Microstructure, Thermal Stability, and Catalytic Activity of Compounds Formed in CaO-SiO2-Cr(NO3)3-H2O System
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Linseis PT1000 instrument (Linseis, Selb, Germany). The operating conditions were: A heating rate of 15 °C/min, temperature range of 30–1000 °C, nitrogen atmosphere, ceramic sample handlers, and crucibles of Pt, and the sample mass was equal to ~13 mg.
- (2)
- In-situ XRD analysis was made with a high-temperature camera MTC-hightemp (Bruker AXS, Karlsruhe, Germany). The measurements were carried out with a step width of 0.02 2θ and 0.6 s/step at a heating rate of 50 °C/min after equilibration for 5 min at the desired temperature.
- (1)
- Scanning electron microscopy was performed by using a JEOL JSM-7600F (JEOL, Tokyo, Japan) instrument at an accelerating voltage of 10 kV, and a working distance of 8.6 mm.
- (2)
- Transmission electron microscopy was performed by using a Tecnai G2 F20 X-TWIN instrument (FEI, Eindhoven, The Netherlands) with a Schottky-type field-emission electron source. The accelerating voltage was 200 kV.
3. Results and Discussions
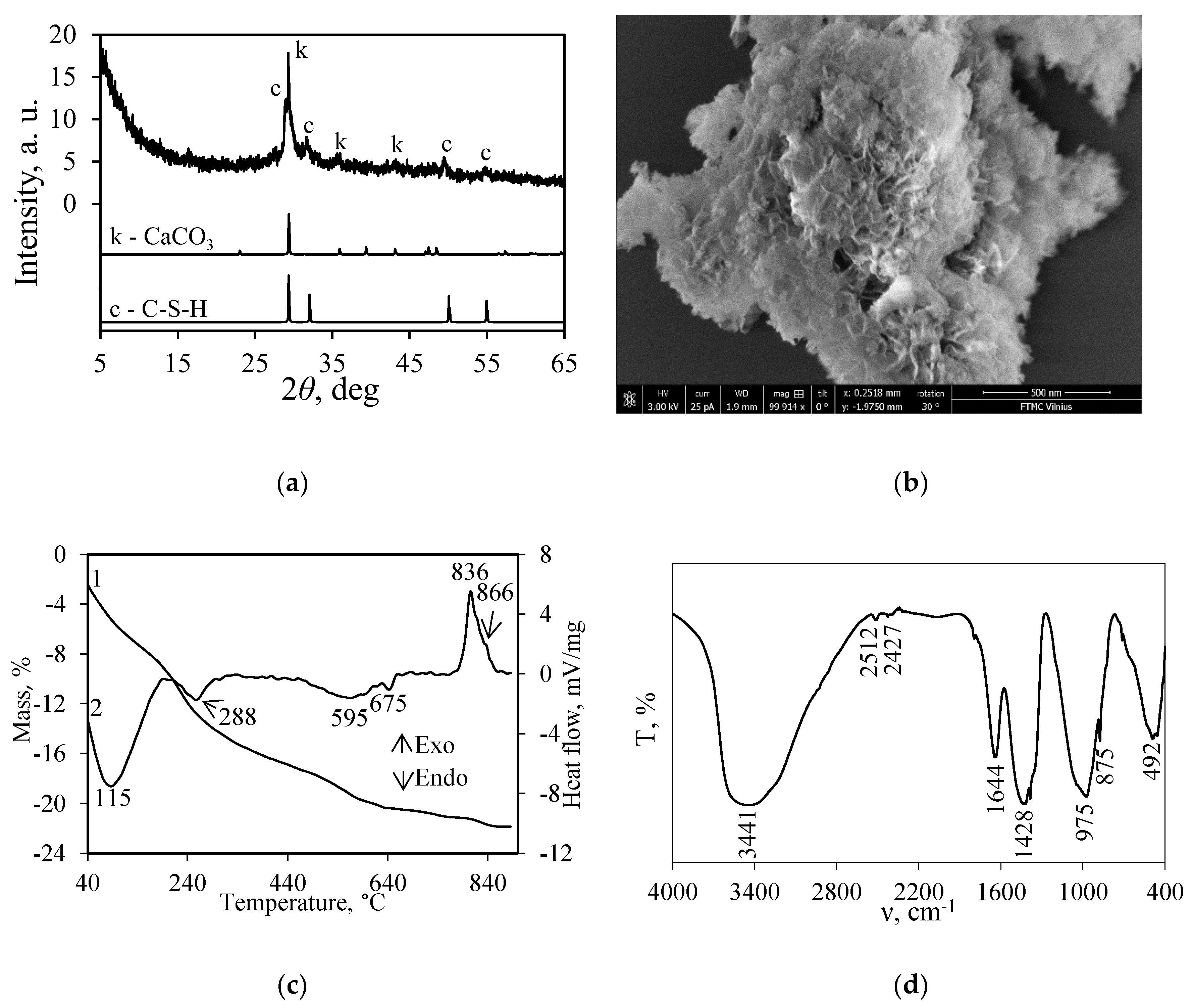
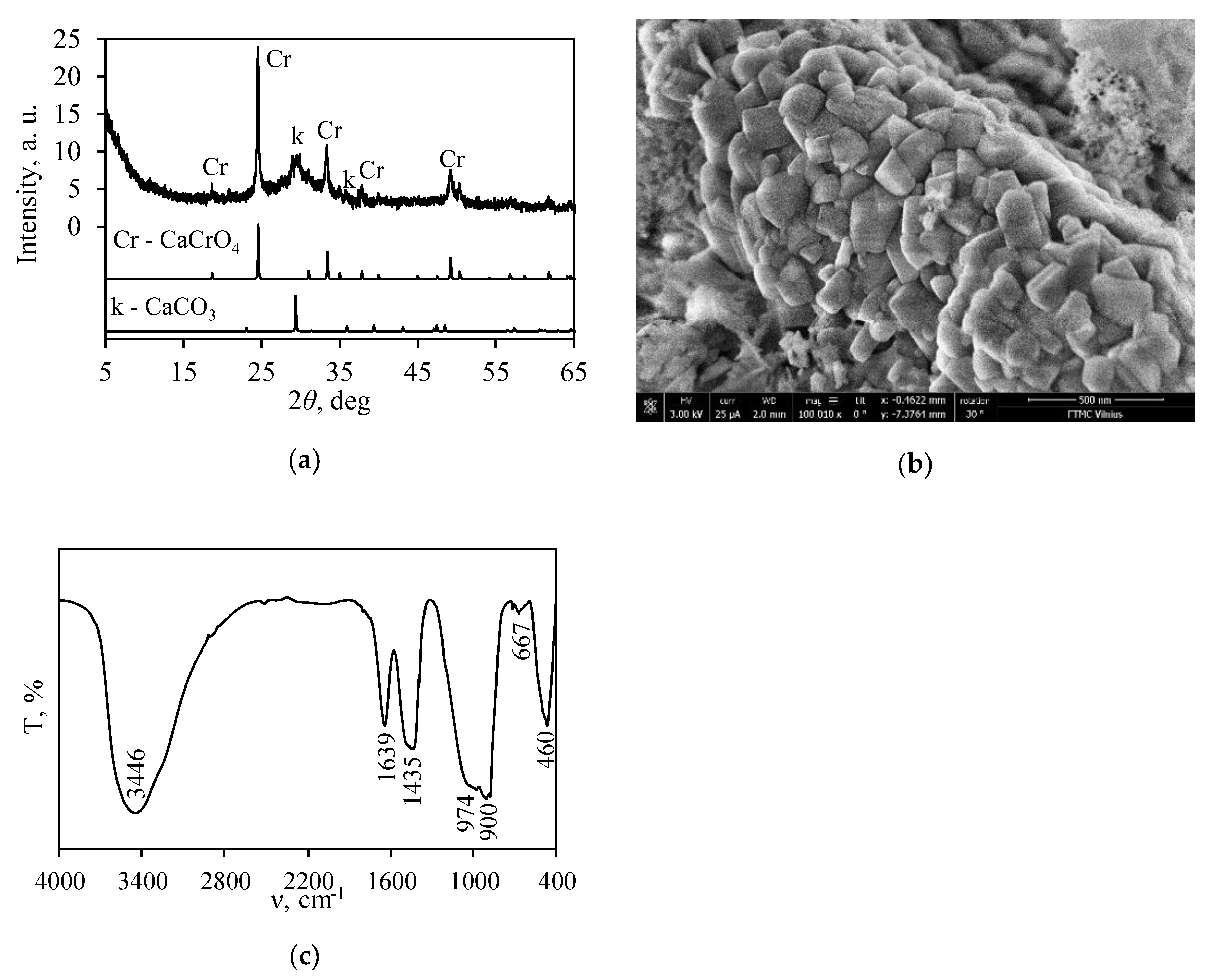
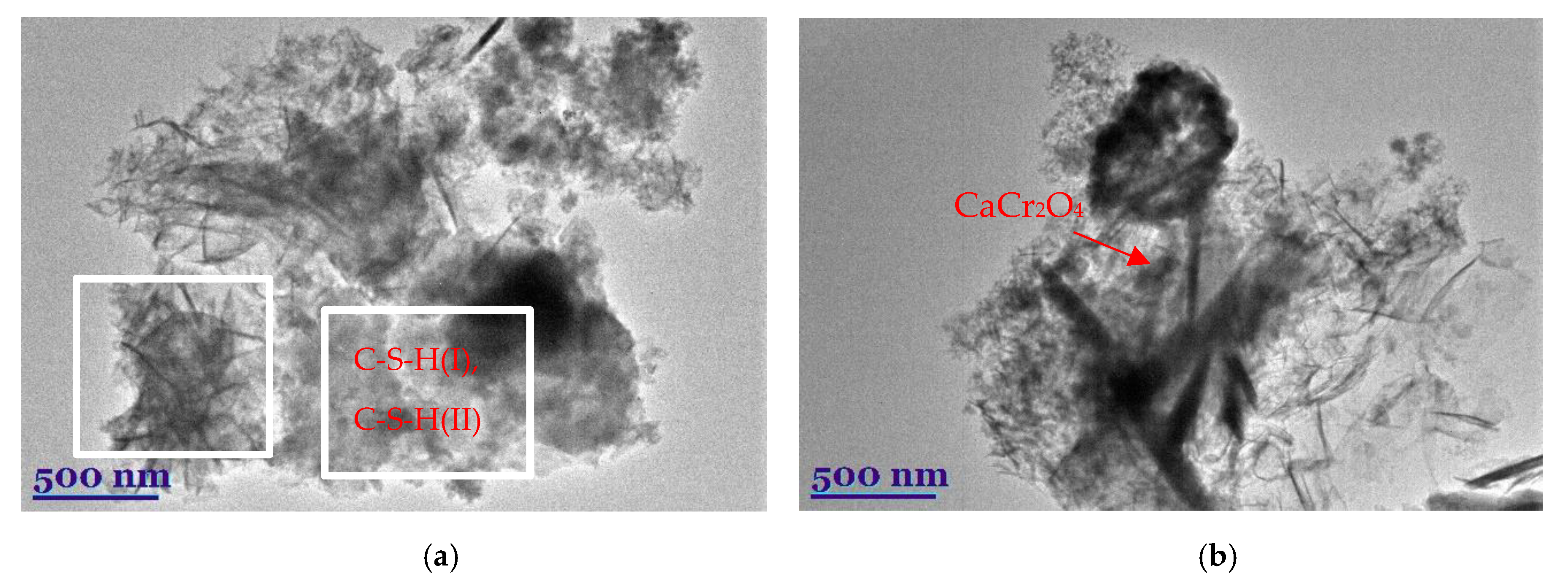
3.1. Synthesis of Calcium Silicate Hydrates with Incorporated Cr3+ Ions
3.2. Thermal Stability of Synthesis Products
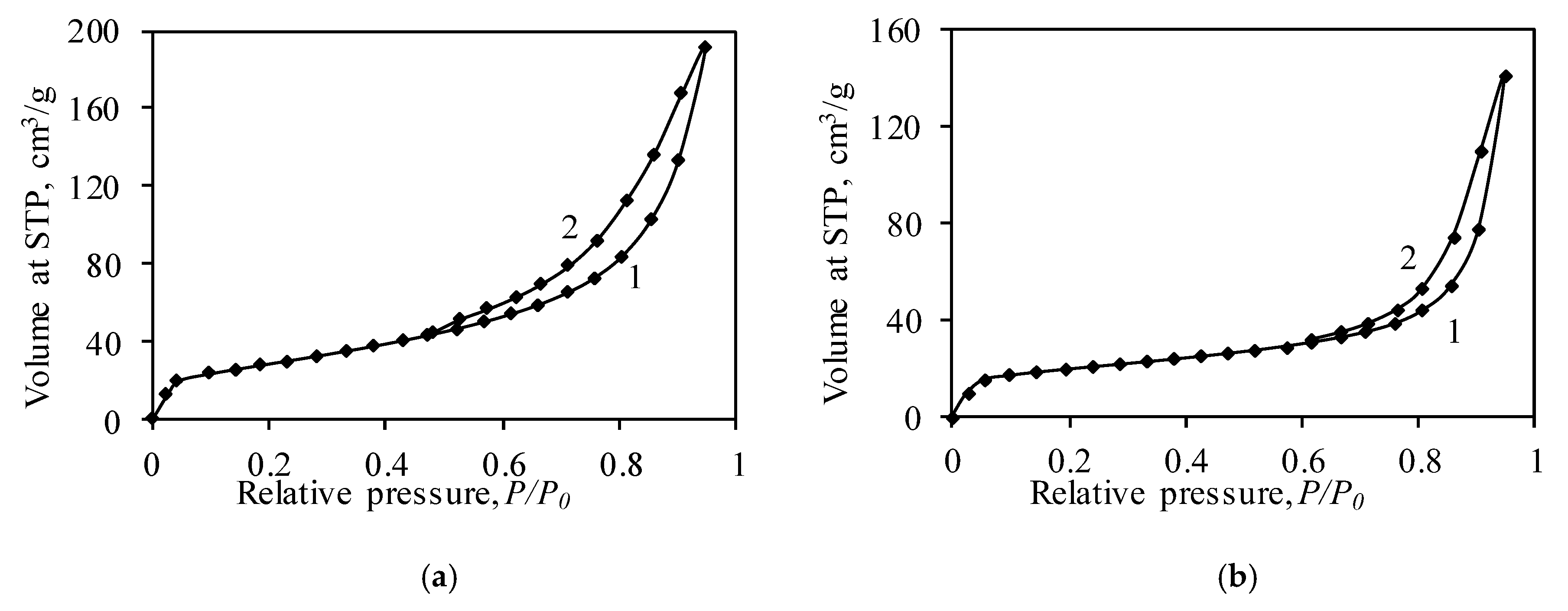
3.3. Porosity of Synthetic and Calcined Products
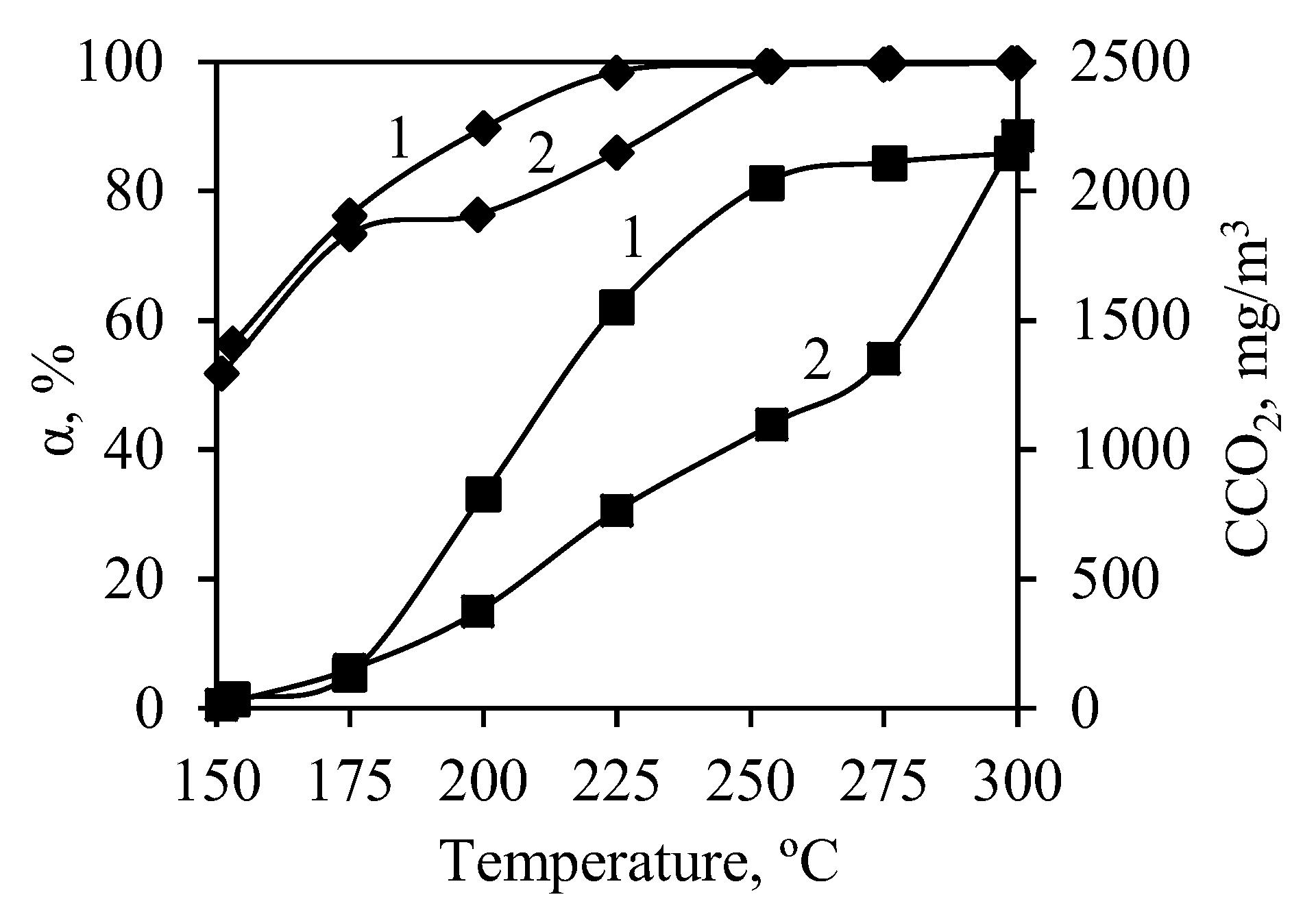
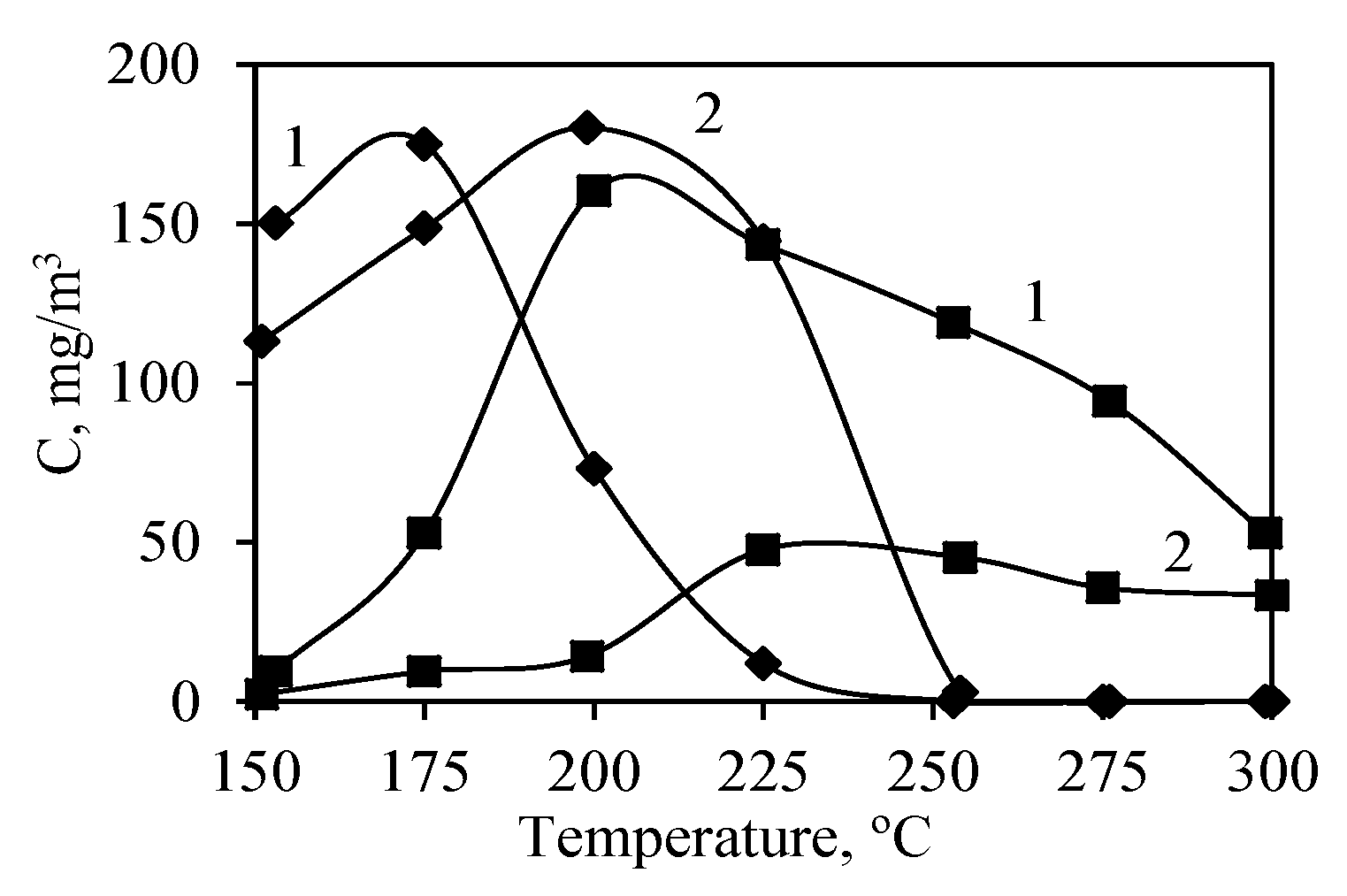
3.4. Catalyst Activity in Oxidation Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Jacobson, M.Z.; Delucchi, M.A.; Bauer, Z.A.; Goodman, S.C.; Chapman, W.E.; Cameron, M.; Bozonnat, C.; Chobadi, L.; Clonts, H.A.; Enevoldsen, P.; et al. 100% Clean and Renewable Wind, Water, and Sunlight All-Sector Energy Roadmaps for 139 Countries of the World. Joule 2017, 1, 108–121. [Google Scholar] [CrossRef]
- Mellmann, D.; Sponholz, P.; Junge, H.; Beller, M. Formic acid as a hydrogen storage material—Development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 2016, 45, 3954–3988. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nie, L.; Li, J.; Wang, Y.; Wang, G.; Wang, J.; Hao, Z. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar] [CrossRef]
