Thermal Annealing Induced Controllable Porosity and Photoactive Performance of 2D ZnO Sheets
Abstract
1. Introduction
2. Materials and Methods
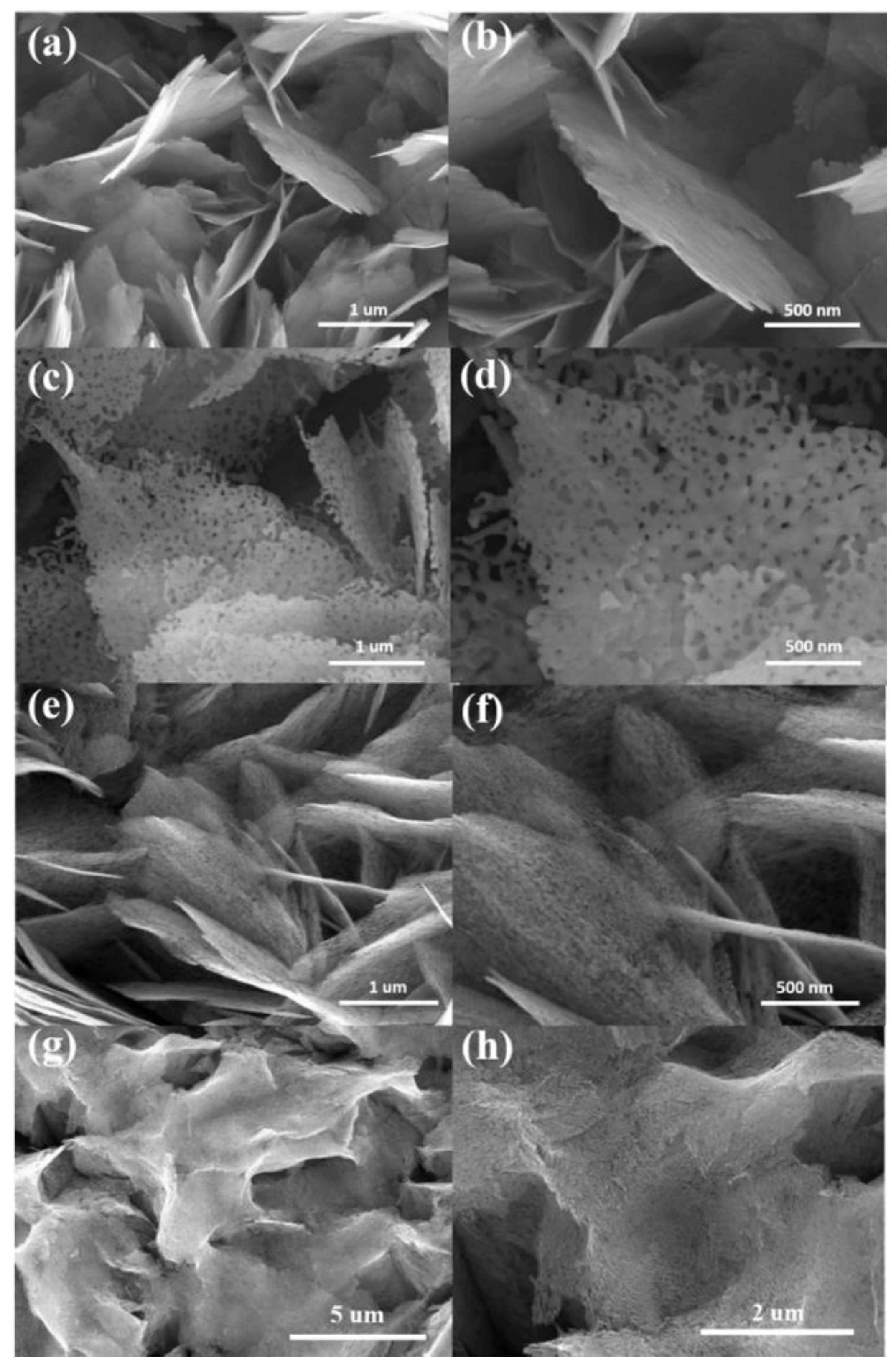
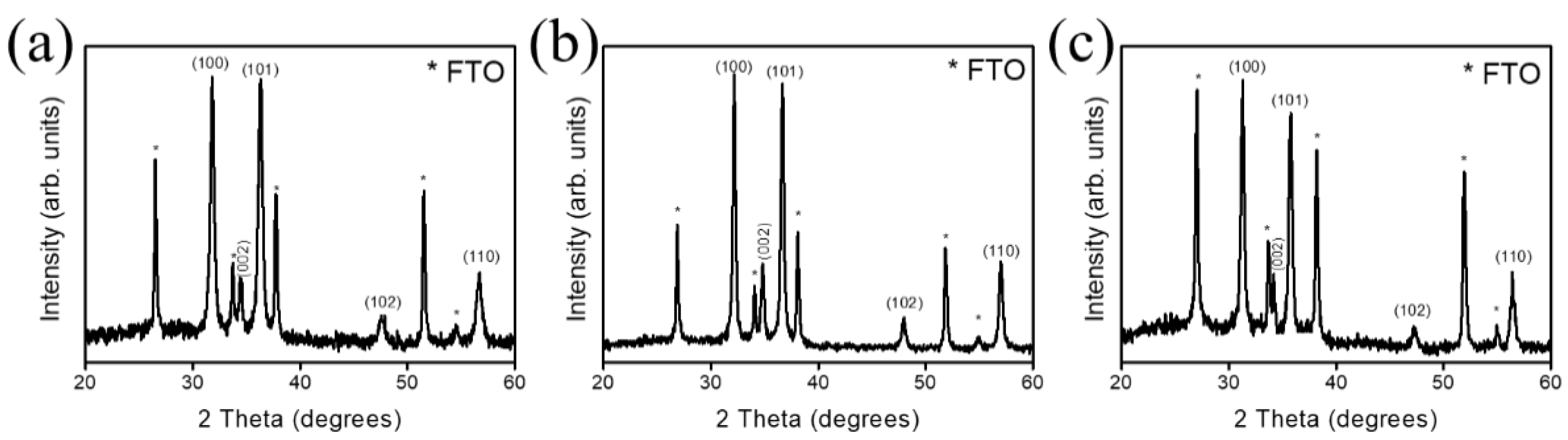
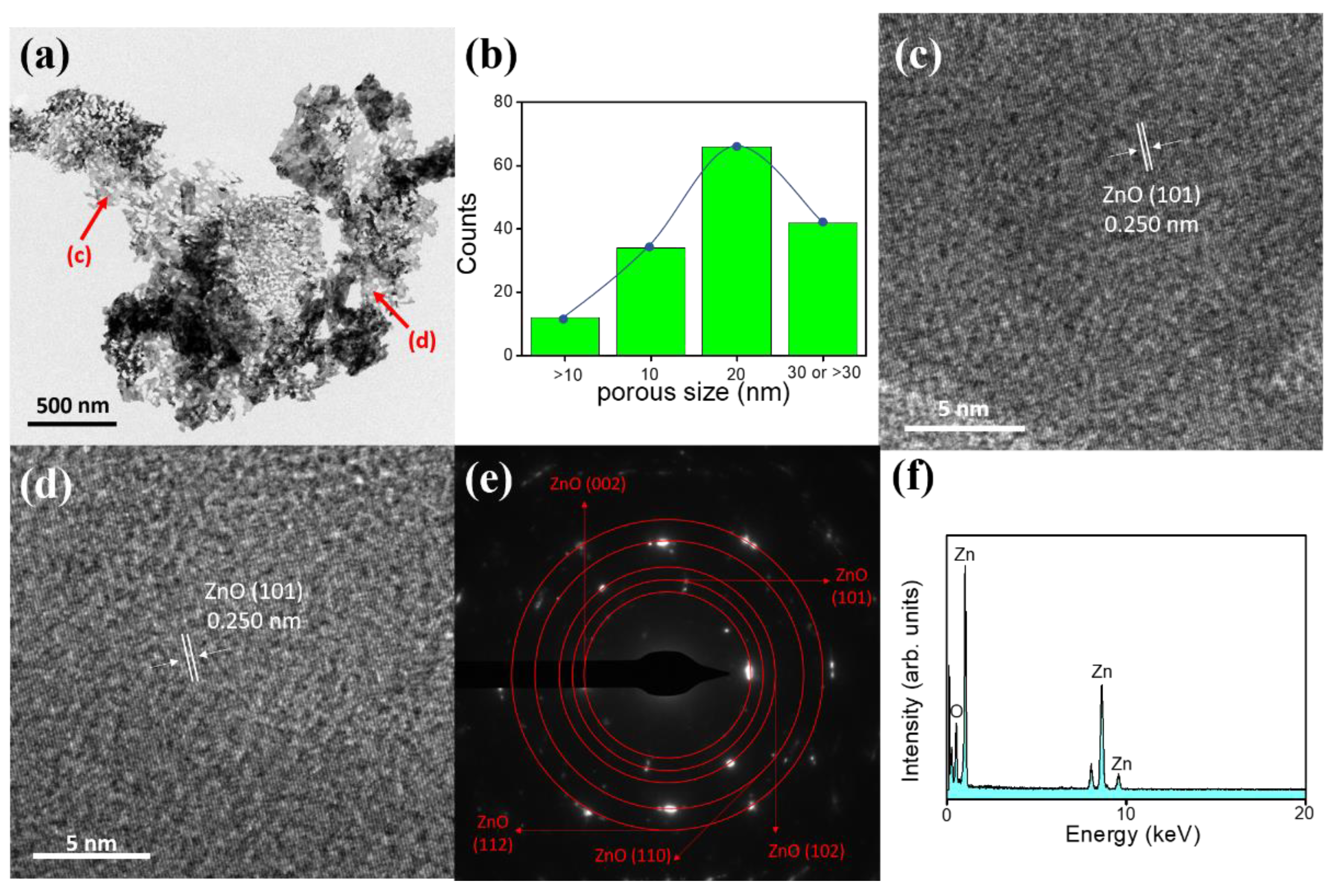
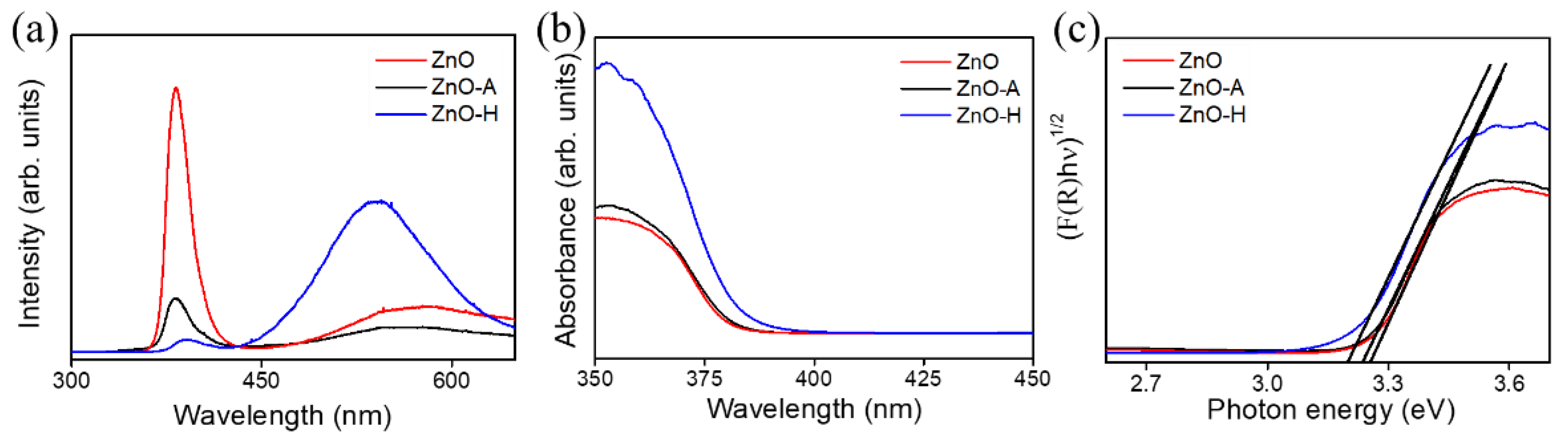
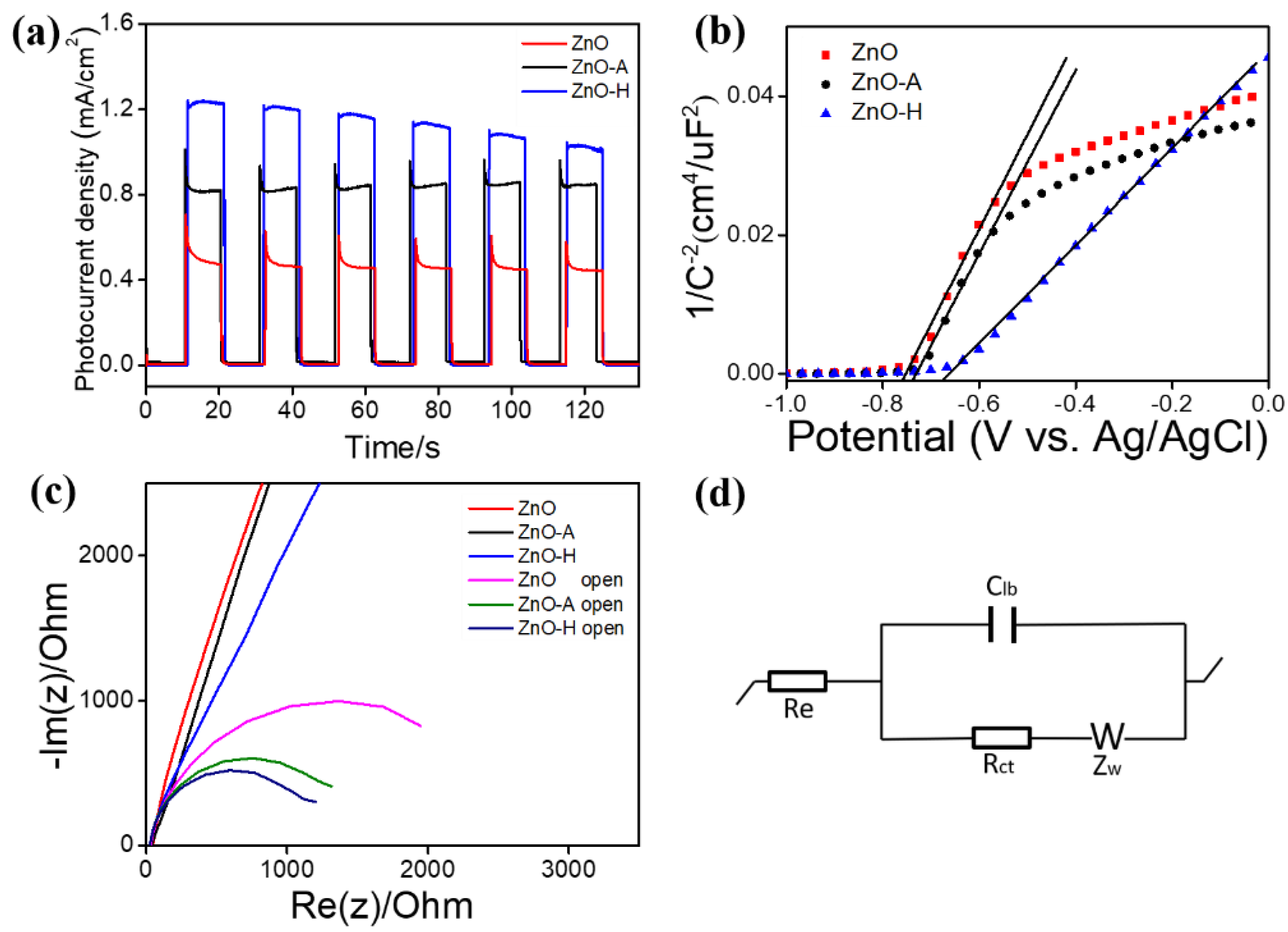
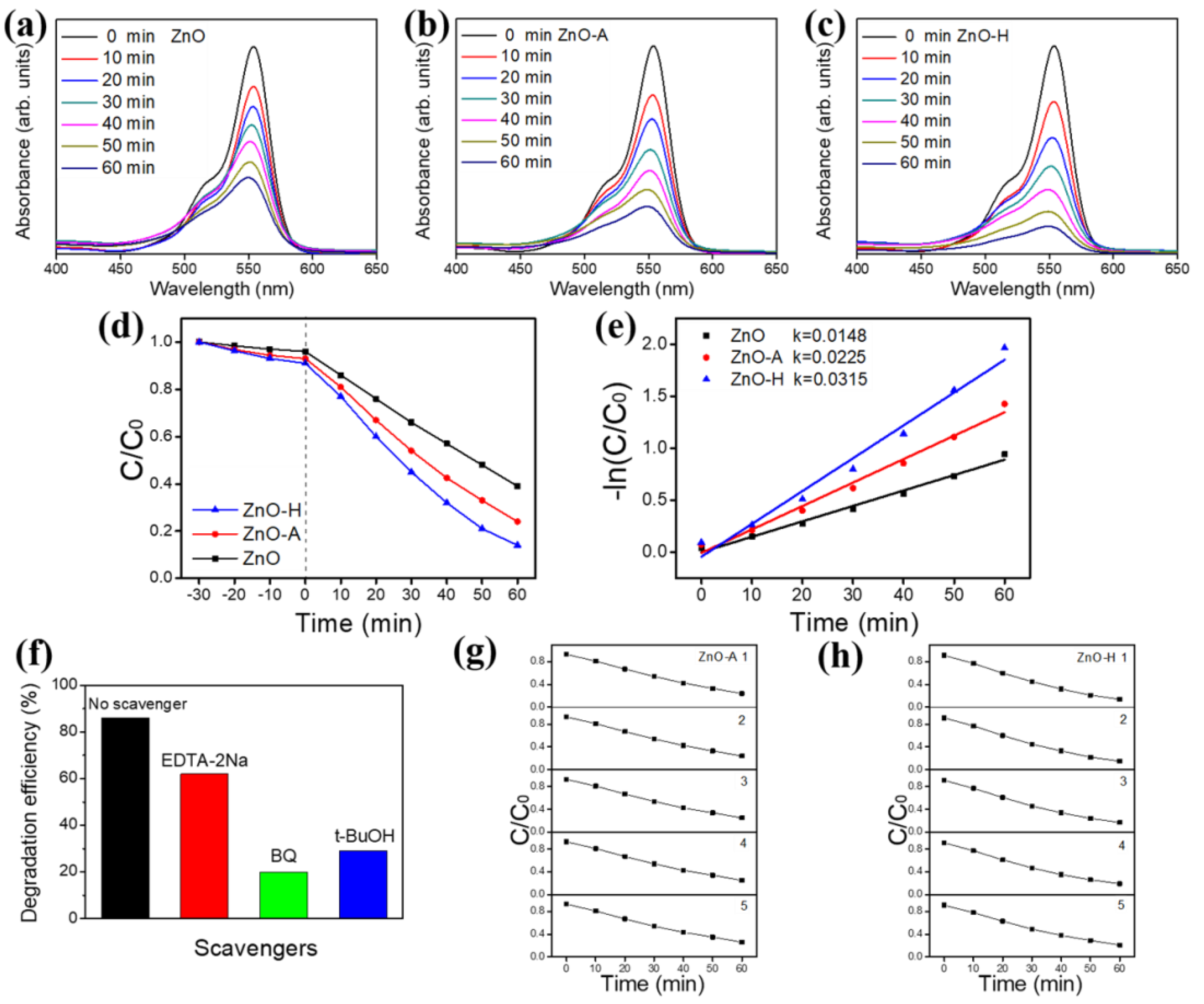
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, D.; Gao, Z.; Xu, F.; Shi, Z.; Tao, W.; Jiang, K. Nanosheet-based hierarchical ZnO structure decorated with TiO2 particles for enhanced performance in dye-sensitized solar cell. CrystEngComm 2012, 14, 7934–7941. [Google Scholar]
- Sun, H.; Yu, Y.; Luo, J.; Ahmad, M.; Zhu, J. Morphology-controlled synthesis of ZnO 3D hierarchical structures and their photocatalytic performance. CrystEngComm 2012, 14, 8626–8632. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, W.; Cao, Y.; Guan, Y.; Sun, Y.; Lu, G. Porous SnO2 hierarchical nanosheets: Hydrothermal preparation, growth mechanism, and gas sensing properties. CrystEngComm 2011, 13, 3718–3724. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, T.; Hu, J.; Li, J. Controlled strategy to synthesize SnO2 decorated SnS2 nanosheets with enhanced visible light photocatalytic activity. CrystEngComm 2012, 14, 5627–5633. [Google Scholar] [CrossRef]
- Dong, J.Y.; Lin, C.H.; Hsu, Y.J.; Lu, S.Y.; Wong, D.S.H. Single-crystalline mesoporous ZnO nanosheets prepared with a green antisolvent method exhibiting excellent photocatalytic efficiencies. CrystEngComm 2012, 14, 4732–4737. