Overcoming the Inflammatory Stage of Non-Healing Wounds: In Vitro Mechanism of Action of Negatively Charged Microspheres (NCMs)
Abstract
1. Introduction
2. Materials and Methods
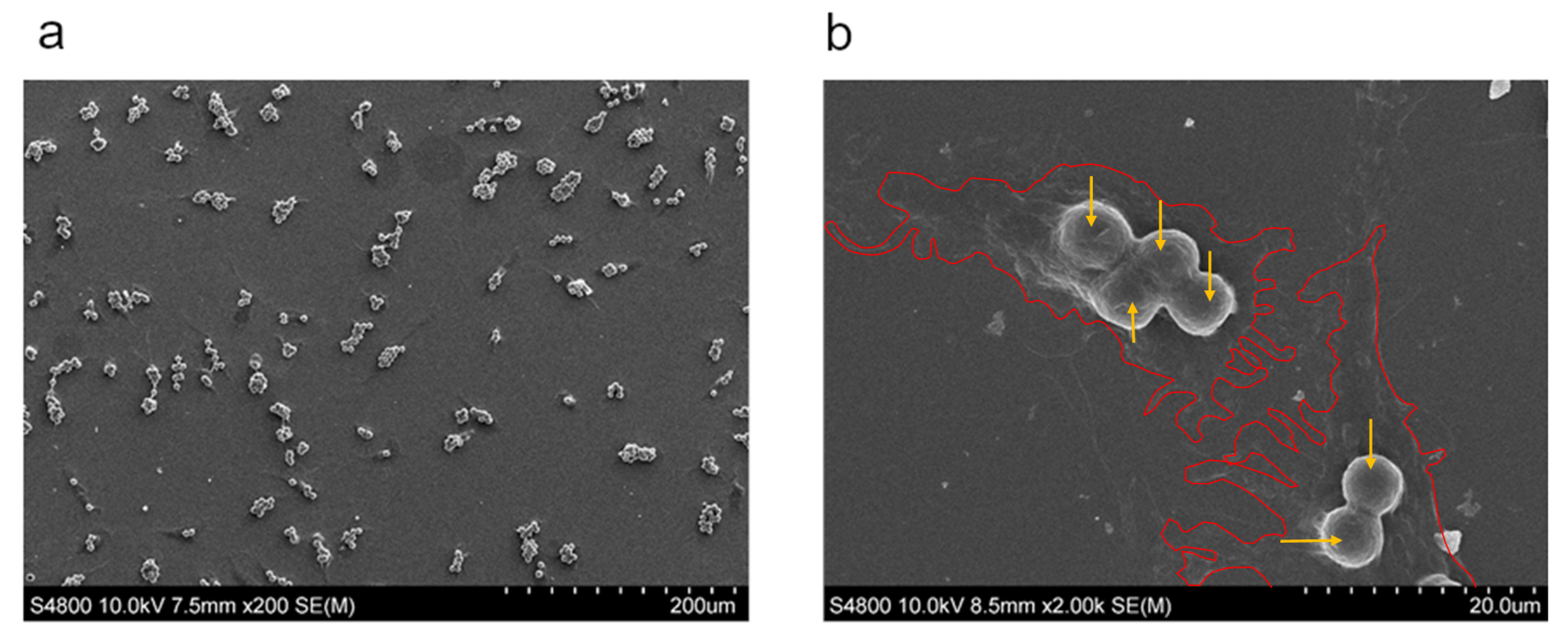
2.1. NCM Samples
2.2. Cell Culture
2.3. Cytotoxicity Assay
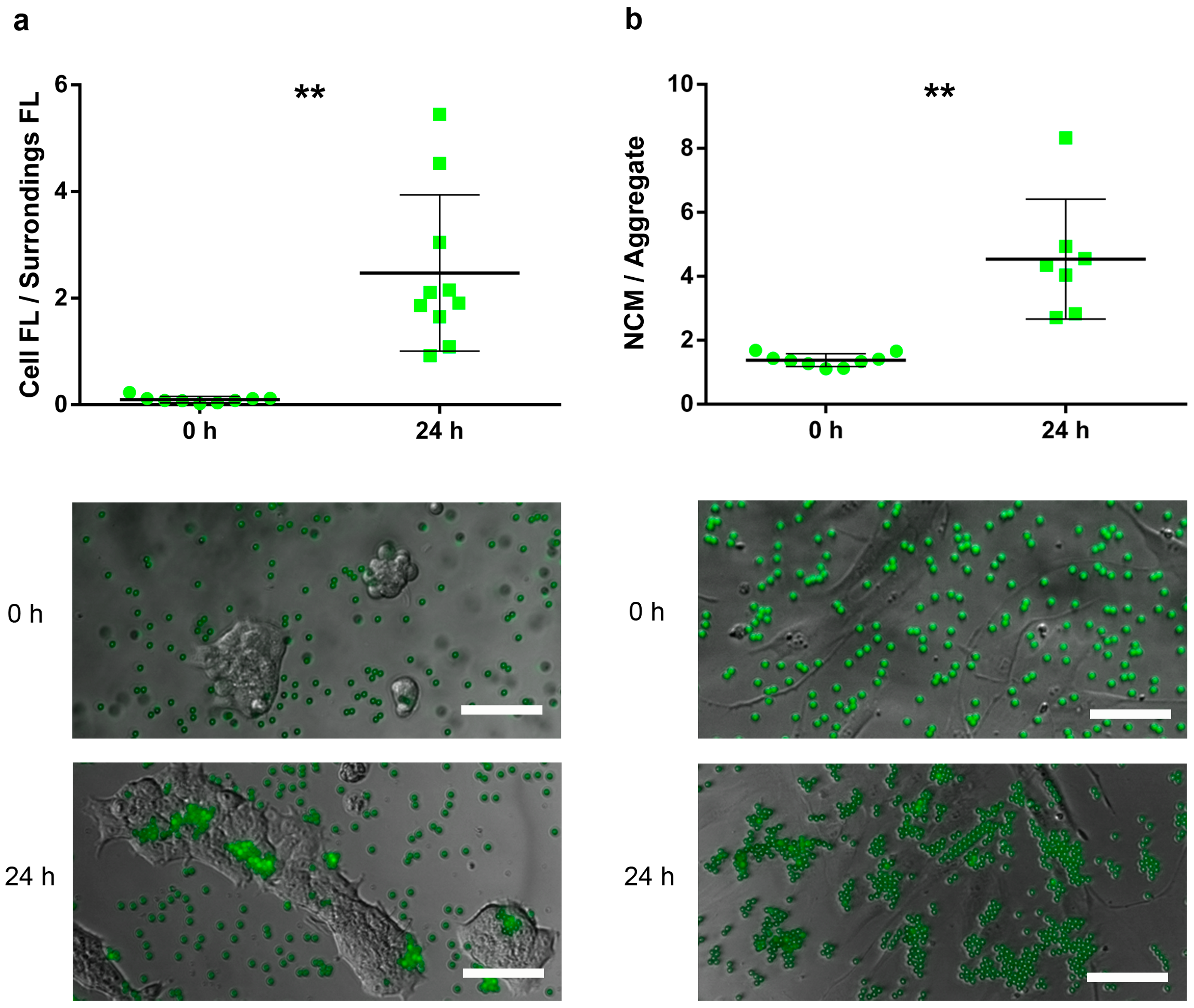
2.4. Attachment Assay
- (i)
- For HaCaT, the attachment capacity of FYG-NCMs was semiquantitatively assessed by calculating the ratio between the fluorescence intensity within the area delimited by a cluster of cells and its bordering area (≈ 50 µm width).
- (ii)
- For HDFa, adhesion of FYG-NCMs was semiquantitatively determined by calculating the particle aggregation factor, namely the average number of FYG-NCMs per aggregate. Briefly, fluorescence intensities obtained from areas with a known number of FYG-NCMs were used to calculate the average fluorescence intensity of a single FYG-NCM (at least 8 fields). Based on these data, the fluorescence intensities of at least 7 blindly taken fields were normalized to find the particle aggregation factor. Only fields with evenly distributed cells were included in the analysis.