- Nematollahi, N.; Kolev, S.D.; Steinemann, A. Volatile chemical emissions from 134 common consumer products. Air Qual. Atmos. Health 2019, 12, 1259–1265. [Google Scholar] [CrossRef]
- Palmisani, J.; Nørgaard, A.W.; Kofoed-Sørensen, V.; Clausen, P.A.; De Gennaro, G.; Wolkoff, P. Formation of ozone-initiated VOCs and secondary organic aerosol following application of a carpet deodorizer. Atmos. Environ. 2020, 222. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs). A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Guiza, M.; Abdedayem, A.; Ghouma, I.; Ouederni, A. Effect of copper and nickel supported activated carbon catalysts on the simultaneous adsorption/ozonation process of nitrobenzene degradation. J. Chem. Technol. Metall. 2017, 52, 836–851. [Google Scholar]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.-W.; et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Zeńczak, K. Activity of chromium oxide deposited on different silica supports in the dehydrogenation of propane with CO2—A comparative study. J. Mol. Catal. A: Chem. 2011, 349, 1–12. [Google Scholar] [CrossRef]
- Al-Awadi, A.S.; El-Toni, A.M.; Alhoshan, M.; Khan, A.; Labis, J.P.; Al-Fatesh, A.S.; Abasaeed, A.E.; Al-Zahrani, S.M. Impact of precursor sequence of addition for one-pot synthesis of Cr-MCM-41 catalyst nanoparticles to enhance ethane oxidative dehydrogenation with carbon dioxide. Ceram. Int. 2019, 45, 1125–1134. [Google Scholar] [CrossRef]
- Lan, J.; Qu, Y.; Zhang, X.; Ma, H.; Xu, P.; Sun, J. A novel water-stable MOF Zn(Py)(Atz) as heterogeneous catalyst for chemical conversion of CO2 with various epoxides under mild conditions. J. CO2 Utili. 2020, 35. [Google Scholar] [CrossRef]
- Ali, M.; Tit, N.; Yamani, Z.H.; Pi, X. First principles study on the functionalization of graphene with Fe catalyst for the detection of CO2: Effect of catalyst clustering. Appl. Surf. Sci. 2020, 502. [Google Scholar] [CrossRef]
- Cheng, G.; Zhao, Y.; Li, W.; Zhang, J.; Wang, X.; Dong, C. Performance enhancement of bipolar membranes modified by Fe complex catalyst. J. Membr. Sci. 2019, 589. [Google Scholar] [CrossRef]
- Ishihara, A.; Andou, A.; Hashimoto, T.; Nasu, H. Steam reforming of ethanol using novel carbon-oxide composite-supported Ni, Co and Fe catalysts. Fuel Process. Technol. 2020, 197. [Google Scholar] [CrossRef]
- Novikov, V.; Xanthopoulou, G.; Knysh, Y.; Amosov, A. Solution Combustion Synthesis of nanoscale Cu-Cr-O spinels: Mechanism, properties and catalytic activity in CO oxidation. Ceram. Int. 2017, 43, 11733–11742. [Google Scholar] [CrossRef]