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, W.; Zhang, X.; Qu, Y.; Wang, L.; Hu, W.; Tian, G. Large-scale synthesis of stable mesoporous black TiO2 nanosheets for efficient solar-driven photocatalytic hydrogen evolution via an earth-abundant low-cost biotemplate. RSC Adv. 2016, 6, 50506–50512. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Tang, D.; Liu, F.; Lai, Y. Characterization of porous bismuth oxide (Bi2O3) nanoplates prepared by chemical bath deposition and post annealing. RSC Adv. 2015, 5, 65591–65594. [Google Scholar]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lin, T.Y.; Lee, C.M. Crystal growth and shell layer crystal feature-dependent sensing and photoactivity performance of zinc oxide–indium oxide core–shell nanorod heterostructures. CrystEngComm 2015, 17, 7948–7955. [Google Scholar] [CrossRef]
- Liang, Y.C. Microstructure and optical properties of electrodeposited Al-doped ZnO nanosheets. Ceram. Int. 2012, 38, 119–124. [Google Scholar] [CrossRef]
- Cao, B.; Cai, W.; Li, Y.; Sun, F.; Zhang, L. Ultraviolet-light-emitting ZnO nanosheets prepared by a chemical bath deposition method. Nanotechnology 2015, 16, 1734–1738. [Google Scholar] [CrossRef]
- Giri, A.K.; Saha, A.; Mondal, A.; Chandra Ghosh, S.; Kundu, S.; Panda, A.B. Rectangular ZnO porous nano-plate assembly with excellent acetone sensing performance and catalytic activity. RSC Adv. 2015, 5, 102134–102142. [Google Scholar] [CrossRef]
- Liu, B.; Xu, J.; Ran, S.; Wang, Z.; Chen, D.; Shen, G. High-performance photodetectors, photocatalysts, and gas sensors based on polyol reflux synthesized porous ZnO nanosheets. CrystEngComm 2012, 14, 4582–4588. [Google Scholar] [CrossRef]
