Sol-Gel Synthesis of Nanocrystalline Mesoporous Li4Ti5O12 Thin-Films as Anodes for Li-Ion Microbatteries
Abstract
1. Introduction
2. Materials and Methods
Analytical Methods
3. Results and Discussion
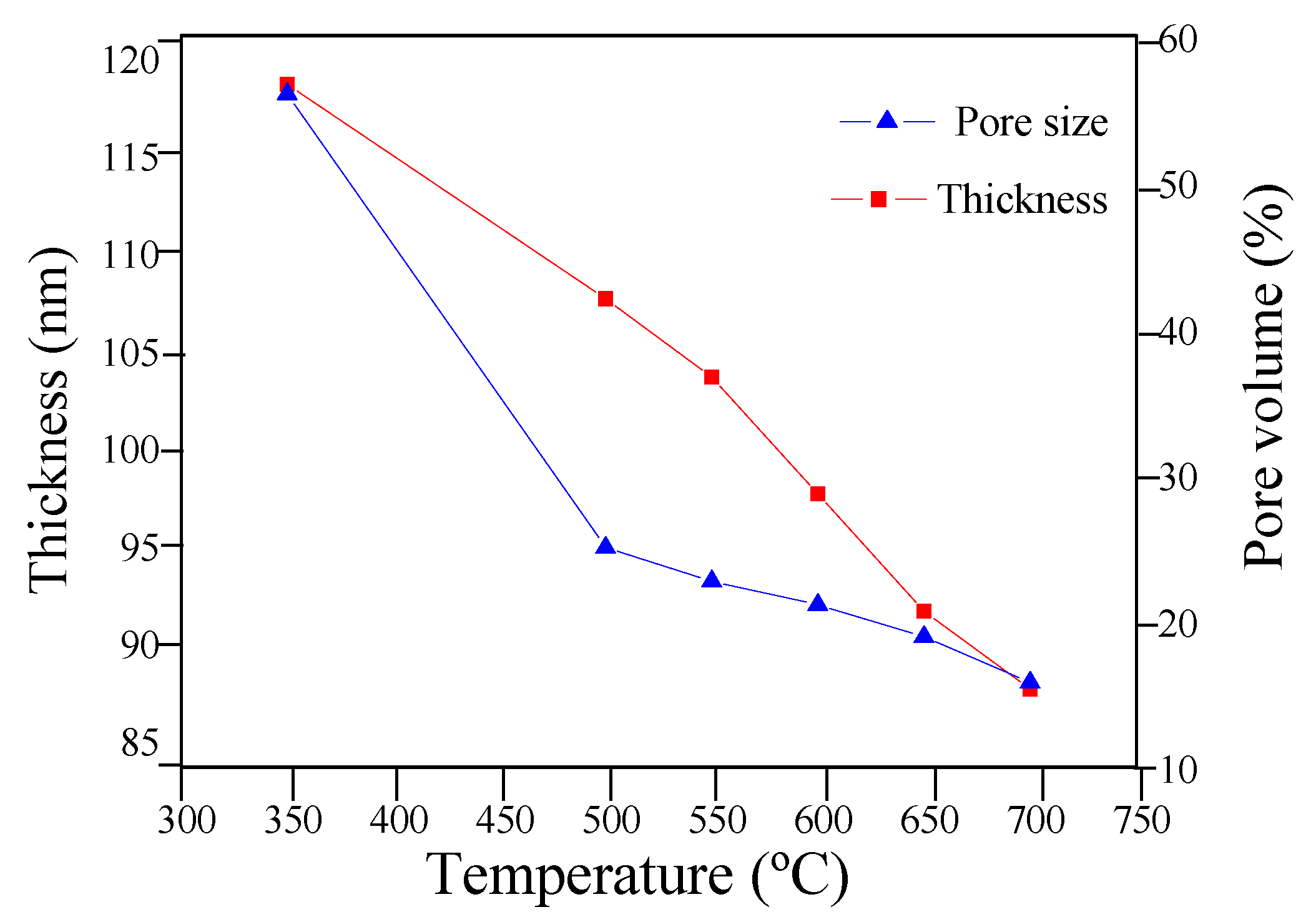
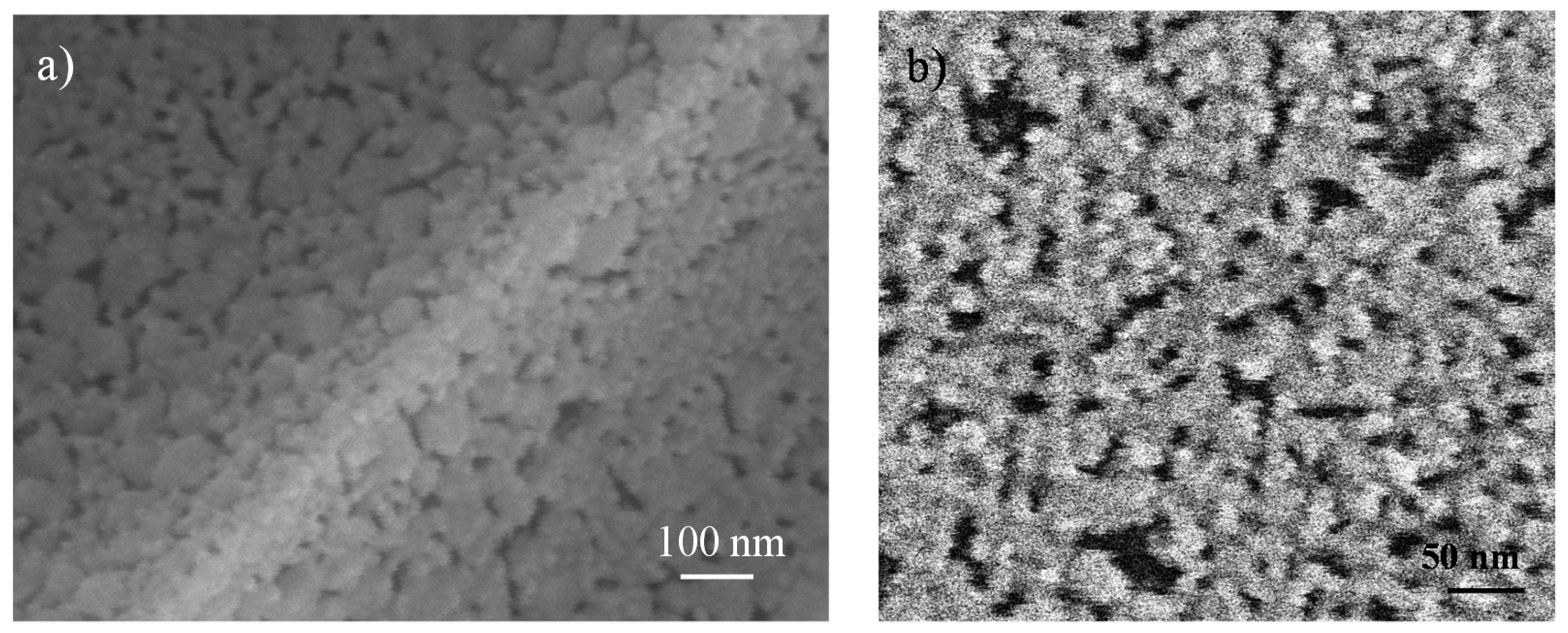
3.1. Li4Ti5O12 Thin-Films
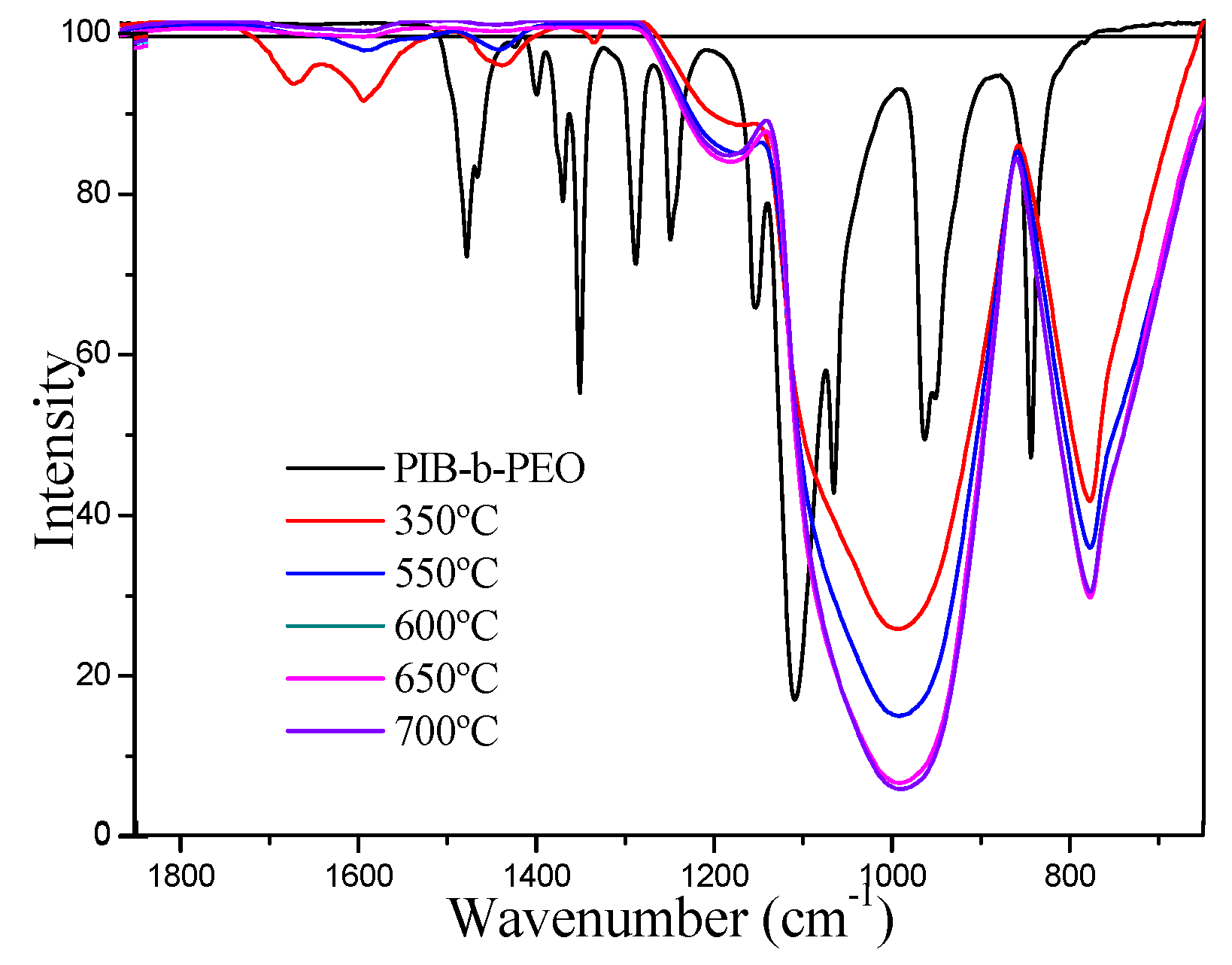
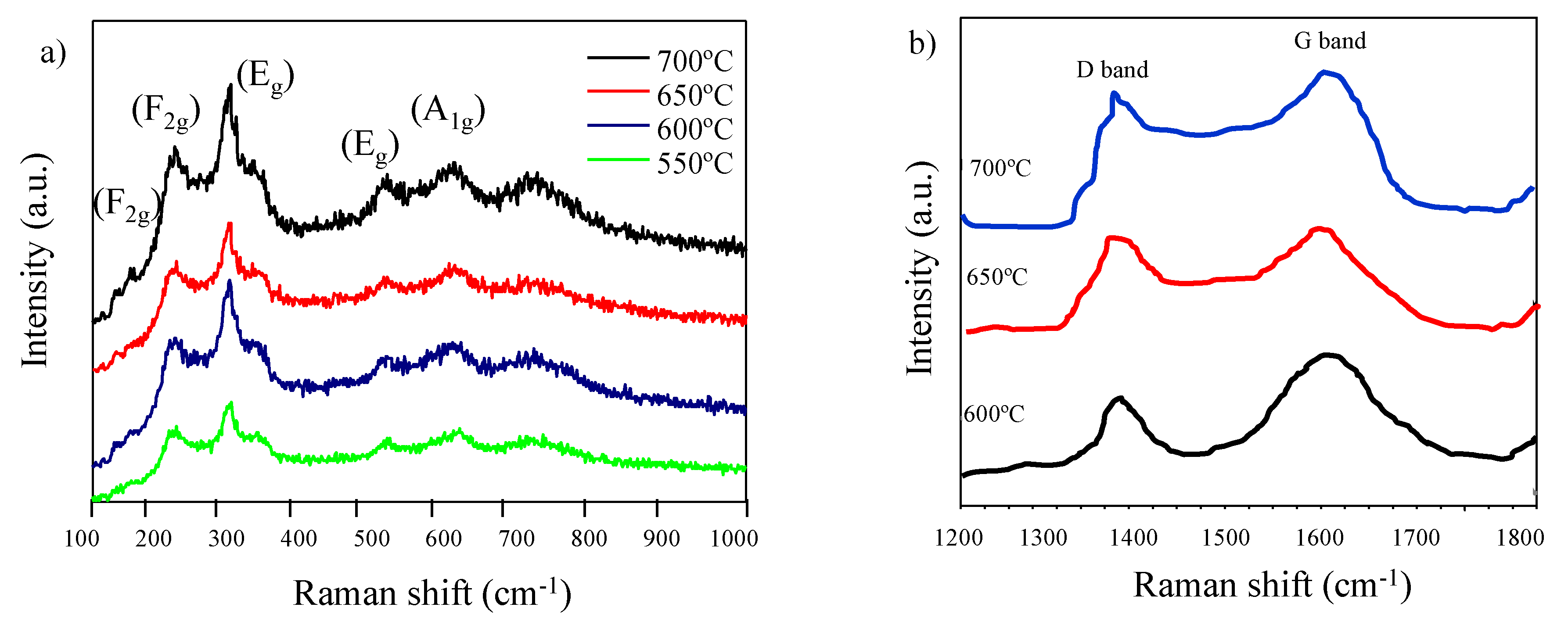
3.2. Structural Characterization of Li4Ti5O12 Thin-Films
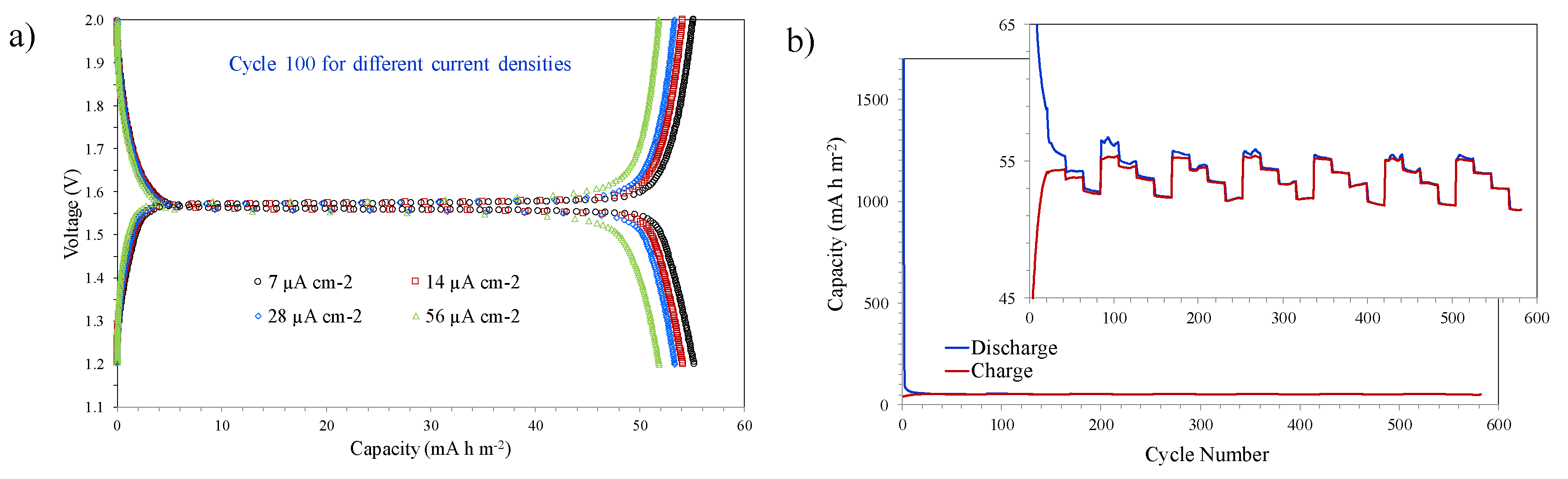
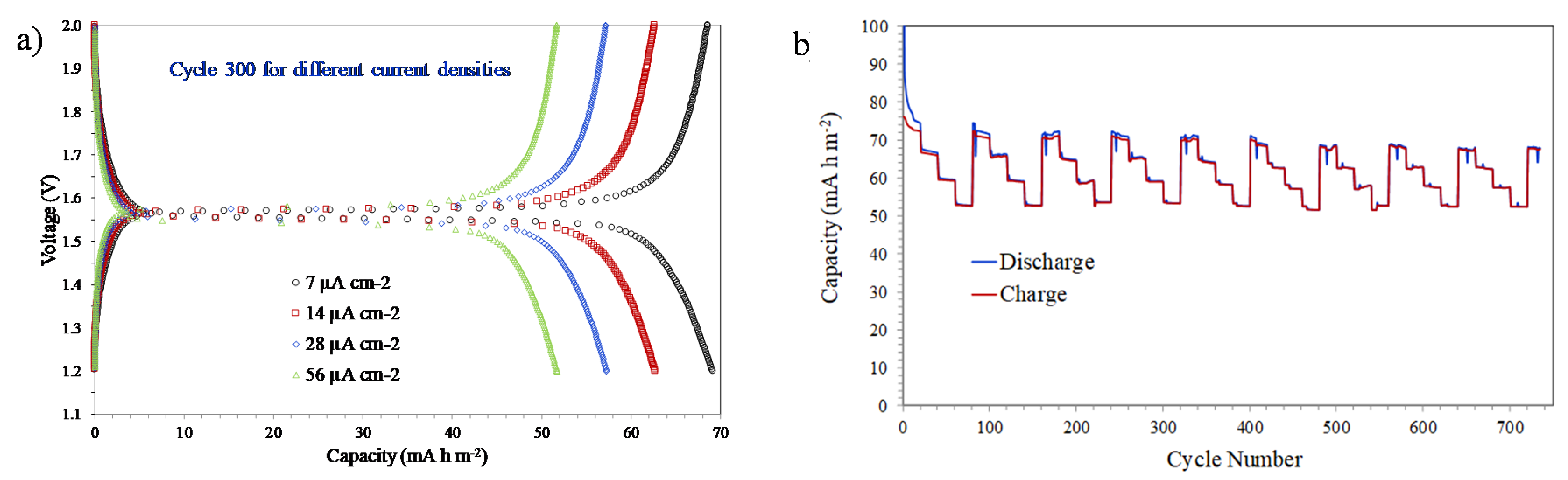
3.3. Electrochemical Characterization of Li4Ti5O12 Thin-Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Xue, M.; Fu, Z. Nanostructured thin film electrodes for lithium storage and all-solid-state thin-film lithium batteries. J. Power Sources 2013, 234, 310–332. [Google Scholar] [CrossRef]
- Borgia, E. The Internet of Things vision: Key features, applications and open issues. Comput. Commun. 2014, 54, 1–31. [Google Scholar] [CrossRef]
- Glenneberg, J.; Andre, F.; Bardenhagen, I.; Langer, F.; Schwenzel, J.; Kun, R. A concept for direct deposition of thin film batteries on flexible polymer substrate. J. Power Sources 2016, 324, 722–728. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Daniel, Z.; Xiao, J. Lithium and lithium ion batteries for applications in microelectronic devices: A review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.M.; Baggetto, L.; Notten, P.H.L. All-Solid-State Lithium-Ion Microbatteries: A Review of Various Three-Dimensional Concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Larfaillou, S.; Guy-Bouyssou, D.; Cras, F.; Franger, S. Comprehensive characterization of all-solid-state thin films commercial microbatteries by Electrochemical Impedance Spectroscopy. J. Power Sources 2016, 319, 139–146. [Google Scholar] [CrossRef]
