Rutin-Loaded Poloxamer 407-Based Hydrogels for In Situ Administration: Stability Profiles and Rheological Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
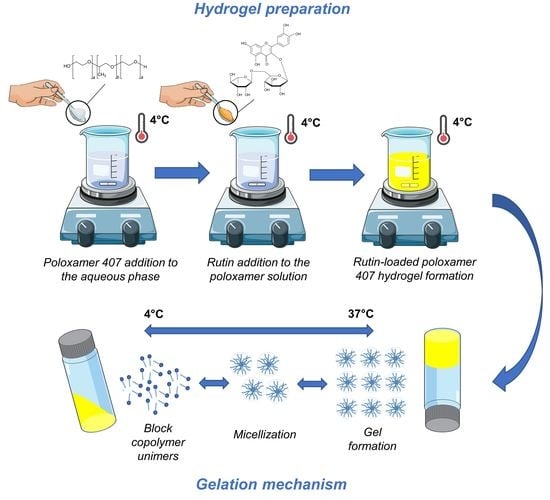
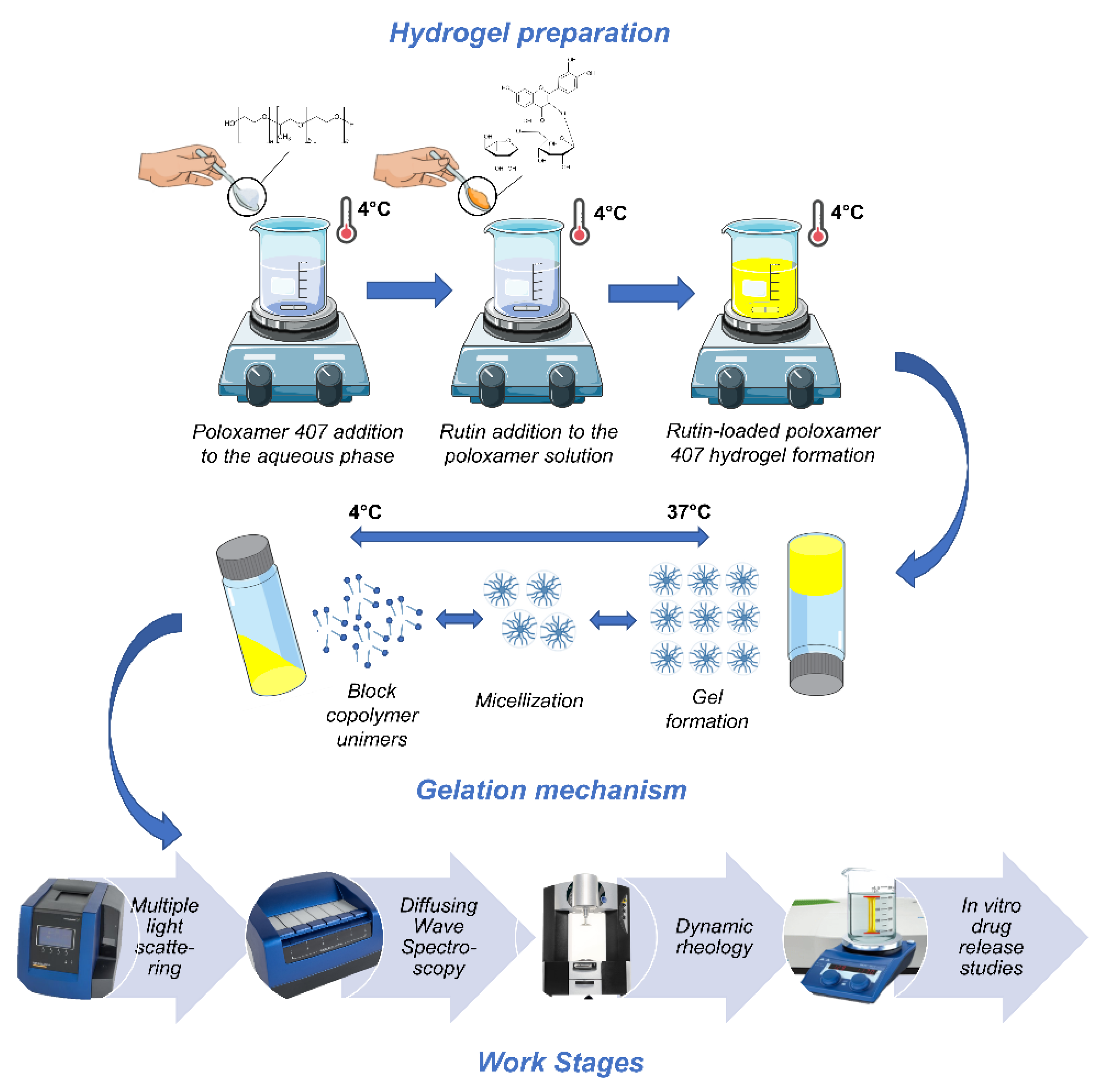
2.2. Hydrogel Preparation
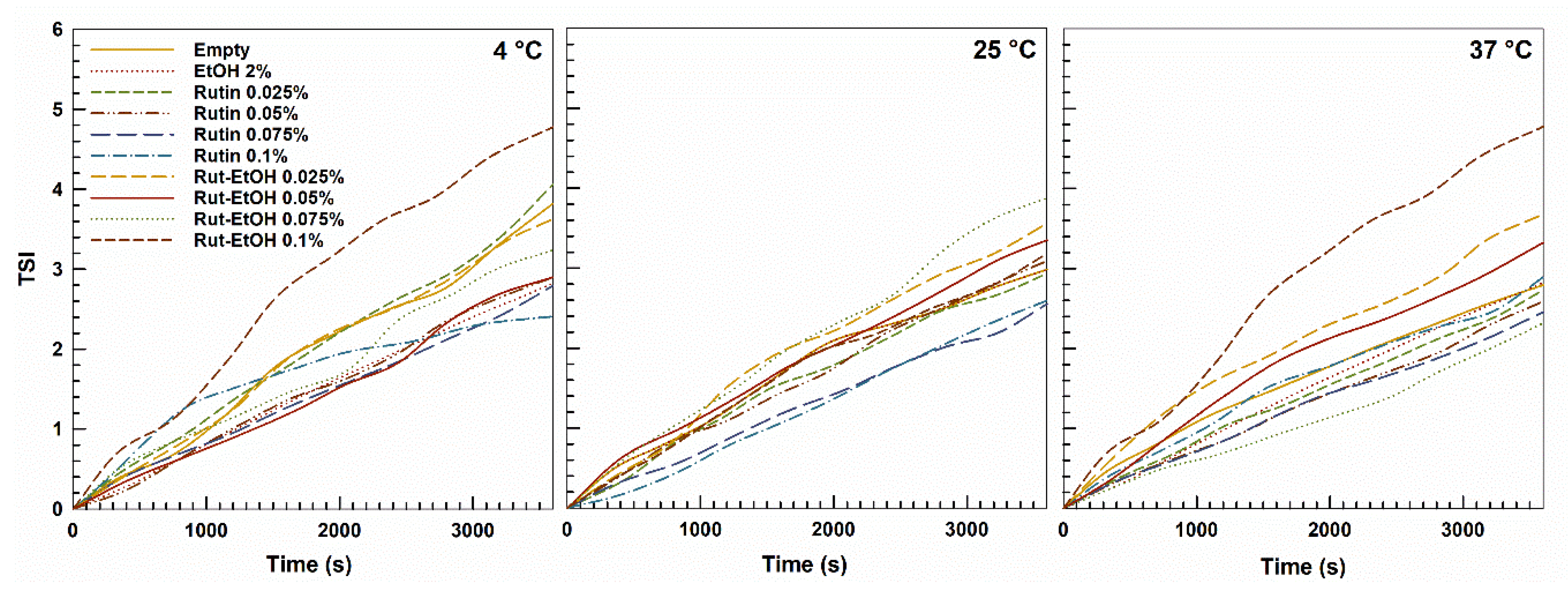
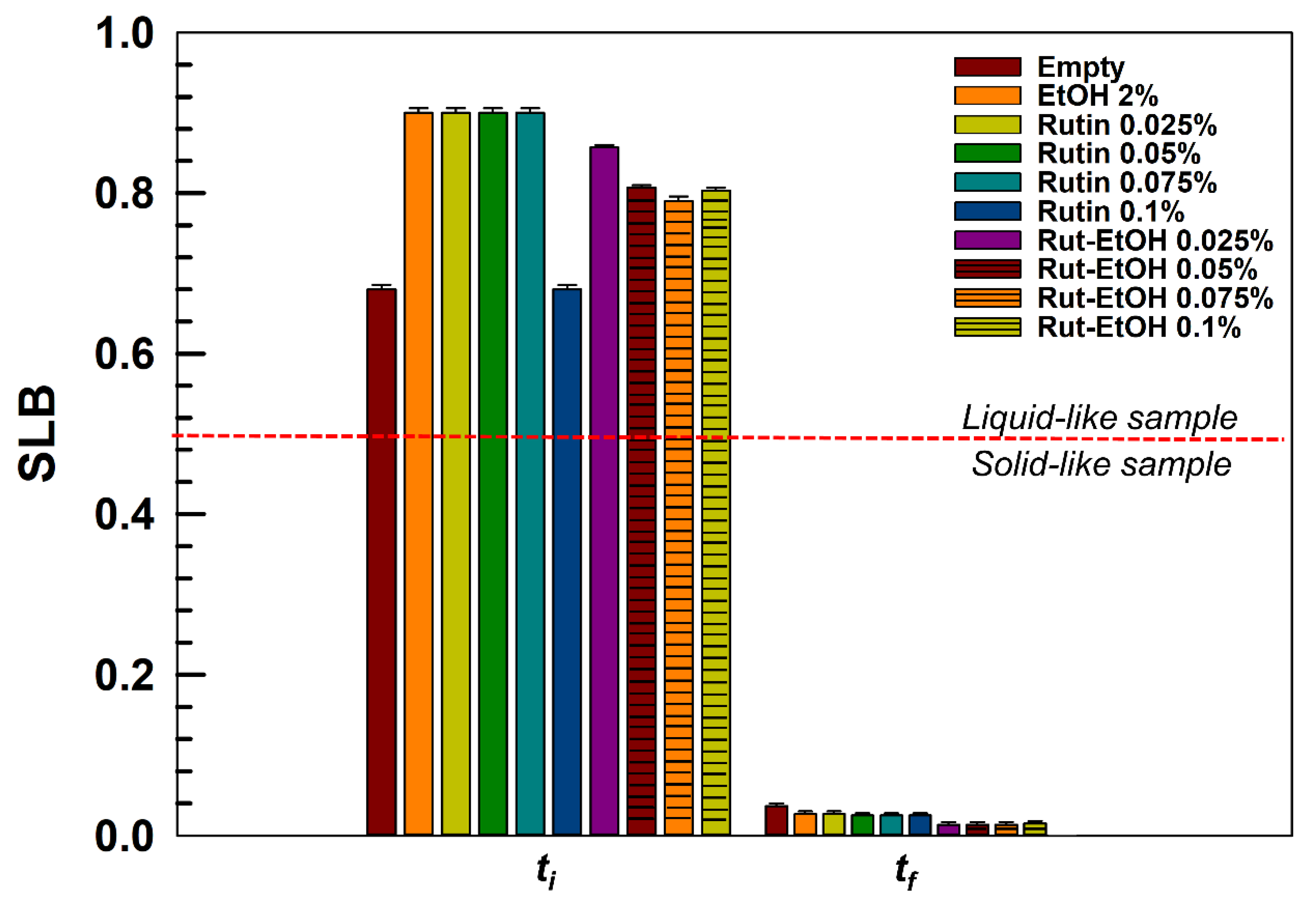
2.3. Stability Evaluation—Multiple Light Scattering
2.4. Rheological Analyses
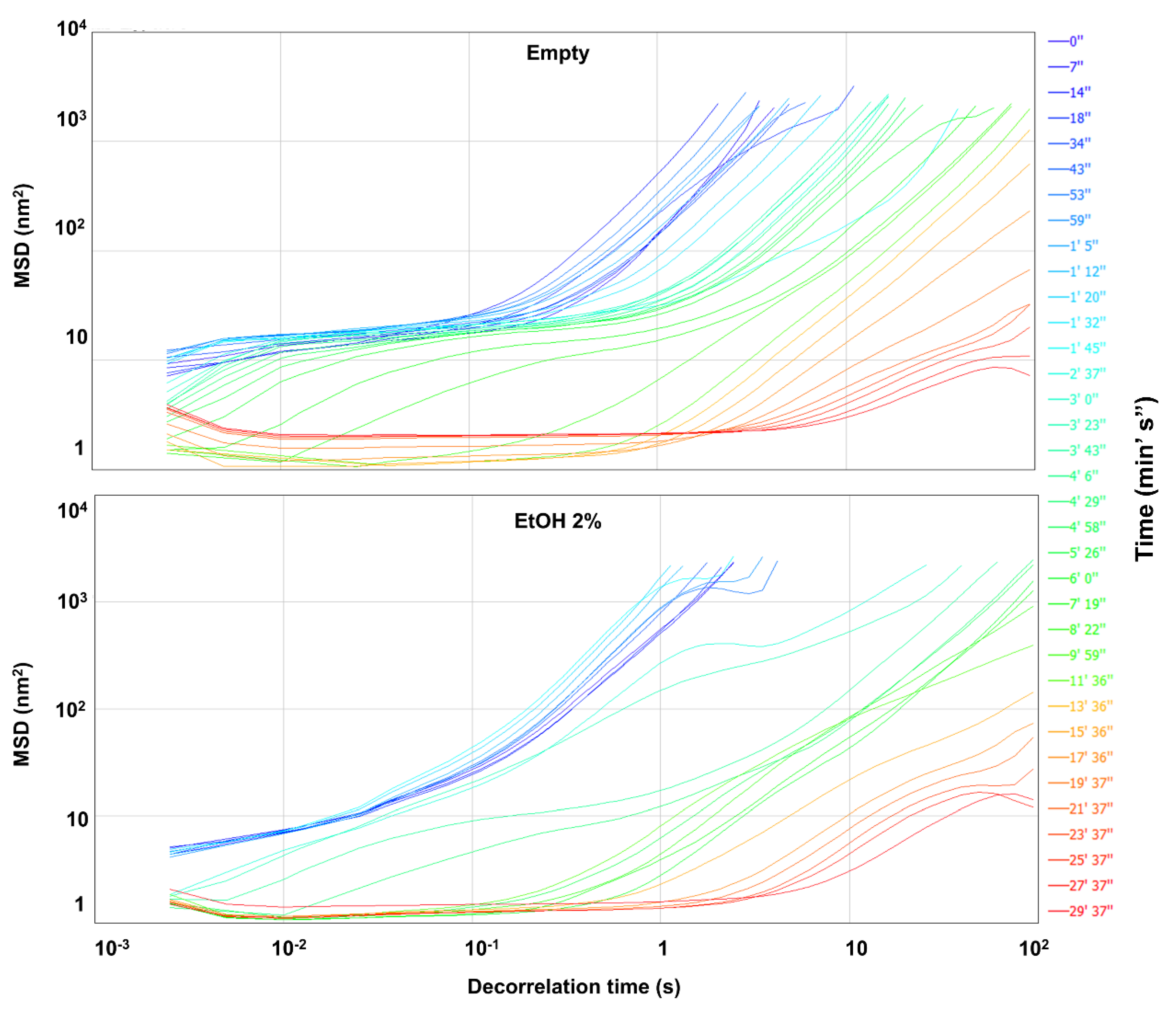
2.4.1. Diffusing Wave Spectroscopy
2.4.2. Dynamic Rheology
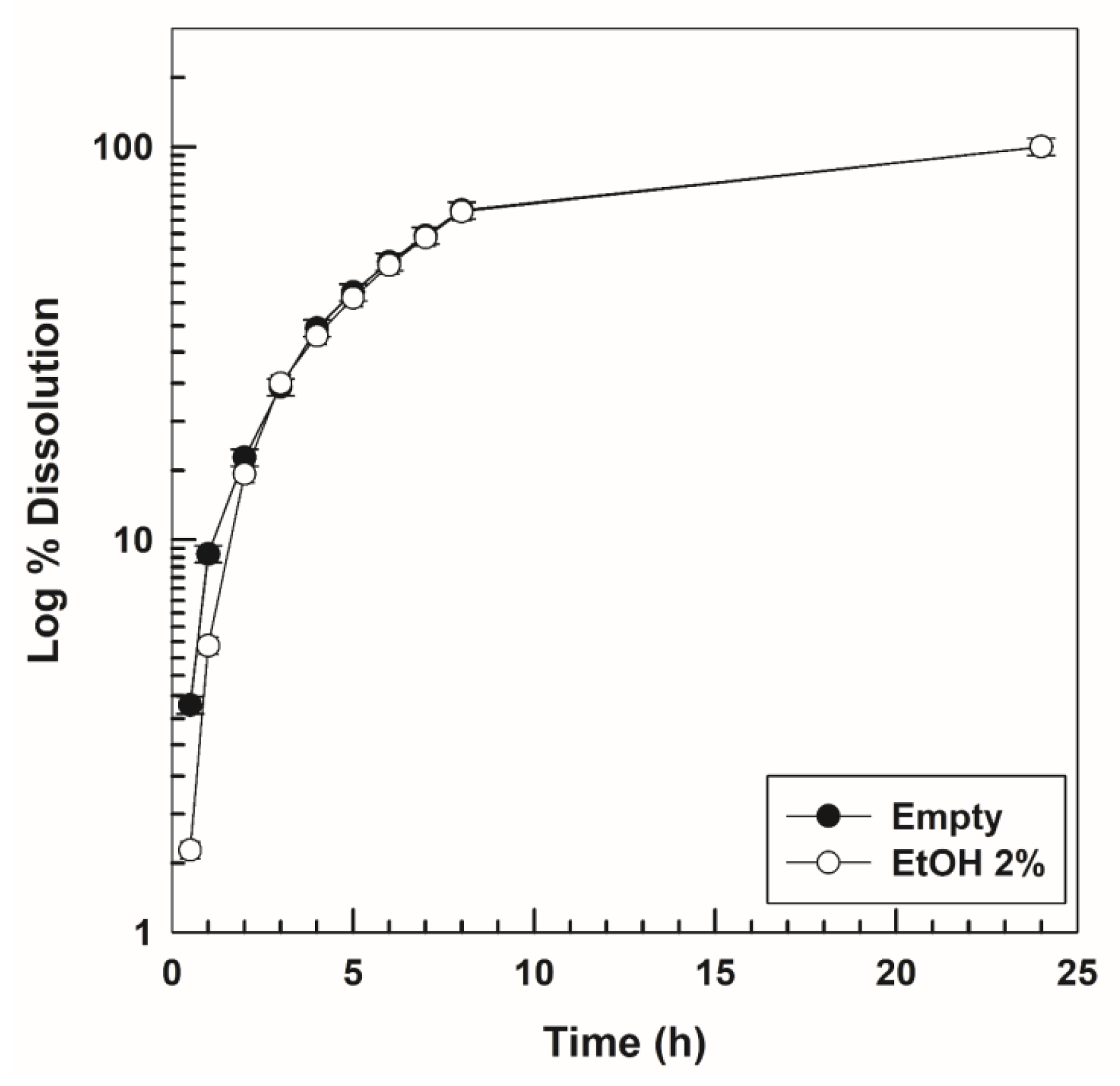
2.5. In Vitro Gel Dissolution Test
2.6. Evaluation of the Drug-Release Profiles
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Stability Evaluation of P407-Based Hydrogels
3.2. Rheological Evaluation
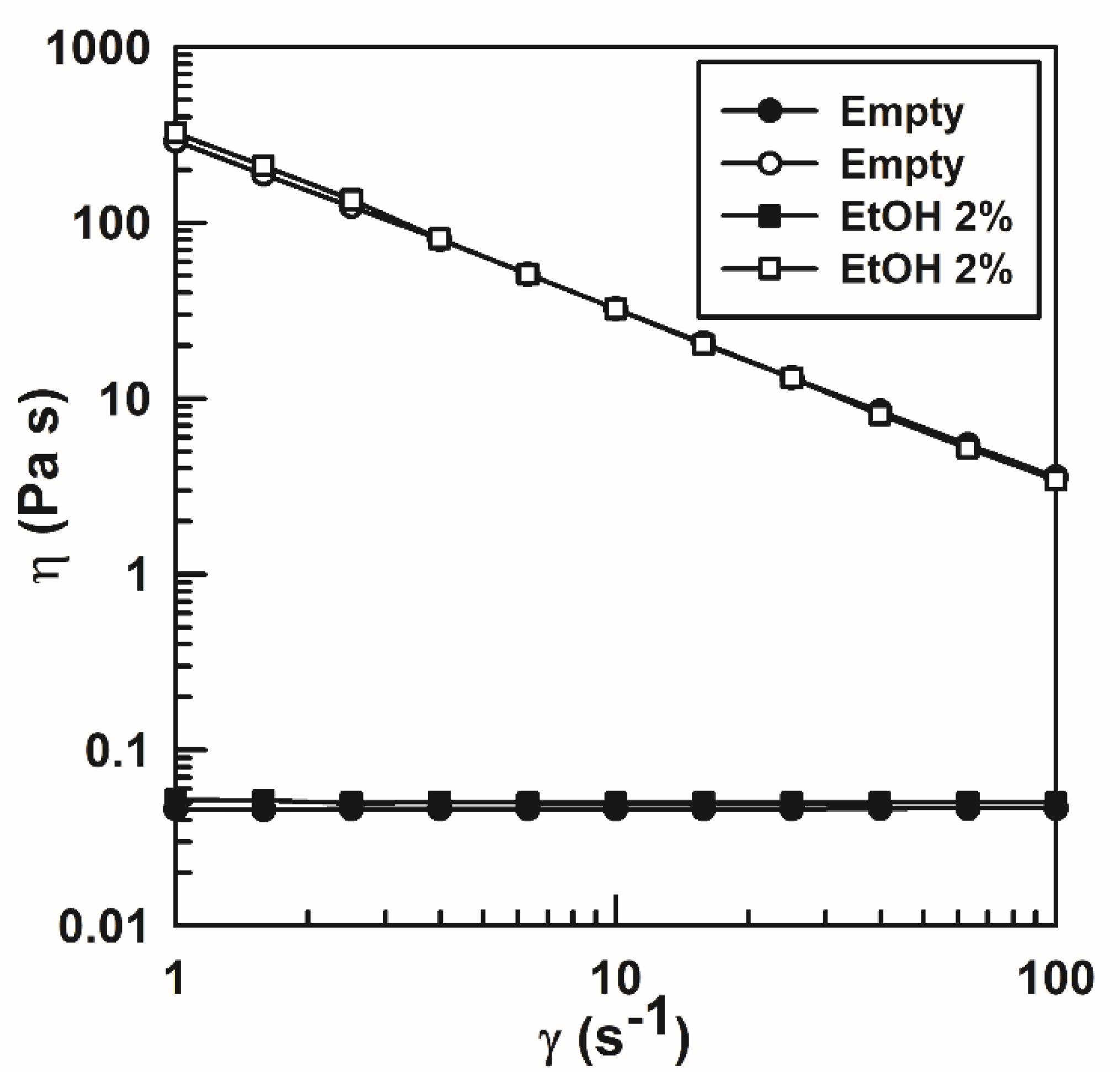
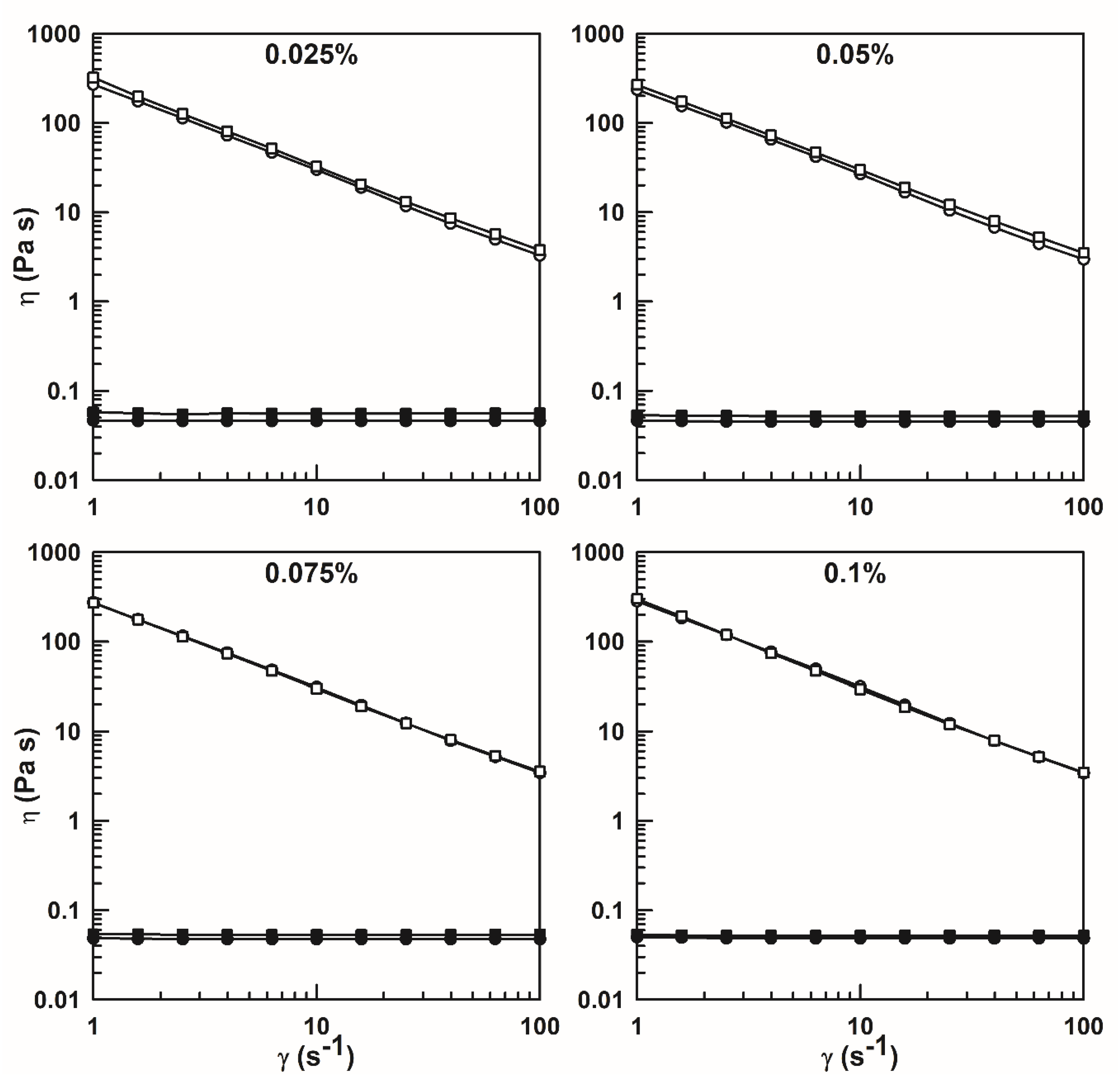
3.2.1. Diffusing Wave Spectroscopy
3.2.2. Dynamic Rheological Measurements
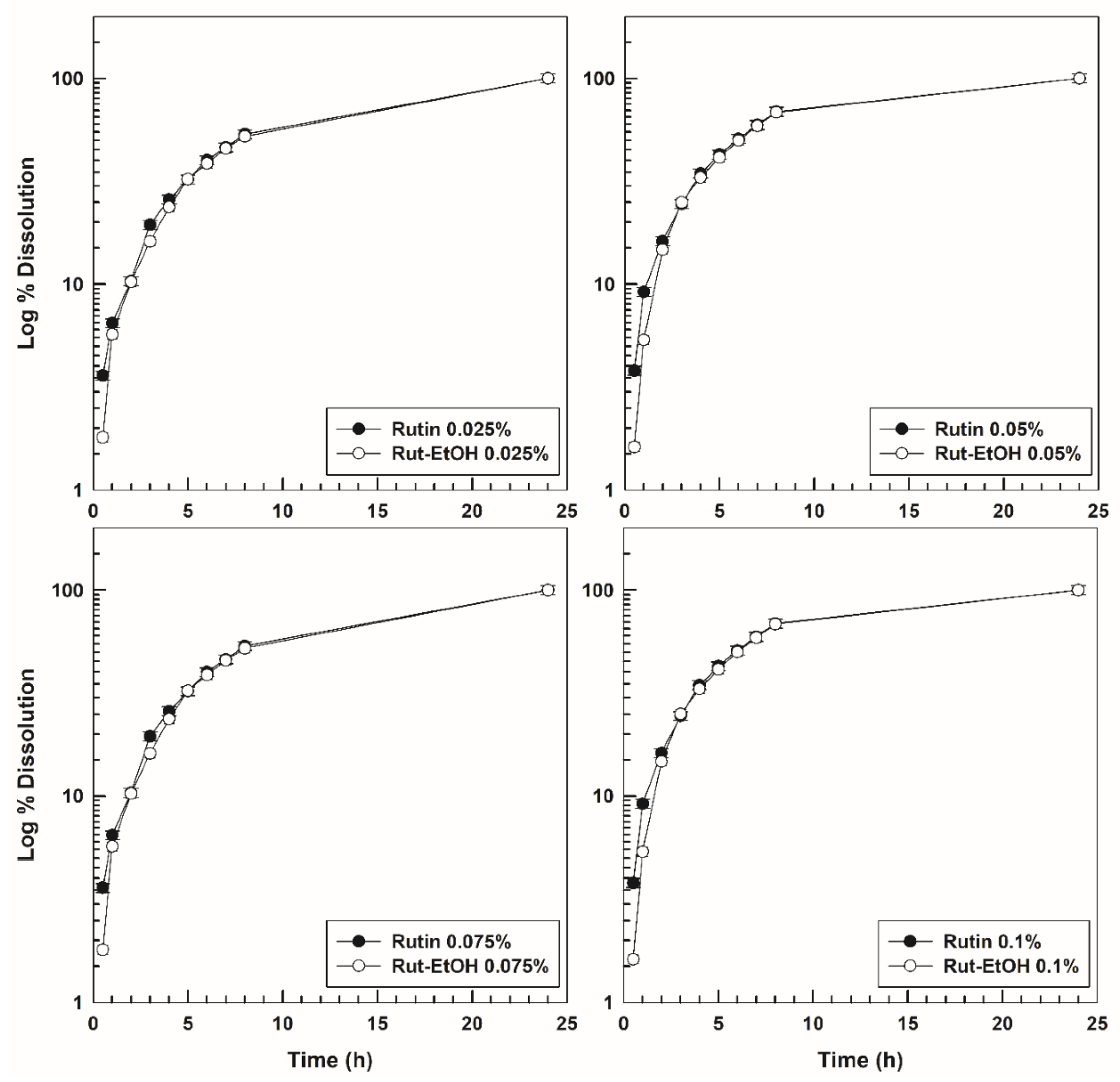
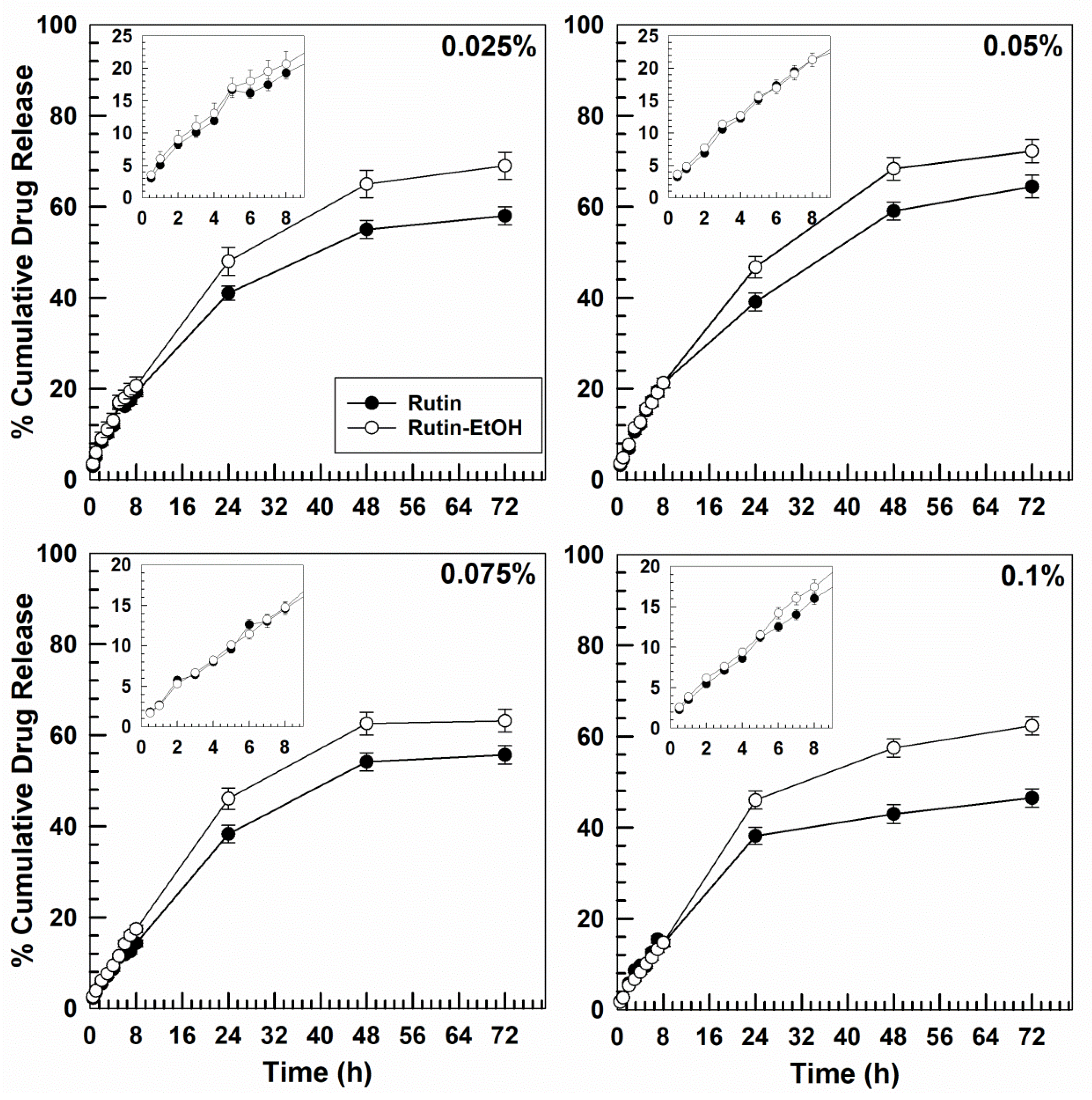
3.3. In Vitro Dissolution and Drug-Release Profiles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Drug-loaded biocompatible nanocarriers embedded in poloxamer 407 hydrogels as therapeutic formulations. Medicines 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for hydrophobic drug delivery. Classification, synthesis and applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mat. Sci. Eng. C Mater. 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Pillai, J.; Thulasidasan, A.K.; Anto, R.; Chithralekha, D.; Narayanan, A.; Kumar, G.S. Folic acid conjugated cross-linked acrylic polymer (FA-CLAP) hydrogel for site specific delivery of hydrophobic drugs to cancer cells. J. Nanobiotechnol. 2014, 12, 25. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.; Cho, H. Hydrogel-based drug delivery systems for poorly water-soluble drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Basso, J.; Miranda, A.; Nunes, S.; Cova, T.; Sousa, J.; Vitorino, C.; Pais, A. Hydrogel-based drug delivery nanosystems for the treatment of brain tumors. Gels 2018, 4, 62. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef]
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.; Douglas, T.E.L.; Volodkin, D.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of hybrid materials—The place of inorganics-in-organics in it, their composition and applications. Front. Chem. 2019, 7, 179. [Google Scholar] [CrossRef]