- Xu, L.; Li, Z.; Cai, Q.; Wang, H.; Gao, H.; Lv, W.; Liu, J. Precursor template synthesis of three-dimensional mesoporous ZnO hierarchical structures and their photocatalytic properties. CrystEngComm 2010, 12, 2166. [Google Scholar] [CrossRef]
- Lv, W.; Wei, B.; Xu, L.; Zhao, Y.; Gao, H.; Liu, J. Photocatalytic properties of hierarchical ZnO flowers synthesized by a sucrose-assisted hydrothermal method. Appl. Surf. Sci. 2012, 259, 557–561. [Google Scholar] [CrossRef]
- Guo, Y.; Lin, S.; Li, X.; Liu, Y. Amino acids assisted hydrothermal synthesis of hierarchically structured ZnO with enhanced photocatalytic activities. Appl. Surf. Sci. 2016, 384, 83–91. [Google Scholar] [CrossRef]
- Gu, C.; Huang, J.; Wu, Y.; Zhai, M.; Sun, Y.; Liu, J. Preparation of porous flower-like ZnO nanostructures and their gas-sensing property. J. Alloys Compd. 2011, 509, 4499–4504. [Google Scholar] [CrossRef]
- Husairi, F.S.; Ali, S.M.; Azlinda, A.; Rusop, M.; Abdullah, S. Special Effect of Urea as a Stabilizer in Thermal Immersion Method to Synthesis Porous Zinc Oxide Nanostructures. J. Nanomater. 2013, 2013, 163527. [Google Scholar] [CrossRef]
- Dollimore, D.; France, J.A.; Krupay, B.W.; Whitehead, R. Kinetic aspects of the thermal decomposition of zinc carbonate. Thermochim. Acta 1980, 36, 343–349. [Google Scholar] [CrossRef]
- Park, K.-S.; Choi, Y.-J.; Ahn, M.-W.; Kim, D.-W.; Sung, Y.-M.; Park, J.-G.; Choi, K.J. Enhancement of Field-Emission Properties in ZnO Nanowire Array by Post-Annealing in H2 Ambient. J. Nanosci. Nanotechnol. 2009, 9, 4328–4332. [Google Scholar] [CrossRef]