- Ahn, B.Y.; Duoss, E.B.; Motala, M.J.; Guo, X.; Park, S.-I.; Xiong, Y.; Yoon, J.; Nuzzo, R.G.; Rogers, J.A.; Lewis, J.A. Omnidirectional printing of flexible, stretchable, and spanning silver microelectrodes. Science 2009, 323, 1590–1593. [Google Scholar] [CrossRef]
- Esmaeilzadeh, H.; Blem, E.; Amant, R.S.; Sankaralingam, K.; Burger, D. Dark silicon and the end of multicore scaling. In Proceedings of the 38th Annual International Symposium on Computer Architecture (ISCA), San Jose, CA, USA, 4–8 June 2011; pp. 365–376. [Google Scholar]
- Sun, K.; Wei, T.-S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D printing of interdigitated Li-ion microbattery architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Roozeboom, F.; Niessen, R.A.H.; Baggetto, L. 3-D Integrated All-Solid-State Rechargeable Batteries. Adv. Mater. 2007, 19, 4564–4567. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Guo, Y.-G.; Hu, J.-S.; Wan, L.-J. Nanostructured materials for electrochemical energy conversion and storage devices. Adv. Mater. 2008, 20, 2878–2887. [Google Scholar] [CrossRef]
- Scrosati, B. Paper powers battery breakthrough. Nat. Nanotechnol. 2007, 2, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Smart, M.C.; Ratnakumar, B.V.; Surampudi, S.; Wang, Y.; Zhang, X.; Greenbaum, S.G.; Hightower, A.; Ahn, C.C.; Fultz, B. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. J. Electrochem. Soc. 1999, 146, 3963–3969. [Google Scholar] [CrossRef]
- Murphy, D.W.; Cava, R.J.; Zahurak, S.M.; Santoro, A. Ternary LixTiO2 phases from insertion reactions. Solid State Ion. 1983, 9, 413–417. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Amamoto, N. Zero-Strain Insertion Material of Li [ Li1 / 3 Ti5 / 3] O4 for Rechargeable Lithium Cells. J. Electrochem. Soc. 1995, 142, 1431–1435. [Google Scholar] [CrossRef]
