Synthetic Tuning of CoII-Doped Silica Nanoarchitecture Towards Electrochemical Sensing Ability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
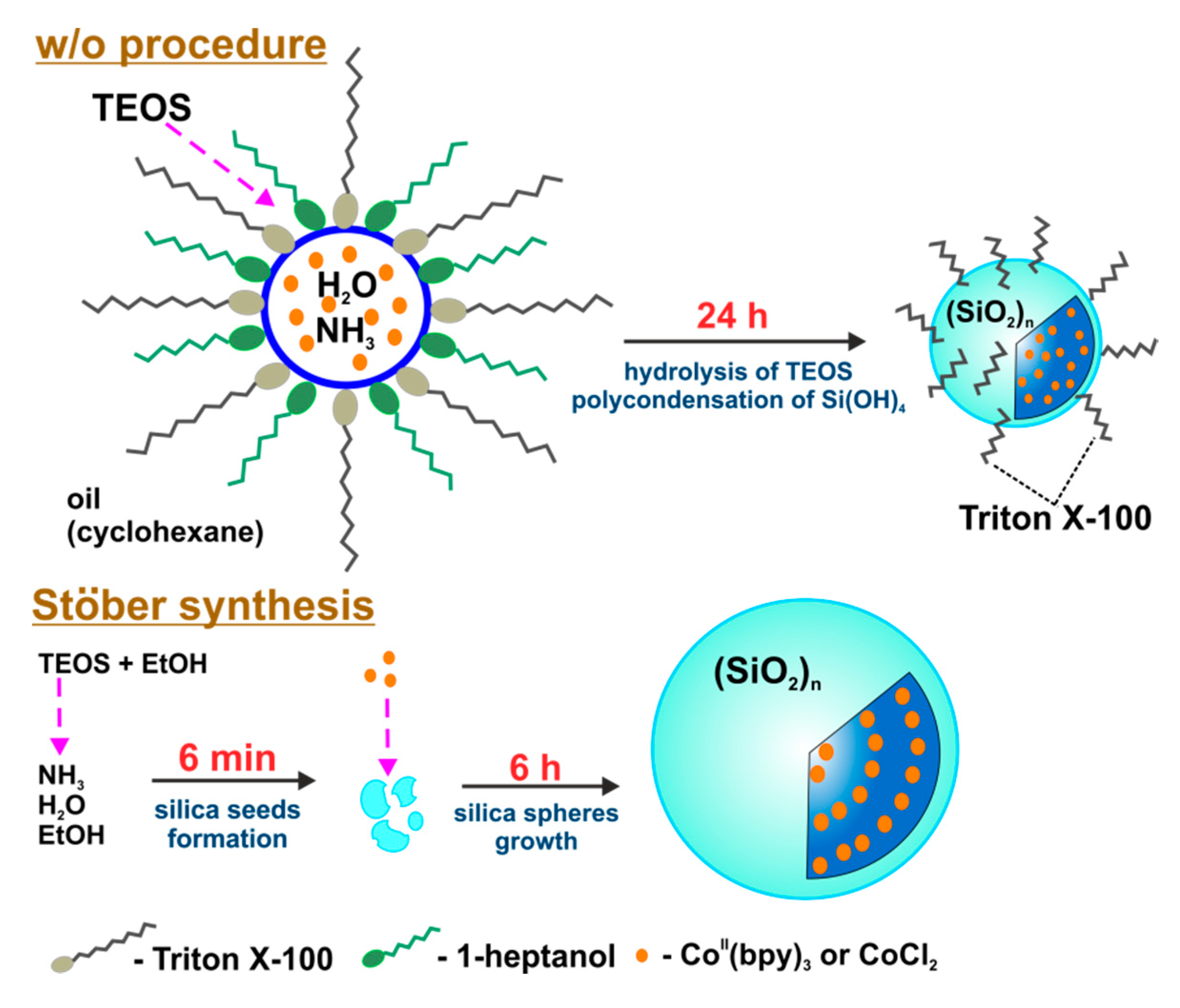
Silica Nanoparticles Preparation
2.2. Samples Characterization
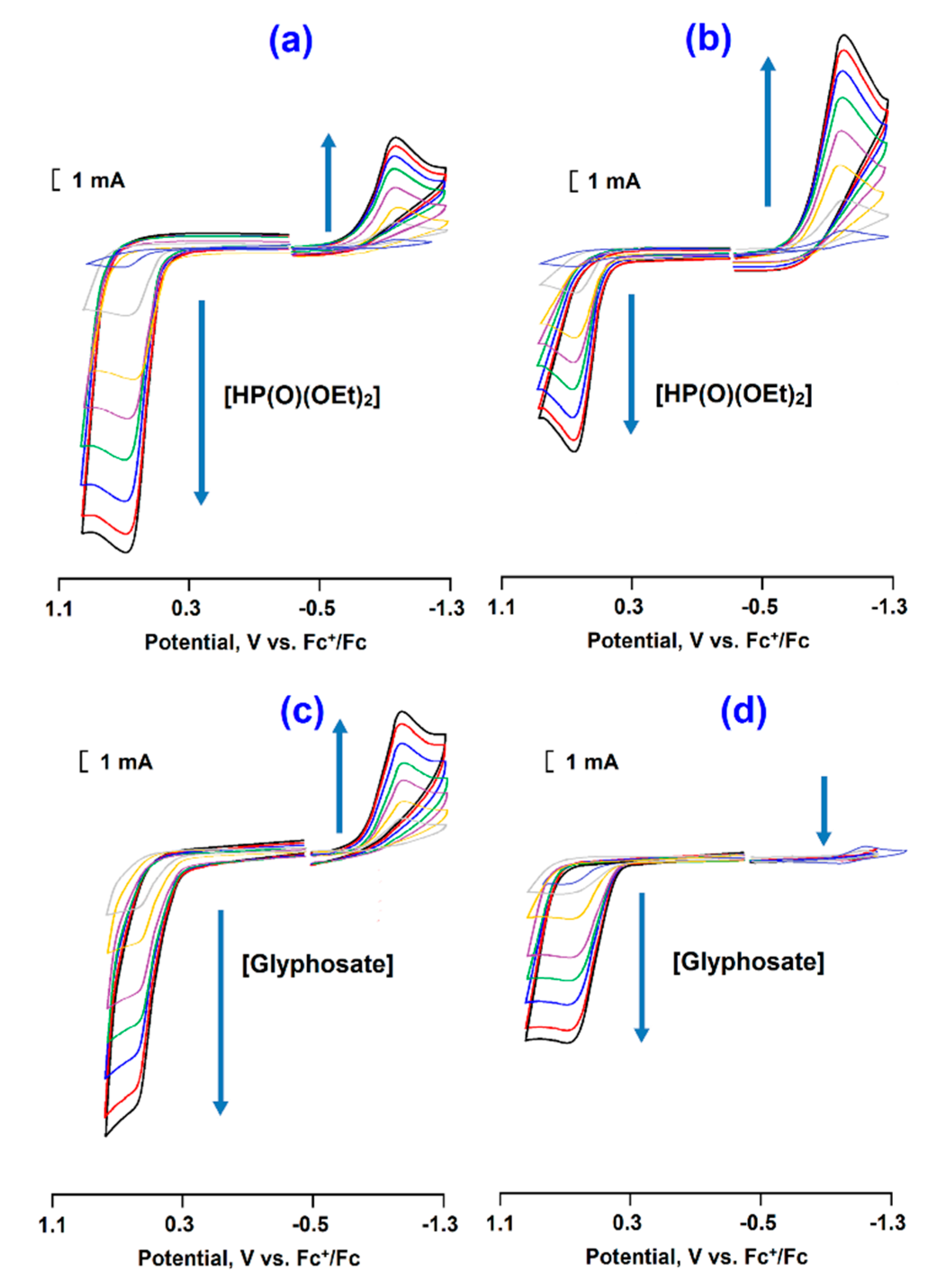
3. Results and Discussion
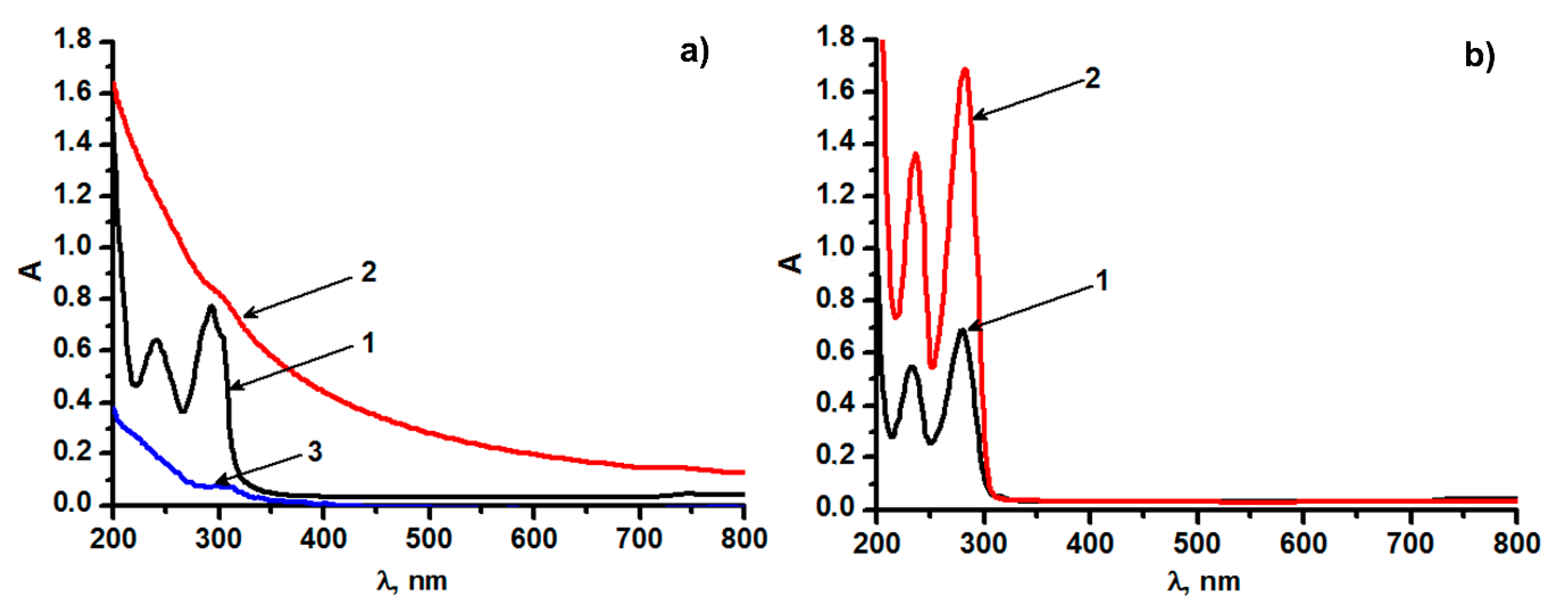
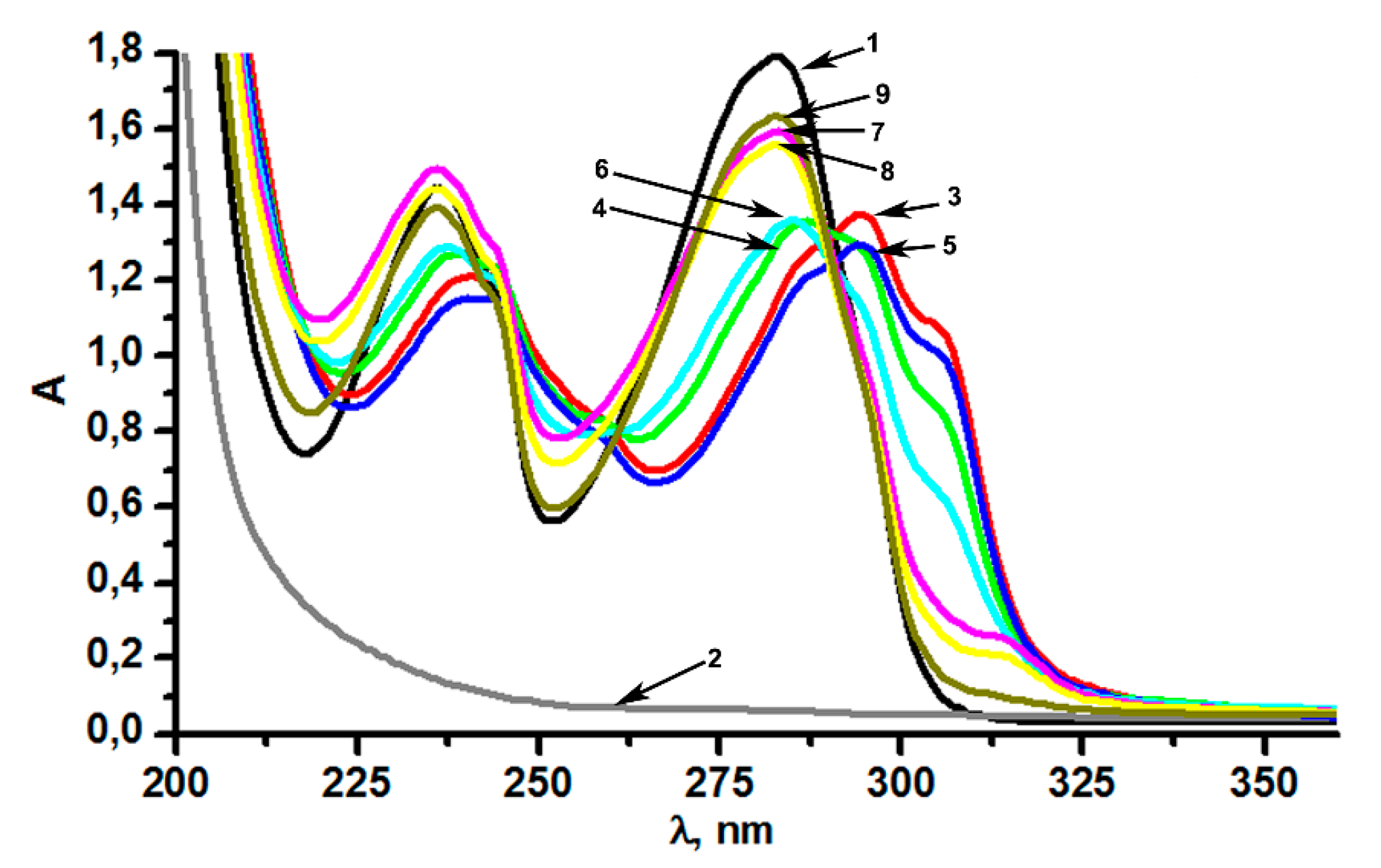
3.1. Size, Cobalt Content, and Spectral Properties of the Hybrid Nanoparticles (CoII@SiO2) in Correlation with the Synthetic Conditions
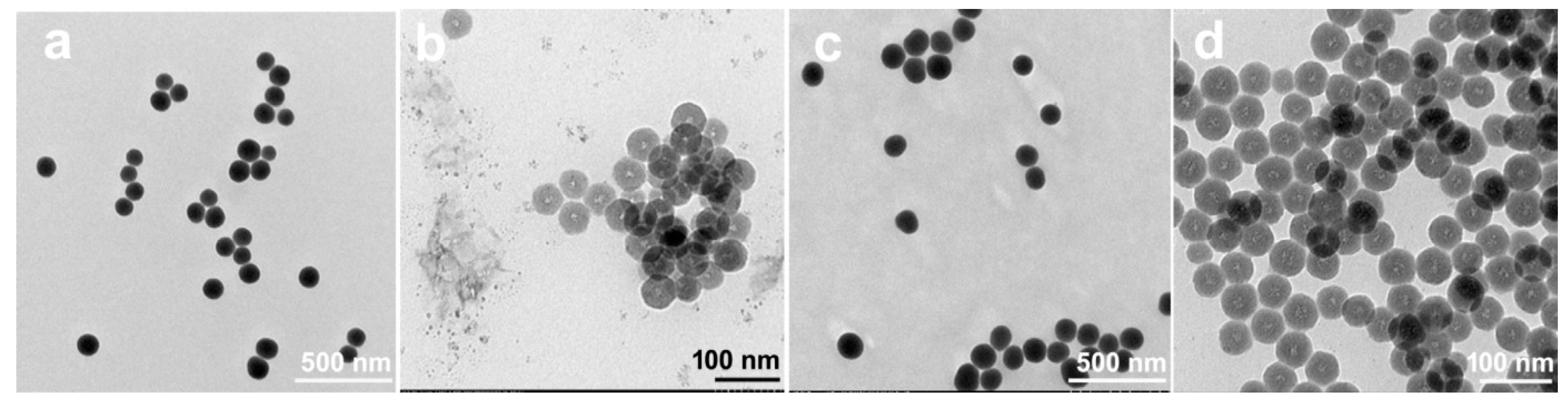
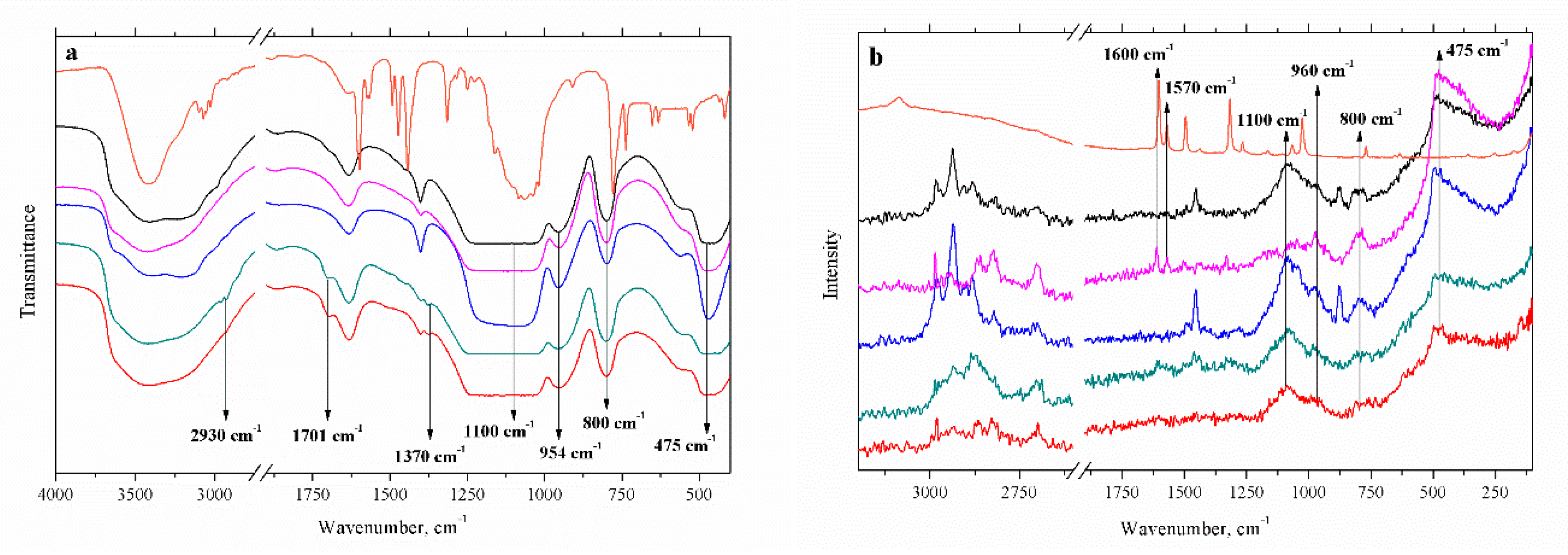
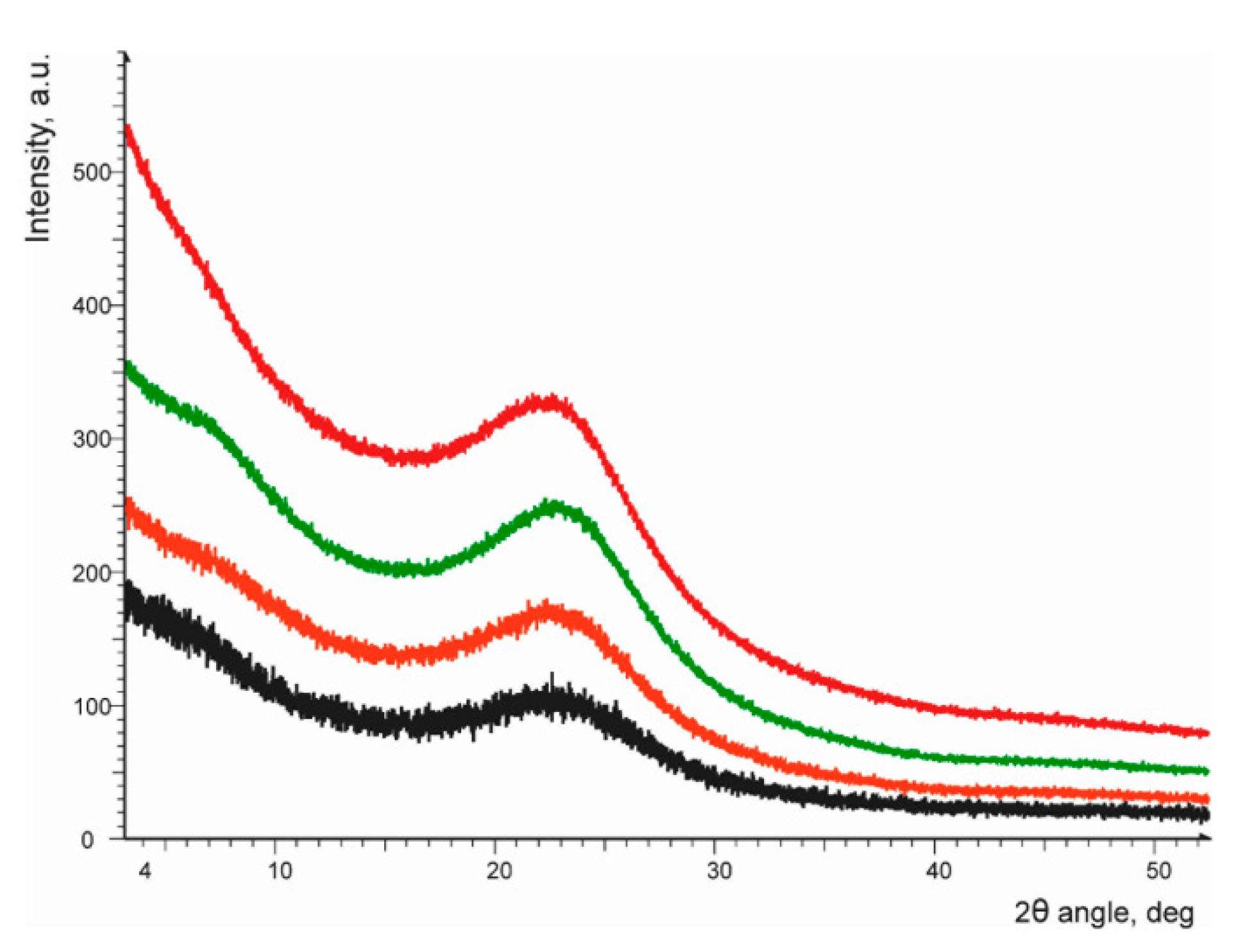
3.2. Nanoarchitecture of CoII@SiO2 and SiO2 in Correlation with the Synthetic Procedure
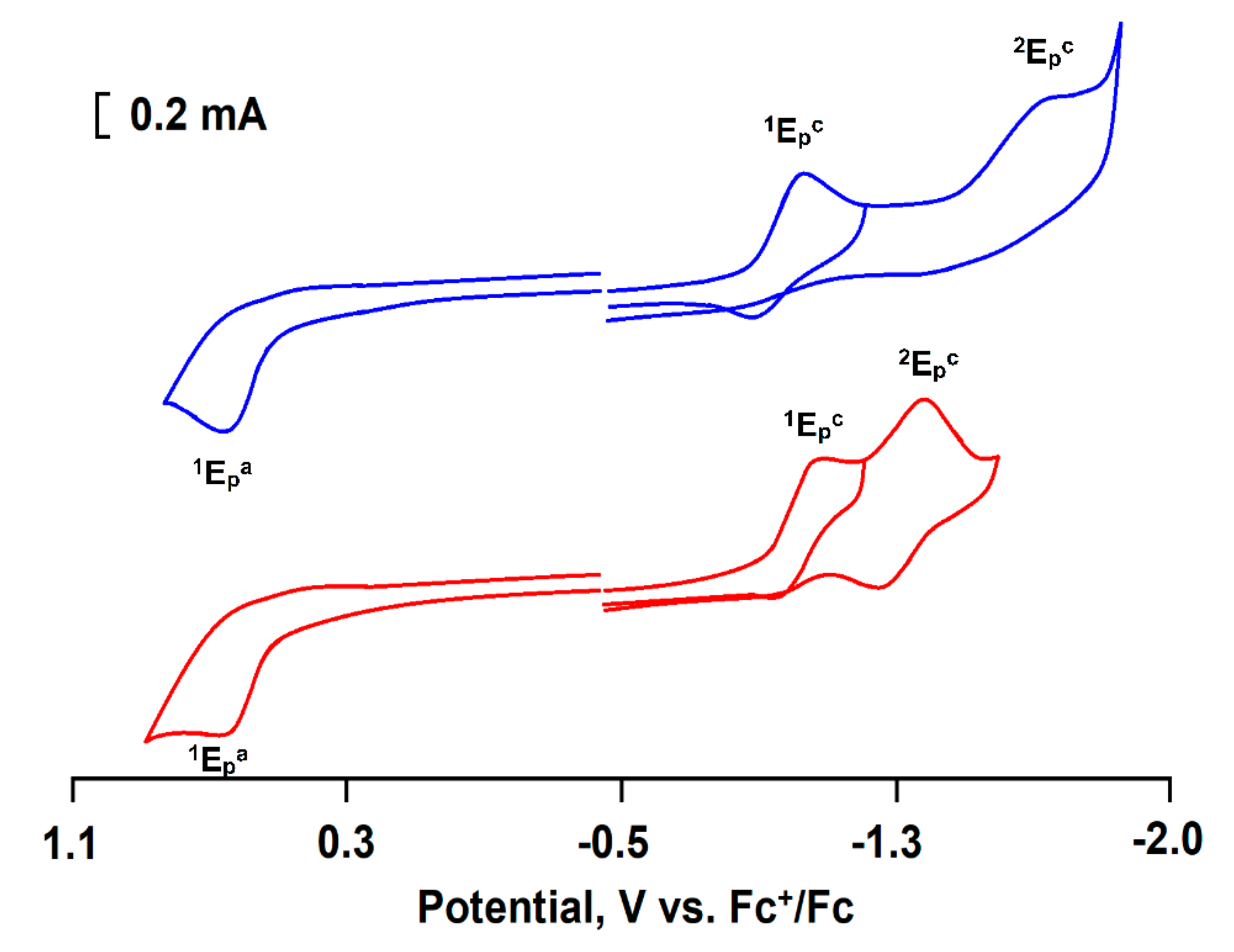
3.3. Electrochemical Behavior of CoII@SiO2
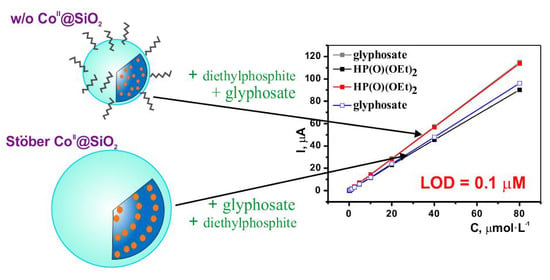
3.4. CoII@SiO2 for Electrochemical Determination of OPC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antony, R.; Manickam, S.T.D.; Balakumar, S. Cu(II), Co(II) and Ni(II) Complexes Installed on Functionalized Silica Surface for Hydrogen Peroxide Assisted Cyclohexane Oxidation. J. Inorg. Organomet. Polym. Mater. 2017, 27, 418–426. [Google Scholar] [CrossRef]
- Vashurin, A.; Marfin, Y.; Tarasyuk, I.; Kuzmin, I.; Znoyko, S.; Goncharenko, A.; Rumyantsev, E. Sulfonated octa-substituted Co(II) phthalocyanines immobilized on silica matrix as catalyst for Thiuram E synthesis. Appl. Organomet. Chem. 2018, 32, e4482. [Google Scholar] [CrossRef]
- Faraji, A.R.; Mosazadeh, S.; Ashouri, F. Synthesis and characterization of cobalt-supported catalysts on modified magnetic nanoparticle: Green and highly efficient heterogeneous nanocatalyst for selective oxidation of ethylbenzene, cyclohexene and oximes with molecular oxygen. J. Colloid Interface Sci. 2017, 506, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Luque, R.; Clark, J.H.; Karimi, B.; Macquarrie, D.J. A silica supported cobalt (II) Salen complex as efficient and reusable catalyst for the selective aerobic oxidation of ethyl benzene derivatives. Catal. Commun. 2011, 12, 510–513. [Google Scholar] [CrossRef]
