Nanomaterials and Nanotechnology-Associated Innovations against Viral Infections with a Focus on Coronaviruses
Abstract
1. Introduction
2. Nanotechnology against Viral Infections with Focus on CoVs
2.1. Nano-Based Advances for Viral Detection
Detection of SARS-CoV-2: Innovative Nano-Based Discoveries
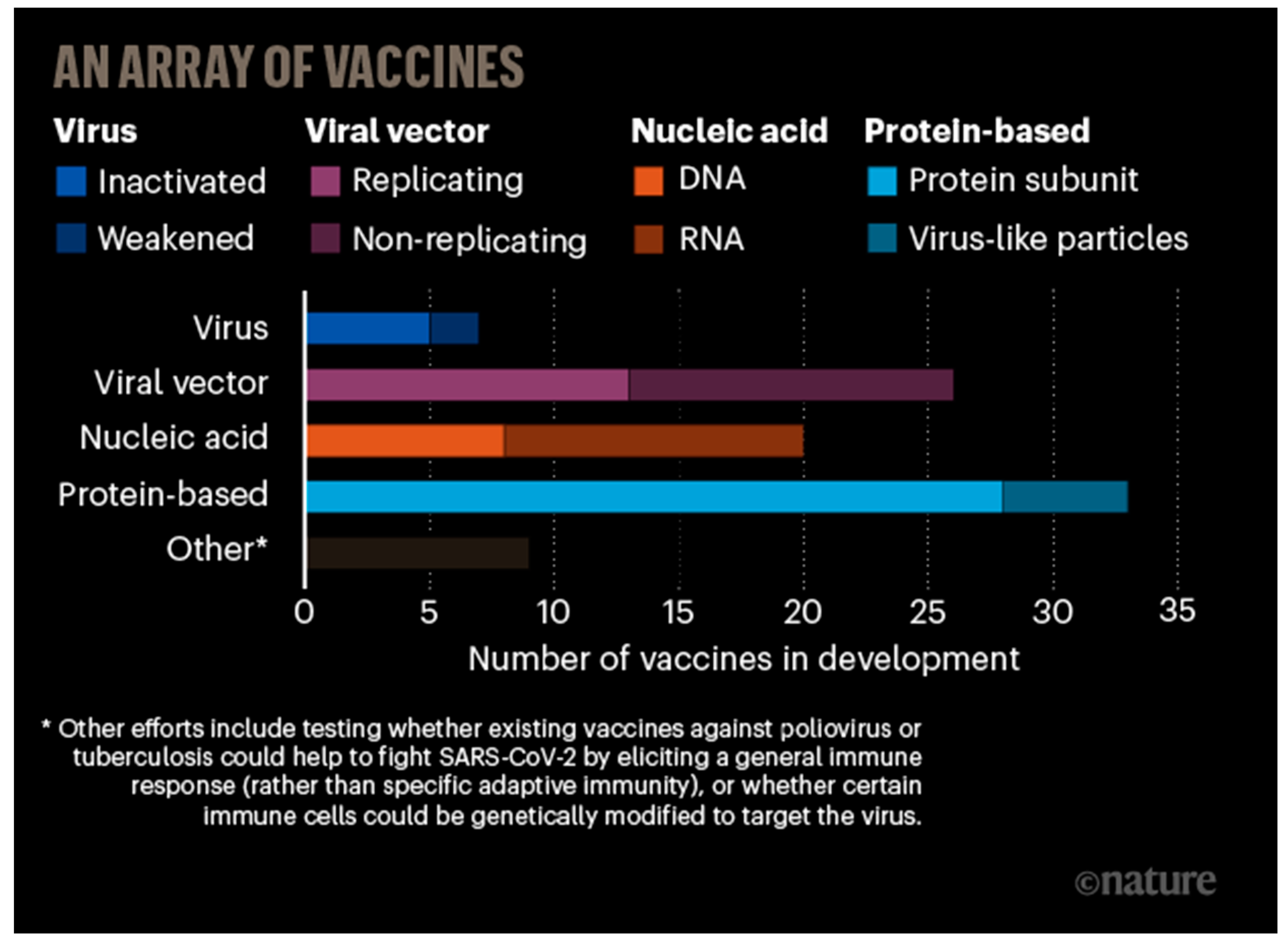
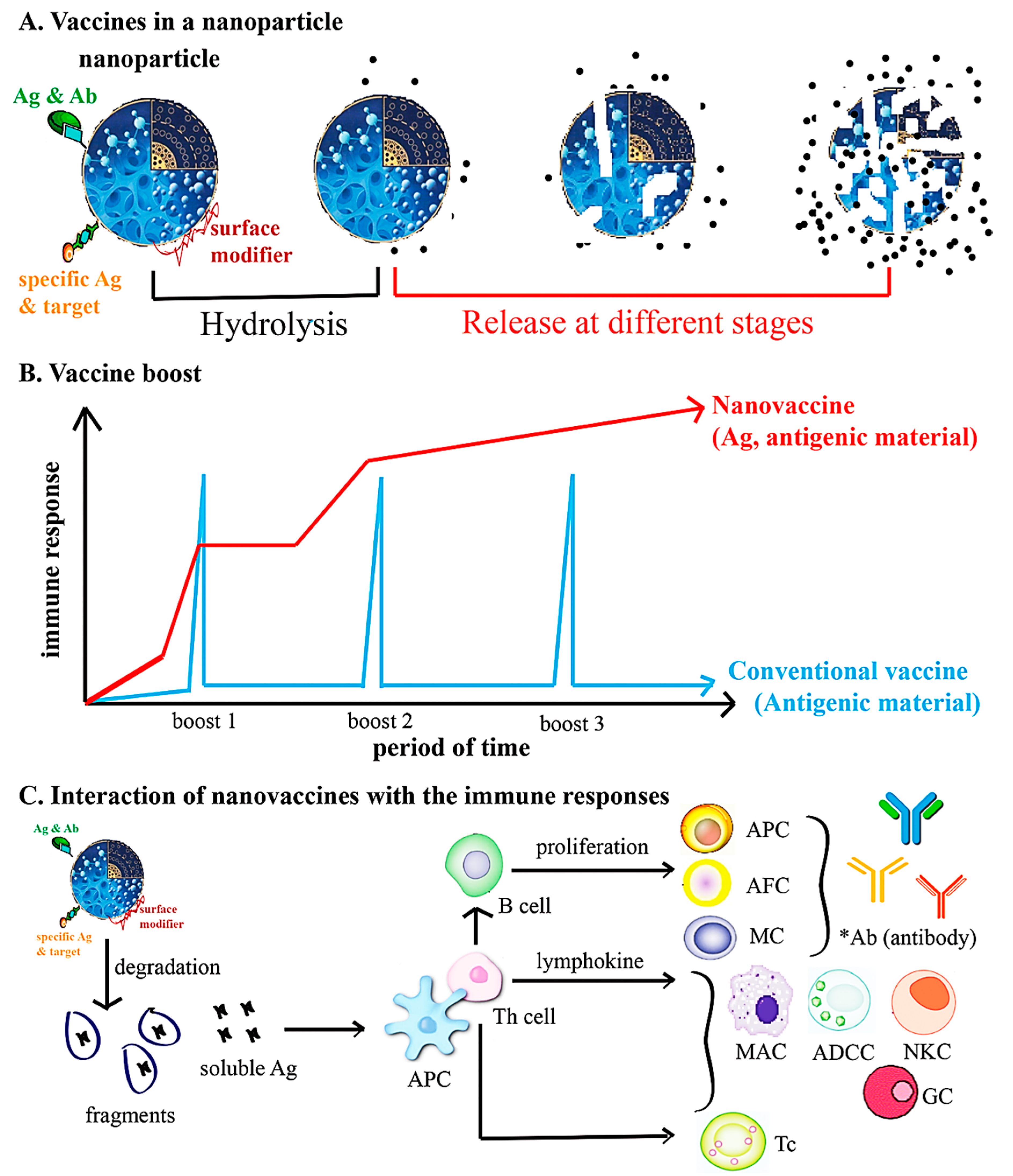
2.2. Nanotechnology for Designing Vaccines
CoVs and Nanovaccines
2.3. QDs and GO against CoVs Infections
3. Current Challenges and Future Prospects
- The effects of metallic NPs (e.g., silver, gold and copper NPs or hybrid nanomaterials) can be evaluated against SARS-CoV-2 [17]. For instance, silver NPs showed inhibition abilities against the viral entrance in host cells, for HIV-1 virus, as these NPs are capable of interacting with the cell receptors [146]. Additionally, gold NPs stabilized by biocompatible polymers demonstrated antiviral activity against HIV-1 and some subtypes of influenza virus (e.g., H1N1, H3N2, H5N1) [17,146].
- Biodegradable nanocarriers and carbon nanotubes can be proposed as potential antiviral agent carriers. For instance, antiviral drugs such as ribavirin and isoprinosine have been chemically linked on a single-walled carbon nanotube surface to transmit these antiviral compounds across biological membranes [148].
