Al-Based Nano-Sized Composite Energetic Materials (Nano-CEMs): Preparation, Characterization, and Performance
Abstract
1. Introduction
2. Preparation Methods of Nano-CEMs
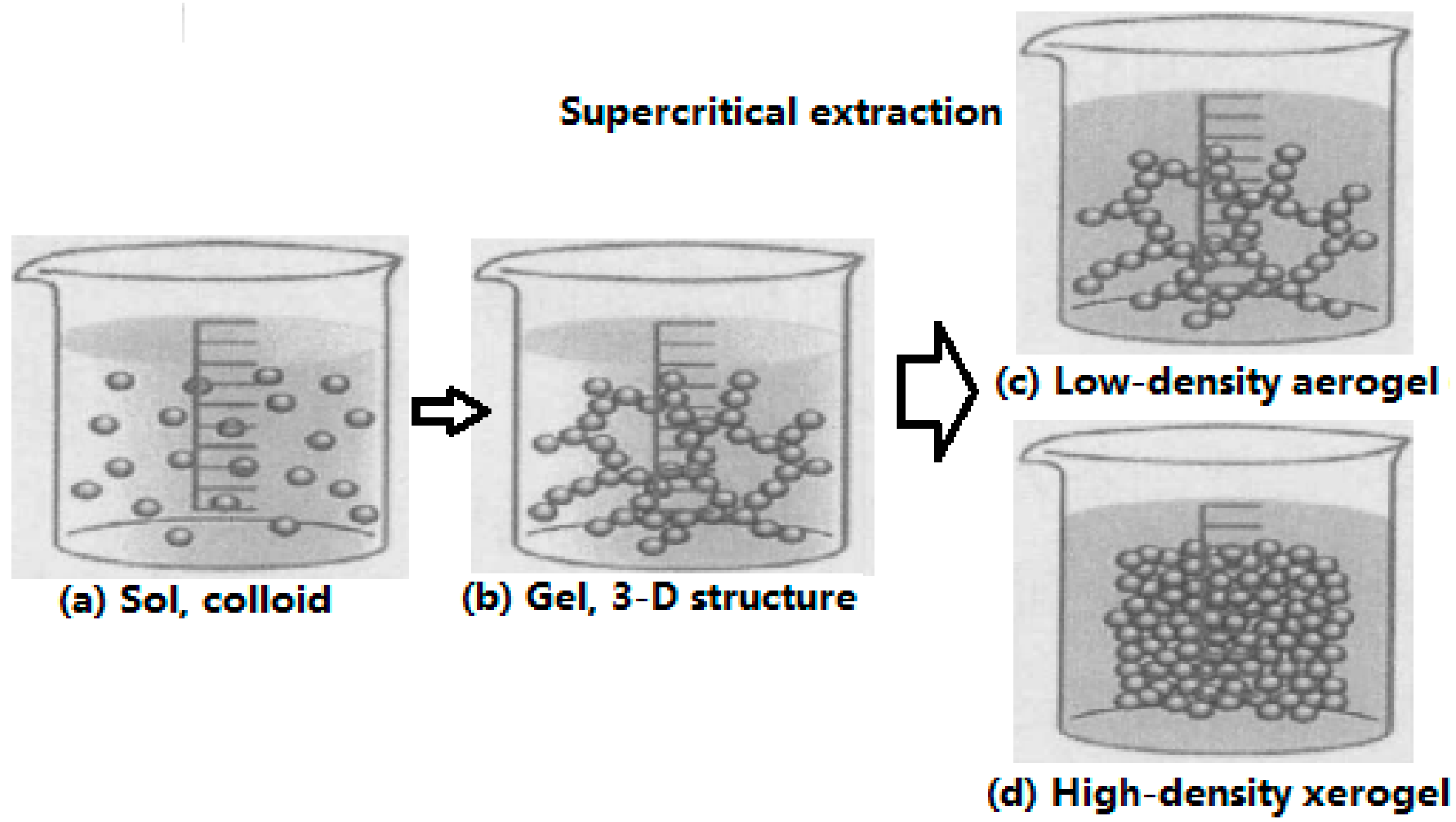
2.1. Sol–Gel Method
2.2. Solvent/Nonsolvent Method
2.3. High-Energy Ball Milling Method
2.4. Spray Drying Method
2.5. Other Methods
3. Characteristics and Performance of Nano-CEMs
3.1. Al-Based Binary System
3.1.1. Nano-Sized Al/Fe2O3 Energetic Composite
3.1.2. Nano-Sized Al/NC Energetic Composite
3.1.3. Nano-Sized Al/MnO2 Energetic Composite
3.1.4. Nano-Sized Al/CuO Energetic Composite
3.1.5. Nano-Sized Al/Co3O4 Composite
3.1.6. Nano-Sized Al/MoO3 Energetic Composite
3.1.7. Nano-Sized Al/Ni Energetic Composite
3.1.8. Nano-Sized Al/Fatty Acid Energetic Composite
3.1.9. Nano-Sized Al/Polymer Energetic Composite
3.2. Al-Based Ternary System
3.2.1. Nano-Sized Al/B/Fe2O3 Energetic Composite
3.2.2. Nano-Sized Al/CuO/KClO4 Energetic Composite
3.2.3. Nano-Sized Al/B/Ni Energetic Composite
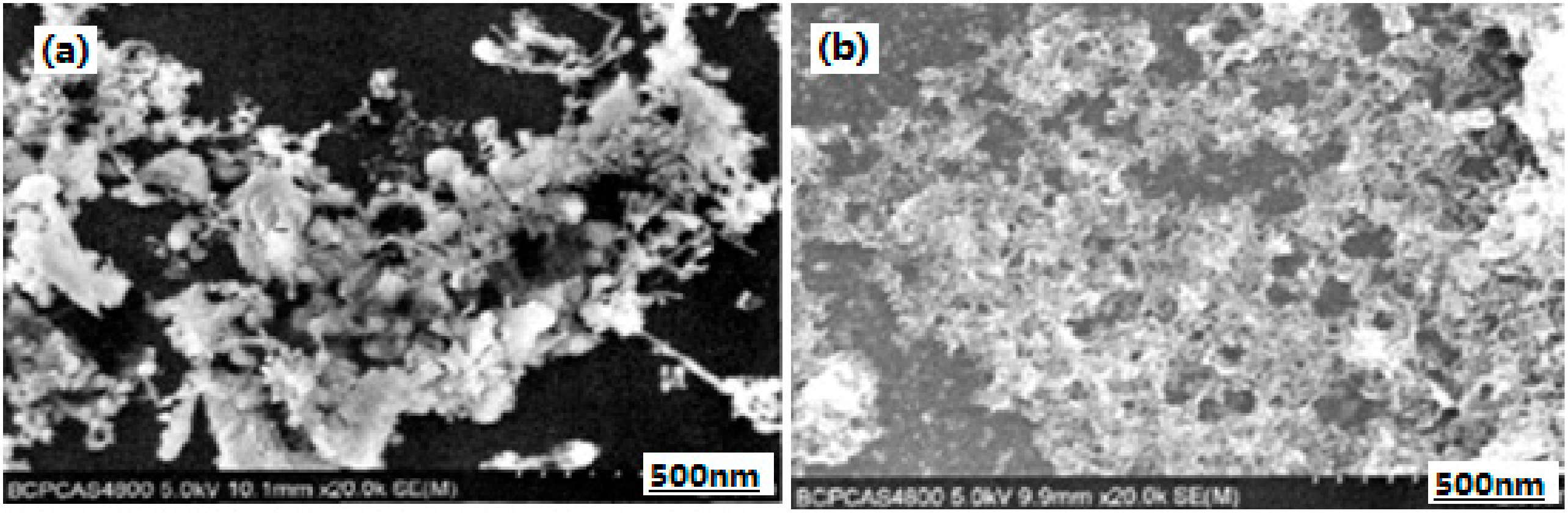
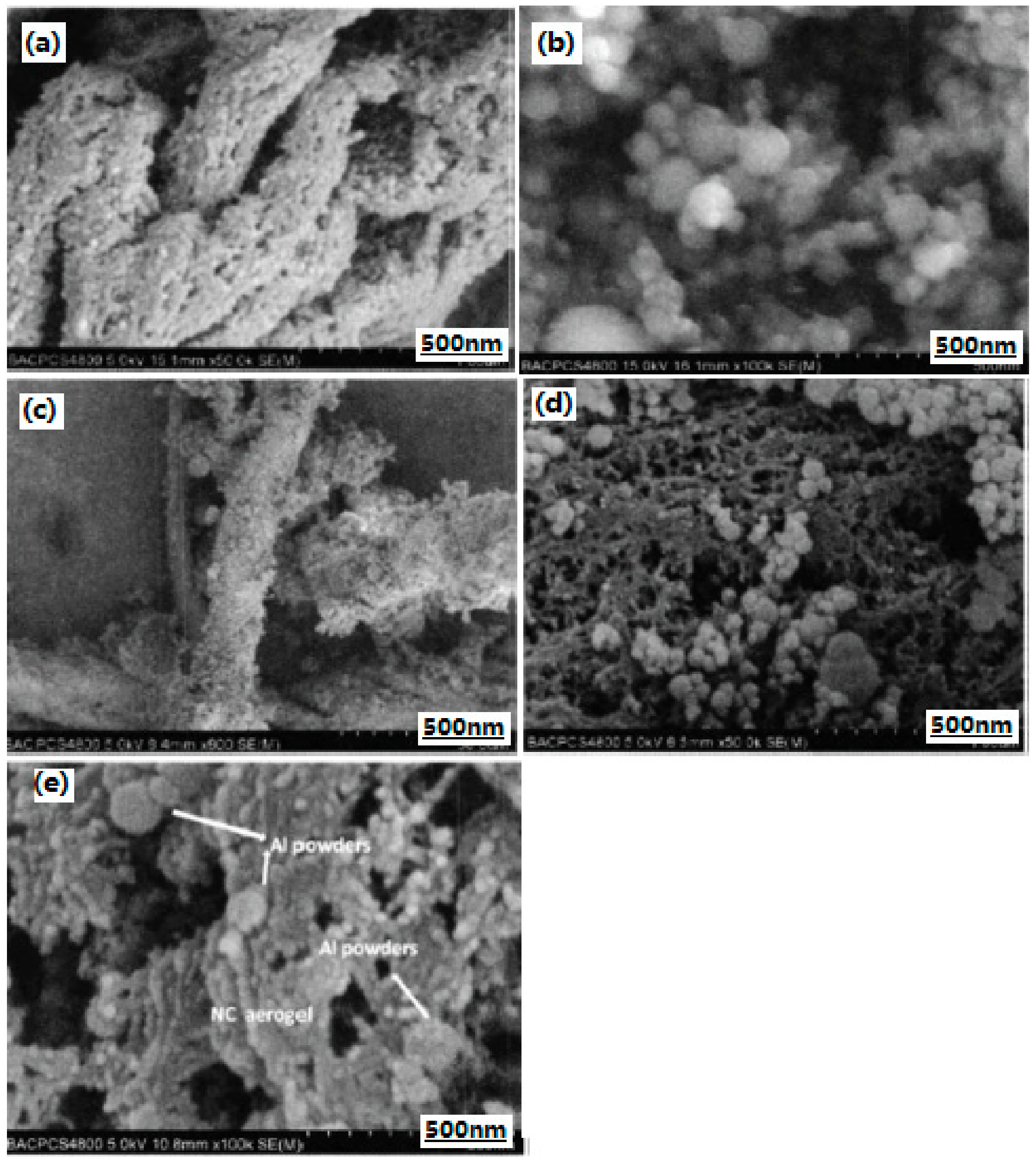
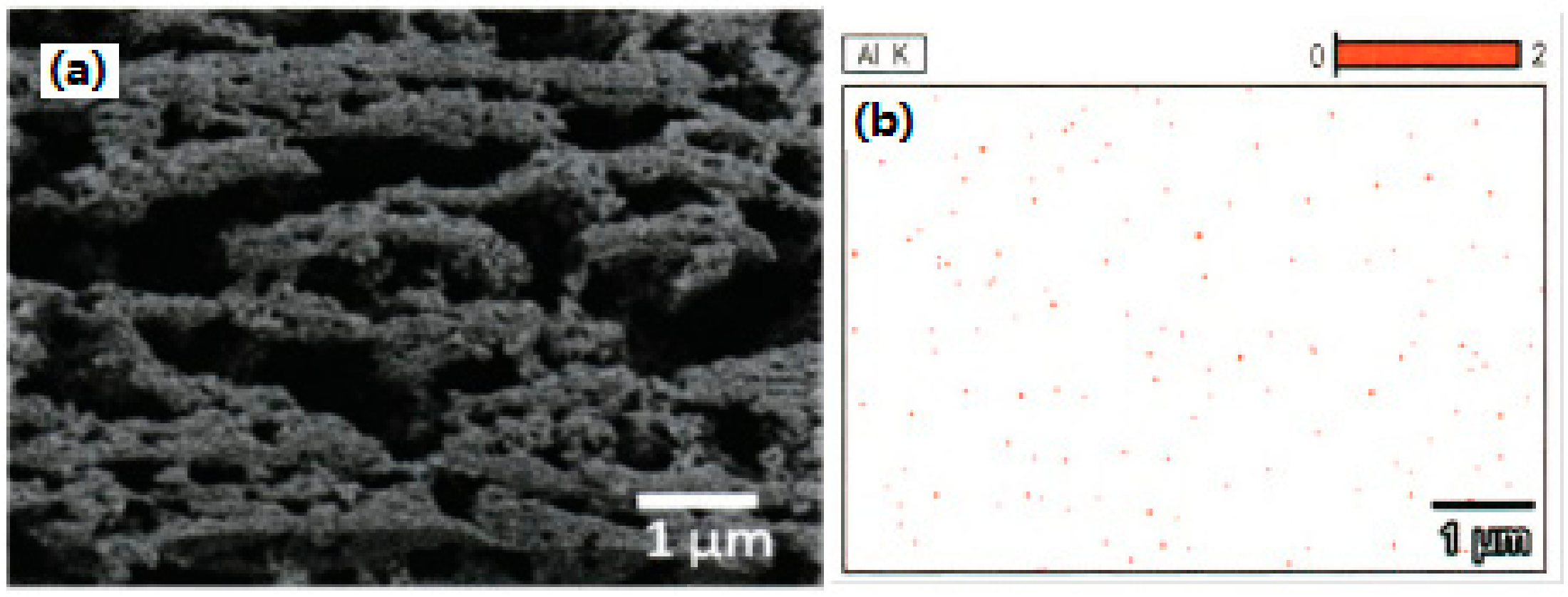
3.2.4. Nano-Sized Al/RDX/Fe2O3 Energetic Composite
3.2.5. Nano-Sized Al/RDX/SiO2 Energetic Composite
3.3. Properties and Applications of Typical Nano-CEMs
4. Conclusions and Future Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, W.; Liu, P.-J.; He, G.-Q.; Go, M.; Yan, Q.-L. Highly reactive metastable intermixed composites (MICs): Preparations and characterizations. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.-Q.; Fan, X.-Z.; Zhang, Z.-P. Nano-Sized Metal Powder: Preparation, Characterization and Energetic Application; National Defense Industry Press: Beijing, China, 2016. [Google Scholar]
- An, T.; Zhao, F.-Q.; Hao, H.-X.; Ma, H.-X.; Yao, E.-G.; Yang, Y.; Tan, Y. Effect of thermites on laser ignition characteristics of double base propellants. Chin. J. Explos. Propellants 2011, 34, 67–72. [Google Scholar]