- Chen, L.; Li, B.-D.; Xu, Q.-X.; Liu, D.-B. A silica gel supported cobalt(II) Schiff base complex as efficient and recyclable heterogeneous catalyst for the selective aerobic oxidation of alkyl aromatics. Chin. Chem. Lett. 2013, 24, 849–852. [Google Scholar] [CrossRef]
- Islam, S.M.; Ghosh, K.; Molla, R.A.; Roy, A.S.; Salam, N.; Iqubal, M.A. Synthesis of a reusable polymer anchored cobalt(II) complex for the aerobic oxidation of alkyl aromatics and unsaturated organic compounds. J. Organomet. Chem. 2014, 774, 61–69. [Google Scholar] [CrossRef]
- Marfin, Y.S.; Vashurin, A.S.; Rumyantsev, E.V.; Puhovskaya, S.G. Sol–gel synthesis of highly effective catalyst based on cobalt tetrasulfophthalocyanine complex and silicon oxide. J. Sol Gel Sci. Technol. 2013, 66, 306–311. [Google Scholar] [CrossRef]
- Ranaweera, S.A.; Rowe, M.D.; Walters, K.B.; Henry, W.P.; White, M.G.; Rodriguez, J.M. Synthesis, characterization and catalytic activity of a cobalt catalyst: Silica-supported, bis(1,5-diphenyl-1,3,5-pentanetrionato)dicobalt(II)[Co2(dba)2]. Appl. Catal. A Gen. 2017, 529, 108–117. [Google Scholar] [CrossRef]
- Li-Juan, C.; Fu-Ming, M.; Guang-Xing, L. Co(II) Schiff base complexes on silica by sol–gel method as heterogeneous catalysts for oxidative carbonylation of aniline. Catal. Commun. 2009, 10, 981–985. [Google Scholar] [CrossRef]
- Trujillano, R.; Lambert, J.-F.; Louis, C. Chemistry of Silica-Supported Cobalt Catalysts Prepared by Cation Adsorption. 2. Neoformation of Cobalt Phyllosilicate. J. Phys. Chem. C 2008, 112, 18551–18558. [Google Scholar] [CrossRef]
- Yakubovich, T.N.; Ermokhina, N.I.; Bratushko, Y.I.; Zub, Y.L.; Chuiko, A.A. Cobalt complex with 2.2′-bipyridyl immobilized on disperse silica surface in the reaction of p-hydroquinone oxidation. Comparison of the homogeneous and heterogeneous catalysis results. In Dioxygen Activation and Homogeneous Catalytic Oxidation; Simindi, L.I., Ed.; Elsevier Science Publishers, B.V.: Amsterdam, The Netherlands, 1991; pp. 179–188. [Google Scholar]
- Rautiainen, A.; Lindblad, M.; Backman, L.B.; Puurunen, R.L. Preparation of silica-supported cobalt catalysts through chemisorption of cobalt(II) and cobalt(III) acetylacetonate. Phys. Chem. Chem. Phys. 2002, 4, 2466–2472. [Google Scholar] [CrossRef]