- Zaghib, K.; Simoneau, M.; Armand, M.; Gauthier, M. Electrochemical study of Li4Ti5O12 as negative electrode for Li-ion polymer rechargeable batteries. J. Power Sources 1999, 81–82, 300–305. [Google Scholar] [CrossRef]
- Mosa, J.; Vélez, J.F.; Lorite, I.; Arconada, N.; Aparicio, M. Film-shaped sol–gel Li4Ti5O12 electrode for lithium-ion microbatteries. J. Power Sources 2012, 205, 491–494. [Google Scholar] [CrossRef]
- Mosa, J.; Vélez, J.F.; Reinosa, J.J.; Aparicio, M.; Yamaguchi, A.; Tadanaga, K.; Tatsumisago, M. Preparation of Li4Ti5O12 electrode thin films by a mist CVD process with aqueous precursor solution. J. Power Sources 2013, 244, 482–487. [Google Scholar] [CrossRef]
- García-Herbosa, G.; Aparicio, M.; Mosa, J.; Cuevas, J.V.; Torroba, T. Choosing the best molecular precursor to prepare Li4Ti5O12 by the sol–gel method using 1H NMR: Evidence of [Ti3(OEt)13]− in solution†. Dalton Trans. 2016, 45, 13888–13898. [Google Scholar] [CrossRef]
- Tadanaga, K.; Yamaguchi, A.; Hayashi, A.; Tatsumisago, M.; Mosa, J.; Aparicio, M. Preparation of Li4Ti5O12 electrode thin films by a mist CVD process with aqueous precursor solution. J. Asian Ceram. Soc. 2015, 3, 88–91. [Google Scholar] [CrossRef]
- Mosa, J.; Aparicio, M.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Li4Ti5O12 thin-film electrodes by in-situ synthesis of lithium alkoxide for Li-ion microbatteries. Electrochim. Acta 2014, 9, 293–299. [Google Scholar] [CrossRef]
- Shao, D.; He, J.; Luo, Y.; Liu, W.; Yu, X.; Fang, Y. Synthesis and electrochemical performance of nanoporous Li4Ti5O12 anode material for lithium-ion batteries. J. Solid State Electrochem. 2012, 16, 2047–2053. [Google Scholar] [CrossRef]