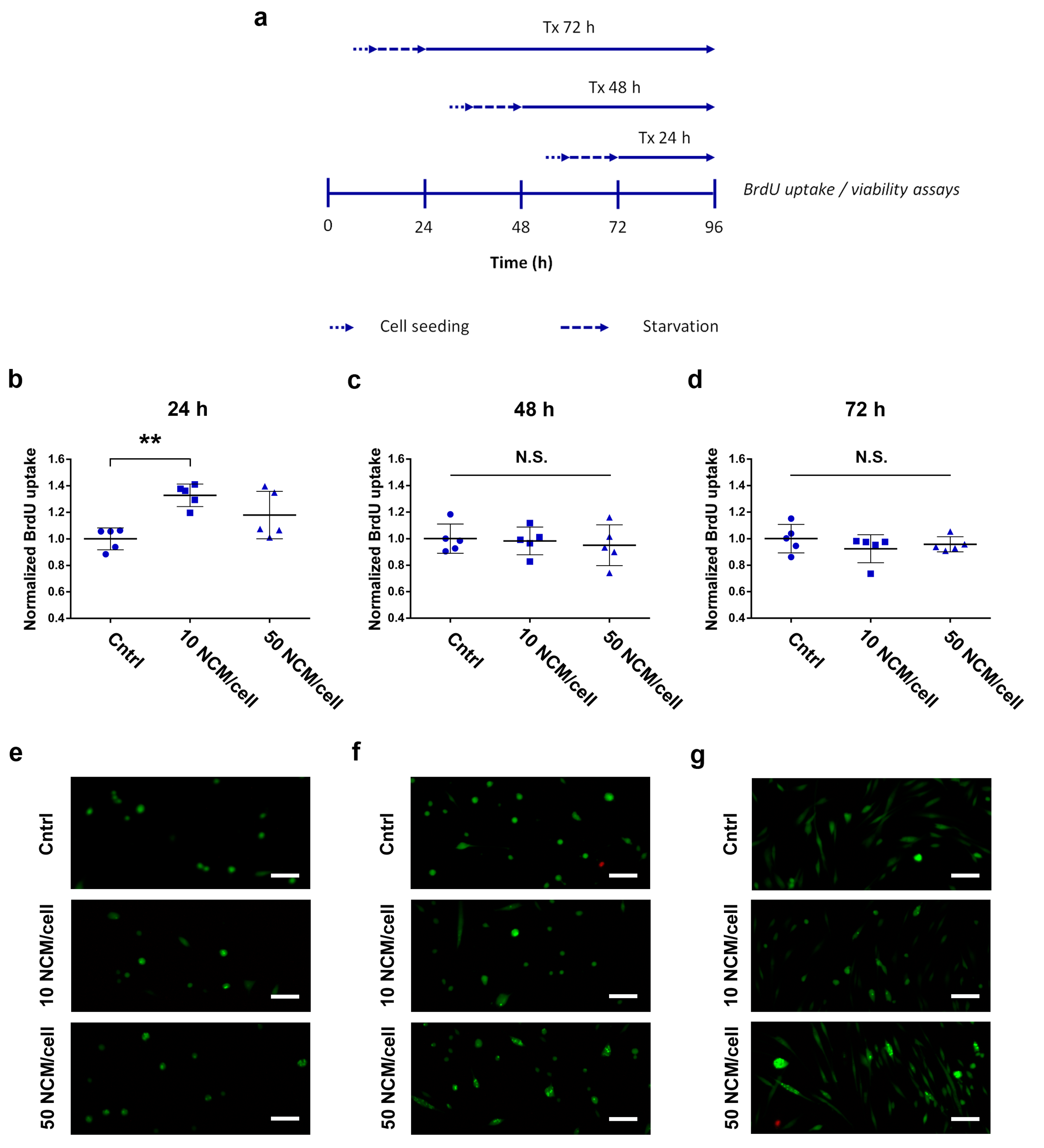
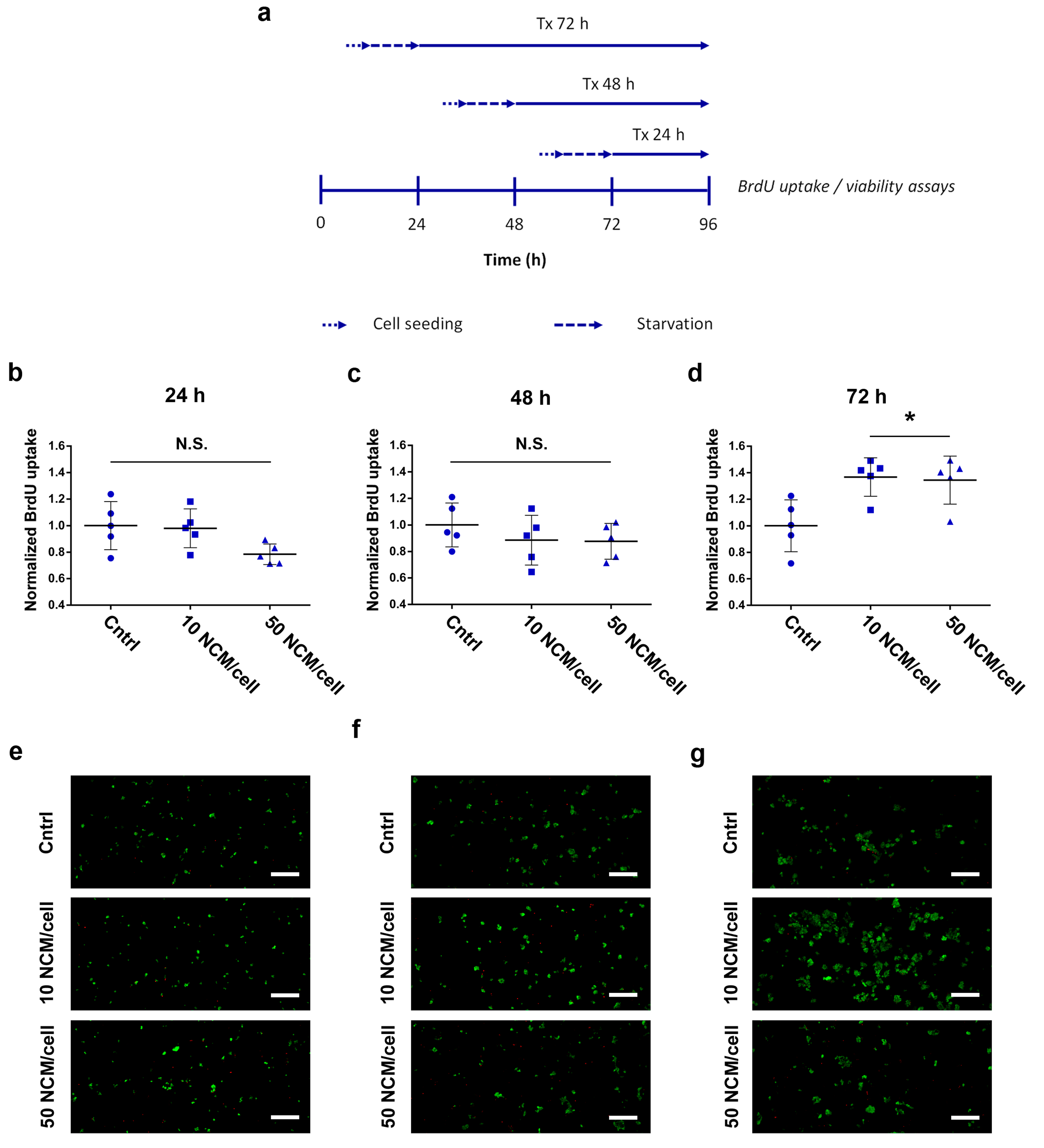
2.5. Proliferation and Viability Assays
2.6. Macrophage Phagocytosis Determination
2.7. Macrophage Polarization Assay
2.8. Statistical Analysis
3. Results
3.1. Cytotoxicity Study
3.2. Attachment Assay
3.3. Proliferation and Viability Assays
3.4. Phagocytosis Determination
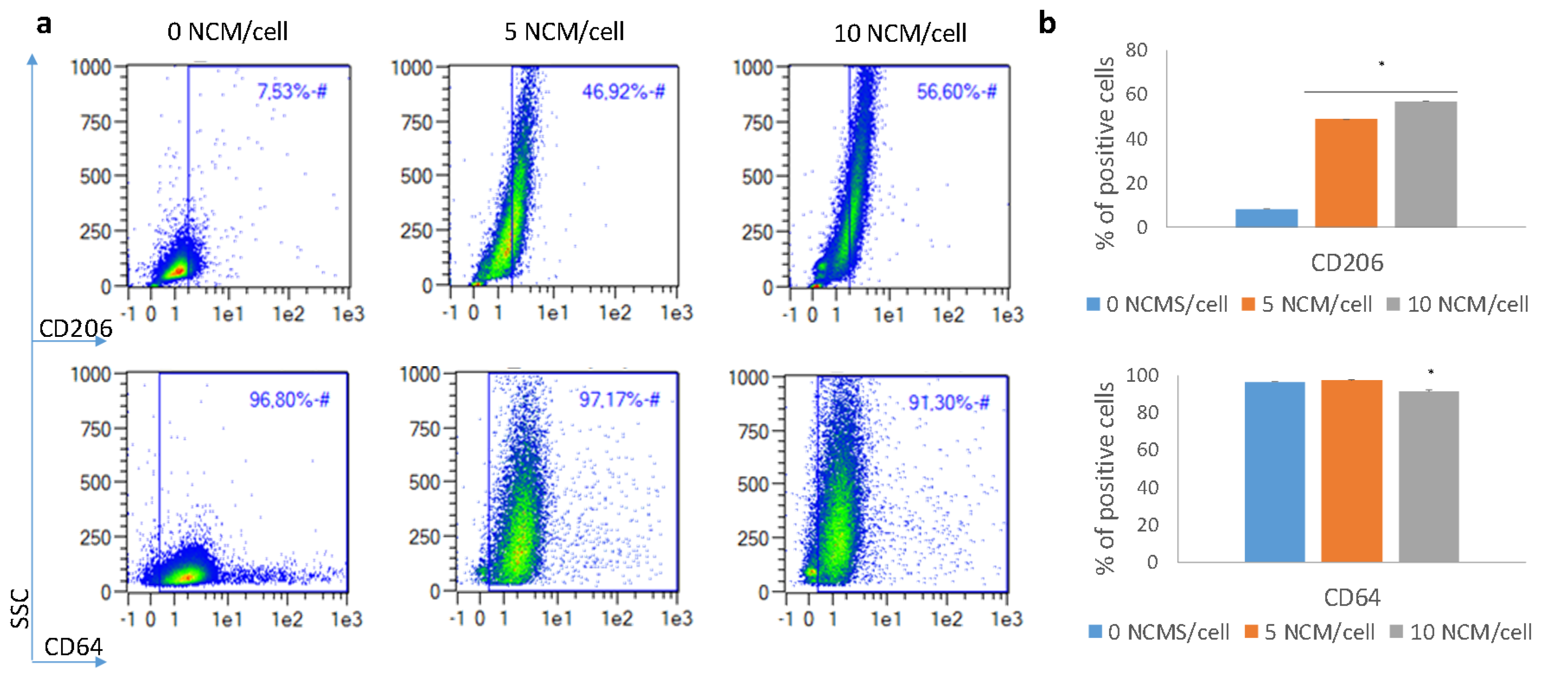
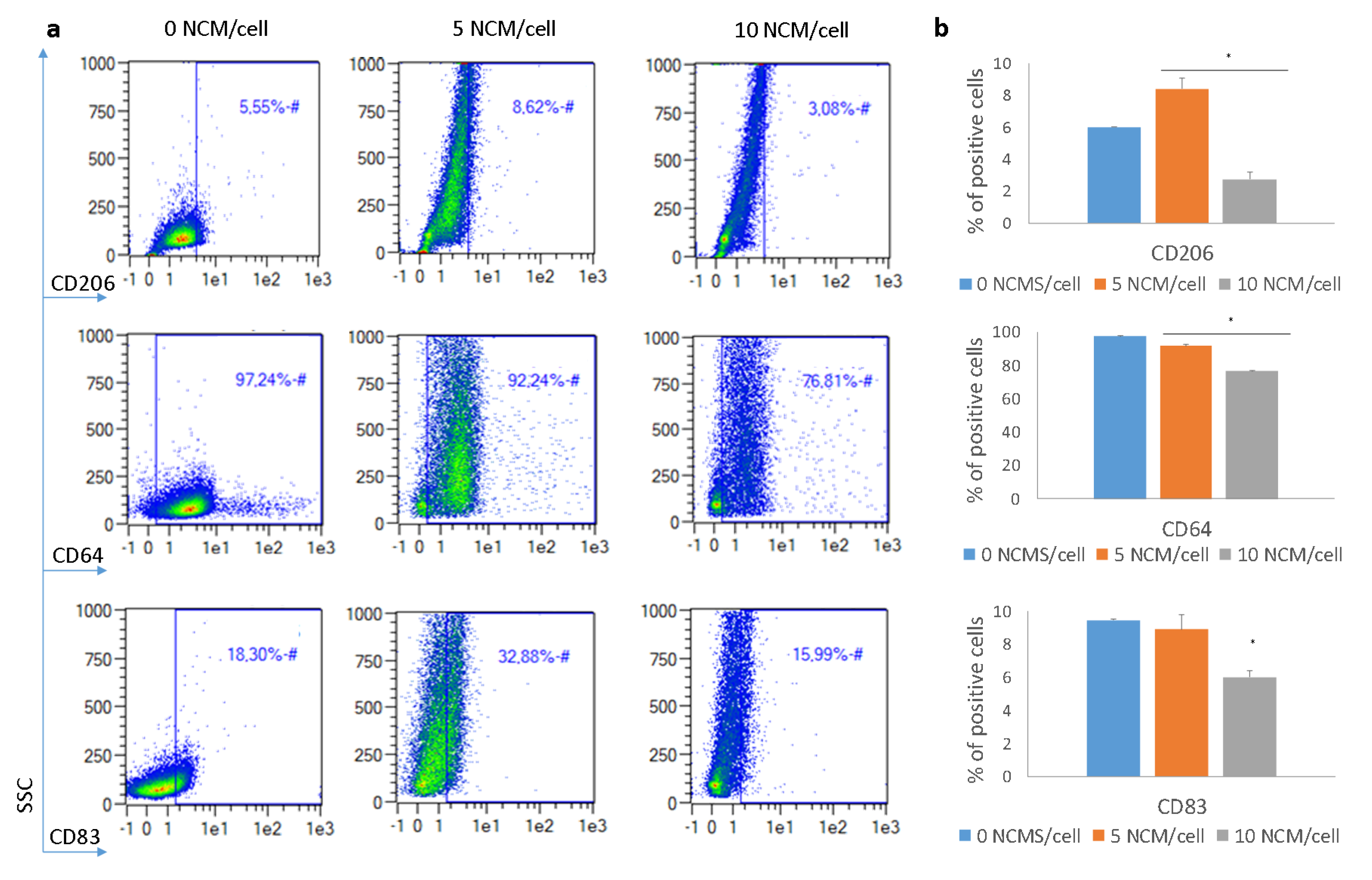
3.5. Polarization Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Heher, P.; Muhleder, S.; Mittermayr, R.; Redl, H.; Slezak, P. Fibrin-based delivery strategies for acute and chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.E.; Gerecht, S. Engineered biopolymeric scaffolds for chronic wound healing. Front. Physiol. 2016, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.; Okai, B.; Hoare, J.I.; Erwig, L.P.; Wilson, H.M. SOCS3 is a modulator of human macrophage phagocytosis. J. Leukoc. Biol. 2016, 100, 771–780. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, J.; Ma, Z.; Iwashina, T.; Tredget, E.E. Systemic depletion of macrophages in the subacute phase of wound healing reduces hypertrophic scar formation. Wound Repair Regen. 2016, 24, 644–656. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. (Maywood) 2016, 241, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, W.G.; Nakayama, Y.; Matsuda, T.; Colton, E.; Ziats, N.P.; Anderson, J.M. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine 2002, 18, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Govrin, J.; Leonid, K.; Luger, E.; Tamir, J.; Zeilig, G.; Shafir, R. New method for treating hard-to-heal wounds: Clinical experience with charged polystyrene microspheres. Wounds UK 2010, 6, 52–61. [Google Scholar]