- Yuanfa, Y.; Renxian, Z.; Shaofen, Z.; Qiaoling, L.; Xiaoming, Z. Silica-supported poly-γ-aminopropylsilane Ni2+, Cu2+, Co2+ complexes: Efficient catalysts for Heck vinylation reaction. J. Mol. Catal. A Chem. 2003, 192, 303–306. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Fedorenko, S.V.; Strekalova, S.O.; Kholin, K.V.; Mustafina, A.R.; Zhilkin, M.Y.; Khrizanforova, V.V.; Osin, Y.N.; Salnikov, V.V.; Gryaznova, T.V.; et al. A Ni(III) complex stabilized by silica nanoparticles as an efficient nanoheterogeneous catalyst for oxidative C–H fluoroalkylation. Dalton Trans. 2016, 45, 11976–11982. [Google Scholar] [CrossRef] [PubMed]
- Khrizanforov, M.N.; Fedorenko, S.V.; Mustafina, A.R.; Khrizanforova, V.V.; Kholin, K.V.; Nizameev, I.R.; Gryaznova, T.V.; Grinenko, V.V.; Budnikova, Y.H. Nano-architecture of silica nanoparticles as a tool to tune both electrochemical and catalytic behavior of NiII@SiO2. RSC Adv. 2019, 9, 22627–22635. [Google Scholar] [CrossRef]
- Budnikova, Y.; Bochkova, O.; Khrizanforov, M.; Nizameev, I.; Kholin, K.; Gryaznova, T.; Laskin, A.; Dudkina, Y.; Strekalova, S.; Fedorenko, S.; et al. Selective C(sp2)-H Amination Catalyzed by High-Valent Cobalt(III)/(IV)-bpy Complex Immobilized on Silica Nanoparticles. ChemCatChem 2019, 11, 5615–5624. [Google Scholar] [CrossRef]
- Ryabchuk, P.; Agostini, G.; Pohl, M.-M.; Lund, H.; Agapova, A.; Junge, H.; Junge, K.; Beller, M. Intermetallic nickel silicide nanocatalyst-A non-noble metal–based general hydrogenation catalyst. Sci. Adv. 2018, 4, eaat0761. [Google Scholar] [CrossRef]
- Dudkina, Y.B.; Gryaznova, T.V.; Osin, Y.N.; Salnikov, V.V.; Davydov, N.A.; Fedorenko, S.V.; Mustafina, A.R.; Vicic, D.A.; Sinyashin, O.G.; Budnikova, Y.H. Nanoheterogeneous catalysis in electrochemically induced olefin perfluoroalkylation. Dalton Trans. 2015, 44, 8833–8838. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Karimi-Maleh, H.; Tahernejad-Javazmi, F. Fabrication of an electroanalytical sensor for determination of deoxyepinephrine in the presence of uric acid using CuFe2O4 nanoparticle/ionic liquid amplified sensor. J. Electrochem. Soc. 2019, 166, H218. [Google Scholar] [CrossRef]