- Lai, C.; He, T.; Li, X.; Chen, F.; Yue, L.; Hou, Z. Catalytic wet air oxidation of phenols over porous plate Cu-based catalysts. Appl. Clay Sci. 2019, 181. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.J. Mn-based catalysts for sulfate radical-based advanced oxidation processes: A review. Environ. Int. 2019, 133. [Google Scholar] [CrossRef]
- Zapata, P.M.C.; Nazzarro, M.S.; Gonzo, E.E.; Parentis, M.L.; Bonini, N.A. Cr/SiO2 mesoporous catalysts: Effect of hydrothermal treatment and calcination temperature on the structure and catalytic activity in the gas phase dehydration and dehydrogenation of cyclohexanol. Catal. Today 2016, 259, 39–49. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, K.; Cai, J.; Hou, S.; Wang, Y.; Jiang, P.; Liang, M. Silica oxide encapsulated natural zeolite for high efficiency removal of low concentration heavy metals in water. Coll. Surf. A: Physicochem. Eng. Asp. 2019, 561, 388–394. [Google Scholar] [CrossRef]
- Fridman, V.Z.; Xing, R.; Severance, M. Investigating the CrOx/Al2O3 dehydrogenation catalyst model: I. identification and stability evaluation of the Cr species on the fresh and equilibrated catalysts. Appl. Catal. A: Gen. 2016, 523, 39–53. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O.; Romero, M.J.V.; Hernandez, S.; Nieto, J.M.L.; Rodríguez-Mirasol, J.; Cordero, T. Lignocellulosic-derived mesoporous materials: An answer to manufacturing non-expensive catalysts useful for the biorefinery processes. Catal. Today 2012, 195, 155–161. [Google Scholar] [CrossRef]
- Ghods, B.; Meshkani, F.; Rezaei, M. Effects of alkaline earth promoters on the catalytic performance of the nickel catalysts supported on high surface area mesoporous magnesium silicate in dry reforming reaction. Int. J. Hydrog. Energy 2016, 41, 22913–22921. [Google Scholar] [CrossRef]
- Nagaraju, N. Alumina and silica supported metal catalysts for the production of carbon nanotubes. J. Mol. Catal. A: Chem. 2002, 181, 57–62. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Kanno, S.; Kon, K.; Siddiki, S.H.; Tanaka, H.; Sakata, Y. N-alkylation of ammonia and amines with alcohols catalyzed by Ni-loaded CaSiO3. Catal. Today 2014, 232, 134–138. [Google Scholar] [CrossRef]
