Low Water Absorption, High-Strength Polyamide 6 Composites Blended with Sustainable Bamboo-Based Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Bamboo Based Biochar Reinforced Polyamide 6 Composites
2.2.2. Characterization
Melt Flow Rate
Water Absorption
Mechanical Properties and Morphological Evaluation
3. Results and Discussion
3.1. Melt Mass-Flow Rate (MFR)
3.2. Water Absorption
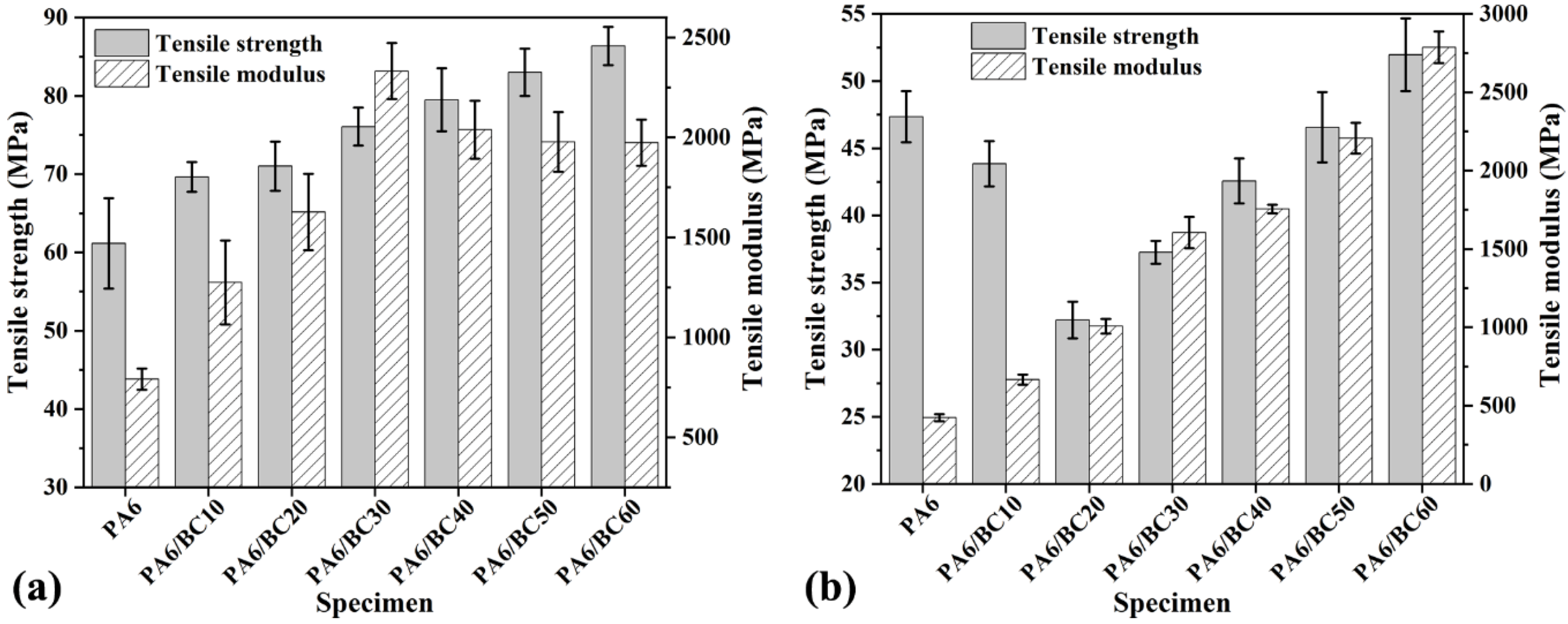
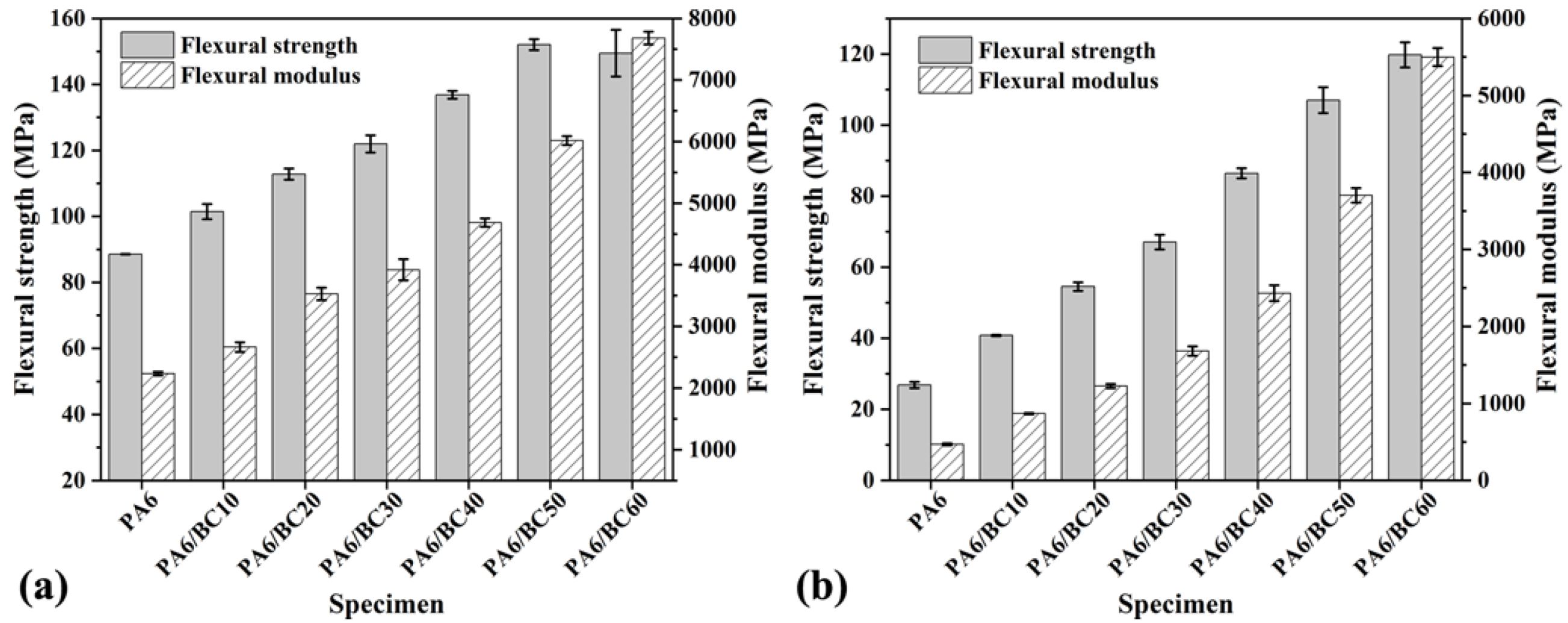
3.3. Mechanical Properties
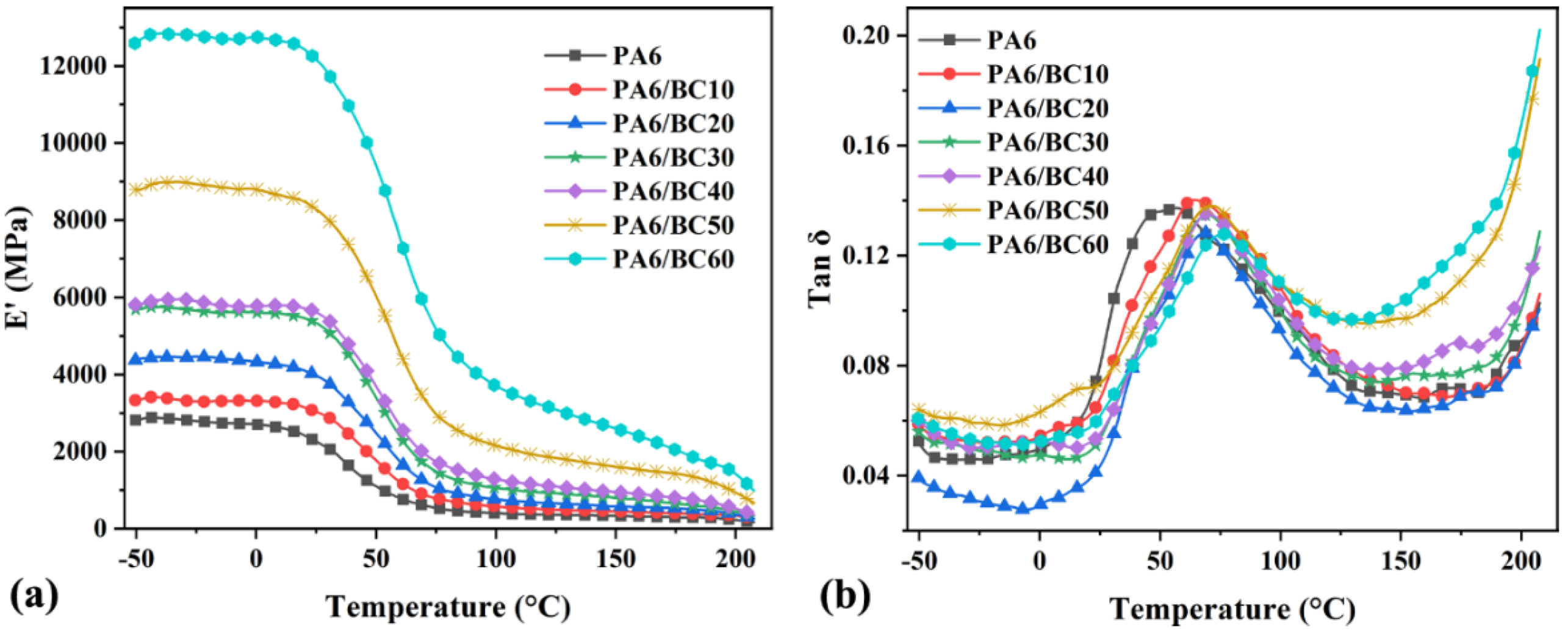
3.4. Dynamic Thermomechanical Analysis (DMA)
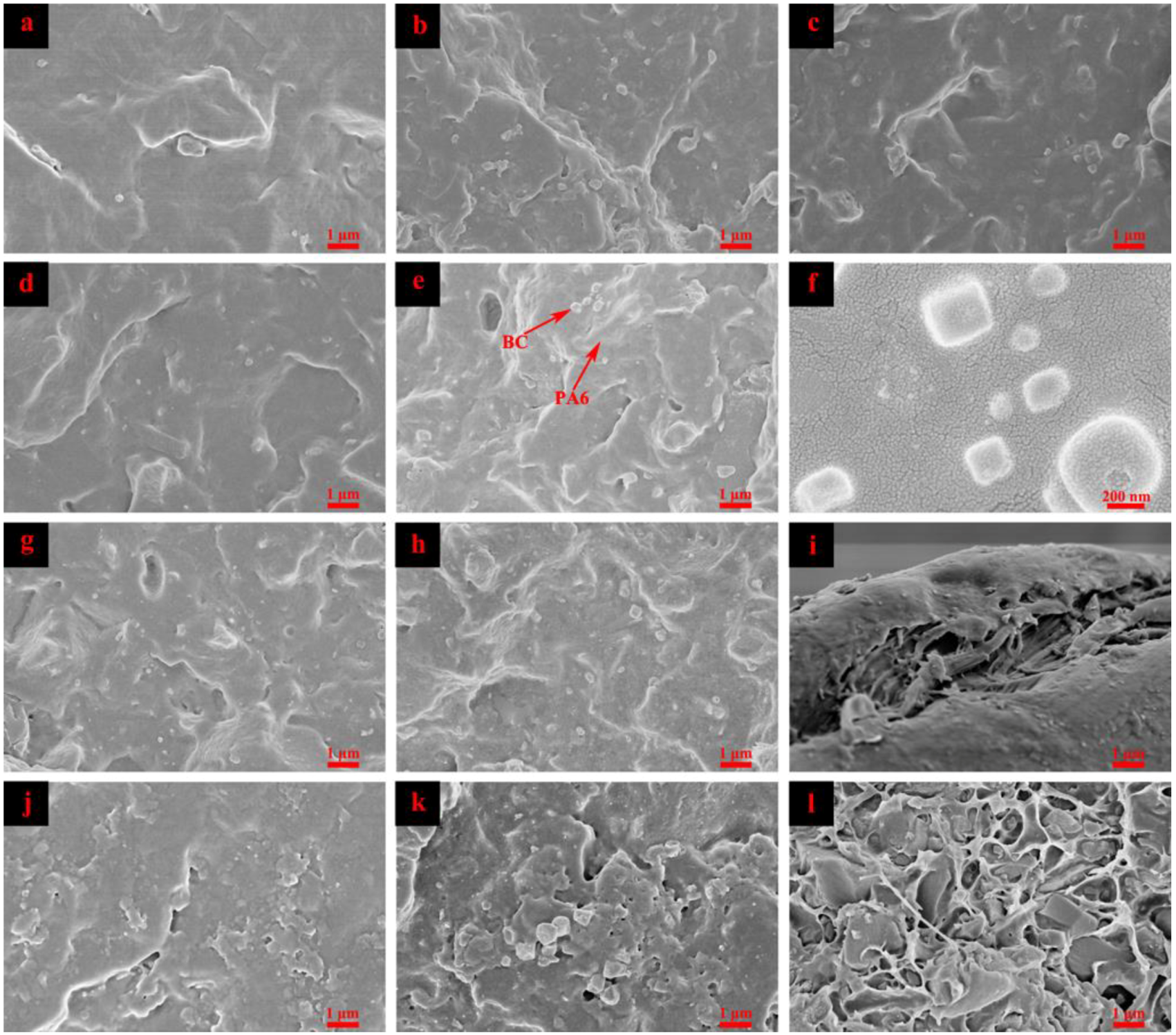
3.5. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moghadam, S.K.; Shamsian, M.; Shahri, H.R. Manufacturing Wood–Plastic Composites from Waste Lignocellulose Plus Haloxylon Species and Recycled Plastics. For. Prod. J. 2019, 69, 205–209. [Google Scholar]
- Bari, E.; Sistani, A.; Taghiyari, H.R.; Morrell, J.J.; Cappellazzi, J. Influence of test method on biodegradation of bamboo-plastic composites by fungi. Maderas Ciencia Y Tecnología 2017, 19, 455–462. [Google Scholar] [CrossRef][Green Version]
- Pan, M.; Mei, C.; Du, J.; Li, G. Synergistic effect of nano silicon dioxide and ammonium polyphosphate on flame retardancy of wood fiber-polyethylene composites. Compos. Part A 2014, 66, 128–134. [Google Scholar] [CrossRef]