- Liu, X. Electrochemical sensor for determination of parathion based on electropolymerization poly (safranine) film electrode. Int. J. Electrochem. 2011, 2011, 986494. [Google Scholar] [CrossRef]
- Cao, Y.; Luona, W.; Chengyin, W.; Xiaoya, H.; Yunling, L.; Guoxiu, W. Sensitive detection of glyphosate based on a Cu-BTC MOF/g-C3N4 nanosheet photoelectrochemical sensor. Electrochim. Acta 2019, 317, 341–347. [Google Scholar] [CrossRef]
- Shelkovnikov, V.V.; Novolokov, K.Y. Voltammetric sensor for determining malathion and diazinon. Anal. Control 2019, 23, 362–369. [Google Scholar] [CrossRef]
- Tadayon, F.; Jahromi, M.N. A sensitive and selective electrochemical determination of Diazinon based on Au–Pd/rGO–MWCNTs composite: Analytical application in water, fruit and vegetable samples. J. Iran. Chem. Soc. 2020, 17, 847–857. [Google Scholar] [CrossRef]
- Song, D.; Li, Y.; Lu, X.; Sun, M.; Liu, H.; Yu, G.; Gao, F. Palladium-copper nanowires-based biosensor for the ultrasensitive detection of organophosphate pesticides. Anal. Chim. Acta 2017, 982, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Zahirifar, F.; Rahimnejad, M.; Abdulkareem, R.A.; Najafpour, G. Determination of Diazinon in fruit samples using electrochemical sensor based on carbon nanotubes modified carbon paste electrode. Biocatal. Agric. Biotechnol. 2019, 20, 101245. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Fakhri, H.; Hajian, A.; Afkhami, A.; Bagheri, H. High-performance electrochemical enzyme sensor for organophosphate pesticide detection using modified metal-organic framework sensing platforms. Bioelectrochemistry 2019, 130, 107348. [Google Scholar] [CrossRef] [PubMed]
- Sok, V.; Fragoso, A. Amperometric biosensor for glyphosate based on the inhibition of tyrosinase conjugated to carbon nano-onions in a chitosan matrix on a screen-printed electrode. Microchim. Acta 2019, 186, 569. [Google Scholar] [CrossRef]
