Biotin-Conjugated Cellulose Nanofibers Prepared via Copper-Catalyzed Alkyne-Azide Cycloaddition (CuAAC) “Click” Chemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
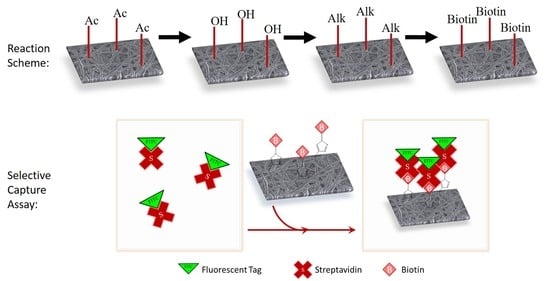
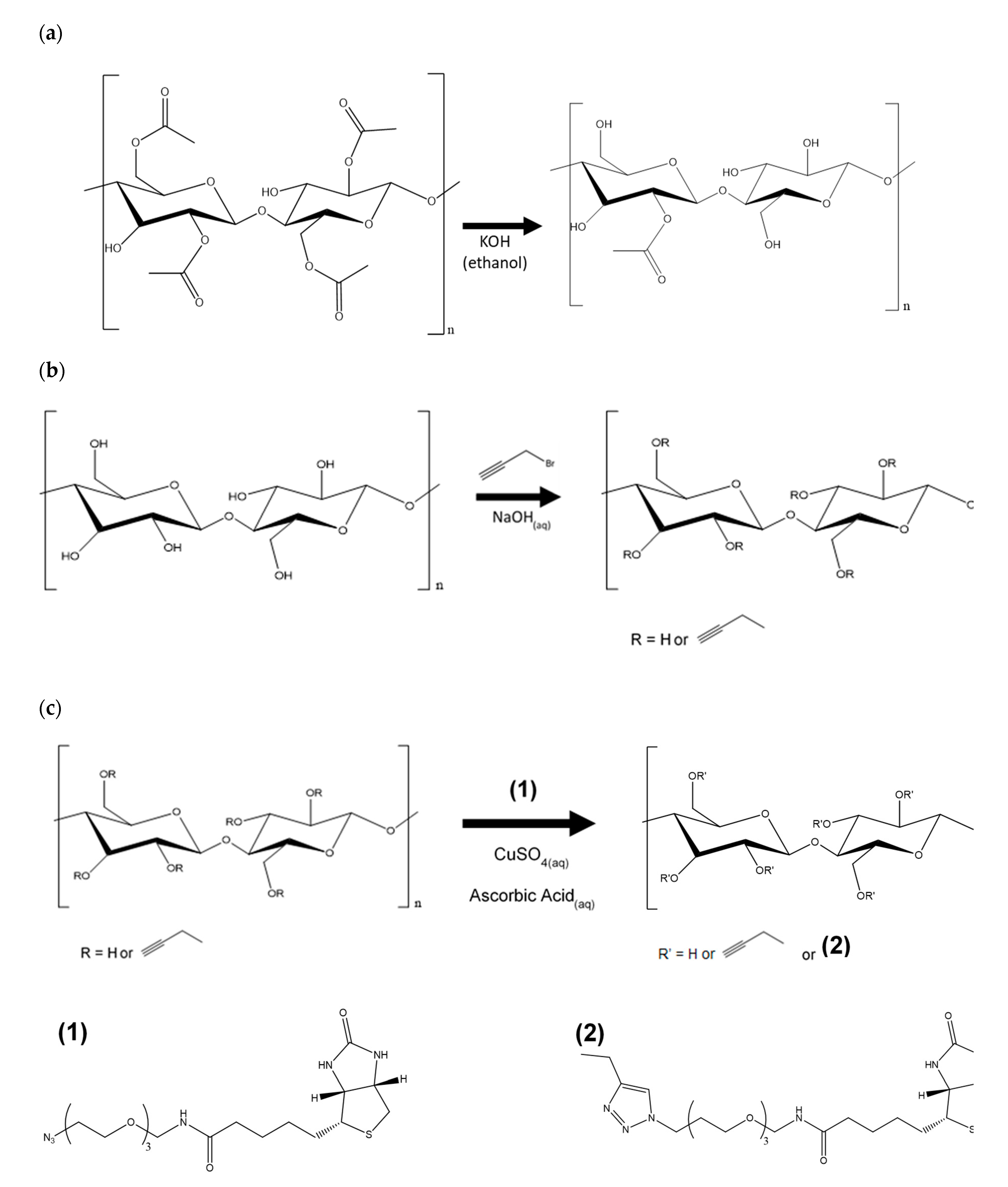
2.3. Regeneration of Cellulose (Scheme 1a)
2.4. Alkyne Substitution (Scheme 1b)
2.5. Click Reaction with Azide-Biotin Conjugate (Scheme 1c)
2.6. Characterization
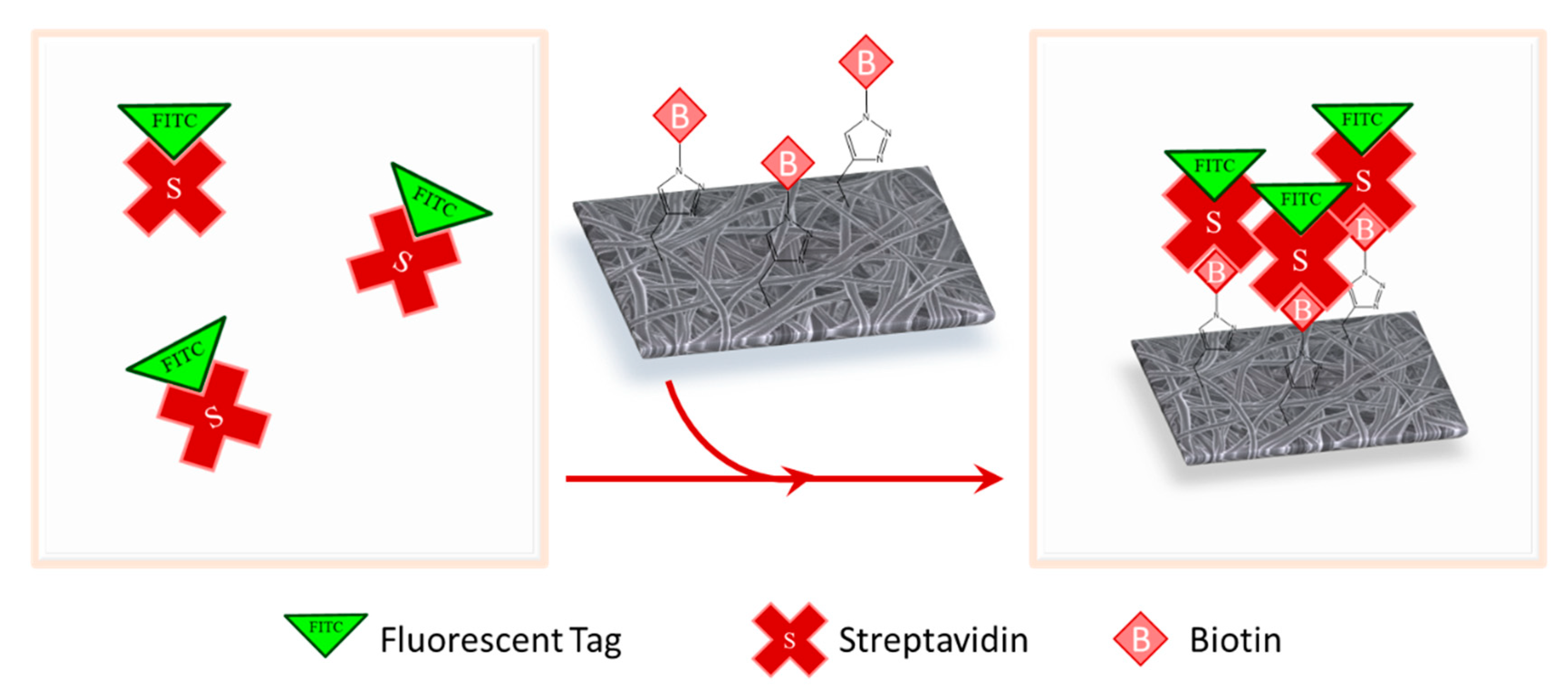
2.7. Streptavidin Binding and Confocal Microscopy
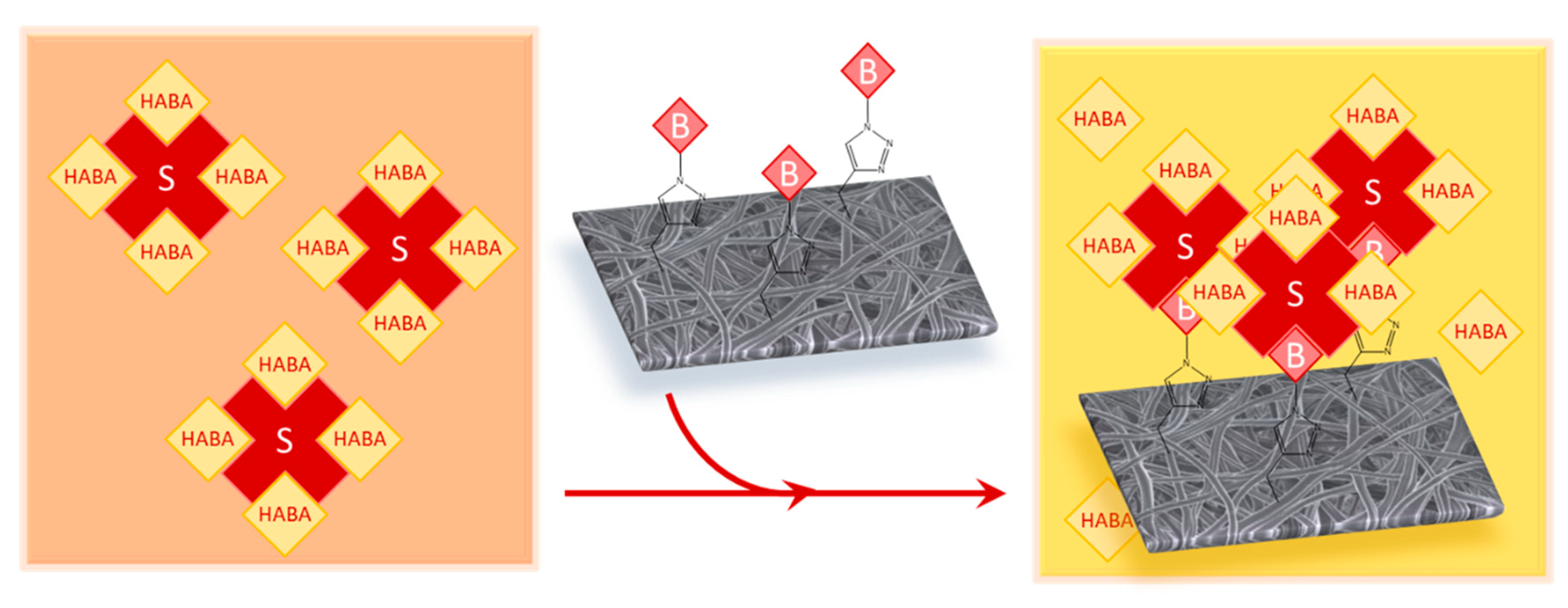
2.8. Surface-Available Biotin Quantification
3. Results
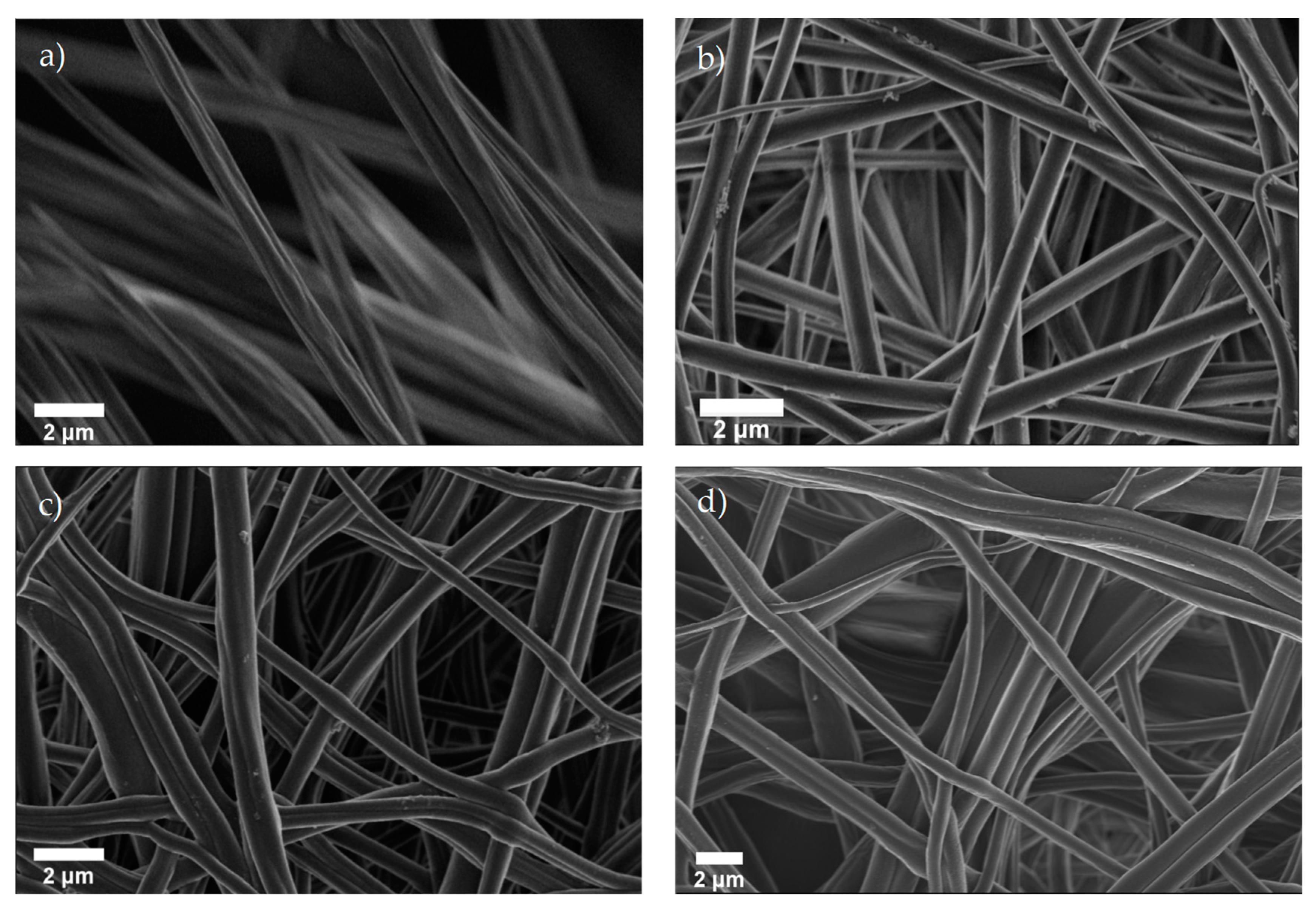
3.1. Morphological Characterization
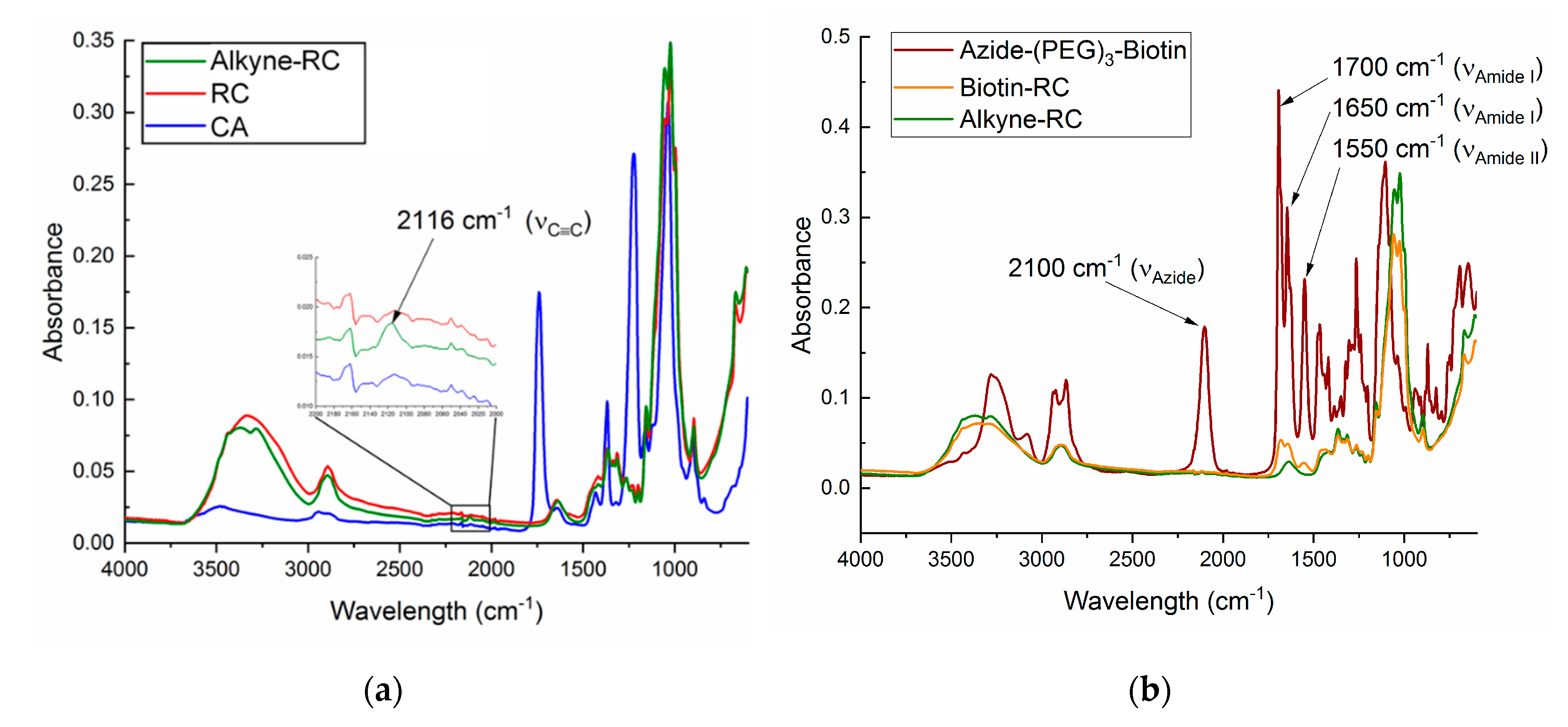
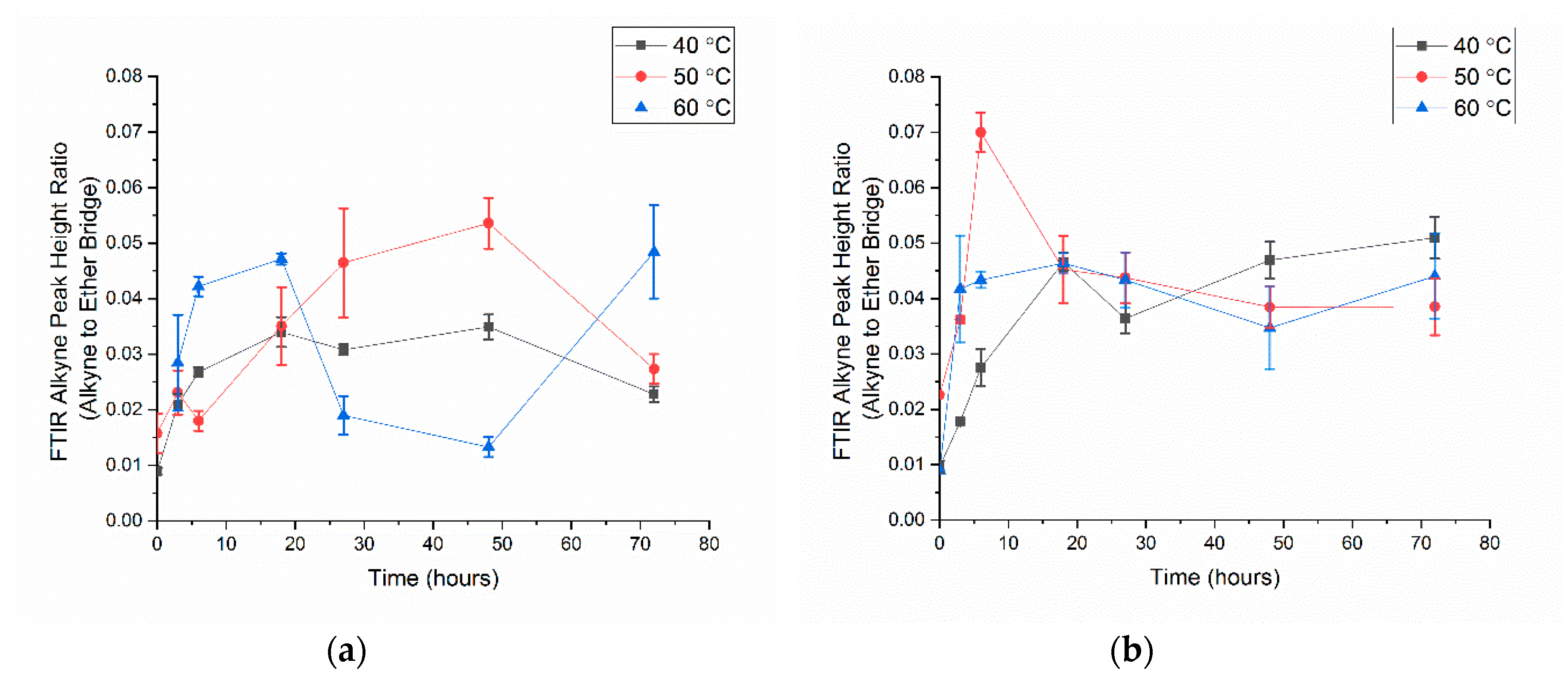
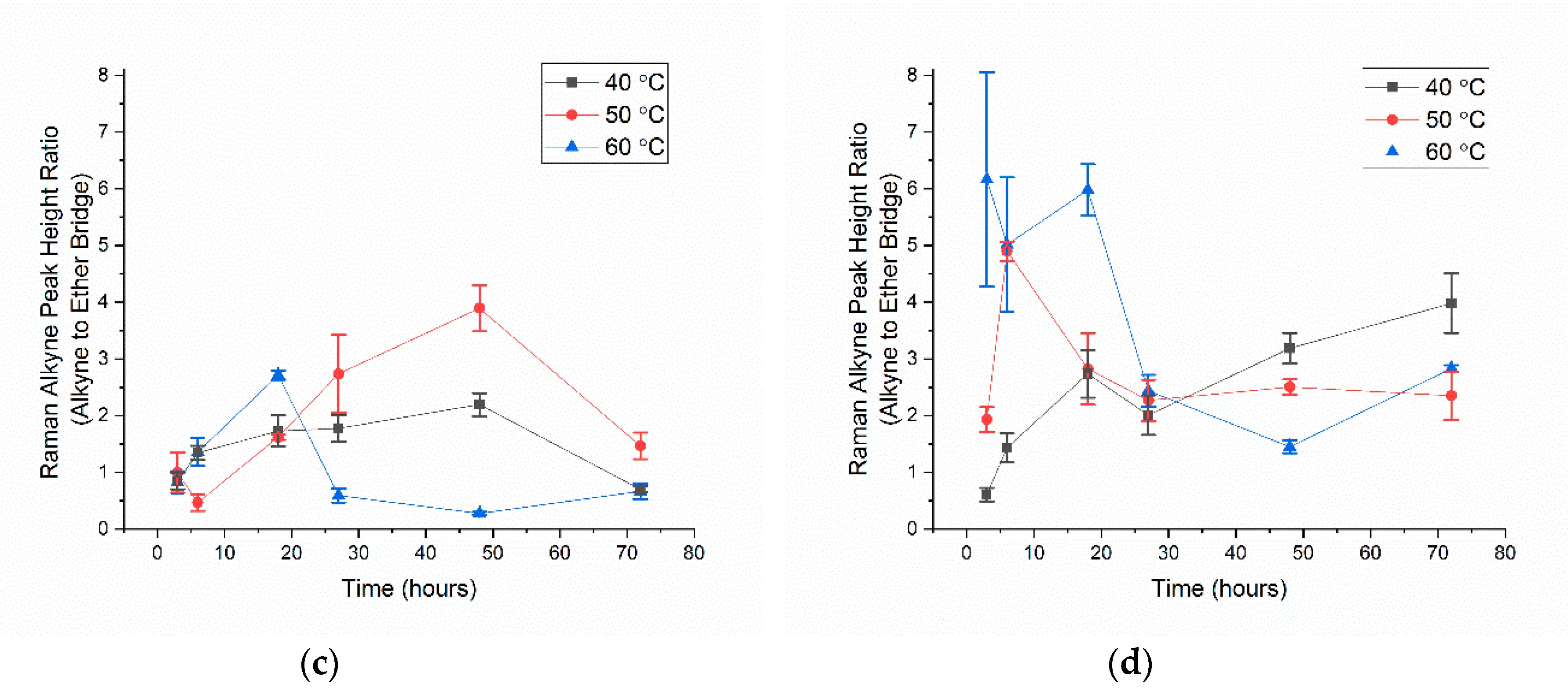
3.2. Alkyne Substitution
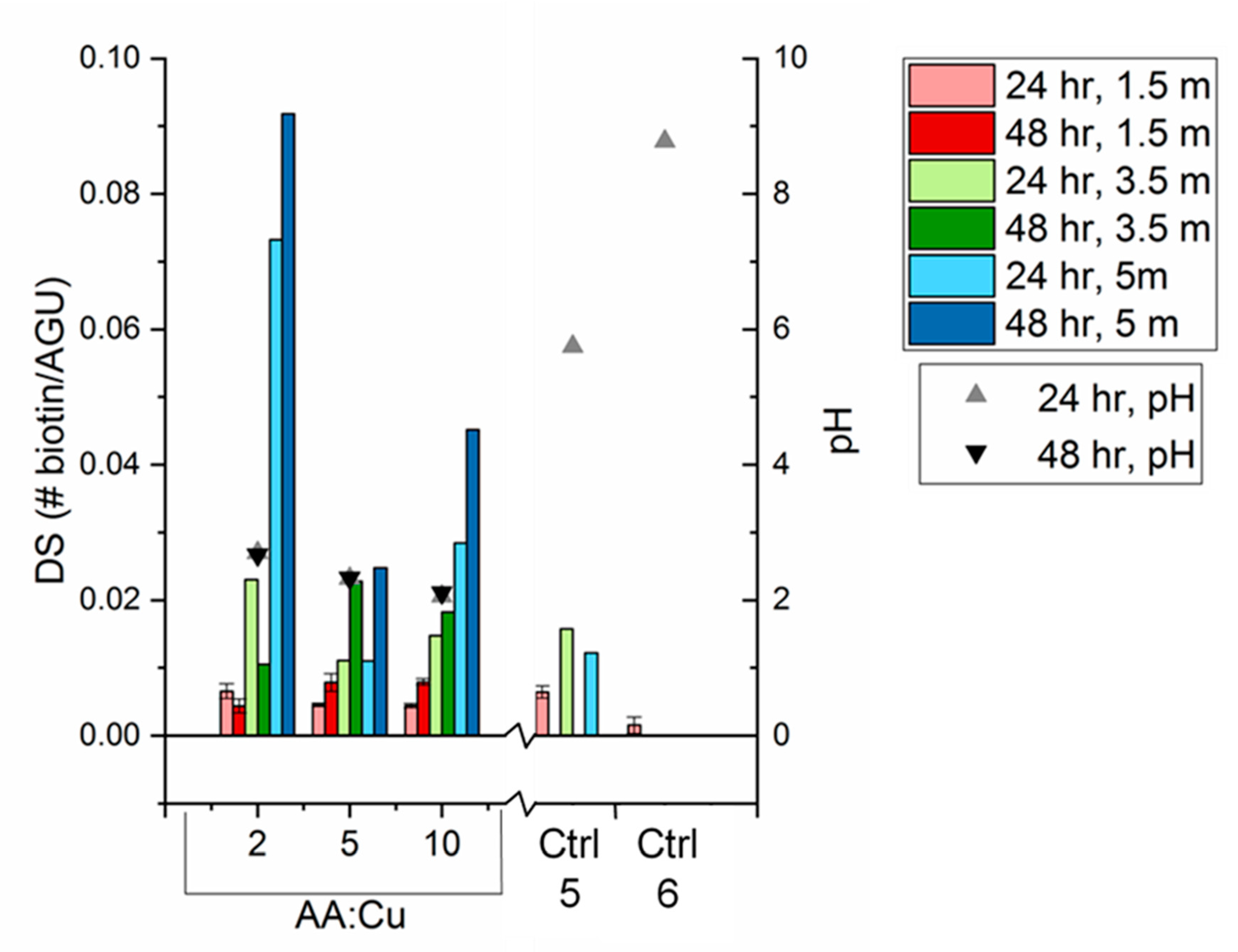
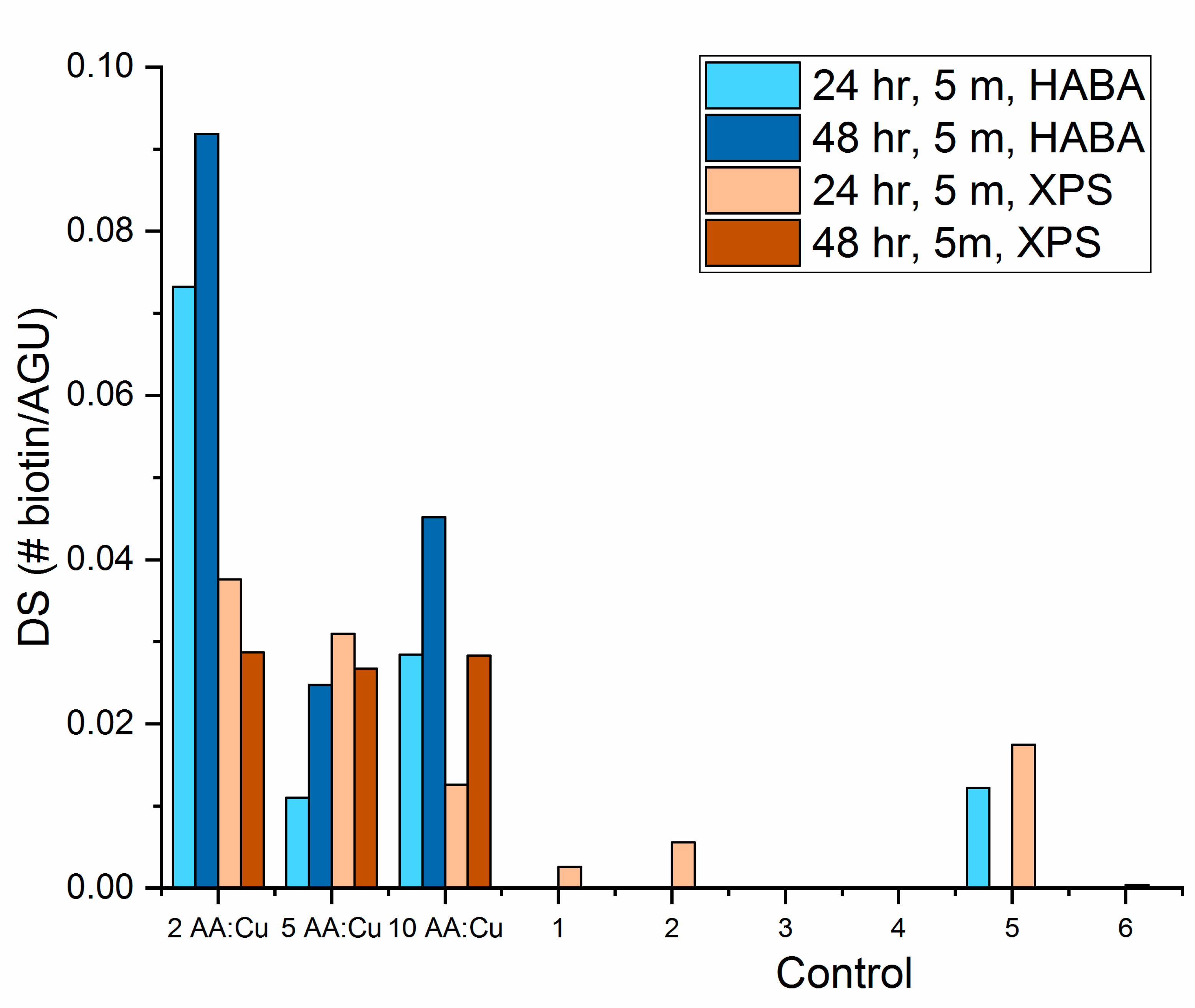
3.3. Biotin Immobilization
4. Conclusions
- (1)
- With all click reaction components, the initial apparent biotin yield was dependent on the ratio of ascorbic acid to copper sulfate, pH, and time.