- Guo, F.; Huang, K.; Niu, J.; Kuang, T.; Zheng, Y.; Gu, Z.; Zou, J. Enhanced osseointegration of double network hydrogels via calcium polyphosphate incorporation for bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Shimano, K.; Okazaki, H.; Kurokawa, T.; Nakajima, T.; Nonoyama, T.; King, D.R.; Gong, J.P. Tough particle-based double network hydrogels for functional solid surface coatings. Adv. Mater. Interfaces 2018, 5, 1801018. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. Applications of poloxamers in ophthalmic pharmaceutical formulations: An overview. Expert Opin. Drug Deliv. 2013, 10, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Schmolka, I.R. Physical basis for poloxamer interactions. Ann. N. Y. Acad. Sci. 1994, 720, 92–97. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef]
- Vashi, A.V.; Keramidaris, E.; Abberton, K.M.; Morrison, W.A.; Wilson, J.L.; O’Connor, A.J.; Cooper-White, J.J.; Thompson, E.W. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials 2008, 29, 573–579. [Google Scholar] [CrossRef]
- Akash, M.S.; Rehman, K. Recent progress in biomedical applications of pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef]
- Cho, H.; Jammalamadaka, U.; Tappa, K.; Egbulefu, C.; Prior, J.; Tang, R.; Achilefu, S. 3D printing of poloxamer 407 nanogel discs and their applications in adjuvant ovarian cancer therapy. Mol. Pharm. 2019, 16, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.; Nazarova, I.; Astafieva, I.; Batrakova, E.; Alakhov, V.; Yaroslavov, A.; Kabanov, V. Micelle formation and solubilization of fluorescent probes in poly(oxyethylene-boxypropilene-b-oxyethylene) solutions. Macromolecules 1995, 28, 2303–2314. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarthf, J.F.; Hatton, T.A. Micellization of poly(ethy1ene oxide)-poly(propy1ene oxide)-poly(ethy1ene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Gu, D.; O’Connor, A.J.; Qiao, G.G.H.; Ladewig, K. Hydrogels with smart systems for delivery of hydrophobic drugs. Expert Opin. Drug Deliv. 2017, 14, 879–895. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Failla, P.; Costa, N.; Pullano, S.; Fiorillo, A.; Mollace, V.; Fresta, M.; Paolino, D. Rutin-loaded chitosan microspheres: Characterization and evaluation of the anti-inflammatory activity. Carbohyd. Polym. 2016, 152, 583–591. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Arasu, M.V.; Park, C.H.; Park, S.U. An up-to-date review of rutin and its biological and pharmacological activities. EXCLI J. 2015, 14, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Tech. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Almeida, J.S.; Lima, F.; Ros, S.D.; Bulhoes, L.O.; de Carvalho, L.M.; Beck, R.C. Nanostructured systems containing rutin: In vitro antioxidant activity and photostability studies. Nanoscale Res. Lett. 2010, 5, 1603–1610. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.S.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef]