- Hua, Q.T.; Ruecha, N.; Hiruta, Y.; Citterio, D. Disposable electrochemical biosensor based on surface-modified screen-printed electrodes for organophosphorus pesticide analysis. Anal. Methods 2019, 11, 3439–3445. [Google Scholar] [CrossRef]
- Hou, W.; Zhang, Q.; Dong, H.; Li, F.; Zhang, Y.; Guo, Y.; Sun, X. Acetylcholinesterase biosensor modified with ATO/OMC for detecting organophosphorus Pesticides. New J. Chem. 2019, 43, 946–952. [Google Scholar] [CrossRef]
- Tefera, M.; Admassie, S.; Tessema, M.; Mehretie, S. Electrochemical sensor for determination of fenitrothion at multi-wall carbon nanotubes modified glassy carbon electrode. Anal. Bioanal. Chem. Res. 2015, 2, 139–150. [Google Scholar] [CrossRef]
- Motaharian, A.; Motaharian, F.; Abnous, K.; Hosseini, M.R.M.; Hassanzadeh-Khayyat, M. Molecularly imprinted polymer nanoparticles-based electrochemical sensor for determination of diazinon pesticide in well water and apple fruit samples. Anal. Bioanal. Chem. 2016, 408, 6769–6779. [Google Scholar] [CrossRef]
- Budnikov, G.K.; Evtyugin, G.A.; Budnikova, Y.G.; Al′fonsov, V.A. Chemically modified electrodes with amperometric response in enantioselective analysis. J. Anal. Chem. 2008, 63, 2–12. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Ghazizadeh, A.J.; Hashemi, P.; Afkhami, A.; Arduini, F.; Bagheri, H. An overview to electrochemical biosensors and sensors for the detection of environmental contaminants. J. Iran. Chem. Soc. 2020, 1–19. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A. Recent advances in voltammetric, amperometric and ion-selective (bio)sensors fabricated by microengineering manufacturing approaches. Curr. Opin. Electrochem. 2020, 23, 21–25. [Google Scholar] [CrossRef]
- Wu., L.; Yan, H.; Wang, J.; Liu, G.; Xie, W. Tyrosinase Incorporated with Au-Pt@SiO2 Nanospheres for Electrochemical Detection of Bisphenol, A. J. Electrochem. Soc. 2019, 166, B562. [Google Scholar] [CrossRef]
- Jeerapan, I.; Poorahong, S. Review-Flexible and Stretchable Electrochemical Sensing Systems: Materials, Energy Sources, and Integrations. J. Electrochem. Soc. 2020, 167, 037573. [Google Scholar] [CrossRef]
- Patel, B.R.; Noroozifar, M.; Kerman, K. Review—Nanocomposite-Based Sensors for Voltammetric Detection of Hazardous Phenolic Pollutants in Water. J. Electrochem. Soc. 2020, 167, 037568. [Google Scholar] [CrossRef]