- Multi-functionalization should be performed for drug delivery platforms, imaging, and detection systems for viral localization and specific tissue or organ targeting. Development of toxicity and the reduction of side effects as well as bioavailability of antiviral drugs can be further explored based on nanotechnological insights; nanotrap particles showed suitable inhibition effects against influenza viruses, which can be explored for other viruses [149].
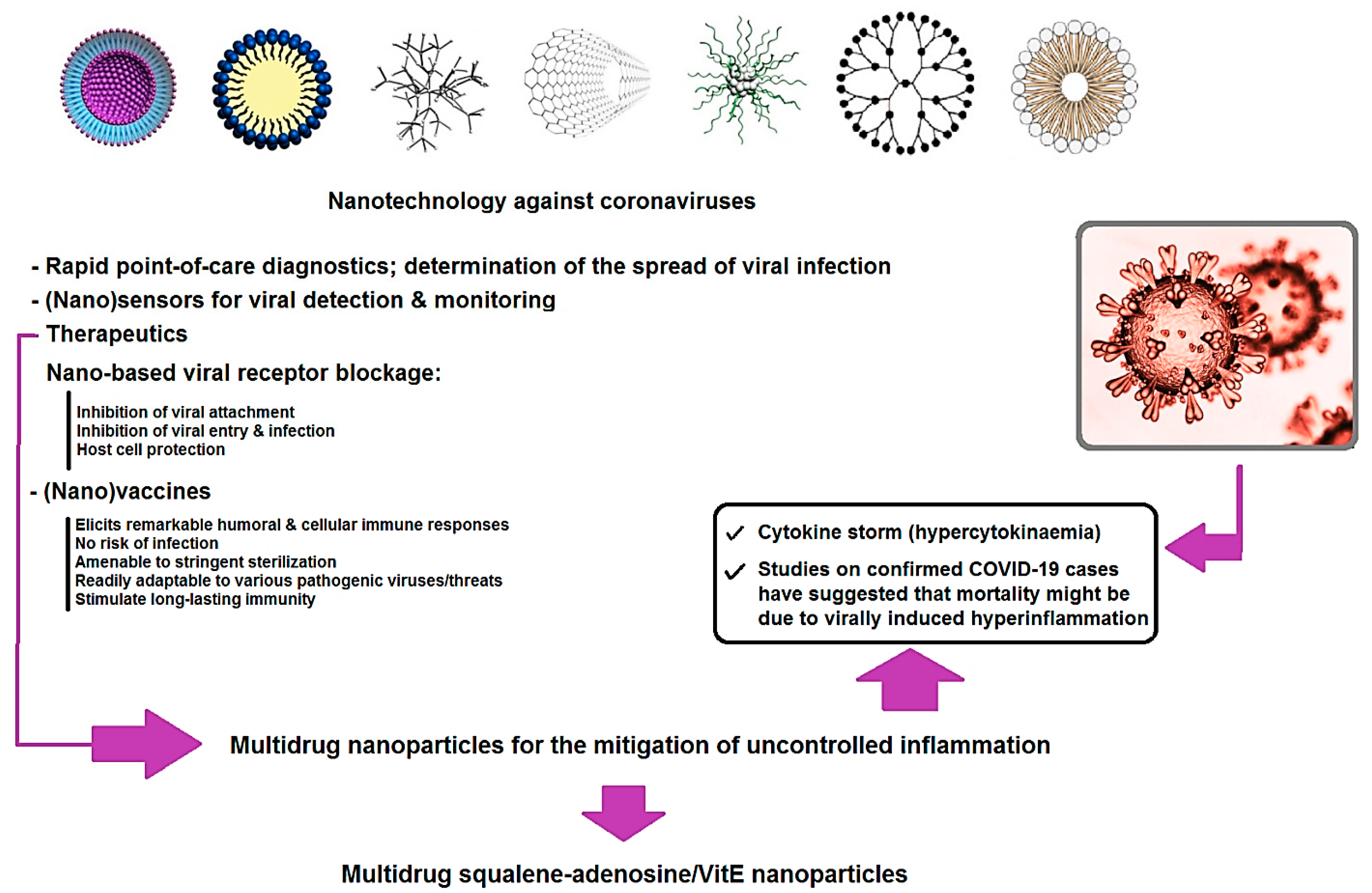
- Importantly, multidrug NPs (with high biocompatibility and drug loading) for the mitigation of uncontrolled inflammation is very promising, especially in the case of COVID-19. In one study, NPs were produced by conjugating squalene, an endogenous lipid, with adenosine, an endogenous immuno-modulator, and then encapsulating α-tocopherol, a natural antioxidant [150]. By using the vascular endothelial barrier dysfunction at sites of acute inflammation, these multidrug NPs could transport the therapeutic agents in a targeted manner and confer remarkable survival advantages to the treated animals in lethal models of endotoxemia. The selective delivery of adenosine and antioxidants together could serve as an innovative method for the treatment of acute inflammation with reduced side effects and significant therapeutic potential, especially in the case of cytokine storm (hypercytokinaemia) ensued by COVID-19 [150].
- Rapid and reliable tests for the new CoV are critical for controlling the pandemic, such as innovative biosensors designed for SARS-CoV-2 detection by applying nano-based technology; the aforementioned biosensor designed by Qui et al. [70] is an example of such.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Financial Disclosure
References
- Kahn, R.E.; Ma, W.; Richt, J.A. Swine and influenza: A challenge to one health research. In Influenza Pathogenesis and Control-Volume I.; Springer: Berlin, Germany, 2014; pp. 205–218. [Google Scholar]
- Qasim, M.; Lim, D.-J.; Park, H.; Na, D. Nanotechnology for diagnosis and treatment of infectious diseases. J. Nanosci. Nanotechnol. 2014, 14, 7374–7387. [Google Scholar] [CrossRef] [PubMed]
- Wejse, C.; Patsche, C.B.; Kühle, A.; Bamba, F.J.V.; Mendes, M.S.; Lemvik, G.; Gomes, V.F.; Rudolf, F. Impact of HIV-1, HIV-2, and HIV-1+ 2 dual infection on the outcome of tuberculosis. Int. J. Infect. Dis. 2015, 32, 128–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Braden, C.R.; Dowell, S.F.; Jernigan, D.B.; Hughes, J.M. Progress in global surveillance and response capacity 10 years after severe acute respiratory syndrome. Emerg. Infect. Dis. 2013, 19, 864. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.M.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef]
- Pavia, A.T. Viral infections of the lower respiratory tract: Old viruses, new viruses, and the role of diagnosis. Clin. Infect. Dis. 2011, 52, S284–S289. [Google Scholar] [CrossRef]
- Walker, T.A.; Khurana, S.; Tilden, S.J. Viral respiratory infections. Pediatric Clin. North Am. 1994, 41, 1365–1381. [Google Scholar] [CrossRef]
- Cojocaru, F.-D.; Botezat, D.; Gardikiotis, I.; Uritu, C.-M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.; Tamba, B.-I.; Mihai, C.-T. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics 2020, 12, 171. [Google Scholar] [CrossRef]
- Wong, S.S.Y.; Yuen, K.-Y. The management of coronavirus infections with particular reference to SARS. J. Antimicrob. Chemother. 2008, 62, 437–441. [Google Scholar] [CrossRef]
- Principi, N.; Piralla, A.; Zampiero, A.; Bianchini, S.; Umbrello, G.; Scala, A.; Bosis, S.; Fossali, E.; Baldanti, F.; Esposito, S. Bocavirus infection in otherwise healthy children with respiratory disease. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
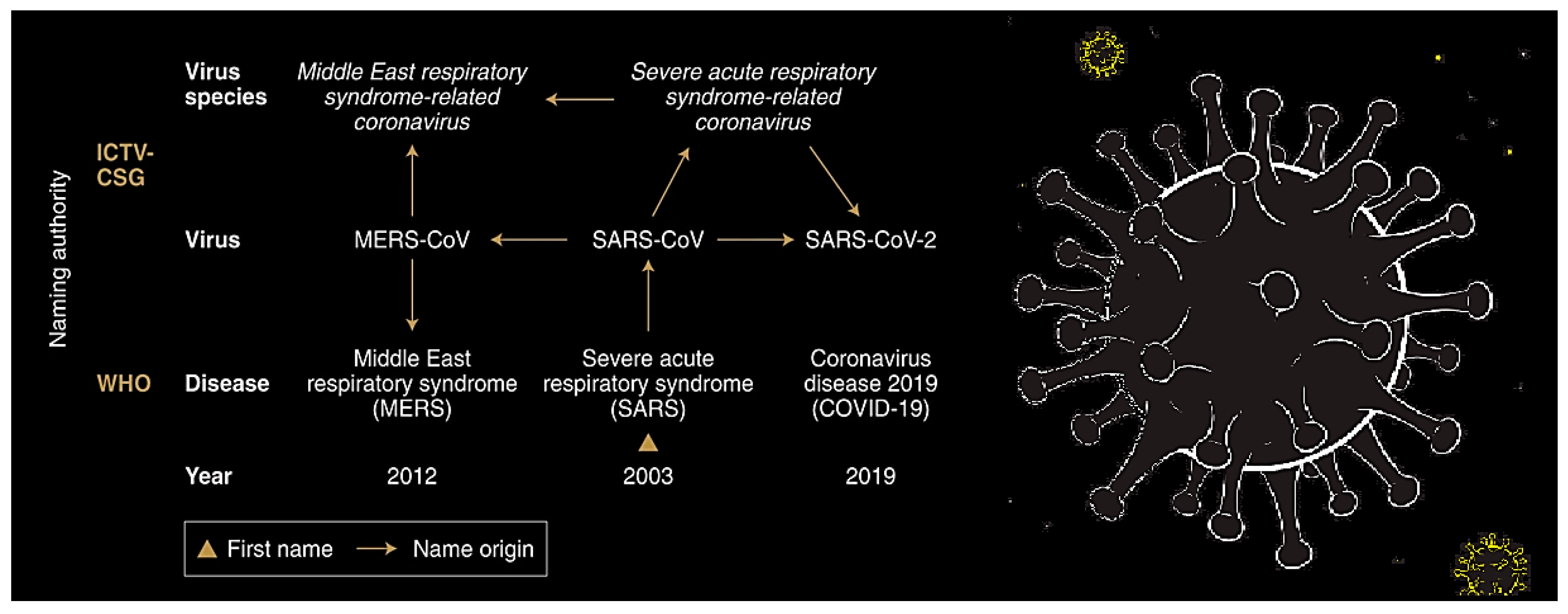
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.I.; Iauber, C.; Ieontovich, A.M.; Neuman, B.W.; et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5. [Google Scholar] [CrossRef]
- Branche, A.R.; Falsey, A.R. Parainfluenza virus infection. Semin. Resp. Crit. Care Med. 2016, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.I.; Higgins, D.; Nunes, M.C.; Melero, J.A.; Langedijk, A.C.; Horsley, N.; Buchholz, U.J.; Openshaw, P.J.; McLellan, J.S.; Englund, J.A. The respiratory syncytial virus vaccine landscape: Lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018, 18, e295–e311. [Google Scholar] [CrossRef]
- Lu, M.-P.; Ma, L.-Y.; Zheng, Q.; Dong, L.-L.; Chen, Z.-M. Clinical characteristics of adenovirus associated lower respiratory tract infection in children. World J. Pediatrics 2013, 9, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Munster, V.J.; Koopmans, M.; van Doremalen, N.; van Riel, D.; de Wit, E. A novel coronavirus emerging in China—key questions for impact assessment. New Engl. J. Med. 2020, 382, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; Ditaranto, N.; Cioffi, N. Can Nanotechnology and Materials Science Help the Fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef]
- Hayden, F. Developing new antiviral agents for influenza treatment: What does the future hold? Clin. Infect. Dis. 2009, 48, S3–S13. [Google Scholar] [CrossRef]
- Little, S.J.; Holte, S.; Routy, J.-P.; Daar, E.S.; Markowitz, M.; Collier, A.C.; Koup, R.A.; Mellors, J.W.; Connick, E.; Conway, B. Antiretroviral-drug resistance among patients recently infected with HIV. N. Engl. J. Med. 2002, 347, 385–394. [Google Scholar] [CrossRef]
- Maseko, S.B.; Natarajan, S.; Sharma, V.; Bhattacharyya, N.; Govender, T.; Sayed, Y.; Maguire, G.E.M.; Lin, J.; Kruger, H.G. Purification and characterization of naturally occurring HIV-1 (South African subtype C) protease mutants from inclusion bodies. Protein Expr. Purif. 2016, 122, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Parboosing, R.; Maguire, G.E.M.; Govender, P.; Kruger, H.G. Nanotechnology and the treatment of HIV infection. Viruses 2012, 4, 488–520. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. An Introduction to Nanotechnology. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 1–27. [Google Scholar]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Cheng, H.-F.; Yang, Y.-C.; Yeh, M.-K. Nanotechnologies applied in biomedical vaccines. Micro Nanotechn. Biotech. 2016. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Dou, H.; Morehead, J.; Rabinow, B.; Gendelman, H.E.; Destache, C.J. Nanotechnology: A focus on nanoparticles as a drug delivery system. J. Neuroimmune Pharmacol. 2006, 1, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Poeschla, E.M. Integrase, LEDGF/p75 and HIV replication. Cell. Mol. Life Sci. 2008, 65, 1403–1424. [Google Scholar] [CrossRef]