- Sakata, Y.; Tamaura, Y.; Imamura, H.; Watanabe, M. Preparation of a New Type of CaSiO3 with High Surface Area and Property as a Catalyst Support. Adv. Pharm. 2006, 162, 331–338. [Google Scholar]
- Richardson, I. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Dambrauskas, T.; Baltakys, K.; Eisinas, A. Formation and thermal stability of calcium silicate hydrate substituted with Al3+ ions in the mixtures with CaO/SiO2 = 1.5. J. Therm. Anal. Calorim. 2017, 131, 501–512. [Google Scholar] [CrossRef]
- Novembre, D.; Pace, C.; Gimeno, D. Synthesis and characterization of wollastonite-2Mby using a diatomite precursor. Miner. Mag. 2018, 82, 95–110. [Google Scholar] [CrossRef]
- Niuniavaite, D.; Baltakys, K.; Dambrauskas, T.; Eisinas, A. Cu2+, Co2+ and Cr3+ adsorption by synthetic dibasic calcium silicate hydrates and their thermal stability in a 25–1000° C temperature range. J. Therm. Anal. Calorim. 2019, 138, 2241–2249. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Peng, H.; Yang, Z.; Zhao, L.; Tang, A. Surface charge of mesoporous calcium silicate and its adsorption characteristics for heavy metal ions. Solid State Sci. 2020, 99. [Google Scholar] [CrossRef]
- Xia, X.; Xu, Y.; Chen, Y.; Liu, Y.; Lu, Y. The distinct catalytic behaviours of calcium silicate hydrate for the high selectivity of 2, 2′-isomer in reaction of phenol with formaldehyde. Catal. Commun. 2019, 118, 15–18. [Google Scholar] [CrossRef]
- Segawa, A.; Nakashima, A.; Nojima, R.; Yoshida, N.; Okamoto, M. Acetaldehyde Production from Ethanol by Eco-Friendly Non-Chromium Catalysts Consisting of Copper and Calcium Silicate. Ind. Eng. Chem. Res. 2018, 57, 11852–11857. [Google Scholar] [CrossRef]
- Zhu, G.; Li, H.; Wang, X.; Hou, X.; Wu, W.; Tang, Q. Synthesis of Calcium Silicate Hydrate in Highly Alkaline System. J. Am. Ceram. Soc. 2016, 99, 2778–2785. [Google Scholar] [CrossRef]
- Qu, X.; Zhao, Z.; Zhao, X. Microstructure and characterization of aluminum-incorporated calcium silicate hydrates (C–S–H) under hydrothermal conditions. R. Soc. Chem. Adv. 2018, 8, 28198–28208. [Google Scholar] [CrossRef]
- El-Korashy, S. Characterization of Cation Exchange and Cesium Selectivity of Synthetic Beta-Dicalcium Silicate Hydrate. J. Korean Chem. Soc. 2002, 46, 515–522. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Jia, H.; Zhang, G.; Sun, Z.; Pan, Y.; Zheng, S. Synthesis and humidity control performances of natural opoka based porous calcium silicate hydrate. Adv. Powder Technol. 2019, 30, 2733–2741. [Google Scholar] [CrossRef]