- Lazaro-Martinez, J.L.; Garcia-Alvarez, Y.; Alvaro-Alfonso, F.J.; Garcia-Morales, E.; Sanz-Corbalan, I.; Molines-Barroso, R.J. Hard-to-heal diabetic foot ulcers treated using negatively charged polystyrene microspheres: A prospective case series. J. Wound Care 2019, 28, 104–109. [Google Scholar] [CrossRef]
- Weissman, O.; Winkler, E.; Teot, L.; Remer, E.; Faber, N.; Bank, J.; Hundeshagen, G.; Zilinsky, I.; Haik, J. Treatment of wounds following breast reduction and mastopexy with subsequent wound dehiscence with charged polystyrene microspheres. Wounds 2014, 26, 37–42. [Google Scholar]
- Mobed-Miremadi, M.; Grandio, D.; Kunkel, J. Polystyrene-based wound healing systems. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 309–334. [Google Scholar]
- Shoham, Y.; Kogan, L.; Weiss, J.; Tamir, E.; Krieger, Y.; Barnea, Y.; Regev, E.; Vigoda, D.; Haikin, N.; Inbal, A.; et al. Wound ’dechronification’ with negatively-charged polystyrene microspheres: A double-blind RCT. J. Wound Care 2013, 22, 144–155. [Google Scholar] [CrossRef]
- Feng, Y.; Borrelli, M.; Meyer-Ter-Vehn, T.; Reichl, S.; Schrader, S.; Geerling, G. Epithelial wound healing on keratin film, amniotic membrane and polystyrene in vitro. Curr. Eye Res. 2014, 39, 561–570. [Google Scholar] [CrossRef]
- Drukala, J.; Bandura, L.; Cieslik, K.; Korohoda, W. Locomotion of human skin keratinocytes on polystyrene, fibrin, and collagen substrata and its modification by cell-to-cell contacts. Cell Transplant. 2001, 10, 765–771. [Google Scholar] [CrossRef]
- Sands, R.W.; Mooney, D.J. Polymers to direct cell fate by controlling the microenvironment. Curr. Opin. Biotechnol. 2007, 18, 448–453. [Google Scholar] [CrossRef][Green Version]