- Vaisanen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: A review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Ashori, A. Wood-plastic composites as promising green-composites for automotive industries! Bioresour. Technol. 2008, 99, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Murayama, K.; Yamamoto, M.; Kobori, H.; Kojima, Y.; Suzuki, S.; Aoki, K.; Okamoto, M. Mechanical and physical properties of wood–plastic composites containing cellulose nanofibers added to wood flour. For. Prod. J. 2018, 68, 398–404. [Google Scholar] [CrossRef]
- Borah, J.S.; Kim, D.S. Recent development in thermoplastic/wood composites and nanocomposites: A review. Korean J. Chem. Eng. 2016, 33, 3035–3049. [Google Scholar] [CrossRef]
- Chan, C.M.; Vandi, L.; Pratt, S.; Halley, P.J.; Richardson, D.; Werker, A.; Laycock, B. Composites of Wood and Biodegradable Thermoplastics: A Review. Polym. Rev. 2018, 58, 444–494. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Codou, A.; Misra, M.; Mohanty, A.K. Thermally Stable Pyrolytic Biocarbon as an Effective and Sustainable Reinforcing Filler for Polyamide Bio-composites Fabrication. J. Polym. Environ. 2018, 26, 3574–3589. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Khan, A.N.; Ahmed, B.A. Comparative study of Polyamide 6 reinforced with glass fibre and montmorillonite. Polym. Bull. 2015, 72, 1207–1216. [Google Scholar] [CrossRef]
- Gutierrezgonzalez, S.; Alonso, M.M.; Gadea, J.; Rodriguez, A.; Calderon, V. Rheological behaviour of gypsum plaster pastes with polyamide powder wastes. Constr. Build. Mater. 2013, 38, 407–412. [Google Scholar] [CrossRef]
- Shen, L.; Lin, Y.; Du, Q.; Zhong, W. Studies on structure–property relationship of polyamide-6/attapulgite nanocomposites. Compos. Sci. Technol. 2006, 66, 2242–2248. [Google Scholar] [CrossRef]