- Dambrauskas, T.; Baltakys, K.; Eisinas, A.; Kitrys, S. The specific surface area and porosity of synthetic and calcined α-C2SH, kilchoanite and hydroxyledgrewite. Powder Technol. 2019, 355, 504–513. [Google Scholar] [CrossRef]
- You, Y.-Z.; Kalebaila, K.K.; Brock, S.L.; Oupicky, D. Temperature-Controlled Uptake and Release in PNIPAM-Modified Porous Silica Nanoparticles. Chem. Mater. 2008, 20, 3354–3359. [Google Scholar] [CrossRef]
- Meng, Y.; Ling, T.-C.; Mo, K.H.; Tian, W. Enhancement of high temperature performance of cement blocks via CO2 curing. Sci. Total Environ. 2019, 671, 827–837. [Google Scholar] [CrossRef]
- Chen, Q.; Hills, C.D.; Yuan, M.; Liu, H.; Tyrer, M. Characterization of carbonated tricalcium silicate and its sorption capacity for heavy metals: A micron-scale composite adsorbent of active silicate gel and calcite. J. Hazard. Mater. 2008, 153, 775–783. [Google Scholar] [CrossRef]
- Polychroniadis, E.K.; Oral, A.Y.; Ozer, M. Proceedings of 2nd International Multidisciplinary Microscopy and Microanalysis Congress; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Berei, E.; Ştefănescu, O.; Muntean, C.; Ţăranu, B.; Ştefănescu, M. Study on the formation of CoCr2O4/SiO2 nanocomposite obtained from Co(II) carboxylate and ammonium dichromate. Therm. Anal. Calorim. 2019, 138, 1863–1870. [Google Scholar] [CrossRef]
- He, Y.; Zhao, X.; Lu, L.; Struble, L.J.; Hu, S. Effect of C/S ratio on morphology and structure of hydrothermally synthesized calcium silicate hydrate. J. Wuhan Univ. Technol. Sci. Ed. 2011, 26, 770–773. [Google Scholar] [CrossRef]
- El-Sheikh, S.; Rabah, M. Optical properties of calcium chromate 1D-nanorods synthesized at low temperature from secondary resources. Opt. Mater. 2014, 37, 235–240. [Google Scholar] [CrossRef]
- Chang, S.K.; Seong, I.W. Characterization of Cr/silica ethylene polymerization catalyst by TPO/TPR and FT—IR. J. Mol. Catal. 1992, 73, 249–263. [Google Scholar] [CrossRef]
- Shao, C.; Chen, M. In situ FT-IR studies on the CO2 hydrogenation over the SiO2-supported RhM (M=Cr, Mo, W) complex catalysts. J. Mol. Catal. A: Chem. 2001, 166, 331–335. [Google Scholar] [CrossRef]
- Liu, B.; Yu, S.; Li, L.; Zhang, Q.; Zhang, F.; Jiang, K. Morphology control of stolzite microcrystals with high hierarchy in solution. Angew. Chem. 2004, 43, 4745–4750. [Google Scholar] [CrossRef]