- Zhu, N.; Cai, H.; He, P.; Fang, Y. Tris(2,2′-bipyridyl)cobalt(III)-doped silica nanoparticle DNA probe for the electrochemical detection of DNA hybridization. Anal. Chim. Acta 2003, 481, 181–189. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Xu, J.; Liang, J.; Li, J.; Yang, W. Tuning the Emission Properties of Ru(phen)32+ Doped Silica Nanoparticles by Changing the Addition Time of the Dye during the Stöber Process. Langmuir 2010, 26, 6657–6662. [Google Scholar] [CrossRef]
- Lian, W.; Litherland, S.A.; Badrane, H.; Tan, W.; Wu, D.; Baker, H.V.; Gulig, P.A.; Lim, D.V.; Jin, S. Ultrasensitive detection of biomolecules with fluorescent dye-doped nanoparticles. Anal. Biochem. 2004, 334, 135–144. [Google Scholar] [CrossRef]
- Ahkam, Q.M.; Khan, E.U.; Iqbal, J.; Murtaza, A.; Khan, M.T. Synthesis and characterization of cobalt-doped SiO2 nanoparticles. Phys. B Condens. Matter 2019, 572, 161–167. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Env. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Gryaznova, T.; Sinyashin, O.; Budnikova, Y. Aromatic perfluoroalkylation with metal complexes in electrocatalytic conditions. J. Organomet. Chem. 2012, 718, 101–104. [Google Scholar] [CrossRef]
- Mustafina, A.R.; Fedorenko, S.V.; Konovalova, O.D.; Menshikova, A.Y.; Shevchenko, N.N.; Soloveva, S.E.; Konovalov, A.I.; Antipin, I.S. Novel Highly Charged Silica-Coated Tb(III) Nanoparticles with Fluorescent Properties Sensitive to Ion Exchange and Energy Transfer Processes in Aqueous Dispersions. Langmuir 2009, 25, 3146–3151. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Arkhipova, D.M.; Shekurov, R.P.; Gerasimova, T.P.; Ermolaev, V.V.; Islamov, D.R.; Miluykov, V.A.; Kataeva, O.N.; Khrizanforova, V.V.; Sinyashin, O.G.; et al. Novel paste electrodes based on phosphonium salt room temperature ionic liquids for studying the redox properties of insoluble compounds. J. Solid State Electrochem. 2015, 19, 2883–2890. [Google Scholar] [CrossRef]
- DIFFRAC Plus Evaluation package EVA; Version 11; User’s Manual; Bruker AXS: Karlsruhe, Germany, 2005.