- Khan, S.; Ul-Islam, M.; Ikram, M.; Islam, S.U.; Ullah, M.W.; Israr, M.; Jang, J.H.; Yoon, S.; Park, J.K. Preparation and structural characterization of surface modified microporous bacterial cellulose scaffolds: A potential material for skin regeneration applications in vitro and in vivo. Int. J. Biol. Macromol. 2018, 117, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.; Luo, G.; He, W.; Xu, K.; Xu, R.; Lei, Q.; Tan, J.; Wu, J.; Xing, M. In-situ-generated vasoactive intestinal peptide loaded microspheres in mussel-inspired polycaprolactone nanosheets creating spatiotemporal releasing microenvironment to promote wound healing and angiogenesis. ACS Appl. Mater. Interfaces 2016, 8, 7411–7421. [Google Scholar] [CrossRef] [PubMed]
- Cereceres, S.; Touchet, T.; Browning, M.B.; Smith, C.; Rivera, J.; Höök, M.; Whitfield-Cargile, C.; Russell, B.; Cosgriff-Hernandez, E. Chronic wound dressings based on collagen-mimetic proteins. Adv. Wound Care (New Rochelle) 2015, 4, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Garate, A.; Santos, E.; Pedraz, J.L.; Hernandez, R.M.; Orive, G. Evaluation of different RGD ligand densities in the development of cell-based drug delivery systems. J. Drug Target. 2015, 23, 808–812. [Google Scholar] [CrossRef]
- Neff, J.A.; Tresco, P.A.; Cadwell, K.D. Surface modification for controlled studies of cell-ligand interactions. Biomaterials 1999, 20, 2377–2393. [Google Scholar] [CrossRef]
- Santos, E.; Garate, A.; Pedraz, J.L.; Orive, G.; Hernandez, R.M. The synergistic effects of the RGD density and the microenvironment on the behavior of encapsulated cells: In vitro and in vivo direct comparative study. J. Biomed. Mater. Res. 2014, 102, 3965–3972. [Google Scholar] [CrossRef]