- Xiang, J.-H.; Yu, S.-H.; Xu, Z. Polymorph and Phase Discrimination of Lead Chromate Pigments by a Facile Room Temperature Precipitation Reaction. Cryst. Growth Des. 2004, 4, 1311–1315. [Google Scholar] [CrossRef]
- Kobayashi, M.; Miho, Y.; Shibasaki, N.; Ogura, K. Electrochemical Oxidative Decomposition of Aliphatic Amino Acids. Nippon KAGAKU KAISHI 1998, 1998, 442–446. [Google Scholar] [CrossRef]
- Driscoll, S.; Ozkan, U. Isotopic Labeling Studies on Oxidative Coupling of Methane over Alkali Promoted Molybdate Catalysts. Adv. Pharm. 1994, 82, 367–375. [Google Scholar]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near-, mid-, and far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Rotter, H.; Landau, M.V.; Carrera, M.; Goldfarb, D.; Herskowitz, M. High surface area chromia aerogel efficient catalyst and catalyst support for ethylacetate combustion. Appl. Catal. B: Environ. 2004, 47, 111–126. [Google Scholar] [CrossRef]
- Kang, M.; Chang-Ha, L. Methylene chloride oxidation on oxidative carbon-supported chromium oxide catalyst. Appl. Catal. A: Gen. 2004, 263, 163–172. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Naderi, M. Chapter Fourteen—Surface Area: Brunauer–Emmett–Teller (BET). In Progress in Filtration and Saperation; Academic Press: Cambridge, MA, SUA, 2015; pp. 585–608. [Google Scholar]
- Babaee, M.; Castel, A. Water vapor sorption isotherms, pore structure, and moisture transport characteristics of alkali-activated and Portland cement-based binders. Cem. Concr. Res. 2018, 113, 99–120. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: Cambridge, MA, SUA, 2013. [Google Scholar]
- Sun, Z.; Huang, D.; Duan, X.; Hong, W.; Liang, J. Functionalized nanoflower-like hydroxyl magnesium silicate for effective adsorption of aflatoxin B1. J. Hazard. Mater. 2020, 387. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, B.J.; Hsiao, J.; Park, J.; Zydney, A.L.; Fissell, W.H.; Roy, S. Slit pores preferred over cylindrical pores for high selectivity in biomolecular filtration. J. Colloid Interface Sci. 2018, 517, 176–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Liu, Z.; Liu, G.; Yang, L. Modelling of diffusion behavior of ions in low-density and high-density calcium silicate hydrate. Constr. Build. Mater. 2017, 155, 965–980. [Google Scholar] [CrossRef]