- Tark, S.J.; Ok, Y.-W.; Kang, M.G.; Lim, H.J.; Kim, W.M.; Kim, D. Effect of a hydrogen ratio in electrical and optical properties of hydrogenated Al-doped ZnO films. J. Electroceram. 2008, 3, 548. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chang, C.W. Improvement of Ethanol Gas-Sensing Responses of ZnO–WO3 Composite Nanorods through Annealing Induced Local Phase Transformation. Nanomaterials 2019, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Lo, Y.J. High-temperature solid-state reaction induced structure modifications and associated photoactivity and gas-sensing performance of binary oxide one-dimensional composite system. RSC Adv. 2017, 7, 29428–29439. [Google Scholar] [CrossRef]
- Liang, Y.C.; Hu, C.Y.; Zhong, H.; Wang, J.L. Crystal synthesis and effects of epitaxial perovskite manganite underlayer conditions on characteristics of ZnO nanostructured heterostructures. Nanoscale 2013, 5, 2346. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Wang, C.C. Surface crystal feature-dependent photoactivity of ZnO–ZnS composite rods via hydrothermal sulfidation. RSC Adv. 2018, 8, 5063–5070. [Google Scholar] [CrossRef]
- Xu, J.-P.; Shi, S.-B.; Li, L.; Zhang, X.-S.; Wang, Y.-X.; Chen, X.-M. Effects of Annealing Temperature on Structural and Optical Properties of ZnO Thin Films. Chin. Phys. Lett. 2010, 27, 047803. [Google Scholar]
- Song, R.Q.; Xu, A.W.; Deng, B.; Li, Q.; Chen, G.Y. From Layered Basic Zinc Acetate Nanobelts to Hierarchical Zinc Oxide Nanostructures and Porous Zinc Oxide Nanobelts. Adv. Funct. Mater. 2007, 17, 296–306. [Google Scholar] [CrossRef]