- An, T.; Zhao, F.-Q.; Gao, H.-X.; Hao, H.-X.; Ma, H.-X. Sensitivity characteristics of double base propellants containing super thermites. J. Propuls. Technol. 2013, 34, 129–134. [Google Scholar]
- Dilip, S.; Vigor, Y.; Richard, A.Y. Metal-based nanoenergetic materials: Synthesis, properties, and applications. Prog. Energy Combust. Sci. 2017, 61, 293–365. [Google Scholar]
- Zhang, X.-T.; Song, W.-L.; Guo, L.-G.; Hu, M.-L.; Xie, C.-S. Preparation of carbon-coated Al nanopowders by laser-induction complex heating method. J. Propuls. Technol. 2007, 28, 333–338. [Google Scholar]
- Zhou, J.; Ding, L.; An, J.; Zhu, Y.; Liang, Y. Study on the thermal behaviors of nano-Al based fuel air explosive. J. Therm. Anal. Calorim. 2017, 130, 1111–1116. [Google Scholar] [CrossRef]
- de Luca, L.T.; Galfetti, L.; Severini, F.; Meda, L.; Marra, G.; Vorozhtsov, A.B.; Sedoi, V.S.; Babuk, V.A. Burning of nano-aluminized composite rocket propellants. Combust. Explos. Shock Waves 2005, 41, 680–692. [Google Scholar]
- Paravan, C.; Verga, A.; Maggi, F.; Galfetti, L. Accelerated ageing of micron- and nano-sized aluminum powders: Metal content, composition and non-isothermal oxidation reactivity. Acta Astronaut. 2019, 158, 397–406. [Google Scholar] [CrossRef]
- Bockmon, B.S.; Son, S.F.; Asay, B.W.; Busse, J.R.; Mang, J.T.; Peterson, P.D.; Pantoya, M.L. Combustion performance of metastable intermolecular composites (MIC). Cpia Publ. 2002, 712, 613–624. [Google Scholar]
- Borgonovo, C.; Apelian, D. Manufacture of Aluminum nanocomposites: A critical review. Mater. Sci. Forum 2011, 678, 1–22. [Google Scholar] [CrossRef]
- Simpson, R.L.; Tillotson, T.M.; Hrubesh, L.W.; Gash, A.E. Nanostructured energetic materials derived from sol-gel chemistry. J. Non Cryst. Solid 2001, 285, 338–345. [Google Scholar]
- Guo, Q.-X.; Nie, F.-D.; Yang, G.-C.; Li, J.-S.; Chu, S.-J. Preparation of RDX/resorcinol-formaldehyde (RF) nano-composite energetic materials by sol-gel method. Chin. J. Energetic Mater. 2006, 14, 268–271. [Google Scholar]
- Zhou, C.; Li, G.-P.; Luo, Y.-J. Preparation of Fe2O3/Al nano composite by sol-gel method. Chin. J. Explos. Propellants 2010, 33, 1–4. [Google Scholar]