- Celik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Antioxidant capacity of quercetin and its glycosides in the presence of β-cyclodextrins: Influence of glycosylation on inclusion complexation. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 309–319. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Piotrowska, H.; Szymanowska-Powałowska, D.; Lewandowska, K.; Goscianska, J.; Pietrzak, R.; Bednarski, W.; Majka, Z.; Cielecka-Piontek, J. Complex of rutin with β-cyclodextrin as potential delivery system. PLoS ONE 2015, 10, e0120858. [Google Scholar] [CrossRef]
- Tran, N.Q.; Joung, Y.K.; Lih, E.; Park, K.D. In situ forming and rutin-releasing chitosan hydrogels as injectable dressings for dermal wound healing. Biomacromolecules 2011, 12, 2872–2880. [Google Scholar] [CrossRef]
- Almeida, J.; Benvegnú, D.; Boufleur, N.; Reckziegel, P.; Barcelos, R.; Coradini, K.; De Carvalho, L.; Bürger, M.; Beck, R. Hydrogels containing rutin intended for cutaneous administration: Efficacy in wound healing in rats. Drug Dev. Ind. Pharm. 2012, 38, 792–799. [Google Scholar] [CrossRef]
- Park, S.N.; Lee, M.H.; Kim, S.J.; Yu, E.R. Preparation of quercetin and rutin-loaded ceramide liposomes and drug-releasing effect in liposome-in-hydrogel complex system. Biochem. Biophys. Res. Commun. 2013, 435, 361–366. [Google Scholar] [CrossRef]
- Soni, H.; Malik, J.; Singhai, A.; Sharma, S. Antimicrobial and antiinflammatory activity of the hydrogels containing rutin delivery. Asian J. Chem. 2013, 25, 8371–8373. [Google Scholar] [CrossRef]
- Schmolka, I.R. Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Gagliardi, A.; Bonacci, S.; Paolino, D.; Celia, C.; Procopio, A.; Fresta, M.; Cosco, D. Paclitaxel-loaded sodium deoxycholate-stabilized zein nanoparticles: Characterization and in vitro cytotoxicity. Heliyon 2019, 5, e02422. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Wu, T.; Liang, B.; Shi, C.; Zhang, M. Effect of superfine grinding on the structural and physicochemical properties of whey protein and applications for microparticulated proteins. Food Sci. Biotechnol. 2015, 24, 1637–1643. [Google Scholar] [CrossRef]
- Rohart, A.; Michon, C.; Confiac, J.; Bosc, V. Evaluation of ready-to-use SMLS and DWS devices to study acid-induced milk gel changes and syneresis. Dairy Sci. Technol. 2016, 96, 459–475. [Google Scholar] [CrossRef]
- Brunel, L. Method for the Rheological Charaterisation of a Complex Medium. WO2010130766, 18 November 2010. [Google Scholar]