- Raj, H.; Saxena, S.; Sil, A. Improved electrochemical performance of Li4Ti5O12 by reducing rutile TiO2 phase impurity and particle size. Mater. Technol. 2017, 32, 196–201. [Google Scholar] [CrossRef]
- Saxena, S.; Sil, A. Nanoporous Li4Ti5O12 Material for the Electrode of Lithium Ion Battery. IETE Tech. Rev. 2016, 33, 60–63. [Google Scholar] [CrossRef]
- Ping, L.; Zhi-an, Z.; Jie, L.; Yan-qing, L. Effects of carbon sources on electrochemical performance of Li4Ti5O12/C composite anode material. J. Cent. South Univ. Technol. 2010, 17, 1207–1210. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Li, J.; Stach, E.A.; Tong, X.; Takeuchi, E.S.; Takeuchi, K.J.; Liu, P.; Marschilok, A.C.; Wong, S.S. Structural and Electrochemical Characteristics of Ca-Doped “Flower-like” Li4Ti5O12 Motifs as High-Rate Anode Materials for Lithium-Ion Batteries. Chem. Mater. 2018, 30, 671–684. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Li, B.; Kang, S.; Li, X.; Wang, Y. Preparation and electrochemical properties of Ca-doped Li4Ti5O12 as anode materials in lithium-ion battery. Electrochim. Acta 2013, 98, 146–152. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Allen, J.L. Electrical conductivity and charge compensation in Ta doped Li4Ti5O12. J. Power Sources 2008, 180, 582–585. [Google Scholar] [CrossRef]
- Hu, G.-R.; Zhang, X.-L.; Peng, Z.-D. Preparation and electrochemical performance of tantalum-doped lithium titanate as anode material for lithium-ion battery. Trans. Nonferrous Metals Soc. China 2011, 21, 2248–2253. [Google Scholar] [CrossRef]