- Jiang, X.-B.; Liang, Y.-Q.; Zhang, J.-L.; Chen, J.-S. Preparation of RDX/SiO2 booster membrane by sol-gel method. Chin. J. Energetic Mater. 2009, 17, 689–694. [Google Scholar]
- Gash, A.E.; Tillotson, T.M.; Sateher, J.H., Jr.; Poco, J.F.; Hrubesh, L.W.; Simpson, R.L. Use of epoxides in the sol-gel synthesis of porous Fe2O3 monliths from Fe (III) salts. Chem. Mater. 2001, 13, 999–1007. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Li, F.-S.; Chen, A.-S.; Song, H.-C. Preparation and characterization of Al/ammonium perchlorate composite particles. J. Propuls. Technol. 2004, 25, 373–376. [Google Scholar]
- Ma, Z.-Y.; Li, F.-S.; Chen, A.-S.; Bai, H.-P. Preparation and thermal decomposition behavior of Fe2O3/ammonium perchlorate composite nanoparticles. Acta Chim. Sin. 2004, 62, 1252–1255. [Google Scholar]
- Xu, G.-C.; Zhang, L.-D. Nano Composite Materials; Chemical Industry Press: Beijing, China, 2002. [Google Scholar]
- Xie, Q.-D. Preparation of Carbide and Nanocomposites by High Energy Ball Milling; Zhejiang University: Zhejiang, China, 2003. [Google Scholar]
- Shi, X.-F. Preparation and Properties of Micro-Spherical Energetic Nanocompoistes Prepared by Spray Drying; North University of China: Taiyuan, China, 2015. [Google Scholar]
- Yusoff, M.S.M.; Paulus, W.; Muslim, M. Synthesis of nano-sized alpha alumina using solvothermal and hydrothermal methods: A comparative study. In Proceedings of the 2012 International Conference on Enabling Science and Nanotechnology (ESciNano), IEEE, Johor Bahru, Malaysia, 5–7 January 2012. [Google Scholar]
- Chen, D.; Zhao, Y.; Zhu, H.; Zheng, M.; Chen, G. Microstructure and mechanism of in-situ Al2O3(p)/Al nano-composites synthesized by sonochemistry melt reaction. Trans. Nonferrous Met. Soc. China 2012, 22, 36–41. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Martin Joe, A.; Welch Larry, H. Composition and Method for Preparing Oxidizer Matrix Containing Dispersed Metal Particles. WO Patent WO 2001/38264 Al, 27 August 2001. [Google Scholar]