- Sun, C.; Liu, R.; Wu, T.; Liang, B.; Shi, C.; Cong, X.; Hou, T.; Zhang, M. Combined superfine grinding and heat-shearing treatment for the microparticulation of whey proteins. Food Bioprocess Tech. 2016, 9, 378–386. [Google Scholar] [CrossRef]
- Xu, D.; Aihemaiti, Z.; Cao, Y.; Teng, C.; Li, X. Physicochemical stability, microrheological properties and microstructure of lutein emulsions stabilized by multilayer membranes consisting of whey protein isolate, flaxseed gum and chitosan. Food Chem. 2016, 202, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Oh, Y.-K.; Choi, H.-g.; Kim, Y.B.; Kim, C.-K. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int. J. Pharm. 2002, 241, 155–163. [Google Scholar] [CrossRef]
- Xuan, J.-J.; Balakrishnan, P.; Oh, D.H.; Yeo, W.H.; Park, S.M.; Yong, C.S.; Choi, H.-G. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int. J. Pharm. 2010, 395, 317–323. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef]
- Ur-Rehman, T.; Tavelin, S.; Grobner, G. Effect of DMSO on micellization, gelation and drug release profile of Poloxamer 407. Int. J. Pharm. 2010, 394, 92–98. [Google Scholar] [CrossRef]
- Hemelryck, S.V.; Dewulf, J.; Niekus, H.; van Heerden, M.; Ingelse, B.; Holm, R.; Mannaert, E.; Langguth, P. In vitro evaluation of poloxamer in situ forming gels for bedaquiline fumarate salt and pharmacokinetics following intramuscular injection in rats. Int. J. Pharm. X 2019, 1, 100016. [Google Scholar] [CrossRef]
- Nie, S.; Hsiao, W.L.W.; Pan, W.; Yang, Z. Thermoreversible Pluronic F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 2011, 6, 151–166. [Google Scholar] [CrossRef]
- Ban, E.; Park, M.; Jeong, S.; Kwon, T.; Kim, E.-H.; Jung, K.; Kim, A. Poloxamer-based thermoreversible gel for topical delivery of emodin: Influence of P407 and P188 on solubility of emodin and its application in cellular activity screening. Molecules 2017, 22, 246. [Google Scholar] [CrossRef]
- Vyas, V.; Sancheti, P.; Karekar, P.; Shah, M.; Pore, Y. Physicochemical characterization of solid dispersion systems of tadalafil with poloxamer 407. Acta Pharm. 2009, 59, 453–461. [Google Scholar] [CrossRef]
- Amiji, M.M.; Lai, P.-K.; Shenoy, D.B.; Rao, M. Intratumoral administration of paclitaxel in an in situ gelling poloxamer 407 formulation. Pharm. Dev. Technol. 2002, 7, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liu, M.; Fu, H.; Zhang, W.; Peng, G.; Zhang, Y.; Cao, H.; Luo, L. Novel thermosensitive in situ gel based on poloxamer for uterus delivery. Eur. J. Pharm. 2015, 77, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.P.; Guo, Y.S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.H.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef]