- Small Angle X-ray Scattering; Version 4.0; Software Reference Manual, M86-E00005-0600; Bruker AXS Inc.: Madison, WI, USA, 2000.
- Konarev, P.V.; Volkov, V.V.; Sokolova, A.V.; Koch, M.H.J.; Svergun, D.I. PRIMUS: A Windows PC-based system for small-angle scattering data analysis. J. Appl. Cryst. 2003, 36, 1277–1282. [Google Scholar] [CrossRef]
- Arriagada, F.J.; Osseo-Asare, K. Controlled hydrolysis of tetraethoxysilane in a nonionic water-in-oil microemulsion: A statistical model of silica nucleation. Colloids Surf. A Physicochem. Eng. Asp. 1999, 154, 311–326. [Google Scholar] [CrossRef]
- Liver, E. Elektronnaya Spektroskopiya Neorganicheskih Soedineniy (Electron Spectroscopy of Inorganic Compounds), 2nd ed.; Mir: Moscow, Russia, 1987. [Google Scholar]
- Alexandru, M.-G.; Velickovic, M.; Hrubaru, K.M.-M.; Draghici, C. Two complexes of Co(II) and Pd(II) formed in reaction with a mono-T.C. oxazoline derivative. Spectroscopic characterization and cytotoxic evaluation. J. Mol. Struct. 2013, 1041, 55–60. [Google Scholar] [CrossRef]
- Verberckmoes, A.A.; Weckhuysen, B.M.; Schoonheydt, R.A. Spectroscopy and coordination chemistry of cobalt in molecular sieves. Microporous Mesoporous Mater. 1998, 22, 165–178. [Google Scholar] [CrossRef]
- Gerasimova, T.; Shekurov, R.; Gilmanova, L.; Laskin, A.; Katsyuba, S.; Kovalenko, V.; Khrizanforov, M.; Milyukov, V.; Sinyashin, O. IR and UV study of reversible water-induced structural transformations of poly(manganese 1,10 -ferrocenediyl-bis(Hphosphinate)) and poly(cobalt 1,10-ferrocenediyl-bis(H-phosphinate)). J. Mol. Struct. 2018, 1166, 237–242. [Google Scholar] [CrossRef]
- Shekurov, R.; Khrizanforova, V.; Gilmanova, L.; Khrizanforov, M.; Miluykov, V.; Kataeva, O.; Yamaleeva, Z.; Burganov, T.; Gerasimova, T.; Khamatgalimov, A.; et al. Zn and Co redox active coordination polymers as efficient electrocatalysts. Dalton Trans. 2019, 48, 3601–3609. [Google Scholar] [CrossRef]
- Yousef, T.A.; El-Reash, G.M.A.; El-Gammal, O.A.; Bedier, R.A. Co(II), Cu(II), Cd(II), Fe(III) and U(VI) complexes containing a NSNO donor ligand: Synthesis, characterization, optical band gap, in vitro antimicrobial and DNA cleavage studies. J. Mol. Struct. 2012, 1029, 149–160. [Google Scholar] [CrossRef]
- Kang, W.; Spanjers, C.S.; Rioux, R.M.; Hoefelmeyer, J.D. Synthesis of brookite TiO2nanorods with isolated Co(II) surface sites and photocatalytic degradation of 5,8-dihydroxy-1,4-naphthoquinone dye. J. Mater. Chem. A 2013, 1, 7717–7728. [Google Scholar] [CrossRef]
- Islam, S.; Bakhtiar, H.; Aziz, M.S.B.A.; Duralima, M.B.; Riaz, S.; Naseemb, S.; Abdullha, M.B.; Osman, S.S. CR incorporation in mesoporous silica matrix for fiber optic pH sensing. Sens. Actuators A Phys. 2018, 280, 429–436. [Google Scholar] [CrossRef]
- Islam, S.; Bakhtiar, H.; Naseem, S.; Aziz, M.S.B.A.; Biden, N.; Riaz, S.; Ali, J. Surface functionality and optical properties impact of phenol red dye on mesoporous silica matrix for fiber optic pH sensing. Sens. Actuators A Phys. 2018, 276, 267–277. [Google Scholar] [CrossRef]
- Chen, C.; Xu, J.; Zhang, Q.; Ma, H.; Miao, H.; Zhou, L. Direct synthesis of bifunctionalized hexagonal mesoporous silicas and its catalytic performance for aerobic oxidation of cyclohexane. J. Phys. Chem. C 2009, 113, 2855–2860. [Google Scholar] [CrossRef]
- Fouad, O.A.; Makhlouf, S.A.; Ali, G.A.M.; El-Sayed, A.Y. Cobalt/silica nanocomposite via thermal calcination-reduction of gel precursors. Mater. Chem. Phys. 2011, 128, 70–76. [Google Scholar] [CrossRef]
- Genovese, D.; Rampazzo, E.; Bonacchi, S.; Montalti, M.; Zaccheroni, N.; Prodi, L. Energy transfer processes in dye-doped nanostructures yield cooperative and versatile fluorescent probes. Nanoscale 2014, 6, 3022–3036. [Google Scholar] [CrossRef] [PubMed]
- Koirala, R.; Safonova, O.V.; Pratsinis, S.E.; Baiker, A. Effect of cobalt loading on structure and catalytic behavior of CoOx/SiO2 in CO2-assisted dehydrogenation of ethane. Appl. Catal. A 2018, 552, 77–85. [Google Scholar] [CrossRef]