- Dossi, S.; Reina, A.; Maggi, F.; DeLuca, T.L. Innovative metal fuels for solid rocket propulsion. Int. J. Energ. Mater. Chem. Propul. 2012, 11, 299–322. [Google Scholar]
- Sullivan, K.T.; Chiou, W.; Fiore, R.; Zachariah, M.R. In situ microscopy of rapidly heated nano-Al and nano-Al/WO3 thermites. Appl. Phys. Lett. 2010, 97, 133104. [Google Scholar]
- Jian, G.; Piekiel, N.W.; Zachariah, M.R. Time-resolved mass spectrometry of nano-Al and nano-Al/CuO thermite under rapid heating: A mechanistic study. J. Phys. Chem. C 2012, 116, 26881–26887. [Google Scholar] [CrossRef]
- Wang, H.; Zachariah, M.R.; Xie, L.; Rao, G. Ignition and combustion characterization of nano-Al-AP and nano-Al-CuO-AP micro-sized composites produced by electrospray technique. Energy Procedia 2015, 66, 109–112. [Google Scholar] [CrossRef]
- Liu, P.; Liu, J.; Wang, M. Ignition and combustion of nano-sized aluminum particles: A reactive molecular dynamics study. Combust. Flame 2019, 201, 276–289. [Google Scholar]
- Chao, Z.; Guo-Ping, L.; Yun-Jun, L. Preparation and characterization of porous Fe2O3/Al composite energetic material. Chin. J. Explos. Propellants 2010, 33, 1–4. [Google Scholar]
- Tillotson, T.M.; Gash, A.E.; Simpson, R.I.; Hrubesh, L.W.; Satcher, J.J.H.; Poco, J.F. Nanostructured energetic materials using sol-gel methodologies. J. Non Cryst. Solids 2001, 85, 338–345. [Google Scholar]
- Jin, M.-M.; Luo, Y.-J. Preparation and characterization of NC/Al nano-composite energetic materials. Chin. J. Energetic Mater. 2013, 21, 230–234. [Google Scholar]
- Zhu, Y.; Ma, X.-X.; Zhang, K.-L. Study on Nano-structured MnO2/Al Energetic Composite. Initiators Pyrotech. 2018, 1, 23–27. [Google Scholar]
- Ke, X.; Zhou, X.; Gao, H.; Hao, G.Z.; Xiao, L.; Chen, T.; Liu, J.; Jiang, W. Surface functionalized core/shell structured CuO/Al nanothermite with long-term storage stability and steady combustion performance. Mater. Des. 2018, 140, 179–187. [Google Scholar] [CrossRef]