- Cespi, M.; Bonacucina, G.; Pucciarelli, S.; Cocci, P.; Perinelli, D.R.; Casettari, L.; Illum, L.; Palmieri, G.F.; Palermo, F.A.; Mosconi, G. Evaluation of thermosensitive poloxamer 407 gel systems for the sustained release of estradiol in a fish model. Eur. J. Pharm. Biopharm. 2014, 88, 954–961. [Google Scholar] [CrossRef]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.; Marchetti, J.M. Sustained release of lidocaine from poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef]
- Chaibundit, C.; Ricardo, N.M.; Ricardo, N.M.; Muryn, C.A.; Madec, M.B.; Yeates, S.G.; Booth, C. Effect of ethanol on the gelation of aqueous solutions of Pluronic F127. J. Colloid Interface Sci. 2010, 351, 190–196. [Google Scholar] [CrossRef]
- Choi, H.-G.; Lee, M.-K.; Kim, M.-H.; Kim, C.-K. Effect of additives on the physicochemical properties of liquid suppository bases. Int. J. Pharm. 1999, 190, 13–19. [Google Scholar] [CrossRef]
- Abraham, J.; Sharika, T.; Mishra, R.K.; Thomas, S. 14—Rheological characteristics of nanomaterials and nanocomposites. In Micro and Nano Fibrillar Composites (MFCs and NFCs) from Polymer Blends; Mishra, R.K., Thomas, S., Kalarikkal, N., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 327–350. [Google Scholar] [CrossRef]
- Furst, E.M.; Squires, T.M. Microrheology; Oxford University Press: Oxford, UK, 2017; pp. 1–3. [Google Scholar]
- Zhu, Q.; Qiu, S.; Zhang, H.; Cheng, Y.; Yin, L. Physical stability, microstructure and micro-rheological properties of water-in-oil-in-water (W/O/W) emulsions stabilized by porcine gelatin. Food Chem. 2018, 253, 63–70. [Google Scholar] [CrossRef]
- Sun, C.; Wu, T.; Liu, R.; Liang, B.; Tian, Z.; Zhang, E.; Zhang, M. Effects of superfine grinding and microparticulation on the surface hydrophobicity of whey protein concentrate and its relation to emulsions stability. Food Hydrocolloids 2015, 51, 512–518. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT Food Sci. Technol. 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Ghica, M.V.; Hirjau, M.; Lupuleasa, D.; Dinu-Pirvu, C.E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef]
- Yong, C.S.; Sah, H.; Jahng, Y.; Chang, H.W.; Son, J.-K.; Lee, S.H.; Jeong, T.C.; Rhee, J.-D.; Baek, S.H.; Kim, C.-K. Physicochemical characterization of diclofenac sodium-loaded poloxamer gel as a rectal delivery system with fast absorption. Drug Dev. Ind. Pharm. 2003, 29, 545–553. [Google Scholar] [CrossRef]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef]
- Jalaal, M.; Cottrell, G.; Balmforth, N.; Stoeber, B. On the rheology of Pluronic F127 aqueous solutions. J. Rheol. 2017, 61, 139–146. [Google Scholar] [CrossRef]