- Adesina, S.K.; Akala, E.O. Nanotechnology approaches for the delivery of exogenous siRNA for HIV therapy. Mol. Pharm. 2015, 12, 4175–4187. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yan, Y.; Liu, J.; Wang, B.; Li, P.; Hu, Q.; Chen, W. Target delivery of small interfering RNAs with vitamin E-coupled nanoparticles for treating hepatitis C. Sci. Rep. 2016, 6, 24867. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2016, 8, 3040–3048. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Zhao, M.; Guo, M.; Xu, T.; Wang, C.; Xia, H.; Zhu, B. Reversal of H1N1 influenza virus-induced apoptosis by silver nanoparticles functionalized with amantadine. RSC Adv. 2016, 6, 89679–89686. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Guo, M.; Zhao, M.; Xia, Y.; Wang, C.; Xu, T.; Zhu, B. Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. Int. J. Nanomed. 2018, 13, 2005. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Guo, M.; Xia, Y.; Zhao, M.; Wang, C.; Xu, T.; Chen, T.; Zhu, B. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int. J. Nanomed. 2017, 12, 5733. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Chen, Y.-T.; Fang, Z.-S.; Chang, W.-S.; Chen, H.-W. Antiviral efficacy of nanoparticulate vacuolar ATPase inhibitors against influenza virus infection. Int. J. Nanomed. 2018, 13, 8579. [Google Scholar] [CrossRef]
- Yang, X.; Shah, S.J.; Wang, Z.; Agrahari, V.; Pal, D.; Mitra, A.K. Nanoparticle-based topical ophthalmic formulation for sustained release of stereoisomeric dipeptide prodrugs of ganciclovir. Drug Deliv. 2016, 23, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, T.; Ames, J.; Agelidis, A.; Suryawanshi, R.; Jaishankar, D.; Hopkins, J.; Thakkar, N.; Koujah, L.; Shukla, D. Drug-encapsulated carbon (DECON): A novel platform for enhanced drug delivery. Sci. Adv. 2019, 5, eaax0780. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.A.; Kırımlıoğlu, G.Y. Preparation and in vitro of characterization lamivudine loaded nanoparticles prepared by acid or ester terminated PLGA for effective oral antiretroviral therapy. J. Res. Pharm. 2019, 23, 897–913. [Google Scholar]
- Venkatesh, D.N.; Baskaran, M.; Karri, V.V.S.R.; Mannemala, S.S.; Radhakrishna, K.; Goti, S. Fabrication and in vivo evaluation of Nelfinavir loaded PLGA nanoparticles for enhancing oral bioavailability and therapeutic effect. Saudi Pharm. J. 2015, 23, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Shah, L.K.; Amiji, M.M. Intracellular delivery of saquinavir in biodegradable polymeric nanoparticles for HIV/AIDS. Pharm. Res. 2006, 23, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liang, Y.; Liu, X.; Zhou, S.; Liu, L.; Zhang, F.; Xie, C.; Cai, S.; Wei, J.; Zhu, Y. PLGA-PEG nanoparticles coated with anti-CD45RO and loaded with HDAC plus protease inhibitors activate latent HIV and inhibit viral spread. Nanoscale Res. Lett. 2015, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sankar, V.; Keerthi, M.L.; Parmar, N. Formation and In-vitro Evaluation of Zidovudine-Lamivudine Nanoparticles. Ind. J. Pharma. Educ. Res. 2012, 46, 192–196. [Google Scholar]
- Fiandra, L.; Colombo, M.; Mazzucchelli, S.; Truffi, M.; Santini, B.; Allevi, R.; Nebuloni, M.; Capetti, A.; Rizzardini, G.; Prosperi, D. Nanoformulation of antiretroviral drugs enhances their penetration across the blood brain barrier in mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1387–1397. [Google Scholar] [CrossRef]
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-loaded chitosan nanospheres from nano-emulsion templating for the topical treatment of herpesviruses infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef]
- Lauster, D.; Glanz, M.; Bardua, M.; Ludwig, K.; Hellmund, M.; Hoffmann, U.; Hamann, A.; Böttcher, C.; Haag, R.; Hackenberger, C.P.R. Multivalent Peptide–Nanoparticle Conjugates for Influenza-Virus Inhibition. Angew. Chem. Int. Ed. 2017, 56, 5931–5936. [Google Scholar] [CrossRef]
- Freeling, J.P.; Koehn, J.; Shu, C.; Sun, J.; Ho, R.J.Y. Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates. AIDS Res. Hum. Retrovir. 2015, 31, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Freeling, J.P.; Koehn, J.; Shu, C.; Ho, R.J.Y. Evaluation of atazanavir and darunavir interactions with lipids for developing pH-responsive anti-HIV drug combination nanoparticles. J. Pharm. Sci. 2014, 103, 2520–2529. [Google Scholar] [CrossRef]
- Raskin, M.M.; Schlachet, I.; Sosnik, A. Mucoadhesive nanogels by ionotropic crosslinking of chitosan-g-oligo (NiPAam) polymeric micelles as novel drug nanocarriers. Nanomedicine 2016, 11, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Nagy, E.; Neethirajan, S. Self-assembled star-shaped chiroplasmonic gold nanoparticles for ultrasensitive chiro-immunosensor of viruses. RSC Adv. 2017, 7, 40849–40857. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Hsueh, Y.-H.; Hsieh, C.-T.; Tzou, D.-Y.; Chang, P.-L. Antiviral activity of graphene-silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Env. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef]
- Layqah, L.A.; Eissa, S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Mikrochim. Acta 2019, 186, 224. [Google Scholar] [CrossRef]
- Sekimukai, H.; Iwata-Yoshikawa, N.; Fukushi, S.; Tani, H.; Kataoka, M.; Suzuki, T.; Hasegawa, H.; Niikura, K.; Arai, K.; Nagata, N. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2020, 64, 33–51. [Google Scholar] [CrossRef]
- Ting, D.; Dong, N.; Fang, L.; Lu, J.; Bi, J.; Xiao, S.; Han, H. Multisite Inhibitors for Enteric Coronavirus: Antiviral Cationic Carbon Dots Based on Curcumin. ACS Appl. Nano Mater. 2018, 1, 5451–5459. [Google Scholar] [CrossRef]
- Du, T.; Liang, J.; Dong, N.; Lu, J.; Fu, Y.; Fang, L.; Xiao, S.; Han, H. Glutathione-Capped Ag2S Nanoclusters Inhibit Coronavirus Proliferation through Blockage of Viral RNA Synthesis and Budding. ACS Appl. Mater. Interfaces 2018, 10, 4369–4378. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Kang, S.W.; Oh, S.; Lee, J.; Neethirajan, S. Chiral zirconium quantum dots: A new class of nanocrystals for optical detection of coronavirus. Heliyon 2018, 4, e00766. [Google Scholar] [CrossRef]