- Veksha, A.; Moo, J.G.S.; Krikstolaityte, V.; Oh, W.; Udayanga, W.D.C.; Giannis, A.; Lisak, G. Synthesis of CaCr2O4/carbon nanoplatelets from non-condensable pyrolysis gas of plastics for oxygen reduction reaction and charge storage. J. Electroanal. Chem. 2019, 849. [Google Scholar] [CrossRef]
- Lapham, D.P.; Lapham, J.L. BET surface area measurement of commercial magnesium stearate by krypton adsorption in preference to nitrogen adsorption. Int. J. Pharm. 2019, 568. [Google Scholar] [CrossRef]
- Lapham, D.P.; Lapham, J.L. Gas adsorption on commercial magnesium stearate: Effects of degassing conditions on nitrogen BET surface area and isotherm characteristics. Int. J. Pharm. 2017, 530, 364–376. [Google Scholar] [CrossRef]
- Anokhin, A.; Chernova, E.S.; Strelnikova, S.S.; Andrianov, N.T.; Ashmarin, A.A.; Zheleznyi, M.V. Influence of additives of aluminum, magnesium and calcium on synthesis and sintering of lanthanum chromite. Inorg. Mater. Appl. Res. 2014, 5, 323–329. [Google Scholar] [CrossRef]
- Roosz, C.; Gaboreau, S.; Grangeon, S.; Prêt, D.; Montouillout, V.; Maubec, N.; Ory, S.; Blanc, P.; Vieillard, P.; Henocq, P. Distribution of Water in Synthetic Calcium Silicate Hydrates. Langmuir 2016, 32, 6794–6805. [Google Scholar] [CrossRef]
- Morales-Flórez, V.; Findling, N.; Brunet, F. Changes on the nanostructure of cementitius calcium silicate hydrates (C–S–H) induced by aqueous carbonation. J. Mater. Sci. 2011, 47, 764–771. [Google Scholar] [CrossRef]
- Zdujić, M.; Lukic, I.; Kesić, Ž.; Janković-Častvan, I.; Marković, S.; Jovalekić, Č.; Skala, D. Synthesis of CaO SiO2 compounds and their testing as heterogeneous catalysts for transesterification of sunflower oil. Adv. Powder Technol. 2019, 30, 1141–1150. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Hu, Y.; Sun, J.; Tong, X.; Li, Q.; Zhou, Z. Novel low cost Li4SiO4-based sorbent with naturally occurring wollastonite as Si-source for cyclic CO2 capture. Chem. Eng. J. 2019, 374, 328–337. [Google Scholar] [CrossRef]
- Pal, N. Nanoporous metal oxide composite materials: A journey from the past, present to future. Adv. Colloid Interface Sci. 2020, 280. [Google Scholar] [CrossRef]
- Zukal, A.; Shamzhy, M.V.; Kubů, M.; Čejka, J. The effect of pore size dimensions in isoreticular zeolites on carbon dioxide adsorption heats. J. CO2 Utili. 2018, 24, 157–163. [Google Scholar] [CrossRef]