- (2)
- Click reaction controls confirmed the selectivity and specificity of the reaction; catalysis is possible without a reducing agent, as seen with Control 5 (all click reaction components except ascorbic acid).
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rebelo, R.; Barbosa, A.I.; Caballero, D.; Kwon, I.K.; Oliveira, J.M.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. 3D biosensors in advanced medical diagnostics of high mortality diseases. Biosens. Bioelectron. 2019, 130, 20–39. [Google Scholar] [CrossRef]
- Mahmoudifard, M.; Vossoughi, M.; Soudi, S.; Soleimani, M. Electrospun polyethersolfone nanofibrous membrane as novel platform for protein immobilization in microfluidic systems. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1108–1120. [Google Scholar] [CrossRef]
- Rodriguez-deLuna, S.E.; Moreno-Cortez, I.E.; Garza-Navarro, M.A.; Lucio-Porto, R.; Lopez Pavon, L.; Gonzalez-Gonzalez, V.A. Thermal stability of the immobilization process of horseradish peroxidase in electrospun polymeric nanofibers. J. Appl. Polym. Sci. 2017, 134, 44811. [Google Scholar] [CrossRef]
- Afshari, E.; Mazinani, S.; Ranaei-Siadat, S.-O.; Ghomi, H. Surface modification of polyvinyl alcohol/malonic acid nanofibers by gaseous dielectric barrier discharge plasma for glucose oxidase immobilization. Appl. Surf. Sci. 2016, 385, 349–355. [Google Scholar] [CrossRef]
- Shepherd, L.M.; Gonzalez, E.; Chen, E.X.; Frey, M.W. Increasing Stability of Biotin Functionalized Electrospun Fibers for Biosensor Applications. ACS Appl. Mater. Interfaces 2017, 9, 1968–1974. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.A.; Teo, W.E.; Lim, T.C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Najafi, M.; Frey, M.W. Electrospun Nanofibers for Chemical Separation. Nanomaterials 2020, 10. [Google Scholar] [CrossRef]
- Chen, K.; Chou, W.; Liu, L.; Cui, Y.; Xue, P.; Jia, M. Electrochemical Sensors Fabricated by Electrospinning Technology: An Overview. Sensors 2019, 19. [Google Scholar] [CrossRef]
- Frey, M.W. Electrospinning Cellulose and Cellulose Derivatives. Polym. Rev. 2008, 48, 378–391. [Google Scholar] [CrossRef]