- Mo, Y.; Fisher, D. Review of Treatment Modalities Of Middle Repsiratorey Syndroms. J. Antimicrob. Chemother. 2016, 71, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Erlandson, K.J.; Korch, G.; O’Hara, M.; Wathen, M.; Hu-Primmer, J.; Hojvat, S.; Stemmy, E.J.; Donabedian, A. Evelopment of Medical Countermeasures to Middle East Respiratory Syndrome Coronavirus. Emerg. Infect. Dis. 2016, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Chan, J.F.W.; Azhar, E.I.; Hui, D.S.C.; Yuen, K.-Y. Coronavirus-Drug Discovery and Therapeutic Options. Nat. Rev 2016, 15, 327–347. [Google Scholar]
- Łoczechin, A.; Séron, K.; Barras, A.; Giovanelli, E.; Belouzard, S.; Chen, Y.T.; Metzler-Nolte, N.; Boukherroub, R.; Dubuisson, J.; Szunerits, S. Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus. ACS Appl. Mater. Interfaces 2019, 11, 42964–42974. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.; Zhou, Y.; Lu, L.; Li, F.; Jiang, S. ERS-Cov Spike Protein: A Key Target for Antivirals. Exp. Opin. Ther. Targets 2017, 21, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, Q.; Zhu, Y.; Chan, K.-H.; Qin, L.; Li, Y.; Wang, Q.; Chan, J.F.W.; Du, L.; Yu, F.; et al. Sructures-Based Discovery Of Middle East Repiratory Syndrome Coronavirus Fusion Inhibitor. Nat. Commun. 2014, 5, 1–15. [Google Scholar]
- Szunerits, S.; Barras, A.; Khanal, M.; Pagneux, Q.; Boukherroub, R. Nanostructures for the Inhibition of Viral Infections. Molecules 2015, 20, 14051–14081. [Google Scholar] [CrossRef]
- Huang, X.; Li, M.; Xu, Y.; Zhang, J.; Meng, X.; An, X.; Sun, L.; Guo, L.; Shan, X.; Ge, J.; et al. Novel Gold Nanorod-Based HR1 Peptide Inhibitor for Middle East Respiratory Syndrome Coronavirus. ACS Appl. Mater. Interfaces 2019, 11, 19799–19807. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, X.; Tong, T.; Fang, L.; Wu, Y.; Liang, J.; Xiao, S. High antiviral activity of mercaptoethane sulfonate functionalized Te/BSA nanostars against arterivirus and coronavirus. RSC Adv. 2020, 10, 14161–14169. [Google Scholar] [CrossRef]
- Walsh, J.H.; Yalow, R.S.; Berson, S.A. Radioimmunoassay of Australia antigen. VoxSanguinis 1970, 19, 217–224. [Google Scholar]
- Bosch, A. Diagnostic Virology Protocols; John, R., Stephenson, A.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 2, p. 56. [Google Scholar]
- Zehbe, I.; Hacker, G.W.; Su, H.; Hauser-Kronberger, C.; Hainfeld, J.F.; Tubbs, R. Sensitive in situ hybridization with catalyzed reporter deposition, streptavidin-Nanogold, and silver acetate autometallography: Detection of single-copy human papillomavirus. Am. J. Pathol. 1997, 150, 1553. [Google Scholar] [PubMed]
- Wang, X.; Liu, L.-H.; Ramstroem, O.; Yan, M. Engineering nanomaterial surfaces for biomedical applications. Exp. Biol. Med. 2009, 234, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, P. The use of nanocrystals in biological detection. Nat. Biotechnol. 2004, 22, 47–52. [Google Scholar] [CrossRef]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.S.; Shafiee, H. Applications of gold nanoparticles in virus detection. Theranostics 2018, 8, 1985. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano. 2020. [Google Scholar] [CrossRef]
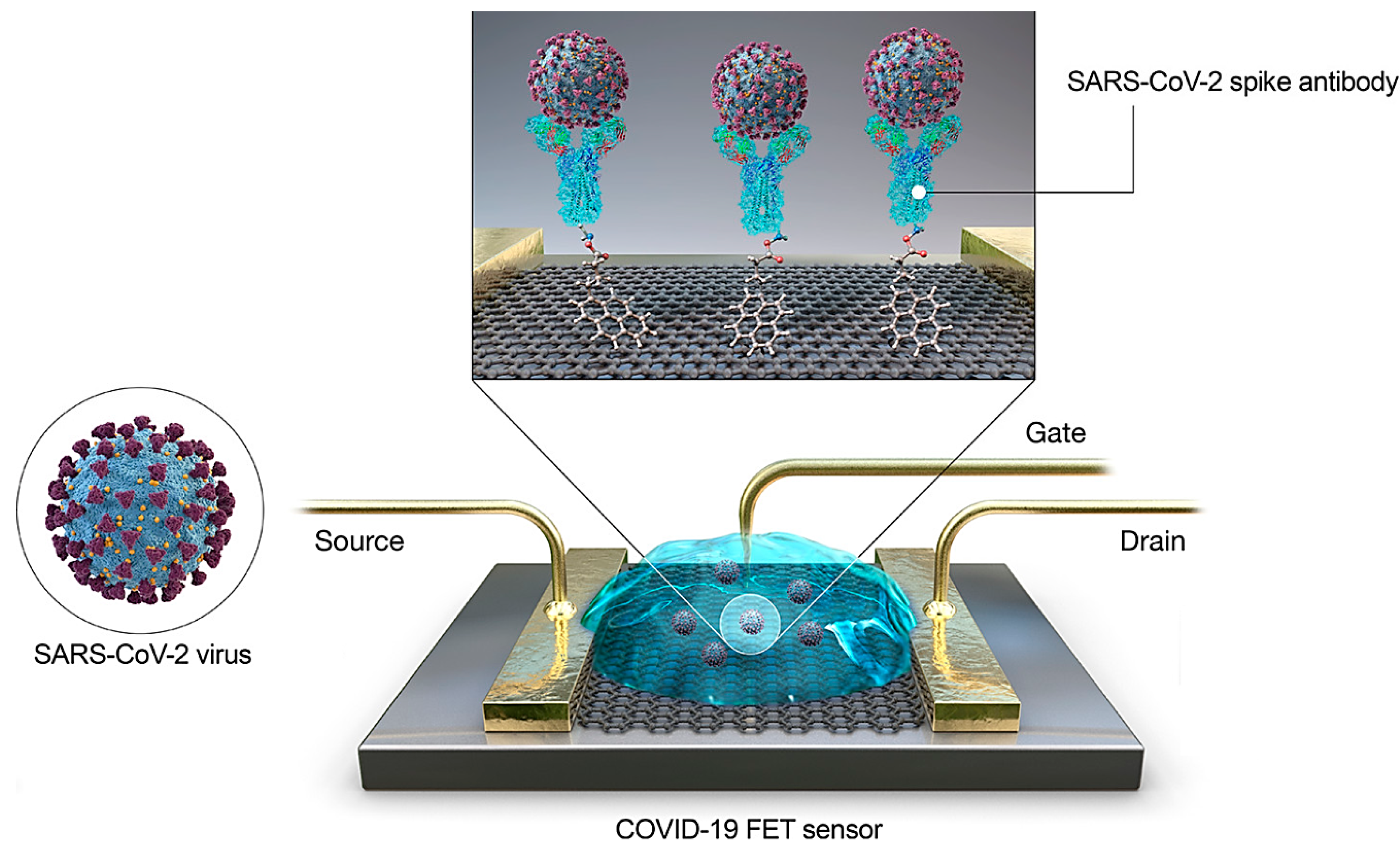
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano. 2020. [Google Scholar] [CrossRef]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Callaway, E. The race for coronavirus vaccines: A graphical guide. Nature 2020, 580, 576–577. [Google Scholar] [CrossRef]
- Shang, W.; Yang, Y.; Rao, Y.; Rao, X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccin. 2020, 5. [Google Scholar] [CrossRef]
- Amanat, F.; Krammer, F. SARS-CoV-2 Vaccines: Status Report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Naiman, B.M.; Alt, D.; Bolin, C.A.; Zuerner, R.; Baldwin, C.L. Protective killed Leptospira borgpetersenii vaccine induces potent Th1 immunity comprising responses by CD4 and γδ T lymphocytes. Infect. Immun. 2001, 69, 7550–7558. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Chowdhury, M.I.; Nazim, M.; Alam, M.M.; Ahmed, T.; Hossain, M.B.; Hore, S.K.; Sultana, G.N.N.; Svennerholm, A.-M.; Qadri, F. Vaccine specific immune response to an inactivated oral cholera vaccine and EPI vaccines in a high and low arsenic area in Bangladeshi children. Vaccine 2013, 31, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Scallan, C.D.; Tingley, D.W.; Lindbloom, J.D.; Toomey, J.S.; Tucker, S.N. An adenovirus-based vaccine with a double-stranded RNA adjuvant protects mice and ferrets against H5N1 avian influenza in oral delivery models. Clin. Vaccine Immunol. 2013, 20, 85–94. [Google Scholar] [CrossRef]
- Epstein, J.E.; Tewari, K.; Lyke, K.E.; Sim, B.K.L.; Billingsley, P.F.; Laurens, M.B.; Gunasekera, A.; Chakravarty, S.; James, E.R.; Sedegah, M. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 2011, 334, 475–480. [Google Scholar] [CrossRef]
- Norton, E.B.; Lawson, L.B.; Mahdi, Z.; Freytag, L.C.; Clements, J.D. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens. Infect. Immun. 2012, 80, 2426–2435. [Google Scholar] [CrossRef]
- Tevi-Benissan, M.C.; Moturi, E.; Anya, B.-P.M.; Aschalew, T.; Dicky, A.B.; Nyembo, P.A.; Mbulu, L.K.; Okeibunor, J.; Mihigo, R.; Zawaira, F. Contribution of polio eradication initiative to effective new vaccine introduction in Africa, 2010–2015. Vaccine 2016, 34, 5193–5198. [Google Scholar] [CrossRef]
- Nuismer, S.L.; Althouse, B.M.; May, R.; Bull, J.J.; Stromberg, S.P.; Antia, R. Eradicating infectious disease using weakly transmissible vaccines. Proc. Biol. Sci. 2016, 283, 20161903. [Google Scholar] [CrossRef]
- Staroverov, S.A.; Vidyasheva, I.V.; Gabalov, K.P.; Vasilenko, O.A.; Laskavyi, V.N.; Dykman, L.A. Immunostimulatory effect of gold nanoparticles conjugated with transmissible gastroenteritis virus. Bull. Exp. Biol. Med. 2011, 151, 436. [Google Scholar] [CrossRef]
- Kim, Y.S.; Son, A.; Kim, J.; Kwon, S.B.; Kim, M.H.; Kim, P.; Kim, J.; Byun, Y.H.; Sung, J.; Lee, J.; et al. Chaperna-Mediated Assembly of Ferritin-Based Middle East Respiratory Syndrome-Coronavirus Nanoparticles. Front. Immunol. 2018, 9, 1093. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.W.; Huang, C.Y.; Yao, B.Y.; Lin, J.C.; Agrawal, A.; Algaissi, A.; Peng, B.H.; Liu, Y.H.; Huang, P.H.; Juang, R.H.; et al. Viromimetic STING agonist-loaded hollow polymeric nanoparticles for safe and effective vaccination against Middle East respiratory syndrome coronavirus. Adv. Funct. Mater. 2019, 29, 1807616. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Kang, K.W.; Lee, E.Y.; Seo, D.W.; Kim, H.L.; Kim, H.; Kwon, T.; Park, H.L.; Kim, H.; Lee, S.M.; et al. Heterologous prime–boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East Respiratory syndrome coronavirus. Vaccine 2018, 36, 3468–3476. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.M.; Graham, D.Y.; Opekun, A.R.; Gilger, M.A.; Guerrero, R.A.; Estes, M.K. Recombinant Norwalk virus–like particles given orally to volunteers: Phase I study. Gastroenterology 1999, 117, 40–48. [Google Scholar] [CrossRef]