- Okamoto, Y.; Nagata, K.; Adachi, T.; Imanaka, T.; Inamura, K.; Takyu, T. Preparation and Characterization of Highly Dispersed Cobalt Oxide and Sulfide Catalysts Supported on SiO2. J. Phys. Chem. 1991, 95, 310–319. [Google Scholar] [CrossRef]
- Masalov, V.M.; Kudrenko, E.A.; Grigoryeva, N.A.; Ezdakova, K.V.; Roddatis, V.V.; Sukhinina, N.S.; Arefev, M.V.; Mistonov, A.A.; Grigoriev, S.V.; Emelchenko, G.A. Direct observation of the shell-like structure of SiO2 partices synthesized by the multistage Stober method. Nano Brief Rep. Rev. 2013, 8, 1350036. [Google Scholar] [CrossRef]
- Fouilloux, S.; Désert, A.; Taché, O.; Spalla, O.; Daillant, J.; Thill, A. SAXS exploration of the synthesis of ultra monodisperse silica nanoparticles and quantitative nucleation growth modeling. J. Colloid Interface Sci. 2010, 346, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Fouilloux, S.; Daillant, J.; Thill, A. Single step synthesis of 5–30 nm monodisperse silica nanoparticles: Important experimental parameters and modeling. Colloids Surf. A Physicochem. Eng. Asp. 2012, 393, 122–127. [Google Scholar] [CrossRef]
- De Almeida, L.K.S.; Chigome, S.; Torto, N.; Frost, C.L.; Pletschke, B.I. A novel colorimetric sensor strip for the detection of glyphosate in water. Sens. Actuators B Chem. 2015, 206, 357–363. [Google Scholar] [CrossRef]


| Elements | C | H | B | Co | F | N |
|---|---|---|---|---|---|---|
| Anal. Calc., % | 51.39 | 3.45 | 3.08 | 8.41 | 21.68 | 11.99 |
| Found, % | 51.41 | 3.41 |
| Synthetic Method, Precursor | d, nm | Si:Co Molar Ratio, % | D, nm | PDI | ζ, mV |
|---|---|---|---|---|---|
| Stöber, CoII(bpy)3 | 109 ± 20 | 100:0.243 | 225 ± 2 | 0.139 | −51 ± 1 |
| w/o, CoII(bpy)3 | 50 ± 5 1 | 100:0.680 | 144 ± 1 | 0.114 | −29 ± 1 |
| Stöber, CoCl2 | 118 ± 10 | 100:0.130 | 154 ± 1 | 0.144 | −51 ± 1 |
| w/o, CoCl2 | 43 ± 3 | 100:0.578 | 118± 2 | 0.117 | −38 ± 1 |
| Sample (Technique, Precursor) | Rg, Å | Rg*, Å | Dmax, nm | Vpart, Å3 | I0, a.u. | ds, nm | FD | dTEM, nm |
|---|---|---|---|---|---|---|---|---|
| w/o, CoCl2 | 131.0 ± 6 | 130.9 ± 7 | 39.4 ± 0.6 | 122 × 105 | 166000 | 33.8 ± 1.8 | 3.41 | 43 ± 3 |
| w/o, CoII(bpy)3 | 145.5 ± 7 | 145.6 ± 5 | 43.4 ± 0.7 | 148 × 105 | 229900 | 37.6 ± 1.3 | 3.29 | 50 ± 5 |
| Stöber, CoCl2 | 164.6 ± 7 | 164.4 ± 8 | 50.1 ± 0.7 | 236 × 105 | 84660 | 41.4 ± 2.1 | 2.61 | 118 ± 10 |
| Stöber, CoII(bpy)3 | 139.4 ± 6 | 139.4 ± 7 | 43.7 ± 0.6 | 133 × 105 | 60640 | 36.0 ± 1.8 | 2.69 | 109 ± 20 |
| w/o, “empty” | 128.0 ± 6 | 127.8 ± 7 | 36.4 ± 0.6 | 114 × 105 | 183600 | 33.0 ± 1.8 | 3.53 | 48 ± 5 |
| Stöber, “empty” | 132.7 ± 8 | 132.6 ± 6 | 37.1 ± 0.6 | 101 × 105 | 143200 | 34.0 ± 1.5 | 3.37 | 97 ± 5 |
| Sample | Reduction | Oxidation | |
|---|---|---|---|
| 1Epc/Epa, V | 2Epc/Epa, V | 1Epa/Epc, V | |
| Stöber CoII@SiO2 | −1.12/−0.90 | −1.69/irrev | 0.71/irrev |
| w/o CoII@SiO2 | −1.15/−0.99 | −1.38/−1.26 | 0.72/irrev |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochkova, O.; Khrizanforov, M.; Gubaidullin, A.; Gerasimova, T.; Nizameev, I.; Kholin, K.; Laskin, A.; Budnikova, Y.; Sinyashin, O.; Mustafina, A. Synthetic Tuning of CoII-Doped Silica Nanoarchitecture Towards Electrochemical Sensing Ability. Nanomaterials 2020, 10, 1338. https://doi.org/10.3390/nano10071338
Bochkova O, Khrizanforov M, Gubaidullin A, Gerasimova T, Nizameev I, Kholin K, Laskin A, Budnikova Y, Sinyashin O, Mustafina A. Synthetic Tuning of CoII-Doped Silica Nanoarchitecture Towards Electrochemical Sensing Ability. Nanomaterials. 2020; 10(7):1338. https://doi.org/10.3390/nano10071338
Chicago/Turabian StyleBochkova, Olga, Mikhail Khrizanforov, Aidar Gubaidullin, Tatiana Gerasimova, Irek Nizameev, Kirill Kholin, Artem Laskin, Yulia Budnikova, Oleg Sinyashin, and Asiya Mustafina. 2020. "Synthetic Tuning of CoII-Doped Silica Nanoarchitecture Towards Electrochemical Sensing Ability" Nanomaterials 10, no. 7: 1338. https://doi.org/10.3390/nano10071338
APA StyleBochkova, O., Khrizanforov, M., Gubaidullin, A., Gerasimova, T., Nizameev, I., Kholin, K., Laskin, A., Budnikova, Y., Sinyashin, O., & Mustafina, A. (2020). Synthetic Tuning of CoII-Doped Silica Nanoarchitecture Towards Electrochemical Sensing Ability. Nanomaterials, 10(7), 1338. https://doi.org/10.3390/nano10071338