- Kubiak, P.; Garcia, A.; Womes, M.; Aldon, L.; Fourcade, J.O.; Lippens, P.E.; Jumas, J.C. Phase transition in the spinel Li4Ti5O12 induced by lithium insertion: Influence of the substitutions Ti/V., Ti/Mn, Ti/Fe. J. Power Sources 2003, 119–121, 626–630. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Lee, U.; Allen, J.L. Electrical conductivity and rate-capability of Li4Ti5O12 as a function of heat-treatment atmosphere. J. Power Sources 2006, 154, 287–289. [Google Scholar] [CrossRef]
- Mosa, J.; Aparicio, M.; Durán, A.; Laberty−Robert, C.; Sanchez, C. Nanocrystalline Mesoporous LiFePO4 thin−films as cathode for Li- ion Microbatteries. J. Mater. Chem. A 2014, 2, 3038–3046. [Google Scholar] [CrossRef]
- Sassoye, C.; Laberty, C.; Khanh, H.L.; Cassaignon, S.; Boissière, C.; Antonietti, M.; Sanchez, C. Block-Copolymer-Templated Synthesis of Electroactive RuO2-Based Mesoporous Thin Films. Adv. Funct. Mater. 2009, 19, 1922–1929. [Google Scholar] [CrossRef]
- Haetge, J.; Hartman, P.; Brezenski, K.; Janek, J.; Brezenski, T. Ordered large-pore mesoporous Li4Ti5O12 spinel thin film electrodes with nanocrystalline framework for high rate rechargeable lithium batteries: Relationships among charge storage, electrical conductivity, and nanoscale structure. Chem. Mater. 2011, 23, 4348–4393. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, K.H.; Park, B.G.; Kim, H.G.; Park, Y.J. Synthesis of Li4Ti5O12 Thin Film with Inverse Hemispheric Structure. Bull. Korean Chem. Soc. 2010, 31, 360–364. [Google Scholar] [CrossRef]
- Rho, Y.H.; Kanamura, K. Preparation of Li4Ti5O12 Thin Film Electrode with PVP Sol-Gel for a Rechargeable Lithium Microbattery. J. Surf. Sci. Soc. Jpn. 2003, 24, 423–428. [Google Scholar] [CrossRef][Green Version]