- Dreizin, E.L.; Schoenitz, M. Nano-composite energetic powders prepared by arrested reactive milling. Thermochim. Acta 2016, 636, 48–56. [Google Scholar]
- Williams, R.A.; Schoenitz, M.; Dreizin, E.L. Validation of the thermal oxidation model for Al/CuO nanocomposite powder. Combust. Sci. Technol. 2014, 186, 46–67. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, B.; Qiao, Z.-J.; Chen, J.; Zhang, L.-Y.; Zhang, L.; Li, Z.-Q.; Zhang, X.-Q.; Yang, G.-C. Construct 3D porous hollow Co3O4 micro-sphere: A potential oxidizer of nano-energetic materials with superior reactivity. Appl. Surf. Sci. 2018, 442, 767–772. [Google Scholar] [CrossRef]
- Zakiyyan, N.; Wang, A.-Q.; Thiruvengadathan, R.; Staley, C.; Mathai, J.; Gangopadhyay, K.; Maschmann, M.R.; Gangopadhyay, S. Combustion of aluminum nanoparticles and exfoliated 2D molybdenum trioxide composites. Combust. Flame 2018, 187, 1–10. [Google Scholar] [CrossRef]
- Dixon, G.P.; Martin, J.A.; Thompson, D. Lead-Free Precussion Peimer Mixes Based on Metastable Interstitial Composite (MIC) Technology. Patent US 5717159, 10 February 1998. [Google Scholar]
- Troy, J.; Barbee, W.; Simpson, R.L.; Gash, A.E.; Joe, S.H., Jr. Nano-Laminate-Based Ignitors. Patent US 2004 0060625, 4 January 2004. [Google Scholar]
- Williams, R.A.; Schoenitz, M.; Ermoline, A.; Dreizin, E.L. Low-temperature exothermic reactions in fully-dense Al/MoO3 nanocomposite powders. Thermochim. Acta 2014, 594, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.-C.; Shen, R.-Q.; Ye, J.-H.; Wang, X.-W. Research progress of nano thermite. Chin. J. Explos. Propellants 2014, 37, 1–9. [Google Scholar]
- Jin, X.-Y.; Hu, Y.; Shen, R.-Q.; Ye, Y.-H. Preparation and laser ignition studies of Al/Ni energetic nanocomposite. Explos. Mater. 2012, 41, 12–15. [Google Scholar]
- Nemtseva, S.Y.; Lebedev, E.A.; Shaman, Y.P.; Lazarenko, P.I.; Ryazanov, R.M.; Gavrilov, S.A.; Gromov, D.G. Nano-sized Al-Ni energetic powder material for heat release element of thermoelectric device. J. Phys. Conf. Ser. 2018, 1124. [Google Scholar] [CrossRef]