- Geldmacher, A.; Skrastina, D.; Borisova, G.; Petrovskis, I.; Krüger, D.H.; Pumpens, P.; Ulrich, R. A hantavirus nucleocapsid protein segment exposed on hepatitis B virus core particles is highly immunogenic in mice when applied without adjuvants or in the presence of pre-existing anti-core antibodies. Vaccine 2005, 23, 3973–3983. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Liu, Y.; Wu, X.; Ji, Y.; Zhao, Y. Surface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012, 12, 2003–2012. [Google Scholar] [CrossRef]
- Tao, W.; Gill, H.S. M2e-immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine 2015, 33, 2307–2315. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hung, Y.-C.; Lin, W.-H.; Huang, G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology 2010, 21, 195101. [Google Scholar] [CrossRef]
- Tao, W.; Hurst, B.L.; Shakya, A.K.; Uddin, M.J.; Ingrole, R.S.J.; Hernandez-Sanabria, M.; Arya, R.P.; Bimler, L.; Paust, S.; Tarbet, E.B. Consensus M2e peptide conjugated to gold nanoparticles confers protection against H1N1, H3N2 and H5N1 influenza A viruses. Antivir. Res. 2017, 141, 62–72. [Google Scholar] [CrossRef]
- Lugade, A.A.; Bharali, D.J.; Pradhan, V.; Elkin, G.; Mousa, S.A.; Thanavala, Y. Single low-dose un-adjuvanted HBsAg nanoparticle vaccine elicits robust, durable immunity. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 923–934. [Google Scholar] [CrossRef]
- Schreiber, H.A.; Prechl, J.; Jiang, H.; Zozulya, A.; Fabry, Z.; Denes, F.; Sandor, M. Using carbon magnetic nanoparticles to target, track, and manipulate dendritic cells. J. Immunol. Method. 2010, 356, 47–59. [Google Scholar] [CrossRef][Green Version]
- Zhao, K.; Chen, G.; Shi, X.-M.; Gao, T.-T.; Li, W.; Zhao, Y.; Zhang, F.-Q.; Wu, J.; Cui, X.; Wang, Y.-F. Preparation and efficacy of a live newcastle disease virus vaccine encapsulated in chitosan nanoparticles. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Jesus, S.; Soares, E.; Costa, J.; Borchard, G.; Borges, O. Immune response elicited by an intranasally delivered HBsAg low-dose adsorbed to poly-ε-caprolactone based nanoparticles. Int. J. Pharm. 2016, 504, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Matsuura, M.; Akagi, T.; Akashi, M.; Tanimoto, T.; Ishikawa, T.; Takahashi, M.; Yamanishi, K.; Mori, Y. Poly (γ-glutamic acid) nano-particles combined with mucosal influenza virus hemagglutinin vaccine protects against influenza virus infection in mice. Vaccine 2009, 27, 5896–5905. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.A.; Carter, D.M.; Daniluk, S.; Toapanta, F.R.; Ahmad, A.; Gavrilov, V.; Massare, M.; Pushko, P.; Mytle, N.; Rowe, T. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 2007, 25, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Parez, N.; Fourgeux, C.; Mohamed, A.; Dubuquoy, C.; Pillot, M.; Dehee, A.; Charpilienne, A.; Poncet, D.; Schwartz-Cornil, I.; Garbarg-Chenon, A. Rectal immunization with rotavirus virus-like particles induces systemic and mucosal humoral immune responses and protects mice against rotavirus infection. J. Virol. 2006, 80, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.A.P.F.; Yan, Z.; Jeffers, S.A.; Holmes, K.V.; Hodges, R.S.; Burkhard, P. Peptide nanoparticles as novel immunogens: Design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem. Biol. Drug Des. 2009, 73, 53–61. [Google Scholar] [CrossRef]
- Borges, O.; Cordeiro-da-Silva, A.; Tavares, J.; Santarém, N.; de Sousa, A.; Borchard, G.; Junginger, H.E. Immune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate coated chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2008, 69, 405–416. [Google Scholar] [CrossRef]
- Thomas, C.; Rawat, A.; Hope-Weeks, L.; Ahsan, F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 2011, 8, 405–415. [Google Scholar] [CrossRef]
- Raghuvanshi, R.S.; Katare, Y.K.; Lalwani, K.; Ali, M.M.; Singh, O.; Panda, A.K. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. Int. J. Pharm. 2002, 245, 109–121. [Google Scholar] [CrossRef]
- Mansoor, F.; Earley, B.; Cassidy, J.P.; Markey, B.; Doherty, S.; Welsh, M.D. Comparing the immune response to a novel intranasal nanoparticle PLGA vaccine and a commercial BPI3V vaccine in dairy calves. BMC Vet. Res. 2015, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.L.; Kelly, S.M.; Kumar, P.; Speckhart, S.; Haughney, S.L.; Henningson, J.; Narasimhan, B.; Sacco, R.E. Efficacy of mucosal polyanhydride nanovaccine against respiratory syncytial virus infection in the neonatal calf. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Francica, J.R.; Lynn, G.M.; Laga, R.; Joyce, M.G.; Ruckwardt, T.J.; Morabito, K.M.; Chen, M.; Chaudhuri, R.; Zhang, B.; Sastry, M. Thermoresponsive polymer nanoparticles co-deliver RSV F trimers with a TLR-7/8 adjuvant. Bioconjugate Chem. 2016, 27, 2372–2385. [Google Scholar] [CrossRef] [PubMed]
- Lynn, G.M.; Laga, R.; Darrah, P.A.; Ishizuka, A.S.; Balaci, A.J.; Dulcey, A.E.; Pechar, M.; Pola, R.; Gerner, M.Y.; Yamamoto, A. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015, 33, 1201. [Google Scholar] [CrossRef]
- Tai, W.; Roberts, L.; Seryshev, A.; Gubatan, J.M.; Bland, C.S.; Zabriskie, R.; Kulkarni, S.; Soong, L.; Mbawuike, I.; Gilbert, B. Multistrain influenza protection induced by a nanoparticulate mucosal immunotherapeutic. Mucosal Immunol. 2011, 4, 197–207. [Google Scholar] [CrossRef]
- Zhao, M.; Li, M.; Zhang, Z.; Gong, T.; Sun, X. Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA. Drug Deliv. 2016, 23, 2596–2607. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Grip, J. PLGA/PLA micro-and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine 2012, 30, 656–667. [Google Scholar] [CrossRef]
- Kumar, A.; Ma, H.; Zhang, X.; Huang, K.; Jin, S.; Liu, J.; Wei, T.; Cao, W.; Zou, G.; Liang, X.-J. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 2012, 33, 1180–1189. [Google Scholar] [CrossRef]
- McNeil, S.E. Unique benefits of nanotechnology to drug delivery and diagnostics. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: Berlin, Germany, 2011; pp. 3–8. [Google Scholar]