- Mani, J.; Katzke, H.; Habouti., S.; Moonoosawmy, K.R.; Dietze, M.; Es-Souni, M. A template-free synthesis and structural characterization of hierarchically nano-structured lithium-titanium-oxide films. J. Mater. Chem. 2012, 22, 6632–6639. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Fang, S.; Hirano, S.; Tachibana, K. Synthesis of hierarchical mesoporous nest-like Li4Ti5O12 for high-rate lithium ion batteries. J. Power Sources 2012, 200, 59–66. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Z.; Yang, T.; Liao, C.; Wang, Z.; Sun, K. Facile preparation of nanocrystalline Li4Ti5O12 and its high electrochemical performance as anode material for lithium-ion batteries. Electrochem. Commun. 2011, 13, 654–656. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Duh, J.-G. Porous Li4Ti5O12 anode material synthesized by one-step solid state method for electrochemical properties enhancement. J. Alloys Comp. 2011, 509, 3682–3685. [Google Scholar] [CrossRef]
- Kang, E.; Juang, Y.S.; Kim, G.-H.; Chun, J.; Weisner, U.; Dillon, A.C.; Kim, J.K.; Lee, J. Highly Improved Rate Capability for a Lithium-Ion Battery Nano-Li4Ti5O12 Negative Electrode via Carbon-Coated Mesoporous Uniform Pores with a Simple Self-Assembly Method. Adv. Funct. Mater. 2011, 21, 4349–4357. [Google Scholar] [CrossRef]
- Bass, J.D.; Boissiere, C.; Nicole, L.; Grosso, D.; Sanchez, C. Thermally Induced Porosity in CSDMgF2-Based Optical Coatings: An Easy Method to Tune the Refractive Index. Chem. Mater. 2008, 17, 5550–5556. [Google Scholar] [CrossRef]