- Lee, H.M.; Yun, J. Preparation of aluminum-oleic acid nano-composite for application to electrode for Si solar cells. Mater. Trans. 2011, 52, 1222–1227. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Liu, Y.; Sun, D.; Guo, W.; Sun, X.; Feng, Y.; Chi, H.; Li, X.; Tian, F.; et al. Performance and microstructure characteristics in polyimide/nano-aluminum composites. Surf. Coat. Technol. 2017, 320, 103–108. [Google Scholar] [CrossRef]
- Shen, L.-H.; Li, G.-P.; Luo, Y.-J.; Gao, K.; Chai, C.-P.; Ge, Z. Preparation of Al/B/Fe2O3 nano-composite energetic materials by high energy ball milling. J. Solid Rocket Technol. 2014, 37, 233–237. [Google Scholar]
- Yang, F.; Kang, X.-L.; Luo, J.-S.; Yi, Z.; Tang, Y.-J. Preparation of core-shell structure KClO4@Al/CuO nano energetic material and enhancement of thermal behavior. Sci. Rep. 2017, 7, 3730. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pan, Z.-H.; Li, L.-X.; Li, Y.-B.; Cao, X.-F. Preparation of nanometer NiB/Al composite and its thermal catalysis effect on AP decomposition. Chin. J. Energetic Mater. 2009, 17, 446–450. [Google Scholar]
- Wang, R.-H.; Zhang, J.-L.; Wang, J.-Y.; Pan, J.; Zhang, J. Preparation and characterization of nano-composite energetic materials Fe2O3/Al/RDX. Chin. J. Energetic Mater. 2011, 19, 739–742. [Google Scholar]
- Wang, R.-H.; Jin, R.-Y.; Wang, J.-Y.; Zhang, J.-L.; Wang, D.-J. Preparation of RDX/B/Fe2O3 nano-composite energetic material with gel-template method. Chin. J. Energetic Mater. 2015, 23, 410–414. [Google Scholar]
- Zhang, D.-D.; Huang, Y.-S.; Li, R.; Li, M.; Wang, J.-J.; Ge, M.-Z.; Zhang, H.-J.; He, Y.-L. Preparation and properties of RDX/Al/SiO2 nano-composite energetic materials. Chin. J. Energetic Mater. 2017, 25, 656–660. [Google Scholar]
- Zhang, D.-D. Preparation and Properties of RDX Based Nanocomposite Energetic Materials; Nanjing University of Science and Technology: Nanjing, China, 2018. [Google Scholar]
- Pan, J.-J.; Zhang, J.-L.; Shang, F.-F.; Song, X.-X. Preparation and characterization of submicron composite energetic materials RDX/AP/Al/SiO2. Shanxi Chem. Ind. 2011, 31, 15–17. [Google Scholar]