- Chiappetta, D.A.; Facorro, G.; de Celis, E.R.; Sosnik, A. Synergistic encapsulation of the anti-HIV agent efavirenz within mixed poloxamine/poloxamer polymeric micelles. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 624–637. [Google Scholar] [CrossRef]
- Santos-Martinez, M.J.; Rahme, K.; Corbalan, J.J.; Faulkner, C.; Holmes, J.D.; Tajber, L.; Medina, C.; Radomski, M.W. Pegylation increases platelet biocompatibility of gold nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1004–1015. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Thalhammer, T.; Tzakos, A.G.; Stojanovska, L. Targeting antigens to dendritic cell receptors for vaccine development. J. Drug Deliv. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, D.; Mishra, V.; Suresh, M.R.; Kaur, K. A simple approach for enhanced immune response using engineered dendritic cell targeted nanoparticles. Vaccine 2012, 30, 7292–7299. [Google Scholar] [CrossRef] [PubMed]
- Misumi, S.; Masuyama, M.; Takamune, N.; Nakayama, D.; Mitsumata, R.; Matsumoto, H.; Urata, N.; Takahashi, Y.; Muneoka, A.; Sukamoto, T. Targeted delivery of immunogen to primate m cells with tetragalloyl lysine dendrimer. J. Immunol. 2009, 182, 6061–6070. [Google Scholar] [CrossRef]
- Lepenies, B.; Lee, J.; Sonkaria, S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv. Drug Deliv. Rev. 2013, 65, 1271–1281. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 180, 1–12. [Google Scholar] [CrossRef]
- Coleman, C.M.; Liu, Y.V.; Mu, H.; Taylor, J.K.; Massare, M.; Flyer, D.C.; Smith, G.E.; Frieman, M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 2014, 32, 3169–3174. [Google Scholar] [CrossRef]
- Liu, Y.V.; Massare, M.J.; Barnard, D.L.; Kort, T.; Nathan, M.; Wang, L.; Smith, G. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine 2011, 29, 6606–6613. [Google Scholar] [CrossRef]
- Coleman, C.M.; Venkataraman, T.; Liu, Y.V.; Glenn, G.M.; Smith, G.E.; Flyer, D.C.; Frieman, M.B. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine 2017, 35, 1586–1589. [Google Scholar] [CrossRef]
- Kato, T.; Takami, Y.; Deo, V.K.; Park, E.Y. Preparation of virus-like particle mimetic nanovesicles displaying the S protein of Middle East respiratory syndrome coronavirus using insect cells. J. Biotechnol. 2019, 306, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Guzman, D.; Le Guen, P.; Villeret, B.; Sola, N.; Le Borgne, R.; Guyard, A.; Kemmel, A.; Crestani, B.; Sallenave, J.M.; Garcia-Verdugo, I. Silver nanoparticle-adjuvanted vaccine protects against lethal influenza infection through inducing BALT and IgA-mediated mucosal immunity. Biomaterials 2019, 217, 119308. [Google Scholar] [CrossRef]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. [Google Scholar] [CrossRef]
- Du, T.; Lu, J.; Liu, L.; Dong, N.; Fang, L.; Xiao, S.; Han, H. Antiviral Activity of Graphene Oxide–Silver Nanocomposites by Preventing Viral Entry and Activation of the Antiviral Innate Immune Response. ACS Appl. Bio Mater. 2018, 1, 1286–1293. [Google Scholar] [CrossRef]
- Sanhai, W.R.; Sakamoto, J.H.; Canady, R.; Ferrari, M. Seven challenges for nanomedicine. Nat. Nanotechnol. 2008, 3, 242. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Gaspar, R. Nanomedicine (s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- De Oliveira, M.P.; Garcion, E.; Venisse, N.; Benoît, J.-P.; Couet, W.; Olivier, J.-C. Tissue distribution of indinavir administered as solid lipid nanocapsule formulation in mdr1a (+/+) and mdr1a (−/−) CF-1 mice. Pharm. Res. 2005, 22, 1898–1905. [Google Scholar] [CrossRef]
- Caron, J.; Reddy, L.H.; Lepêtre-Mouelhi, S.; Wack, S.; Clayette, P.; Rogez-Kreuz, C.; Yousfi, R.; Couvreur, P.; Desmaële, D. Squalenoyl nucleoside monophosphate nanoassemblies: New prodrug strategy for the delivery of nucleotide analogues. Bioorg. Med. Chem. Lett. 2010, 20, 2761–2764. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles–properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef]
- Gagliardi, M. Biomimetic and bioinspired nanoparticles for targeted drug delivery. Ther. Deliv. 2017, 8, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechn. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Mallipeddi, R.; Rohan, L.C. Progress in antiretroviral drug delivery using nanotechnology. Int. J. Nanomed. 2010, 5, 533. [Google Scholar]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Maurer, P.; Jennings, G.T.; Willers, J.; Rohner, F.; Lindman, Y.; Roubicek, K.; Renner, W.A.; Müller, P.; Bachmann, M.F. A therapeutic vaccine for nicotine dependence: Preclinical efficacy, and Phase I safety and immunogenicity. Eur. J. Immunol. 2005, 35, 2031–2040. [Google Scholar] [CrossRef]
- Roldao, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.T.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccin. 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. R. Soc. 2014, 369, 20130433. [Google Scholar] [CrossRef]
- Peek, L.J.; Middaugh, C.R.; Berkland, C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008, 60, 915–928. [Google Scholar] [CrossRef]
- Nandedkar, T.D. Nanovaccines: Recent developments in vaccination. J. Biosci. 2009, 34, 995–1003. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P.J. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef]
- Kerry, R.G.; Malik, S.; Redda, Y.T.; Sahoo, S.; Patra, J.K.; Majhi, S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 196–220. [Google Scholar] [CrossRef] [PubMed]
- Shaligram, S.; Campbell, A. Toxicity of copper salts is dependent on solubility profile and cell type tested. Toxicol. Vitr. 2013, 27, 844–851. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Huang, A.-G.; Huang, J.-Q.; Huang, Y.-Q.; Wang, G.-X. Anti-betanodavirus activity of isoprinosine and improved efficacy using carbon nanotubes based drug delivery system. Aquaculture 2019, 512, 734377. [Google Scholar] [CrossRef]
- Singh, L.; Kruger, H.G.; Maguire, G.E.M.; Govender, T.; Parboosing, R. The role of nanotechnology in the treatment of viral infections. Adv. Infect. Dis. 2017, 4, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Dormont, F.; Brusini, R.; Cailleau, C.; Reynaud, F.; Peramo, A.; Gendron, A.; Mougin, J.; Gaudin, F.; Varna, M.; Couvreur, P. Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation. Sci. Adv. 2020, eaaz5466. [Google Scholar] [CrossRef]



| Nanoplatform | Properties (Shape/Size/Toxicity) | Drug | Virus | Refs. | |
|---|---|---|---|---|---|
| NPs | Silver (Ag) | Monodisperse and uniformly spherical; highly stable Ag@AM (2 nm > 28 days); Ag NPs loaded with AM on their surface less cytotoxic (~90%) than free AgNPs (65%) or AM (56%) | Amantadine (AM) | H1N1 | [30] |