- Hierso, J.; Sel, O.; Ringuede, A.; Laberty-Robert, C.; Bianchi, L.; Grosso, D.; Sanchez, C. Design, Synthesis, Structural and Textural Characterization, and Electrical Properties of Mesoporous Thin Films Made of Rare Earth Oxide Binaries. Chem. Mater. 2009, 21, 2184–2192. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, S.W.; Hoang., H.V.; Doh, C.H.; Kim, D.W. Study on the cycling performance of Li4Ti5O12 electrode in the ionic liquid electrolytes containing an additive. Bull. Korean. Chem. Soc. 2011, 32, 105–108. [Google Scholar] [CrossRef]
- Snyder, M.Q.; DeSisto, W.J.; Tripp, C.P. An infrared study of the surface chemistry of lithium titanate spinel (Li4Ti5O12). Appl. Surf. Sci. 2007, 253, 9336–9341. [Google Scholar] [CrossRef]
- Vikram, B.; Vijaya, B.K.; Vikaya, B.K.; Tewodros, B.G.; Seete, A.L.; Madhavi, D.B.; Sushma, L.M.; Samantha, R.K.; Veeraiah, V. Structural and electrical properties of Li4Ti5O12 anode material for lithium-ion batteries. Results Phys. 2018, 9, 284–289. [Google Scholar] [CrossRef]
- Yu, J.C.; Zhang, L.; Zheng, Z.; Zhao, J. Synthesis and Characterization of Phosphated Mesoporous Titanium Dioxide with High Photocatalytic Activity. Chem. Mater. 2003, 15, 2280–2286. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Kornowski, A.; Weller, H.J. Low-Temperature Synthesis of Soluble and Processable Organic-Capped Anatase TiO2 Nanorods. J. Am. Chem. Soc. 2003, 125, 14539–14548. [Google Scholar] [CrossRef]
- Chen, J.; Zhai, H.; Chen, N.; Wang, X.; Wang, J.; Zhang, R.; Changqing, J. A novel FeAs anode material for lithium ion battery. J. Power Sources 2012, 200, 98–101. [Google Scholar] [CrossRef]
- Pfenninger, R.; Afyon, S.; Garbayo, I.; Struzik, M.; Rupp, J.L.M. Lithium titanate anode thin films for Li ion solid state battery based on garnets. Adv. Funct. Mater. 2018, 28, 1800879. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, W.; Dai, X.; Feng, L.; Zhang, H.; Zhou, A.; Li, J. Solid-state synthesis of graphite carbon-coated Li4Ti5O12 anode for lithium ion batteries. Ionics 2014, 20, 1377–1383. [Google Scholar] [CrossRef]