- Beuguel, Q.; Ville, J.; Crepinleblond, J.; Mederic, P.; Aubry, T. Elaboration and relationships between structure and rheological properties of microtalc or nanotalc/polyamide composites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Fu, X.; Yao, C.; Yang, G. Recent advances in graphene/polyamide 6 composites: A review. RSC Adv. 2015, 5, 61688–61702. [Google Scholar] [CrossRef]
- Wang, R.; Kuan, H.; Qiu, A.; Su, X.; Ma, J. A facile approach to the scalable preparation of thermoplastic/carbon nanotube composites. Nanotechnology 2020, 31, 195706. [Google Scholar] [CrossRef]
- Kausar, A. Emerging trends in poly (methyl methacrylate) containing carbonaceous reinforcements-Carbon nanotube, carbon black, and carbon fiber. J. Plast. Film Sheeting 2020. [Google Scholar] [CrossRef]
- Do, V.T.; Nguyen-Tran, H.D.; Chun, D.M. Effect of polypropylene on the mechanical properties and water absorption of carbon-fiber-reinforced-polyamide-6/polypropylene composite. Compos. Struct. 2016, 150, 240–245. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Tito, A.C. High performance PA6/CNTs nanohybrid fibers prepared in the melt. Compos. Sci. Technol. 2012, 72, 1918–1923. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, F.; Lei, F.; Chen, L.; Zhang, H.; Leng, J.; Sun, D. Graphite fluoride reinforced PA6 composites: Crystallization and mechanical properties. Mater. Today Commun. 2018, 16, 217–225. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Deng, Q.; Li, D. Three kinds of charcoal powder reinforced ultra-high molecular weight polyethylene composites with excellent mechanical and electrical properties. Mater. Des. 2015, 85, 54–59. [Google Scholar] [CrossRef]