- Chan, L.; Cross, H.F.; She, J.K.; Cavalli, G.; Martins, H.F.P.; Neylon, C. Covalent Attachment of Proteins to Solid Supports and Surfaces via Sortase-Mediated Ligation. PLoS ONE 2007, 2. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. Development and Applications of the Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) as a Bioorthogonal Reaction. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2005–2021. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Berg, R.; Straub, B.F. Advancements in the mechanistic understanding of the copper-catalyzed azide–alkyne cycloaddition. Beilstein J. Org. Chem. 2013, 9, 2715–2750. [Google Scholar] [CrossRef]
- Worrell, B.T.; Malik, J.A.; Fokin, V.V. Direct Evidence of a Dinuclear Copper Intermediate in Cu(I)-Catalyzed Azide-Alkyne Cycloadditions. Science 2013, 340, 457–460. [Google Scholar] [CrossRef]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromol. Rapid Commun. 2020, 41, 1900359. [Google Scholar] [CrossRef]
- Meng, X.; Edgar, K.J. “Click” reactions in polysaccharide modification. Prog. Polym. Sci. 2016, 53, 52–85. [Google Scholar] [CrossRef]
- Elchinger, P.-H.; Faugeras, P.-A.; Boëns, B.; Brouillette, F.; Montplaisir, D.; Zerrouki, R.; Lucas, R. Polysaccharides: The “Click” Chemistry Impact. Polymers 2011, 3, 1607–1651. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, X.; Lu, T.; Jing, X. The immobilization of proteins on biodegradable polymer fibers via click chemistry. Biomaterials 2008, 29, 1118–1126. [Google Scholar] [CrossRef]
- Zheng, J.; Hua, G.; Yu, J.; Lin, F.; Wade, M.B.; Reneker, D.H.; Becker, M.L. Post-electrospinning “triclick” functionalization of degradable polymer nanofibers. ACS Macro Lett. 2015, 4, 207–213. [Google Scholar] [CrossRef]
- An, S.; Jeon, B.; Bae, J.H.; Kim, I.S.; Paeng, K.; Kim, M.; Lee, H. Thiol-based chemistry as versatile routes for the effective functionalization of cellulose nanofibers. Carbohydr. Polym. 2019, 226, 115259. [Google Scholar] [CrossRef] [PubMed]