- Comisar, W.A.; Kazmers, N.H.; Mooney, D.J.; Linderman, J.J. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: A combined computational and experimental approach. Biomaterials 2007, 28, 4409–4417. [Google Scholar] [CrossRef]
- Ghadi, R.; Jain, A.; Khan, W.; Domb, A.J. Microparticulate polymers and hydrogels for wound healing. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 203–225. [Google Scholar]
- Khatua, D.; Kwak, B.; Shin, K.; Song, J.M.; Kim, J.S.; Choi, J.H. Influence of charge densities of randomly sulfonated polystyrene surfaces on cell attachment and proliferation. J. Nanosci. Nanotechnol. 2011, 11, 4227–4230. [Google Scholar] [CrossRef]
- Carré, A.; Lacarrière, V. How substrate properties control cell adhesion. A physical–chemical approach. J. Adhes. Sci. Technol. 2010, 24, 815–830. [Google Scholar] [CrossRef]
- Gonzalez-Pujana, A.; Santos-Vizcaino, E.; Garcia-Hernando, M.; Hernaez-Estrada, B.; de Pancorbo, M.M.; Benito-Lopez, F.; Igartua, M.; Basabe-Desmonts, L.; Hernandez, R.M. Extracellular matrix protein microarray-based biosensor with single cell resolution: Integrin profiling and characterization of cell-biomaterial interactions. Sens. Actuators B Chem. 2019, 299, 126954–126965. [Google Scholar] [CrossRef]
- Aiyelabegan, H.T.; Sadroddiny, E. Fundamentals of protein and cell interactions in biomaterials. Biomed. Pharmacother. 2017, 88, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Yoshikawa, C.; Sakakibara, K. Characterization of initial cell adhesion on charged polymer substrates in serum-containing and serum-free media. Langmuir 2018, 34, 4043–4051. [Google Scholar] [CrossRef] [PubMed]
- Chellat, F.; Merhi, Y.; Moreau, A.; Yahia, L. Therapeutic potential of nanoparticulate systems for macrophage targeting. Biomaterials 2005, 26, 7260–7275. [Google Scholar] [CrossRef] [PubMed]
