://doi.org/10.3390/nano10071368
APA StylePanneer Selvam, S., Chinnadayyala, S. R., Cho, S., & Yun, K. (2020). Differential Pulse Voltammetric Electrochemical Sensor for the Detection of Etidronic Acid in Pharmaceutical Samples by Using rGO-Ag@SiO2/Au PCB. Nanomaterials, 10(7), 1368. https://doi.org/10.3390/nano10071368