| Selenium (Se) | Uniformly spherical Se@AM (70 nm)—more stable, superior antiviral effect on kidney cells treated with H1N1 and less cytotoxicity (79.26% viability) than SeNPs (200 nm, 58.8%) and/or free AM (53.23%) | AM | [31] | ||
| Uniformly spherical Se@OTV (100 nm)—superior antiviral effect on kidney cells treated with H1N1 and less cytotoxicity (93% viability) than SeNPs (60%) and/or free OTV (53%) | Oseltamivir (OTV) | [32] | |||
| PEG-PLGA | Uniformly spherical shape; NPs (178 nm & 197 nm) loaded with diphyllin and bafilomycin; high biocompatibility and antiviral activity towards the NP drugs than the free drugs | Diphyllin & Bafilomycin | [33] | ||
| PLGA | Uniform, spherical/smooth surface NPs (116−143 nm); three GCV pro-drugs can be separately loaded on PLGA NPs; non-cytotoxic PLGA NPs (24 h and 48 h contact of three diverse NPs concentrations with HCEC cell) | Gangiclovir (GCV) | HSV a -1 | [34] | |
| HPAC | 99% drug loading efficiency; HPAC (diverse concentrations)—non-cytotoxic towards human epithelial cells (vaginal, corneal), foreskin fibroblasts, HeLa cells: cell viability >75% | Acyclovir (ACV) | HSV | [35] | |
| PLGA | Polydisperse particles loaded with LAM (221−250 nm); slow NPs degradation in simulated intestinal fluid PBS; the molecular interaction between polymer and LAM confirmed by FTIR and DSC | Lamivudine (LAM) | HIV | [36] | |
| PLGA | Spherical/smooth surface NFV NPs (~185 nm); almost narrow distribution | Efavirenz (EFV) | [37] | ||
| PEO-PCL | Spherical PEO-PCL NPs (~200−270 nm)with smooth surface; SQV is encapsulated into NPs | Saquinavir (SQV) | [38] | ||
| PLGA-PEG | Spherical shape (~125 nm) for those loaded with SAHA and NFV; ~119 nm for those loaded with SAHA; ~118 nm for NPs loaded with NFV | Suberoylanilide hydroxamic acid (SAHA) and NFV | [39] | ||
| Hybrid NPs (PLGA, PLA, MMA-SPM, and PMMA) | Almost spherical NPs; PLGA NPs (58~224 nm) and MMA-SPM NPs (91−823 nm); nontoxic NPs (male mice) | LAM+AZT (Zidovudine) | [40] | ||
| PMA coated MNP | Uniformly spherical NPs conjugated with ENF (35.2 nm); nontoxic in vivo and in vitro NPs conjugated with ENF | Enfuvirtide (ENF) | [41] | ||
| Nanospheres (NS) | Chitosan (Cs) | Spherical NS (~200 nm diameter) with smooth surface; polydisperse NS; after contact with NS has a satisfactory Vero cell viability; 86% ACV encapsulation efficiency | ACV | HSV | [42] |
| Dendrimers | PG b | Antiviral effect in vitro; nontoxic peptide-PG conjugates in vitro | Peptides | IAV c | [43] |
| Lipid NPs (LNPs) | DSPC d +MPEG+DSPE | LNPs (52~68 nm); in vivo antiHIV LNPS do not display local reactions, and also animal platelet counts were within normal limits | LPV e +TFV f +RTV g | HIV | [44] |
| PEG and phospholipids | LNPs loaded with drug or drugs (33~68 nm); incorporation efficiency (88~96%) | ATV h +TFV+RTV | [45] | ||
| Micelles | Cs-g-oligo(NiPAam) | Copolymers self-assembled in multimicellar aggregates by hydrodynamic (330~436 nm); high mucoadhesion and cytocompatibility properties | EFV | HIV | [46] |
| NPs | Virus | Mechanistic Aspects | Purpose | Refs. |
|---|---|---|---|---|
| Chiral gold NPs-quantum dot (QD) nanocomposites | CoVs | Chiral plasmon–exciton systems | Viral detection | [47] |
| Graphene oxide (GO) sheets | Feline CoVs | Organism models: fcwf-4 cells, DF-1 cells; association with viral lipid tails leading to aggregation and rupture of the envelop | Viral inhibition | [48] |
| GO sheets with silver particles | Feline CoVs | Association with viral lipid tails leading to aggregation with attachment of silver NPs with –SH group of protein and rupture of the envelop | Viral inhibition | [48] |
| Carbon electrodes modified with gold NPs | MERS CoVs | Recombinant spike protein S1 has been employed as a biomarker; the sensor is based on indirect competition between free virus in the sample and immobilized MERS-CoV protein for a fixed concentration of added antibody to the sample | Viral detection | [49] |
| Gold NP–adjuvanted S protein | SARS-CoVs | Stimulates significant antigen–specific IgG response against SARS–related CoV infection | Development of vaccines against severe pneumonia–associated CoVs | [50] |
| Cationic carbon dots based on curcumin | CoVs; porcine epidemic diarrhea virus (PEDV) | Inhibition of the viral proliferation; the structure of surface protein in viruses has been changed, thus prohibiting viral entry; cationic carbon dots based on curcumin can suppress the synthesis of negative-strand RNA and budding of the virus, and the accumulation of reactive oxygen species by virus. Further, it can suppress viral replication by stimulating the formation of interferon-stimulating genes (ISGs) and pro-inflammatory cytokines | Antiviral properties | [51] |
| Glutathione-capped Ag2S nanoclusters (NCs) | PEDV as a model of CoV | Ag2S NCs treatment prohibited the formation of viral negative-strand RNA and viral budding. It positively regulated the production of IFN-stimulating genes (ISGs) and the expression of pro-inflammation cytokines, which may inhibit the virus infection; inhibition of CoV proliferation | Viral inhibition | [52] |
| Chiral zirconium QDs | CoVs | The fluorescence properties of immuno-conjugated QD-magneto-plasmonic NPs | Viral detection | [53] |
| Virus | Target | Au NP Systems | Assay | Detection Limit & Detection Range | Time (min) | |
|---|---|---|---|---|---|---|
| Shape & Size (nm) | Biomolecule | |||||
| SARS- CoV | NC a protein | Spherical, 70 | DNA | Electrochemical | 2.5 pM, 50–2.5 pM | >120 |
| PP1ab b gene | Spherical, 13 | None | Colorimetric | 60 fmol, ND | 5 | |
| MERS-CoV | E protein gene (upE) and open reading frames (ORF) 1a | Spherical, 19 | DNA | Colorimetric | 1 pmol/μL | 10 |
| HCV | HCV antigen | Spherical, 12 | DNA | Electrical | 1 pg/μL, 10 ng/μL−1 pg/μL | 245 |
| HCV Ab | Spherical, 15 | SPA c | Scanometric | 3 ng/mL, 3 μg/mL−3 ng/mL | 10 | |
| Core gene | Spherical, 8~15 | DNA | DLS/Colorimetric | 0.36 pM, 0.3 μM−0.3 pM | 75 | |
| Full genome | Spherical, 15 | None | Colorimetric | 50 copies, ND | 110 | |
| Spherical, 8 | DNA | Fluorometric | 300 fM, 550−15 pM | 184 | ||
| 5′ UTR d gene | Spherical, 10~15 | DNA | Electrochemical | ~1 pM, 2.0−0.01 nM | 60 | |
| Spherical, 40 | Colorimetric | 2 fmol, 30−2 fmol | 50 | |||
| HBV | Anti-HBV | Spherical, 15 | SPA | Scanometric | 3 ng/mL, 3 μg/mL−3 ng/mL | 10 |
| C e gene | Spherical, 10 | DNA | Scanometric | 1 fM, 10−11−10−15 | 90 | |