- Grassi, G.; Crevatin, A.; Farra, R.; Guarnieri, G.; Pascotto, A.; Rehimers, B.; Lapasin, R.; Grassi, M. Rheological properties of aqueous Pluronic–alginate systems containing liposomes. J. Colloid Interface Sci. 2006, 301, 282–290. [Google Scholar] [CrossRef]
- Antunes, F.E.; Gentile, L.; Rossi, C.O.; Tavano, L.; Ranieri, G.A. Gels of Pluronic F127 and nonionic surfactants from rheological characterization to controlled drug permeation. Colloids Surf. B. Biointerfaces 2011, 87, 42–48. [Google Scholar] [CrossRef]
- Bhat, P.A.; Chat, O.A.; Zhang, Y.; Dar, A.A. An unprecedented dual responsive gelation of Carbopol induced by Pluronic P123 triblock copolymer. Polymer 2016, 102, 153–166. [Google Scholar] [CrossRef]
- El Kechai, N.; Bochot, A.; Huang, N.; Nguyen, Y.; Ferrary, E.; Agnely, F. Effect of liposomes on rheological and syringeability properties of hyaluronic acid hydrogels intended for local injection of drugs. Int. J. Pharm. 2015, 487, 187–196. [Google Scholar] [CrossRef]
- Airey, G.D. Rheological evaluation of ethylene vinyl acetate polymer modified bitumens. Constr. Build. Mater. 2002, 16, 473–487. [Google Scholar] [CrossRef]
- Edsman, K.; Carlfors, J.; Petersson, R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur. J. Pharm. Sci. 1998, 6, 105–112. [Google Scholar] [CrossRef]
- Gagliardi, A.; Froiio, F.; Salvatici, M.C.; Paolino, D.; Fresta, M.; Cosco, D. Characterization and refinement of zein-based gels. Food Hydrocoll. 2020, 101, 105555. [Google Scholar] [CrossRef]
- Pereira, G.G.; Dimer, F.A.; Guterres, S.S.; Kechinski, C.P.; Granada, J.E.; Cardozo, N.S.M. Formulation and characterization of poloxamer 407®: Thermoreversible gel containing polymeric microparticles and hyaluronic acid. Quím. Nova 2013, 36, 1121–1125. [Google Scholar] [CrossRef]
- Park, E.-K.; Song, K.-W. Rheological properties of poloxamer 407 solutions and gels. Annu. Trans. Nord. Rheol. Soc. 2011, 19, 1–5. [Google Scholar]
- Pozzo, D.C.; Walker, L.M. Small-angle neutron scattering of silica nanoparticles templated in PEO-PPO-PEO cubic crystals. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 117–129. [Google Scholar] [CrossRef]
- LaFollette, T.A.; Walker, L.M. Structural and mechanical hysteresis at the order-order transition of block copolymer micellar crystals. Polymers 2011, 3, 281–298. [Google Scholar] [CrossRef]
- Pozzo, D.C.; Hollabaugh, K.R.; Walker, L.M. Rheology and phase behavior of copolymer-templated nanocomposite materials. J. Rheol. 2005, 49, 759. [Google Scholar] [CrossRef]