- Pang, W.-Q.; De Luca, T.L.; Fan, X.-Z.; Wang, K.; Li, J.-Q.; Zhao, F.-Q. Progress on modification of high active aluminum powder and combustion agglomeration in chemical propellants. J. Solid Rocket Technol. 2019, 42, 42–53. [Google Scholar]
- Carole, R. Al-Based Energetic nano Materials: Design, Manufacturing, Properties and Applications; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Puszynski, J.A. Processing and characterization of aluminum-based nanothermites. J. Therm. Anal. Calorim. 2009, 96, 677–685. [Google Scholar] [CrossRef]
- Puszynski, J.A. Reactivity of Nano-Sized Aluminum with Metal Oxides and Water Vapor. Materials Research Society Proceedings; Materials Research Society: Boston, MA, USA, 2003. [Google Scholar]
- Monk, I.; Schoenitz, M.; Jacob, R.J.; Dreizin, E.L.; Zachariah, M.R. Combustion characteristics of stoichiometric Al-CuO nanocomposite thermites prepared by different methods. Combust. Sci. Technol. 2017, 189, 555–574. [Google Scholar] [CrossRef]
- Demitrios, S.; Xianjin, J.; Ervin, B.; Edward, L.D. Aluminum burn rate modifiers based on reactive nanocomposite powders. Propellants Explos. Pyrotech. 2009, 35, 260–267. [Google Scholar]
- Young, G.; Wang, H.; Zachariah, M.R. Application of nano-aluminum/nitrocellulose mesoparticles in composite solid rocket propellants. Propellants Explos. Pyrotech. 2015, 43, 413–418. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Zhang, X.-H.; Qi, X.-F.; Liu, M.; Li, X.-J. Preparation and application of nano-sized composite energetic materials. New Chem. Mater. 2011, 39, 36–39. [Google Scholar]
- Zhang, M.; Wang, Y.; Song, X.-L.; Luo, T.-T. Preparation and characterization of NC/PETN nanocomposite. J. Ordnance Equip. Eng. 2018, 39, 182–186. [Google Scholar]
- Weismiller, M.R.; Malchi, J.Y.; Lee, J.G.; Yetter, R.A.; Foley, T.J. Effects of fuel and oxidizer particle dimensions on the propagation of aluminum containing thermites. Proc. Combust. Inst. 2011, 33, 1989–1996. [Google Scholar] [CrossRef]