- Odinolfi, M.T.; Romanato, A.; Bergamaschi, G.; Strada, A.; Sola, L.; Girella, A.; Milanese, C.; Chiari, M.; Gori, A.; Cretich, M. Clickable cellulosic surfaces for peptide-based bioassays. Talanta 2019, 205, 120152. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.; Abdellatif, F.H.H.; Ali, E.A.; Abdelazeem, R.A.; Soliman, A.A.S.; Abou-Zeid, N.Y. Cellulose-based click-scaffolds: Synthesis, characterization and biofabrications. Carbohydr. Polym. 2018, 199, 610–618. [Google Scholar] [CrossRef]
- Celebioglu, A.; Demirci, S.; Uyar, T. Cyclodextrin-grafted electrospun cellulose acetate nanofibers via Click reaction for removal of phenanthrene. Appl. Surf. Sci. 2014, 305, 581–588. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, Y.-L. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci. B Polym. Phys. 2002, 40, 2119–2129. [Google Scholar] [CrossRef]
- Mangiante, G.; Alcouffe, P.; Burdin, B.; Gaborieau, M.; Zeno, E.; Petit-Conil, M.; Bernard, J.; Charlot, A.; Fleury, E. Green Nondegrading Approach to Alkyne-Functionalized Cellulose Fibers and Biohybrids Thereof: Synthesis and Mapping of the Derivatization. Biomacromolecules 2013, 14, 254–263. [Google Scholar] [CrossRef]