- Glim, J.E.; Niessen, F.B.; Everts, V.; van Egmond, M.; Beelen, R.H. Platelet derived growth factor-CC secreted by M2 macrophages induces alpha-smooth muscle actin expression by dermal and gingival fibroblasts. Immunobiology 2013, 218, 924–929. [Google Scholar] [CrossRef]
- Prechtel, A.T.; Steinkasserer, A. CD83: An update on functions and prospects of the maturation marker of dendritic cells. Arch. Dermatol. Res. 2007, 299, 59–69. [Google Scholar] [CrossRef]
- Guest, J.F.; Sladkevicius, E.; Panca, M. Cost-effectiveness of using Polyheal compared with surgery in the management of chronic wounds with exposed bones and/or tendons due to trauma in France, Germany and the UK. Int. Wound J. 2015, 12, 70–82. [Google Scholar] [CrossRef]
- Ngobili, T.A.; Daniele, M.A. Nanoparticles and direct immunosuppression. Exp. Biol. Med. 2016, 241, 1064–1073. [Google Scholar] [CrossRef]
- Gan, J.; Liu, C.; Li, H.; Wang, S.; Wang, Z.; Kang, Z.; Huang, Z.; Zhang, J.; Wang, C.; Lv, D.; et al. Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials 2019, 219. [Google Scholar] [CrossRef]
- Nosenko, M.A.; Moysenovich, A.M.; Zvartsev, R.V.; Arkhipova, A.Y.; Zhdanova, A.S.; Agapov, I.I.; Vasilieva, T.V.; Bogush, V.G.; Debabov, V.G.; Nedospasov, S.A.; et al. Novel biodegradable polymeric microparticles facilitate scarless wound healing by promoting re-epithelialization and inhibiting fibrosis. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Kotwal, G.J.; Chien, S. Macrophage differentiation in normal and accelerated wound Healing. Results Probl. Cell Differ. 2017, 62, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Ariganello, M.B.; Girard-Lauziere, J.; Hoemann, C.D. Biodegradable chitosan microparticles induce delayed STAT-1 activation and lead to distinct cytokine responses in differentially polarized human macrophages in vitro. Acta Biomater. 