- Li, X.; Lei, B.; Lin, Z.; Huang, L.; Tan, S.; Cai, X. The utilization of bamboo charcoal enhances wood plastic composites with excellent mechanical and thermal properties. Mater. Des. 2014, 53, 419–424. [Google Scholar] [CrossRef]
- Nan, N.; Devallance, D.B.; Xie, X.; Wang, J. The effect of bio-carbon addition on the electrical, mechanical, and thermal properties of polyvinyl alcohol/biochar composites. J. Compos. Mater. 2016, 50, 1161–1168. [Google Scholar] [CrossRef]
- You, Z.; Li, D. The dynamical viscoelasticity and tensile property of new highly filled charcoal powder/ultra-high molecular weight polyethylene composites. Mater. Lett. 2013, 112, 197–199. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, H.; Ren, X.; Kong, L.; Liu, J.; Jiang, X. The Dynamic Mechanical Analysis of Highly Filled Rice Husk Biochar/High-Density Polyethylene Composites. Polymers 2017, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Giorcelli, M.; Savi, P.; Khan, A.A.; Tagliaferro, A. Analysis of biochar with different pyrolysis temperatures used as filler in epoxy resin composites. Biomass Bioenergy 2019, 122, 466–471. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Jadhav, A.; Qureshi, S.S.; Baloch, H.A.; Siddiqui, M.T.H.; Mubarak, N.M.; Griffin, G.; Madapusi, S.; Tanksale, A.; Ahamed, M.I. Synthesis and characterization of polylactide/rice husk hydrochar composite. Sci. Rep. 2019, 9, 5445. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, Y.; Tu, D.; Chen, Y.; Liu, S.; Li, W.; Wang, L. Water Absorption, Mechanical, and Crystallization Properties of High-density Polyethylene filled with Corncob Powder. Bioresources 2018, 13, 3778–3792. [Google Scholar] [CrossRef]
- Cabanelas, J.C.; Prolongo, S.G.; Serrano, B.; Bravo, J.; Baselga, J. Water absorption in polyaminosiloxane-epoxy thermosetting polymers. J. Mater. Process. Technol. 2003, 143, 311–315. [Google Scholar] [CrossRef]
- Xiao, G.; Shanahan, M.E.R. Water absorption and desorption in an epoxy resin with degradation. J. Polym. Sci. Part B 1997, 35, 2659–2670. [Google Scholar] [CrossRef]
- Chunxia, Z.; Mengyun, L.; Yuan, Z.; Suling, Z.; Weixuan, Y. Research on preparation and long-acting water absorption properties of wood flour/HDPE modified composites. J. Funct. Mater. 2015, 11, 11118–11121. [Google Scholar]
- Moghadam, A.D.; Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Mechanical and tribological properties of self-lubricating metal matrix nanocomposites reinforced by carbon nanotubes (CNTs) and graphene—A review. Compos. Part B Eng. 2015, 77, 402–420. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, R. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour. Technol. 2020, 296, 122318. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, T.; Panzacchi, P.; Baratieri, M.; Davies, C.A.; Tonon, G. Effect of pyrolysis temperature on miscanthus (Miscanthus × giganteus) biochar physical, chemical and functional properties. Biomass Bioenergy 2014, 62, 149–157. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Chen, C.; Li, X.; Deng, Q.; Li, D. Mechanical, electrical, and thermal properties of highly filled bamboo charcoal/ultra-high molecular weight polyethylene composites. Polym. Compos. 2018, 39. [Google Scholar] [CrossRef]