- Kumar, S.; Sahare, P.D. Effects of annealing on the surface defects of zinc oxide nanoparticles. Nano 2012, 7, 1250022. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lung, T.W. Growth of Hydrothermally Derived CdS-Based Nanostructures with Various Crystal Features and Photoactivated Properties. Nanoscale Res. Lett. 2016, 11, 264–274. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Xie, S.; Shi, J.; Li, W.; Tong, Y.; Li, Y. Efficient photocatalytic hydrogen evolution over hydrogenated ZnO nanorod arrays. Chem. Commun. 2012, 48, 7717–7719. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Yu, J.; Xiang, Q. Improved visible-light photocatalytic activity of porous carbon self-doped ZnO nanosheet-assembled flowers. CrystEngComm 2011, 13, 2533–2541. [Google Scholar] [CrossRef]
- Vasile, O.R.; Andronescu, E.; Ghitulica, C.; Vasile, B.S.; Oprea, O.; Vasile, E.; Trusca, R. Synthesis and characterization of nanostructured zinc oxide particles synthesized by the pyrosol method. J. Nanoparticle Res. 2012, 14, 1269. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen defect dependent variation of band gap, Urbach energy and luminescence property of anatase, anatase–rutile mixed phase and of rutile phases of TiO2 nanoparticles. Physica E 2014, 56, 364. [Google Scholar] [CrossRef]
- Watanabe, A.; Kozuka, H. Photoanodic Properties of Sol−Gel-Derived Fe2O3 Thin Films Containing Dispersed Gold and Silver Particles. J. Phys. Chem. B 2003, 107, 12713–12720. [Google Scholar] [CrossRef]