2015, 12, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hoppstädter, J.; Seif, M.; Dembek, A.; Cavelius, C.; Huwer, H.; Kraegeloh, A.; Kiemer, A.K. M2 polarization enhances silica nanoparticle uptake by macrophages. Front. Pharmacol. 2015, 6, 55. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Vizcaino, E.; Salvador, A.; Vairo, C.; Igartua, M.; Hernandez, R.M.; Correa, L.; Villullas, S.; Gainza, G. Overcoming the Inflammatory Stage of Non-Healing Wounds: In Vitro Mechanism of Action of Negatively Charged Microspheres (NCMs). Nanomaterials 2020, 10, 1108. https://doi.org/10.3390/nano10061108
Santos-Vizcaino E, Salvador A, Vairo C, Igartua M, Hernandez RM, Correa L, Villullas S, Gainza G. Overcoming the Inflammatory Stage of Non-Healing Wounds: In Vitro Mechanism of Action of Negatively Charged Microspheres (NCMs). Nanomaterials. 2020; 10(6):1108. https://doi.org/10.3390/nano10061108
Chicago/Turabian StyleSantos-Vizcaino, Edorta, Aiala Salvador, Claudia Vairo, Manoli Igartua, Rosa Maria Hernandez, Luis Correa, Silvia Villullas, and Garazi Gainza. 2020. "Overcoming the Inflammatory Stage of Non-Healing Wounds: In Vitro Mechanism of Action of Negatively Charged Microspheres (NCMs)" Nanomaterials 10, no. 6: 1108. https://doi.org/10.3390/nano10061108
APA StyleSantos-Vizcaino, E., Salvador, A., Vairo, C., Igartua, M., Hernandez, R. M., Correa, L., Villullas, S., & Gainza, G. (2020). Overcoming the Inflammatory Stage of Non-Healing Wounds: In Vitro Mechanism of Action of Negatively Charged Microspheres (NCMs). Nanomaterials, 10(6), 1108. https://doi.org/10.3390/nano10061108