- Brotherton, W.S.; Michaels, H.A.; Simmons, J.T.; Clark, R.J.; Dalal, N.S.; Zhu, L. Apparent Copper(II)-Accelerated Azide−Alkyne Cycloaddition. Org. Lett. 2009, 11, 4954–4957. [Google Scholar] [CrossRef]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Ståhl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- Avvakumova, S.; Colombo, M.; Galbiati, E.; Mazzucchelli, S.; Rotem, R.; Prosperi, D. Bioengineered Approaches for Site Orientation of Peptide-Based Ligands of Nanomaterials. In Biomedical Applications of Functionalized Nanomaterials; Elsevier: Amsterdam, the Netherlands, 2018; pp. 139–169. ISBN 978-0-323-50878-0. [Google Scholar]
| Role | Click Molecule | Catalyst | AA:Cu Ratio | Reaction Time (hours) | Confocal Fluorescent Microscopy | |||
|---|---|---|---|---|---|---|---|---|
| Chemical | Alkyne-RC | Azide-Biotin | CuSO4 | Ascorbic Acid | ||||
| Reaction | X | X | X | X | 2, 5, 10 | 24, 48 |  | |
| Control | 1 | X | X | X | - | 24 |  | |
| 2 | X | X | - | 24 |  | |||
| 3 | X | X | X | - | 24 |  | ||
| 4 | X | X | X | - | 24 |  | ||
| 5 | X | X | X | - | 24 |  | ||
| 6 | X | X | - | 24 |  | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodge, K.; Frey, M. Biotin-Conjugated Cellulose Nanofibers Prepared via Copper-Catalyzed Alkyne-Azide Cycloaddition (CuAAC) “Click” Chemistry. Nanomaterials 2020, 10, 1172. https://doi.org/10.3390/nano10061172
Goodge K, Frey M. Biotin-Conjugated Cellulose Nanofibers Prepared via Copper-Catalyzed Alkyne-Azide Cycloaddition (CuAAC) “Click” Chemistry. Nanomaterials. 2020; 10(6):1172. https://doi.org/10.3390/nano10061172
Chicago/Turabian StyleGoodge, Katarina, and Margaret Frey. 2020. "Biotin-Conjugated Cellulose Nanofibers Prepared via Copper-Catalyzed Alkyne-Azide Cycloaddition (CuAAC) “Click” Chemistry" Nanomaterials 10, no. 6: 1172. https://doi.org/10.3390/nano10061172
APA StyleGoodge, K., & Frey, M. (2020). Biotin-Conjugated Cellulose Nanofibers Prepared via Copper-Catalyzed Alkyne-Azide Cycloaddition (CuAAC) “Click” Chemistry. Nanomaterials, 10(6), 1172. https://doi.org/10.3390/nano10061172