| S f gene | Spherical, 8~15 | DNA | Colorimetric | 0.36 pM, 0.3 μM−0.3 pM | 75 | |
| Spherical, 5 | Avidin | Electrochemical | 0.7 ng/mL, 1.47−0.7 ng/mL | 105 | ||
| Spherical, 13 | DNA | Scanometric | 20 fM, At 20 fM | 330 | ||
| HBV DNA | Rod, ND | DNA | Fluorometric | 15 pM, 6.0−0.045 nM | ~60 | |
| HBeAg g | Rod, L = 46, D = 13 | Ab | Fluorometric | 8.3 ng/mL, Up to 264 ng/mL | ND | |
| HBsAg | Spherical, 10, 50, 100 | Ab | DLS h | 0.005 IU/mL, 1−0.005 IU/mL | ˂60 | |
| Spherical, 16 | Ab | Electrochemical | 0.1 ng/mL, 650−0.5 ng/mL | 65 | ||
| Spherical, ~10 | Ab | 2.3 pg/mL, 1.0−0.01 ng/mL | 60 | |||
| Spherical, 16 | Ab | 87 pg/mL, 1500−0.1 ng/mL | ~50 | |||
| Rod, L = 46, D = 13 | Ab | Fluorometric | 9 ng/mL, Up to 288 ng/mL | ND | ||
| Rod, L = 68, D = n30 | Ab | LSPR i | 0.01 IU/mL, 1−0.01 IU/mL | ND | ||
| Spherical, 5.5 | Peptide | Fluorometric | 0.1 pg/mL, 0.1−0.0001 ng/mL | 30 | ||
| H1N1 | Anti-H1N1 | Spherical, 20 | Ab | DLS | ˂100 TCID50/mL, 1.4 × 106−5.5 × 103 TCID50/mL | 30 |
| NA gene | Spherical, ND | DNA | SERS j | 25 nM, 50−25 nM | ND | |
| Fluorometric | ||||||
| HA antigen | Spherical, 25 | Protein A | Fluorometric | 13.9 pg/mL, 800−12.5 ng/mL | ND | |
| M k gene | Spherical, ND | Avidin | Electrochemical | 577 pM, 3−0.001 pmol | 80–50 | |
| H5N1 | NA l gene | Spherical, 15 | DNA | Scanometric | 100 fM &103 TCID50, ND | 150 |
| HA m gene | Spherical, 32 | Ab | Colorimetric | 40–0.1 ng, 100−0.1 ng | ND | |
| Spherical, ND | DNA | Electrochemical | 0.4 pM, 1.0 nM−5.0 pM | 20 | ||
| Spherical, 3 | Daunorubicin | Scanometric | 10 pM, 10 pM−100 nM | 20 | ||
| Spherical, 1.4 | None | 10 pM, 10 pM−100 nM | 90 | |||
| Spherical, 15 | DNA | 100 fM & 103 TCID50 | 150 | |||
| Particles | Spherical, 10 | Pentabody | Colorimetric | 10 ng/mL, 10−1−10−4 μg/mL | 35 | |
| Spherical, 22 | Ab | Fluorometric | 0.09 ng/mL, 12−0.27 ng/mL | 30 | ||
| HSV-2 | Anti-HSV-2 | Spherical, ND | Ab | Colorimetric | ND, ND | 20 |
| HIV | P24 Antigen n | Spherical, 15 | Avidin/DNA | Scanometric | 0.1 pg/mL, 500−0.1 pg/mL | ~360 |
| Spherical, 30 | Avidin/DNA | DLS | 0.2 pM, 31.4 pM−1.6 fM | >170 | ||
| Spherical, 30 | Ab/DNA | IPCRo | 0.1 pg/mL, 1000−0.1 pg/mL | >85 | ||
| Spherical, 30 | Ab/DNA | 1 pg/mL, 10,000−1 pg/mL | >125 | |||
| Polp gene | Spherical, 13 | DNA | DLS | 10 fM, 10 nM−1 fM | 80 | |
| Spherical, 3 | DNA | Electrochemical | 0.34 fM, 1.0 µM−0.1 pM | 50 | ||
| Type of CoVs | NPs | Adjuvant | Findings | Refs. |
|---|---|---|---|---|
| Swine TGEV | Gold NPs | Gold NPs | Acceleration of the peritoneal macrophages respiratory activity and plasma IFN-γ level | [84] |
| SARS-CoV | Gold NPs | Gold NPs | Stimulation of IgG response | [50] |
| MERS-CoV (RBD antigen) | Ferritin-based NPs | - | Stimulation of CD4+ T-cells and IFN-γ TNF-α responses | [85] |
| MERS-CoV (RBD antigen) | Hollow polymeric NPs | STING agonist (cdGMP) | Stimulation of remarkable levels of humoral responses and IgG2a antibodies, with no stimulation in lung eosinophilic immunopathology | [86] |
| MERS-CoV | Spike protein NPs | Aluminum | Stimulation of significant titers of neutralizing antibody and Th2 immune response, with no stimulation of Th1 immune response | [87] |
| Nanocarriers Delivery System | Virus | Antigen/Adjuvant | Disease | Route of Administration | Refs. |
|---|---|---|---|---|---|
| Gold NPs | HIV-1 | Viral plasmid DNA | HIV/AIDS pandemic | Intradermal | [90] |
| Gold NPs | H1N1 | Membrane matrix protein 2 (M2e)/CpG | Influenza | Intranasal | [91] |
| Gold NPs | FMDV a | Viral protein | Foot and mouth disease | Intradermal | [92] |
| Gold NPs | H1N1, H3N2, H5N1 | M2e/CpG | Influenza | Intranasal | [93] |
| Chitosan NPs | HBV b | HBsAg | Hepatitis B | Intraperitoneal | [94] |
| Carbon magnetic NPs | - | Hen egg lysozyme | - | Intravenous | [95] |
| Chitosan NPs | NV c | Live virus vaccine | Newcastle disease | Intranasal or oral | [96] |
| PCL d/chitosan NPs | HBV | HBsAg | Hepatitis B | Intranasal | [97] |
| γ-PGA NPs | H1N1 | Hemagglutinin (HA) | Influenza | Intranasal | [98] |
| VLPs | NV | Capsid protein | Norwalk virus infection | Oral | [88] |
| VLPs | HBV | Nucleocapsid protein | Hepatitis | Intravenous | [89] |
| VLPs | H3N2 | Structural proteins, e.g., HA, neuraminidase, NA, and matrix (M1) | Influenza | Intramuscular | [99] |
| VLPs | RV e 8-2/6/7-VLP | Multiple proteins, e.g., cholera toxin (CT) and Escherichia coli heat-labile toxin (LT) | Rotavirus | Rectal | [100] |
| Polypeptide NPs | CoV | Viral protein (spike) | SARS-CoV | - | [101] |
| Alginate coated chitosan NPs | HBV | HBsAg | Hepatitis B | Intranasal | [102] |
| PLA and PLGA NPs | HBV | Hepatitis B surface antigen | Hepatitis B | Pulmonary or intramuscular | [103] |
| PLA and PLGA nano/micropraticles | TT f | Tetanus toxoid | Tetanus | Intramuscular | [104] |
| PLGA NPs | BPI3V g | BPI3V proteins | Bovine respiratory | Intranasal | [105] |
| Polyanhydride | RSV | F and G glycoproteins | Bovine respiratory syncytial | Intranasal | [106] |
| HPMA/NIPAM h | RSV | F protein/TLR-7/8 agonist | RSV, influenza, HIV-1 | Intramuscular, intranasal, intravenous | [107,108] |
| DLPC i liposomes | H1N1 | M2, HA, NP/MPL j and trehalose 6,6′ dimycolate | Influenza | Intramuscular, intratracheal, intranasal | [109] |
| Cationic nanomicelles based on PSA k | HIV-1 | PSA/mRNA encoding | HIV/AIDS | - | [110] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasrollahzadeh, M.; Sajjadi, M.; Soufi, G.J.; Iravani, S.; Varma, R.S. Nanomaterials and Nanotechnology-Associated Innovations against Viral Infections with a Focus on Coronaviruses. Nanomaterials 2020, 10, 1072. https://doi.org/10.3390/nano10061072
Nasrollahzadeh M, Sajjadi M, Soufi GJ, Iravani S, Varma RS. Nanomaterials and Nanotechnology-Associated Innovations against Viral Infections with a Focus on Coronaviruses. Nanomaterials. 2020; 10(6):1072. https://doi.org/10.3390/nano10061072
Chicago/Turabian StyleNasrollahzadeh, Mahmoud, Mohaddeseh Sajjadi, Ghazaleh Jamalipour Soufi, Siavash Iravani, and Rajender S. Varma. 2020. "Nanomaterials and Nanotechnology-Associated Innovations against Viral Infections with a Focus on Coronaviruses" Nanomaterials 10, no. 6: 1072. https://doi.org/10.3390/nano10061072
APA StyleNasrollahzadeh, M., Sajjadi, M., Soufi, G. J., Iravani, S., & Varma, R. S. (2020). Nanomaterials and Nanotechnology-Associated Innovations against Viral Infections with a Focus on Coronaviruses. Nanomaterials, 10(6), 1072. https://doi.org/10.3390/nano10061072