- Bak, D.; Kim, J.H. Facile fabrication of pseudo-microspherical ZnO/CdS core-shell photocatalysts for solar hydrogen production by water splitting. Ceram. Int. 2017, 43, 13493–13499. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z. Effect of hydrogen treatment on the photoelectrochemical properties of quantum dots sensitized ZnO nanorod array. J. Power Sources 2014, 272, 647–653. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Yao, C.; Wei, B.; Ma, H.; Li, H.; Meng, L.; Zhang, X.; Gong, Q. Enhanced photoelectrochemical performance of hydrogenated ZnO hierarchical nanorod arrays. J. Power Sources 2013, 237, 295–299. [Google Scholar] [CrossRef]
- Mou, H.; Song, C.; Zhou, Y.; Zhang, B.; Wang, D. Design and synthesis of porous Ag/ZnO nanosheets assemblies as super photocatalysts for enhanced visible-light degradation of 4-nitrophenol and hydrogen evolution. Appl. Catal. B Environ. 2018, 221, 565–573. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chiang, K.J. Design and tuning functionality of rod-like titanium dioxide–nickel oxide composites via a combinational methodology. Nanotechnology 2020, 31, 195709. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Liu, Y.C.; Hung, C.S. Sputtering control of Ag2O decoration configurations on ZnO nanorods and their surface arrangement effects on photodegradation ability toward methyl orange. Nanotechnology 2019, 30, 495701. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Huang, C.M.; Chen, H.W.; Yang, H.W. Low temperature solution-processed ZnO nanorod arrays with application to liquid ethanol sensors. Sens. Actuators A Phys. 2013, 189, 307–312. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liu, Y.C. Microstructures and Photodegradation Performance toward Methylene Orange of Sputtering-Assisted Decoration of ZnFe2O4 Crystallites onto TiO2 Nanorods. Nanomaterials 2019, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Han, S.J.; Kwak, S.-Y. Synthesis and photocatalytic activity of mesoporous TiO2 with the surface area, crystallite size, and pore size. J. Colloid Interface Sci. 2007, 316, 85–91. [Google Scholar] [CrossRef]
- Wang, L.; Chang, L.; Zhao, B.; Yuan, Z.; Shao, G.; Zheng, W. Systematic Investigation on Morphologies, Forming Mechanism, Photocatalytic and Photoluminescent Properties of ZnO Nanostructures Constructed in Ionic Liquids. Inorg. Chem. 2008, 47, 1443–1452. [Google Scholar] [CrossRef]
- Han, Z.; Liao, L.; Wu, Y.; Pan, H.; Shen, S.; Chen, J. Synthesis and photocatalytic application of oriented hierarchical ZnO flower-rod architectures. J. Hazard. Mater. 2012, 217–218, 100–106. [Google Scholar] [CrossRef]
- Lin, X.; Huang, T.; Huang, F.; Wang, W.; Shi, J. Photocatalytic activity of a Bi-based oxychloride Bi4NbO8Cl. J. Mater. Chem. 2007, 17, 2145. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, Y. Luminescence and Photocatalytic Activity of ZnO Nanocrystals: Correlation between Structure and Property. Inorg. Chem. 2007, 46, 6675–6682. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Z.-Y.; Peng, Y.; Huang, H.-W.; Li, Y.; Wu, M.; Ke, X.-X.; Van Tendeloo, G.; Su, B.