- Mousa, M.; Dong, Y. Strong Poly (Vinyl Alcohol) (PVA)/Bamboo Charcoal (BC) Nanocomposite Films with Particle Size Effect. ACS Sustain. Chem. Eng. 2018, 6, 467–479. [Google Scholar] [CrossRef]
- Schirp, A.; Wolcott, M.P. Influence of fungal decay and moisture absorption on mechanical properties of extruded wood-plastic composites. Wood Fiber Sci. 2007, 307, 643–652. [Google Scholar]
- Wei, W.; Zhongde, X. Study on the reinforced and toughened PP blends with the rigid nano particles and the elastic rubber-particles. Acta Polym. Sin. 2000, 1, 99–104. [Google Scholar]
- Das, O.; Bhattacharyya, D.; Sarmah, A.K. Sustainable eco–composites obtained from waste derived biochar: A consideration in performance properties, production costs, and environmental impact. J. Clean. Prod. 2016, 129, 159–168. [Google Scholar] [CrossRef]


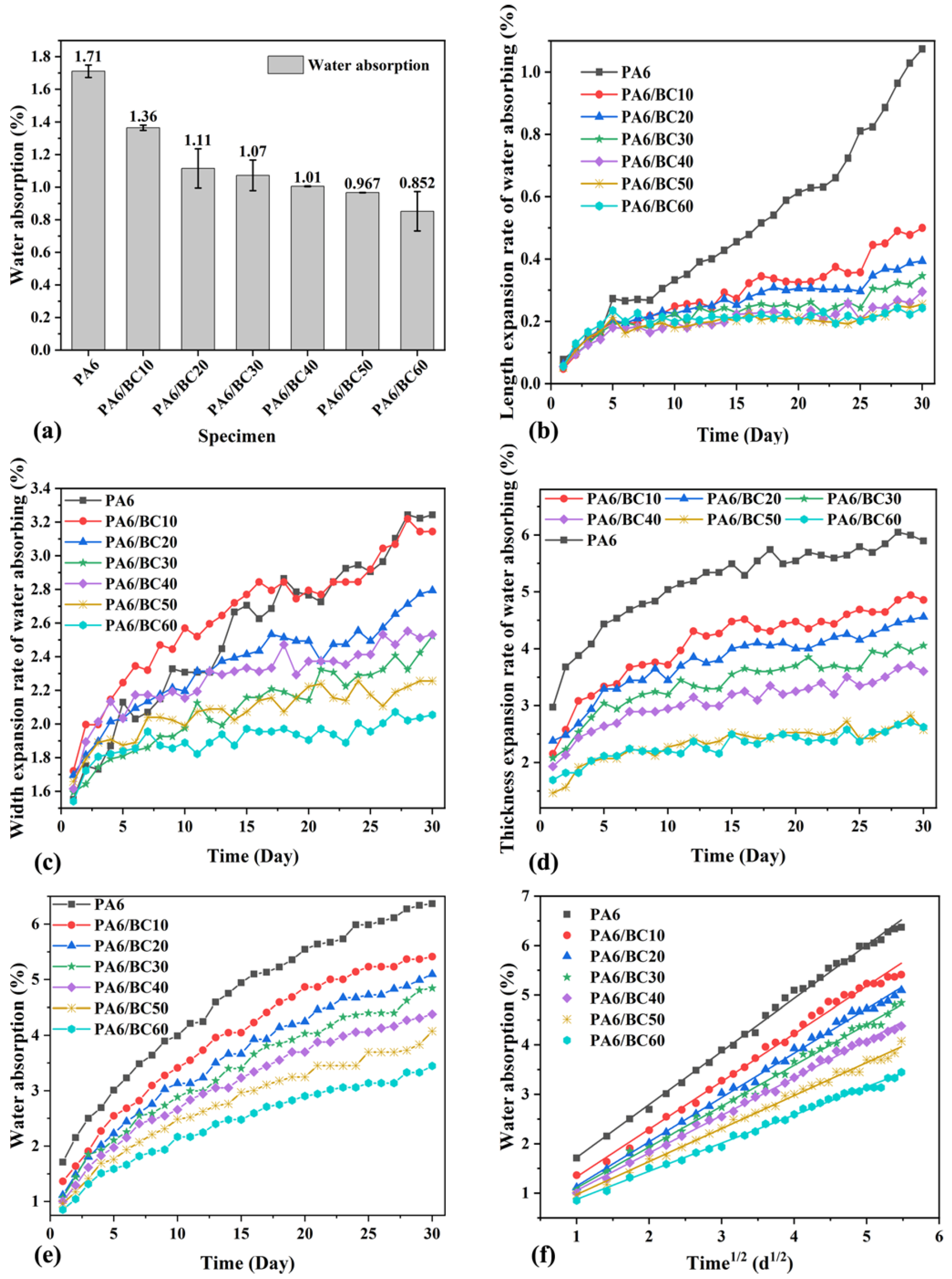

| Specimen | Fitting Curve Equation | R2 value | W30/% | D/ × 10−3 mm2·s−1 |
|---|---|---|---|---|
| PA6 | W = 0.65057 + 1.07107 t1/2 | 0.99674 | 6.37 | 10.27 |
| PA6/BC10 | W = 0.36733 + 0.96303 t1/2 | 0.99344 | 5.41 | 11.52 |
| PA6/BC20 | W = 0.24816 + 0.89537 t1/2 | 0.99653 | 5.10 | 11.2 |
| PA6/BC30 | W = 0.28166 + 0.82569 t1/2 | 0.99517 | 4.84 | 10.58 |
| PA6/BC40 | W = 0.29742 + 0.75818 t1/2 | 0.99708 | 4.38 | 10.89 |
| PA6/BC50 | W = 0.30602 + 0.66704 t1/2 | 0.9953 | 4.07 | 9.76 |