- Borgel, V.; Gershinsky, G.; Hu, T.; Theivanayagam, M.G.; Aurbach, D. LiMn0.8Fe0.2PO4/Li4Ti5O12, a possible Li-ion battery system for load-leveling application. J. Electrochem. Soc. 2013, 160, A650–A657. [Google Scholar] [CrossRef]
- Ding, Z.; Zhao, L.; Suo, L.; Jiao, Y.; Meng, S.; Hu, Y.S.; Wang, Z.; Chen, L. Towards understanding the effects of carbon and nitrogen doped carbon coating on the electrochemical performance of Li4Ti5O12 in lithium ion batteries: A combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2011, 13, 15127–15133. [Google Scholar] [CrossRef] [PubMed]




| Structure | Vibration Modes (cm−1) | |||||
|---|---|---|---|---|---|---|
| Spinel | 165 (F2g) | 233 (F2g) | 276 (F2g) | 334 (Eg) | 526 (Eg) | 654 (A1g) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosa, J.; Aparicio, M. Sol-Gel Synthesis of Nanocrystalline Mesoporous Li4Ti5O12 Thin-Films as Anodes for Li-Ion Microbatteries. Nanomaterials 2020, 10, 1369. https://doi.org/10.3390/nano10071369
Mosa J, Aparicio M. Sol-Gel Synthesis of Nanocrystalline Mesoporous Li4Ti5O12 Thin-Films as Anodes for Li-Ion Microbatteries. Nanomaterials. 2020; 10(7):1369. https://doi.org/10.3390/nano10071369
Chicago/Turabian StyleMosa, Jadra, and Mario Aparicio. 2020. "Sol-Gel Synthesis of Nanocrystalline Mesoporous Li4Ti5O12 Thin-Films as Anodes for Li-Ion Microbatteries" Nanomaterials 10, no. 7: 1369. https://doi.org/10.3390/nano10071369
APA StyleMosa, J., & Aparicio, M. (2020). Sol-Gel Synthesis of Nanocrystalline Mesoporous Li4Ti5O12 Thin-Films as Anodes for Li-Ion Microbatteries. Nanomaterials, 10(7), 1369. https://doi.org/10.3390/nano10071369