-L. 2D ZnO mesoporous single-crystal nanosheets with exposed {0001} polar facets for the depollution of cationic dye molecules by highly selective adsorption and photocatalytic decomposition. Appl. Catal. B Environ. 2016, 181, 138–145. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, H.; Yuan, S.; Jiao, Z.; Zhu, X. Preparation of flower-like ZnO architectures assembled with nanosheets for enhanced photocatalytic activity. J. Colloid Interface Sci. 2016, 462, 9–18. [Google Scholar] [CrossRef]
- Yang, L.; Kong, X.; Wang, J.; Pan, M.; Yang, W.; Yang, J.; Jiang, W. Synthesis and photocatalytic performance of ZnO hollow spheres and porous nanosheets. J. Mater. Sci. Mater. Electron. 2015, 27, 203–209. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.-C.; Hung, C.-S.; Zhao, W.-C. Thermal Annealing Induced Controllable Porosity and Photoactive Performance of 2D ZnO Sheets. Nanomaterials 2020, 10, 1352. https://doi.org/10.3390/nano10071352
Liang Y-C, Hung C-S, Zhao W-C. Thermal Annealing Induced Controllable Porosity and Photoactive Performance of 2D ZnO Sheets. Nanomaterials. 2020; 10(7):1352. https://doi.org/10.3390/nano10071352
Chicago/Turabian StyleLiang, Yuan-Chang, Chen-Shiang Hung, and Wei-Cheng Zhao. 2020. "Thermal Annealing Induced Controllable Porosity and Photoactive Performance of 2D ZnO Sheets" Nanomaterials 10, no. 7: 1352. https://doi.org/10.3390/nano10071352
APA StyleLiang, Y.-C., Hung, C.-S., & Zhao, W.-C. (2020). Thermal Annealing Induced Controllable Porosity and Photoactive Performance of 2D ZnO Sheets. Nanomaterials, 10(7), 1352. https://doi.org/10.3390/nano10071352