- Hosseini, S.; Niaei, A.; Salari, D.; Álvarez-Galván, C.; Fierro, J.L.G. Study of correlation between activity and structural properties of Cu-(Cr, Mn and Co)2 nano mixed oxides in VOC combustion. Ceram. Int. 2014, 40, 6157–6163. [Google Scholar] [CrossRef]

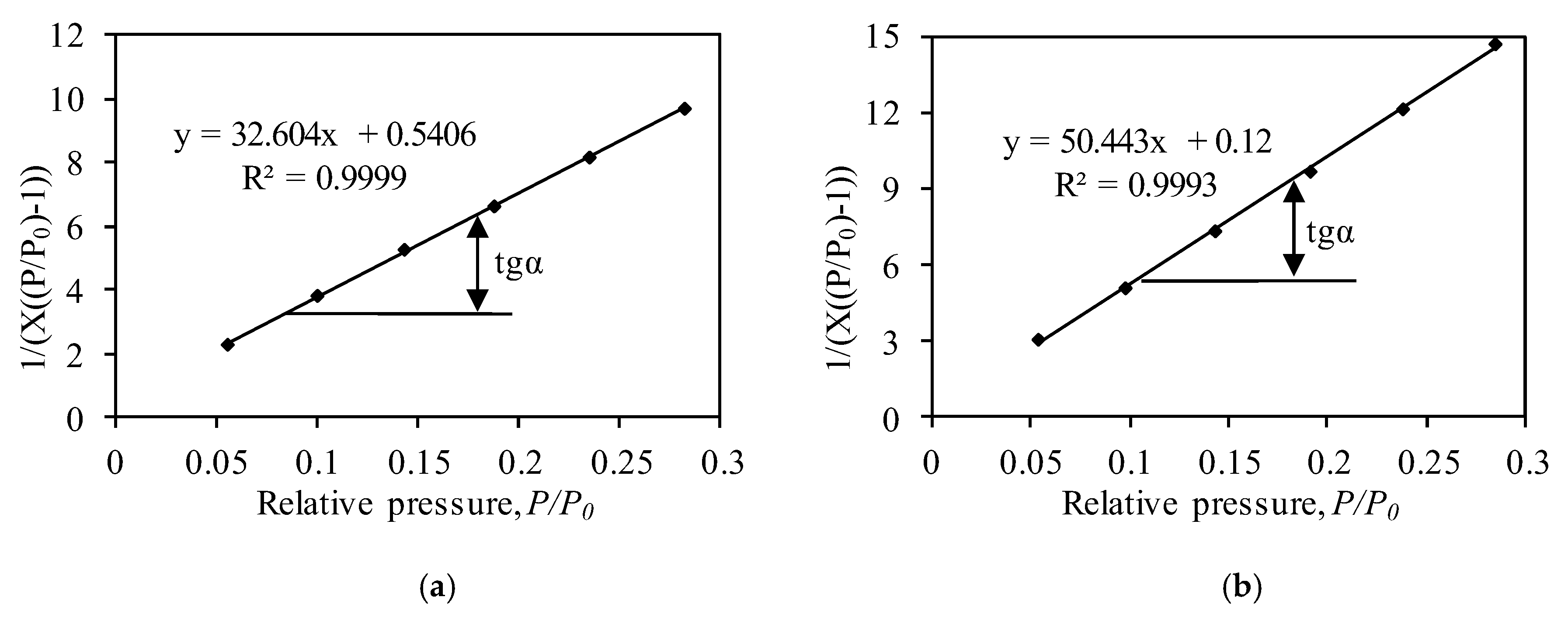
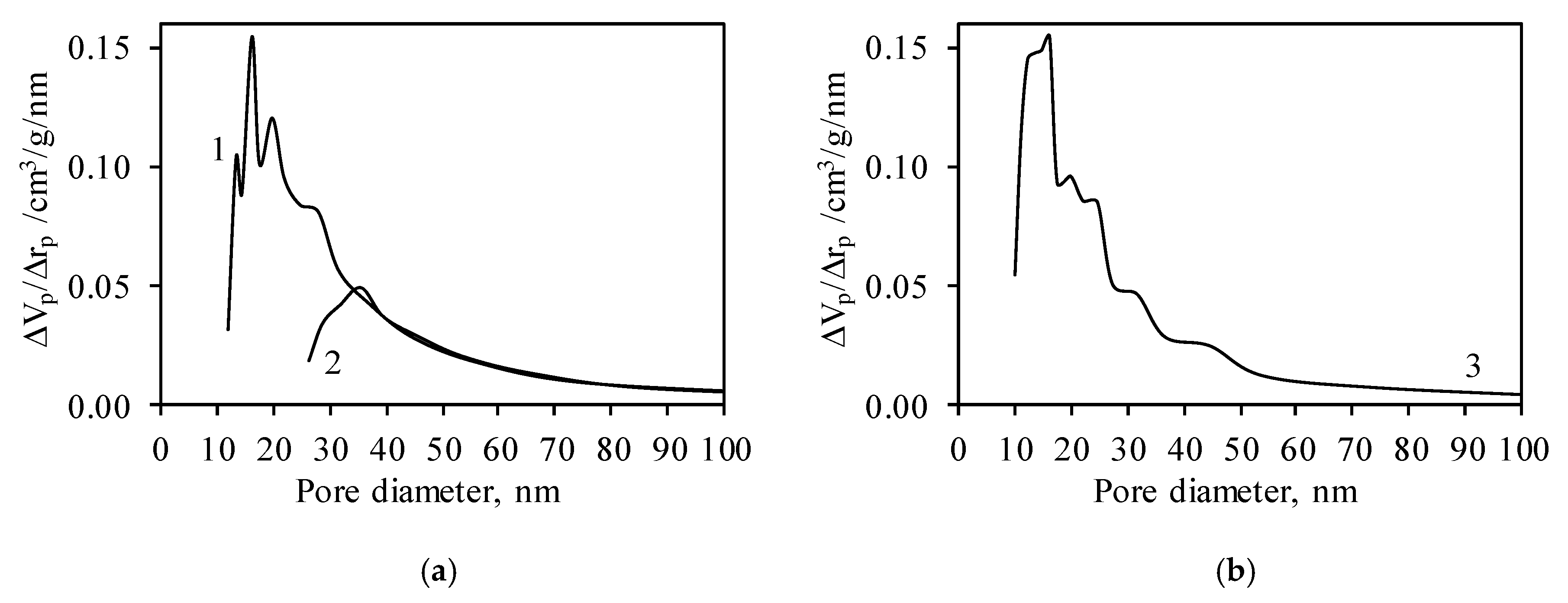
| Sample | BET Equation Constants | Capacity of Mono Layer Xm | SBET, m2/g | CBET Constant | Reliability Coefficient, R2 | |
|---|---|---|---|---|---|---|
| Slope/S = tgα | Intercept/I | |||||
| Synthetic | 32.76 | 0.51 | 0.030 | 104.76 | 65.56 | 0.995 |
| Calcined | 50.44 | 0.12 | 0.020 | 68.92 | 421.30 | 0.995 |
| Sample | SBET, m2/g | Results Obtained by Using the Model of Cylindrical Pores | Results Obtained by Using the Model of Slit-Like Pores | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ΣA, m2/g | |SBET − ΣA|, m2/g | |SBET − ΣA|, % | ΣVP, cm3/g | ΣA, m2/g | |SBET − ΣA|, m2/g | |SBET − ΣA|, % | ΣVP, cm3/g | ||
| Synthetic | 105.14 | 134.46 | 29.32 | 27.89 | 0.320 | 84.46 | 20.68 | 19.67 | 0.278 |
| Calcined | 68.92 | 66.94 | 1.98 | 2.87 | 0.230 | 37.27 | 31.65 | 45.92 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niuniavaite, D.; Baltakys, K.; Dambrauskas, T.; Eisinas, A.; Rubinaite, D.; Jaskunas, A. Microstructure, Thermal Stability, and Catalytic Activity of Compounds Formed in CaO-SiO2-Cr(NO3)3-H2O System. Nanomaterials 2020, 10, 1299. https://doi.org/10.3390/nano10071299
Niuniavaite D, Baltakys K, Dambrauskas T, Eisinas A, Rubinaite D, Jaskunas A. Microstructure, Thermal Stability, and Catalytic Activity of Compounds Formed in CaO-SiO2-Cr(NO3)3-H2O System. Nanomaterials. 2020; 10(7):1299. https://doi.org/10.3390/nano10071299
Chicago/Turabian StyleNiuniavaite, Domante, Kestutis Baltakys, Tadas Dambrauskas, Anatolijus Eisinas, Dovile Rubinaite, and Andrius Jaskunas. 2020. "Microstructure, Thermal Stability, and Catalytic Activity of Compounds Formed in CaO-SiO2-Cr(NO3)3-H2O System" Nanomaterials 10, no. 7: 1299. https://doi.org/10.3390/nano10071299
APA StyleNiuniavaite, D., Baltakys, K., Dambrauskas, T., Eisinas, A., Rubinaite, D., & Jaskunas, A. (2020). Microstructure, Thermal Stability, and Catalytic Activity of Compounds Formed in CaO-SiO2-Cr(NO3)3-H2O System. Nanomaterials, 10(7), 1299. https://doi.org/10.3390/nano10071299