- Bisharat, L.; Perinelli, D.R.; Berardi, A.; Bonacucina, G.; Logrippo, S.; Darwish Elhajji, F.W.; Cespi, M.; Palmieri, G.F. Influence of testing parameters on in vitro tramadol release from poloxamer thermogels using the immersion cell method. AAPS PharmSciTech. 2017, 18, 2706–2716. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.Y.; Wei, G.; Lu, W.Y. Effect of carrageenan on poloxamer-based in situ gel for vaginal use: Improved in vitro and in vivo sustained-release properties. Eur. J. Pharm. Sci. 2009, 37, 306–312. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, W.L.; Wang, J.C.; Zhang, X.; Zhang, H.; Wang, X.Q.; Zhou, T.Y.; Zhang, Q. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic® F127 hydrogel for subcutaneous administration: In vitro and in vivo characterization. J. Control. Release 2007, 117, 387–395. [Google Scholar] [CrossRef]
- Grindel, J.M.; Jaworski, T.; Piraner, O.; Emanuele, R.M.; Balasubramanian, M. Distribution, metabolism, and excretion of a novel surface-active agent, purified poloxamer 188, in rats, dogs, and humans. J. Pharm. Sci. 2002, 91, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.C.M.; Akkari, A.C.S.; Ferreira, I.R.S.; Maruyama, C.R.; Pascoli, M.; Guilherme, V.A.; de Paula, E.; Fraceto, L.F.; de Lima, R.; da Silva Melo, P. Poloxamer-based binary hydrogels for delivering tramadol hydrochloride: Sol-gel transition studies, dissolution-release kinetics, in vitro toxicity, and pharmacological evaluation. Int. J. Nanomed. 2015, 10, 2391. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Pillay, V. A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef]
- Dimitrova, E.; Bogdanova, S.; Mitcheva, M.; Tanev, I.; Minkov, E. Development of model aqueous ophthalmic solution of indomethacin. Drug Dev. Ind. Pharm. 2000, 26, 1297–1301. [Google Scholar] [CrossRef]
- Lukáč, M.; Prokipčák, I.; Lacko, I.; Devínsky, F. Solubilisation of griseofulvin and rutin in aqueous micellar solutions of gemini and heterogemini surfactants and their mixtures. Eur. J. Pharm. Sci. 2011, 44, 194–199. [Google Scholar] [CrossRef]
- Chat, O.A.; Najar, M.H.; Mir, M.A.; Rather, G.M.; Dar, A.A. Effects of surfactant micelles on solubilization and DPPH radical scavenging activity of Rutin. J. Colloid Interface Sci. 2011, 355, 140–149. [Google Scholar] [CrossRef]
- Demirci, S.; Doğan, A.; Karakuş, E.; Halıcı, Z.; Topçu, A.; Demirci, E.; Sahin, F. Boron and poloxamer (F68 and F127) containing hydrogel formulation for burn wound healing. Biol. Trace Elem. Res. 2015, 168, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Kumar, D.; Gopalkrishnan, A.; Pathak, N.N.; Kurade, N.P.; Tandan, S.K.; Kumar, D. Topical pluronic F-127 gel application enhances cutaneous wound healing in rats. Acta Histochem. 