| PA6/BC60 | W = 0.30806 + 0.56831 t1/2 | 0.99569 | 3.45 | 9.86 |
| Specimen | E’max (MPa) | To (°C) | E"p (MPa) | TE"p (°C) | Tg (°C) | Tan δp |
|---|---|---|---|---|---|---|
| PA6 | 2882.00 | 21.20 | 220.00 | 31.80 | 56.90 | 0.137 |
| PA6/BC10 | 3414.00 | 24.10 | 253.00 | 36.50 | 64.40 | 0.140 |
| PA6/BC20 | 4462.00 | 28.20 | 268.00 | 41.70 | 68.40 | 0.128 |
| PA6/BC30 | 5751.00 | 30.90 | 377.00 | 42.90 | 69.50 | 0.135 |
| PA6/BC40 | 5954.00 | 31.50 | 394.00 | 42.80 | 69.70 | 0.135 |
| PA6/BC50 | 8998.00 | 36.50 | 693.00 | 43.10 | 71.50 | 0.138 |
| PA6/BC60 | 12,844.00 | 37.90 | 892.00 | 46.40 | 75.90 | 0.128 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Guo, Y.; Chen, Y.; Liu, S. Low Water Absorption, High-Strength Polyamide 6 Composites Blended with Sustainable Bamboo-Based Biochar. Nanomaterials 2020, 10, 1367. https://doi.org/10.3390/nano10071367
Zhu S, Guo Y, Chen Y, Liu S. Low Water Absorption, High-Strength Polyamide 6 Composites Blended with Sustainable Bamboo-Based Biochar. Nanomaterials. 2020; 10(7):1367. https://doi.org/10.3390/nano10071367
Chicago/Turabian StyleZhu, Shiliu, Yong Guo, Yuxia Chen, and Shengquan Liu. 2020. "Low Water Absorption, High-Strength Polyamide 6 Composites Blended with Sustainable Bamboo-Based Biochar" Nanomaterials 10, no. 7: 1367. https://doi.org/10.3390/nano10071367
APA StyleZhu, S., Guo, Y., Chen, Y., & Liu, S. (2020). Low Water Absorption, High-Strength Polyamide 6 Composites Blended with Sustainable Bamboo-Based Biochar. Nanomaterials, 10(7), 1367. https://doi.org/10.3390/nano10071367