- Rossi, C. Nano-energetic materials for MEMS: A review. J. Micro Electro Mech. Syst. 2007, 16, 919–931. [Google Scholar] [CrossRef]
- Pang, W.-Q.; De Luca, L.T.; Gromov, A.; Cumming, A.S. Innovative Energetic Materials: Properties, Combustion Performance and Application; Springer: Taramani, India, 2020. [Google Scholar]

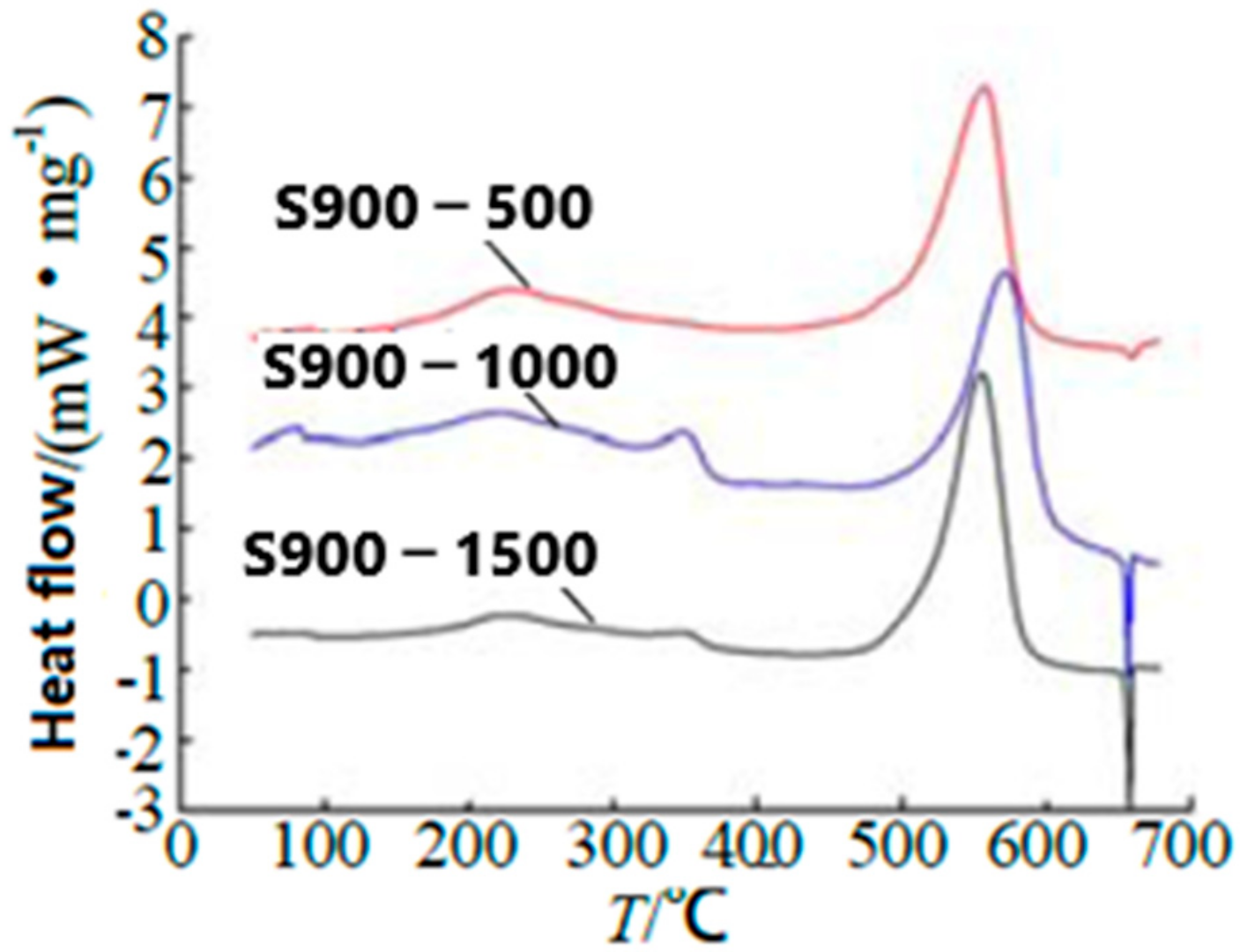
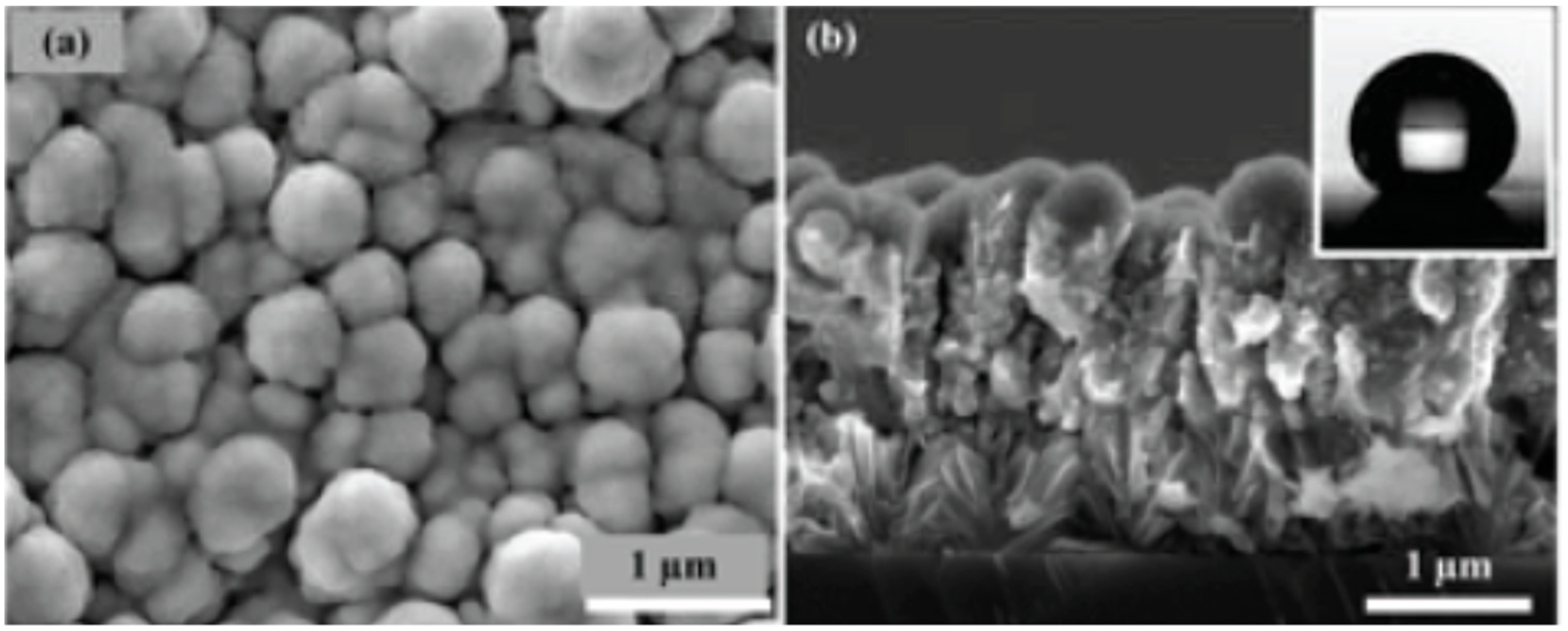
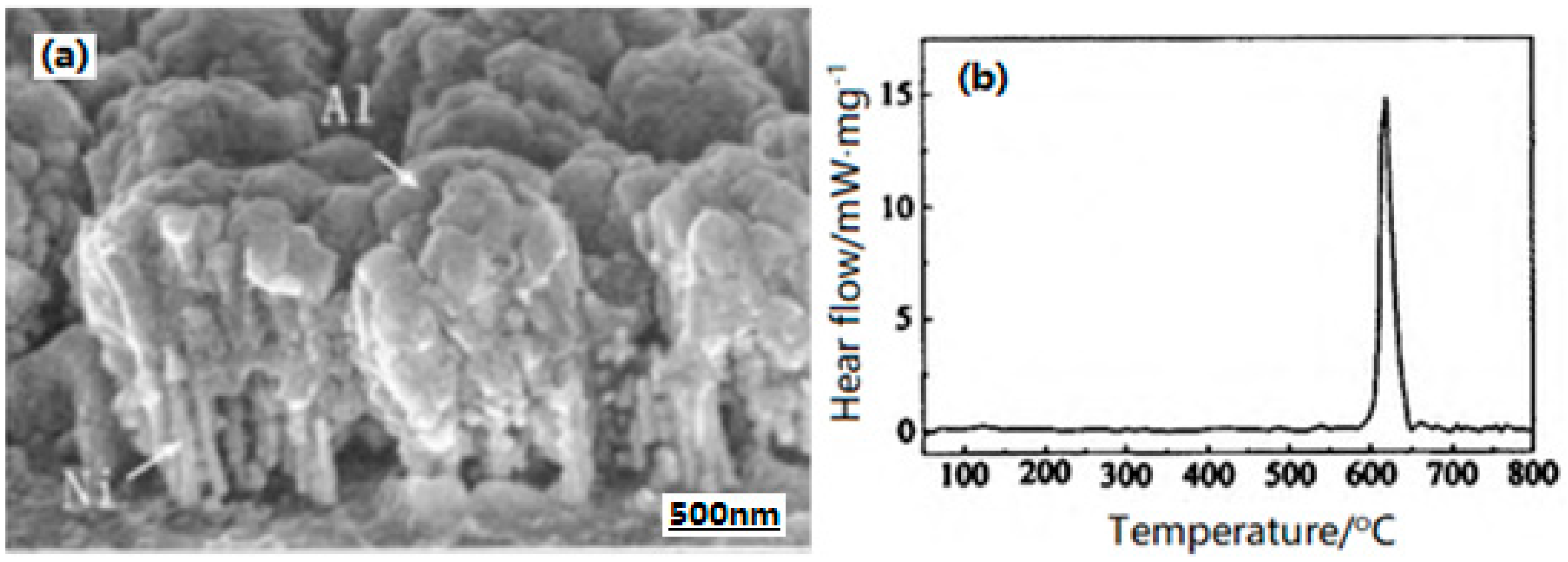
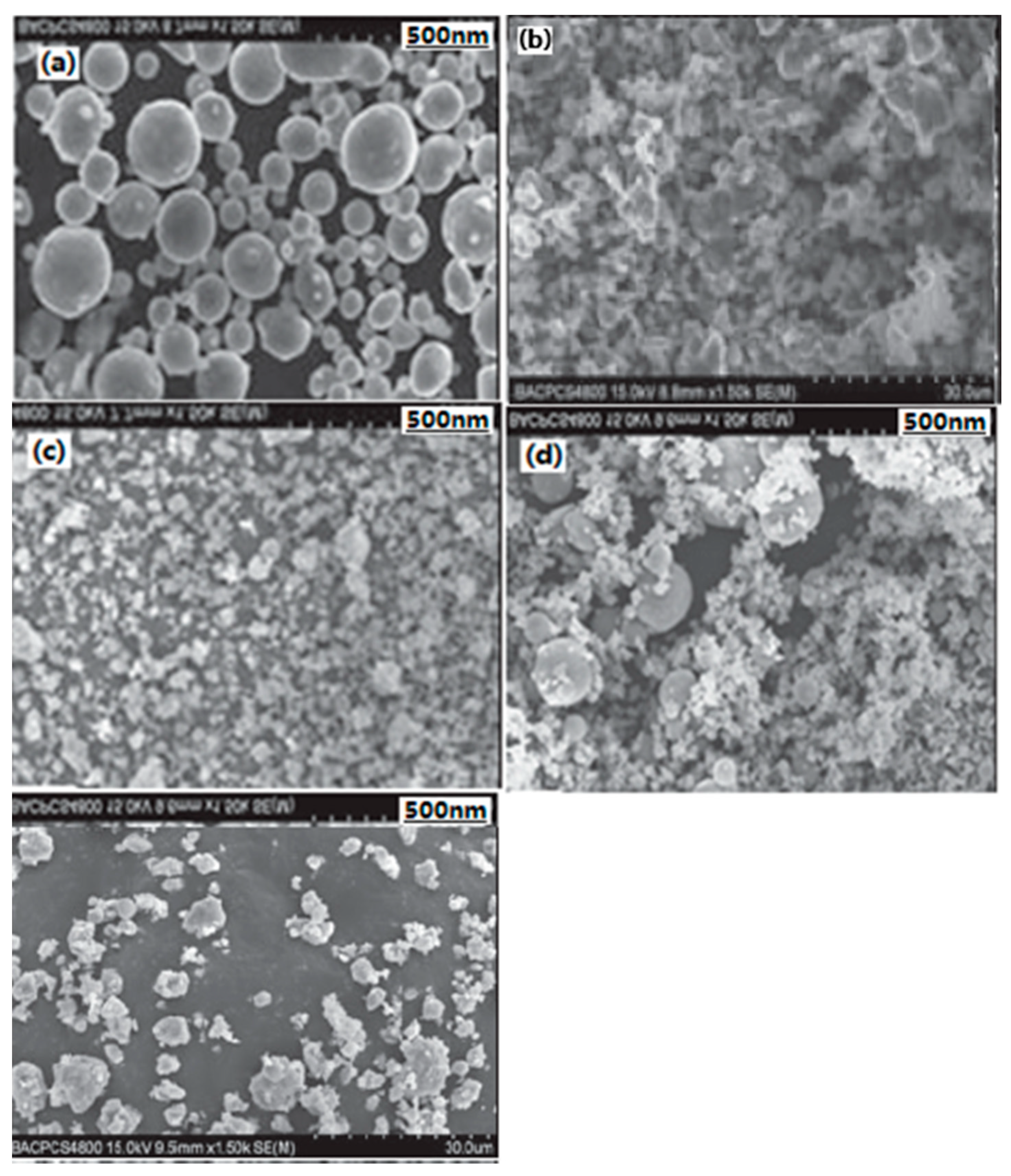
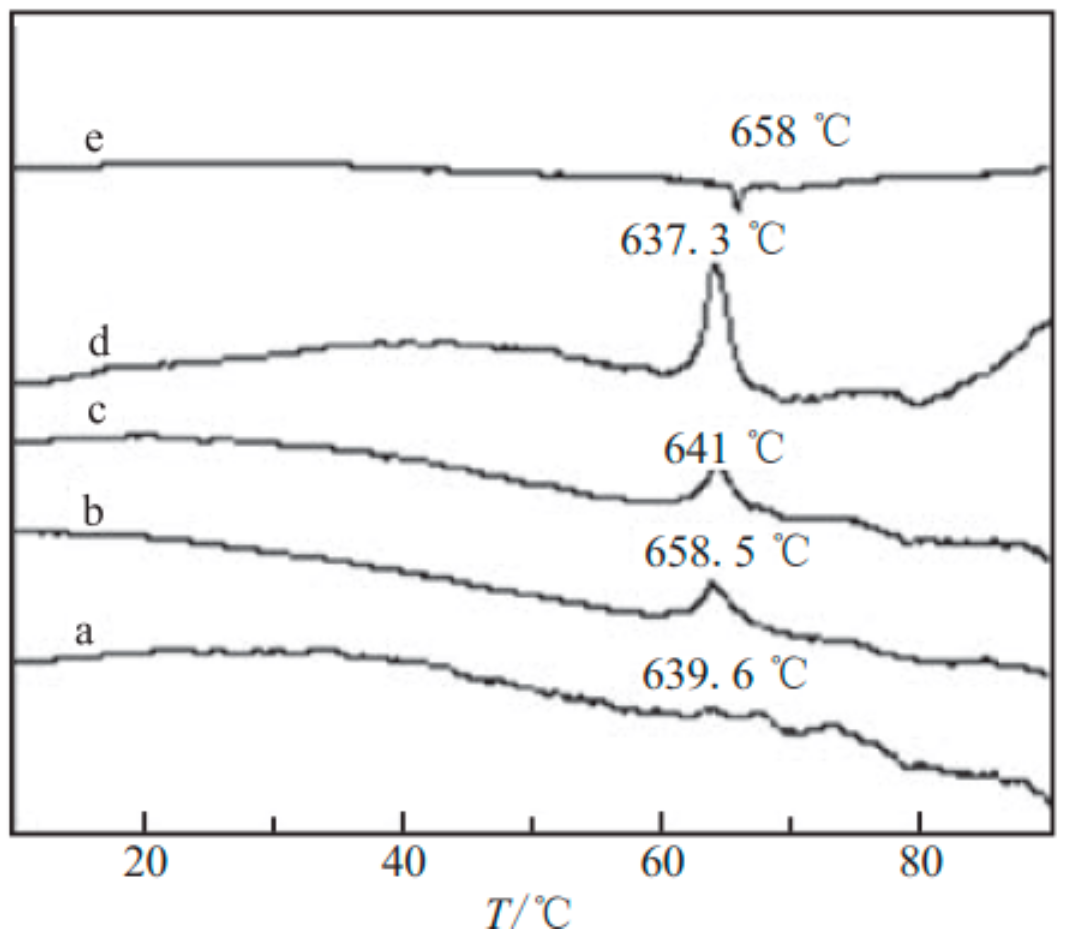
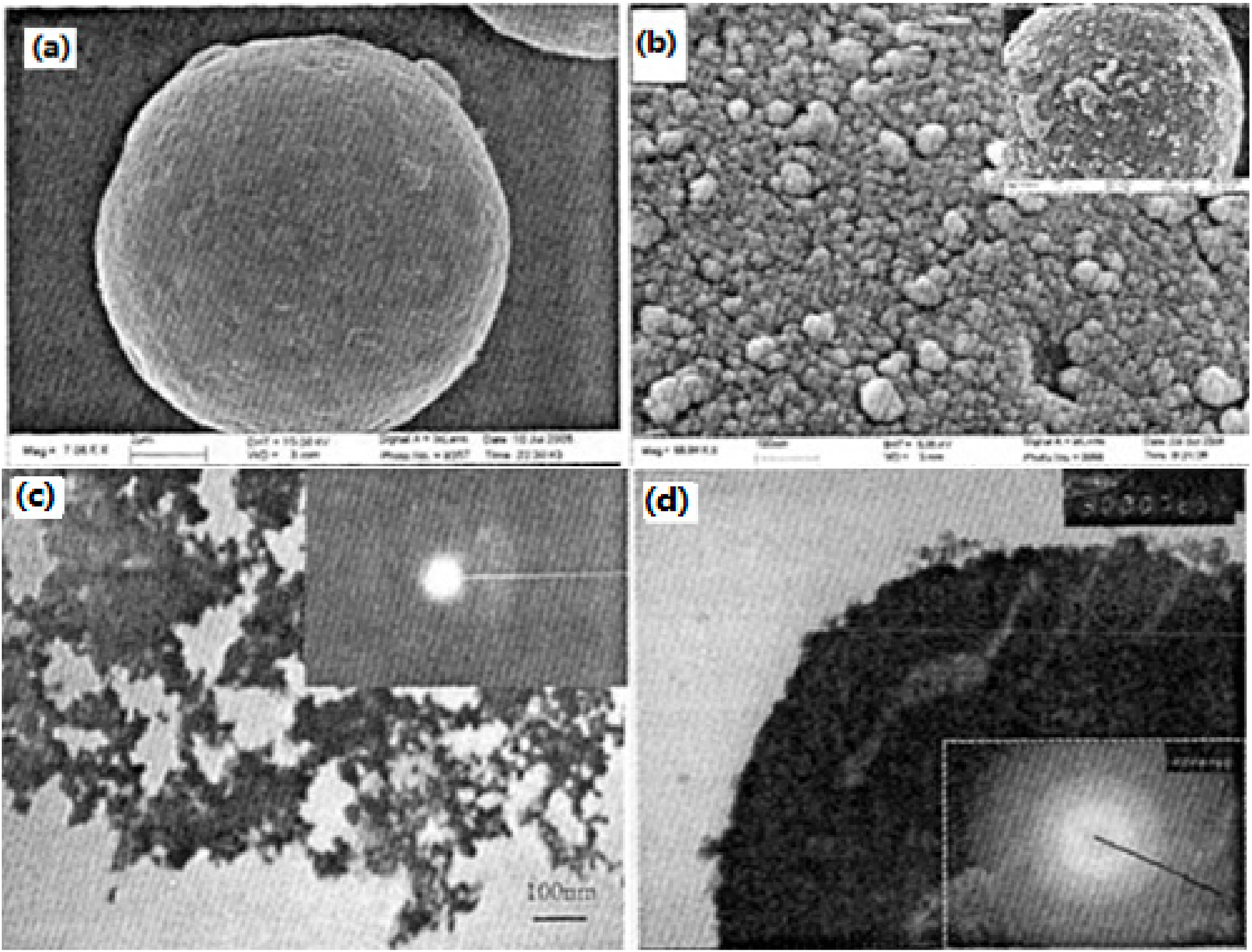
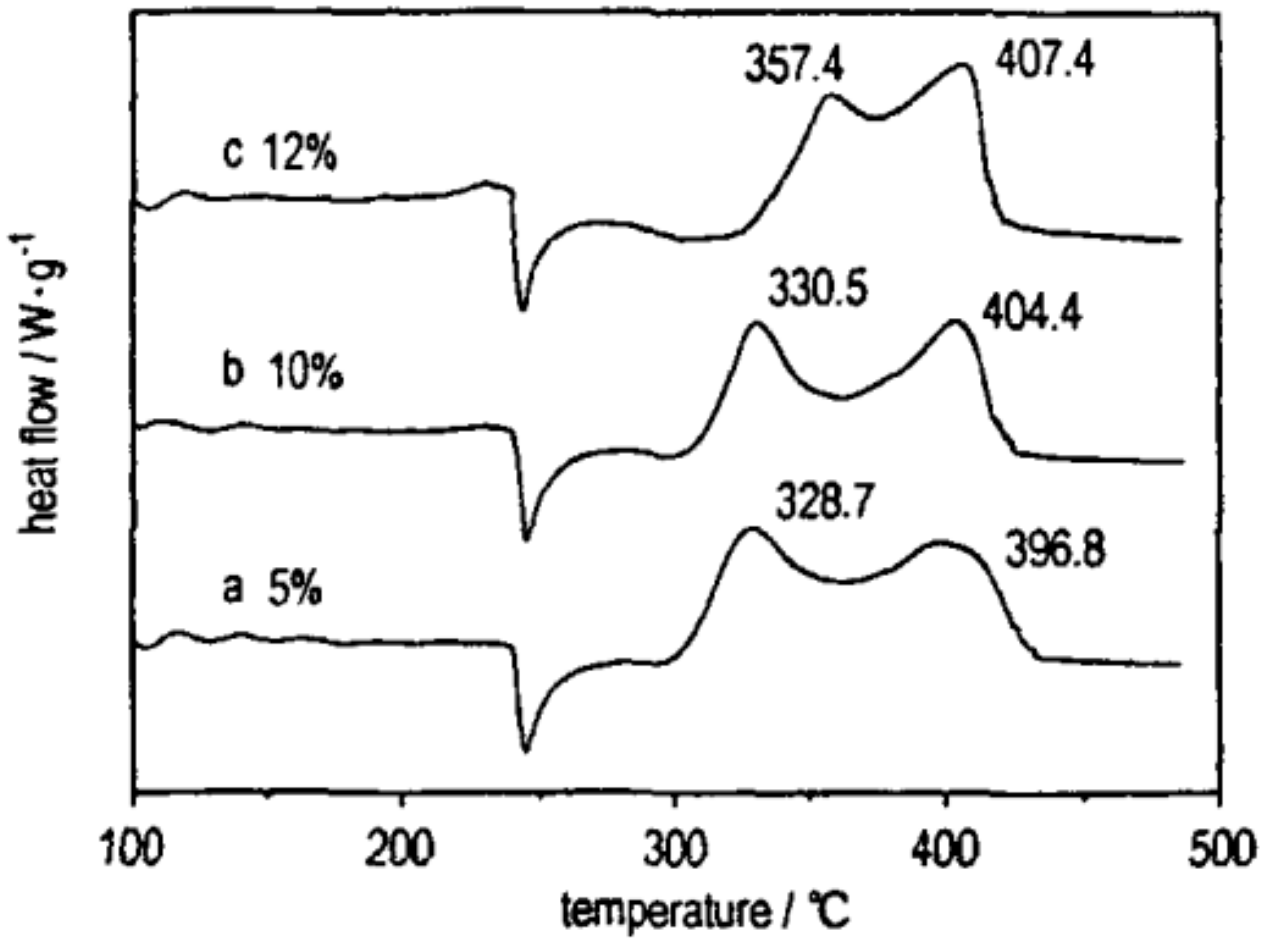
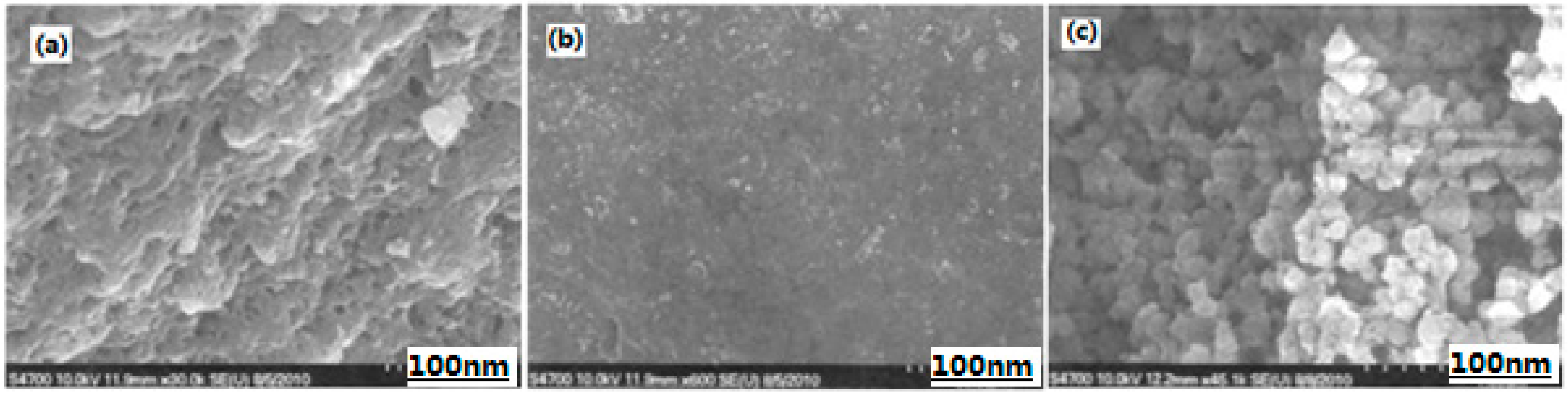
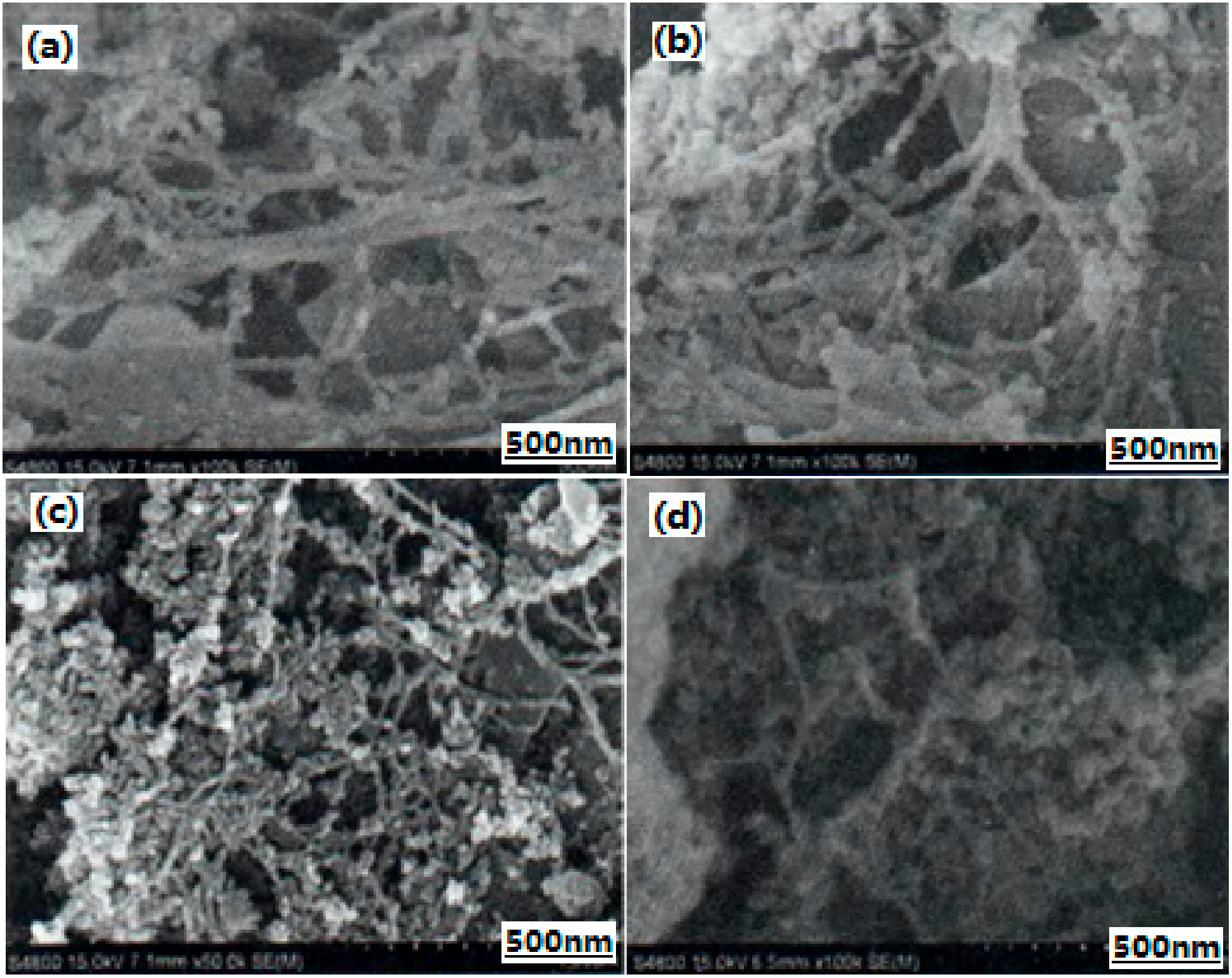

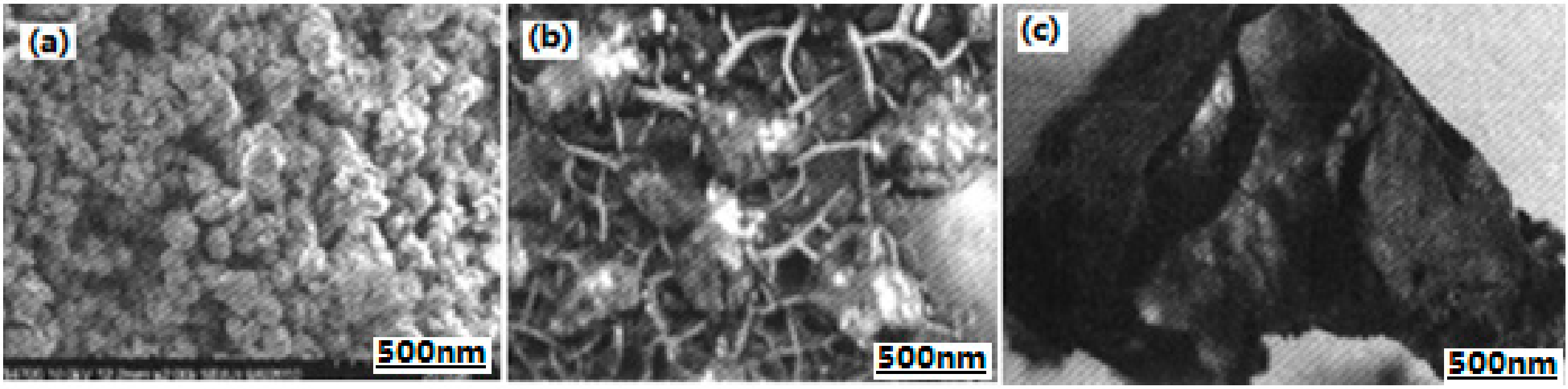
| Samples | Impact Sensitivity/cm | Standard Deviation | Friction Sensitivity/% | Detonation Velocity/m·s−1 |
|---|---|---|---|---|
| RDX | 22.5 | 0.04 | 96 | 6570 |
| Al/RDX/Fe2O3 | 50.2 | 0.05 | 7 | 7185 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, W.; Fan, X.; Wang, K.; Chao, Y.; Xu, H.; Qin, Z.; Zhao, F. Al-Based Nano-Sized Composite Energetic Materials (Nano-CEMs): Preparation, Characterization, and Performance. Nanomaterials 2020, 10, 1039. https://doi.org/10.3390/nano10061039
Pang W, Fan X, Wang K, Chao Y, Xu H, Qin Z, Zhao F. Al-Based Nano-Sized Composite Energetic Materials (Nano-CEMs): Preparation, Characterization, and Performance. Nanomaterials. 2020; 10(6):1039. https://doi.org/10.3390/nano10061039
Chicago/Turabian StylePang, Weiqiang, Xuezhong Fan, Ke Wang, Yimin Chao, Huixiang Xu, Zhao Qin, and Fengqi Zhao. 2020. "Al-Based Nano-Sized Composite Energetic Materials (Nano-CEMs): Preparation, Characterization, and Performance" Nanomaterials 10, no. 6: 1039. https://doi.org/10.3390/nano10061039
APA StylePang, W., Fan, X., Wang, K., Chao, Y., Xu, H., Qin, Z., & Zhao, F. (2020). Al-Based Nano-Sized Composite Energetic Materials (Nano-CEMs): Preparation, Characterization, and Performance. Nanomaterials, 10(6), 1039. https://doi.org/10.3390/nano10061039