2014, 116, 5–13. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Li, N.; Gao, J.-Q.; Sun, H.; Chen, S. Sustained delivery of IL-1Ra from pluronic F127-based thermosensitive gel prolongs its therapeutic potentials. Pharm. Res. 2012, 29, 3475–3485. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chang, J.J.; Chien, C.T.; Yang, M.C.; Chien, H.F. Antioxidant sol-gel improves cutaneous wound healing in streptozotocin-induced diabetic rats. Exp. Diabetes Res. 2012, 2012, 504693. [Google Scholar] [CrossRef]
- Shin, S.-C.; Cho, C.-W.; Oh, I.-J. Enhanced efficacy by percutaneous absorption of piroxicam from the poloxamer gel in rats. Int. J. Pharm. 2000, 193, 213–218. [Google Scholar] [CrossRef]
- Cafaggi, S.; Russo, E.; Caviglioli, G.; Parodi, B.; Stefani, R.; Sillo, G.; Leardi, R.; Bignardi, G. Poloxamer 407 as a solubilising agent for tolfenamic acid and as a base for a gel formulation. Eur. J. Pharm. Sci. 2008, 35, 19–29. [Google Scholar] [CrossRef]


| Formulations | Rutin (%, w/w) | Ethanol (%, w/w) |
|---|---|---|
| Empty | - | - |
| EtOH | - | 2 |
| Rutin | 0.025 | - |
| 0.05 | - | |
| 0.075 | - | |
| 0.1 | - | |
| Rut-EtOH | 0.025 | 2 |
| 0.05 | 2 | |
| 0.075 | 2 | |
| 0.1 | 2 |
| Formulations | Rutin (%, w/w) | Tsol-gel 1 ( °C) |
|---|---|---|
| Empty | - | 24.08 |
| EtOH 2% | - | 24.08 |
| Rutin | 0.025 | 23.99 |
| 0.05 | 24.05 | |
| 0.075 | 24.02 | |
| 0.1 | 24.05 | |
| Rut-EtOH | 0.025 | 24.03 |
| 0.05 | 24.02 | |
| 0.075 | 24.02 | |
| 0.1 | 24.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, E.; Paolino, D.; Cristiano, M.C.; Fresta, M.; Cosco, D. Rutin-Loaded Poloxamer 407-Based Hydrogels for In Situ Administration: Stability Profiles and Rheological Properties. Nanomaterials 2020, 10, 1069. https://doi.org/10.3390/nano10061069
Giuliano E, Paolino D, Cristiano MC, Fresta M, Cosco D. Rutin-Loaded Poloxamer 407-Based Hydrogels for In Situ Administration: Stability Profiles and Rheological Properties. Nanomaterials. 2020; 10(6):1069. https://doi.org/10.3390/nano10061069
Chicago/Turabian StyleGiuliano, Elena, Donatella Paolino, Maria Chiara Cristiano, Massimo Fresta, and Donato Cosco. 2020. "Rutin-Loaded Poloxamer 407-Based Hydrogels for In Situ Administration: Stability Profiles and Rheological Properties" Nanomaterials 10, no. 6: 1069. https://doi.org/10.3390/nano10061069
APA StyleGiuliano, E., Paolino, D., Cristiano, M. C., Fresta, M., & Cosco, D. (2020). Rutin-Loaded Poloxamer 407-Based Hydrogels for In Situ Administration: Stability Profiles and Rheological Properties. Nanomaterials, 10(6), 1069. https://doi.org/10.3390/nano10061069