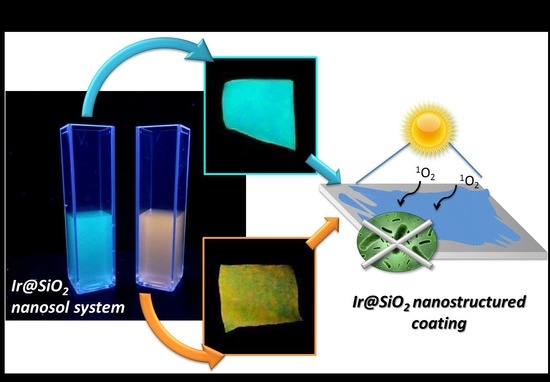
Use of Cotton Textiles Coated by Ir(III) Tetrazole Complexes within Ceramic Silica Nanophases for Photo-Induced Self-Marker and Antibacterial Application
Abstract
1. Introduction
2. Materials and Methods
2.1. General Considerations
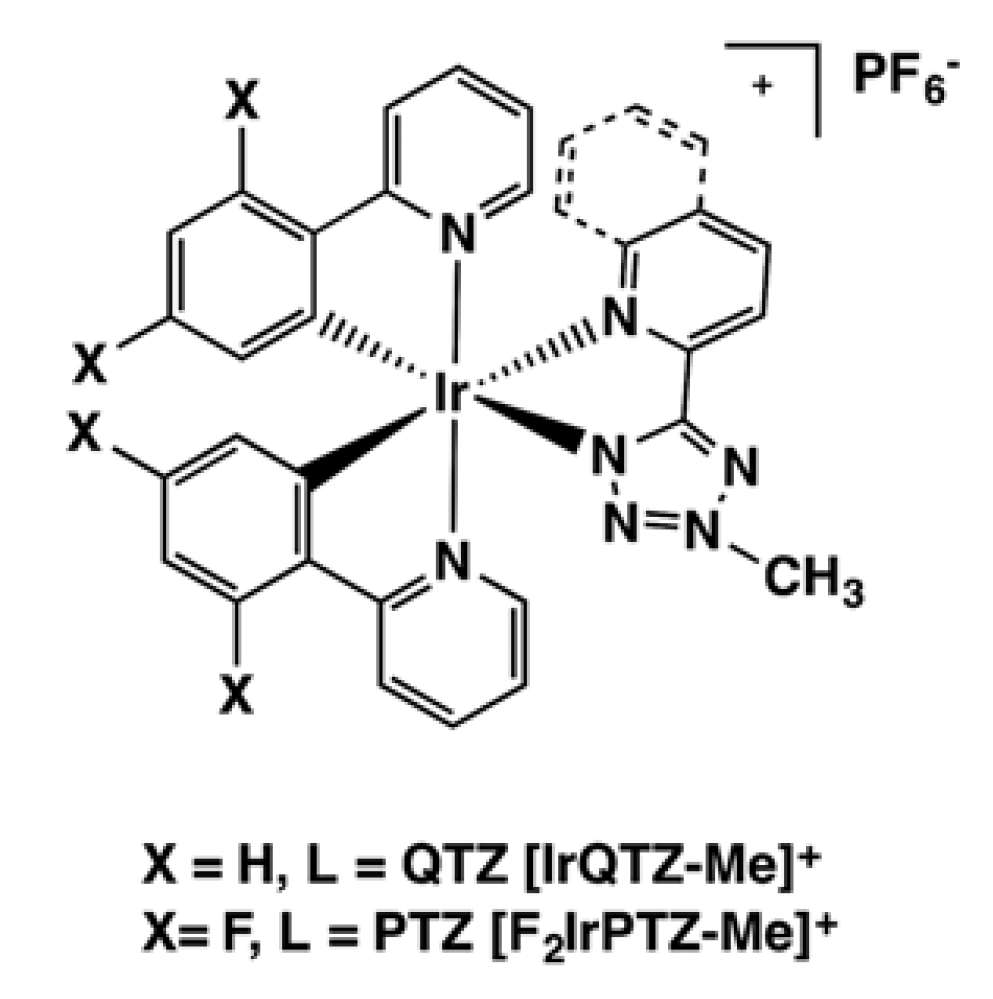
2.2. General Procedure for the Preparation of the Cationic Ir(III) Complexes
2.3. General Procedure for the Colloidal Physical Encapsulation
2.4. General Procedure for the Dip-Pad-Dry-Cure Process for Fabrics
2.5. Colloidal Characterization
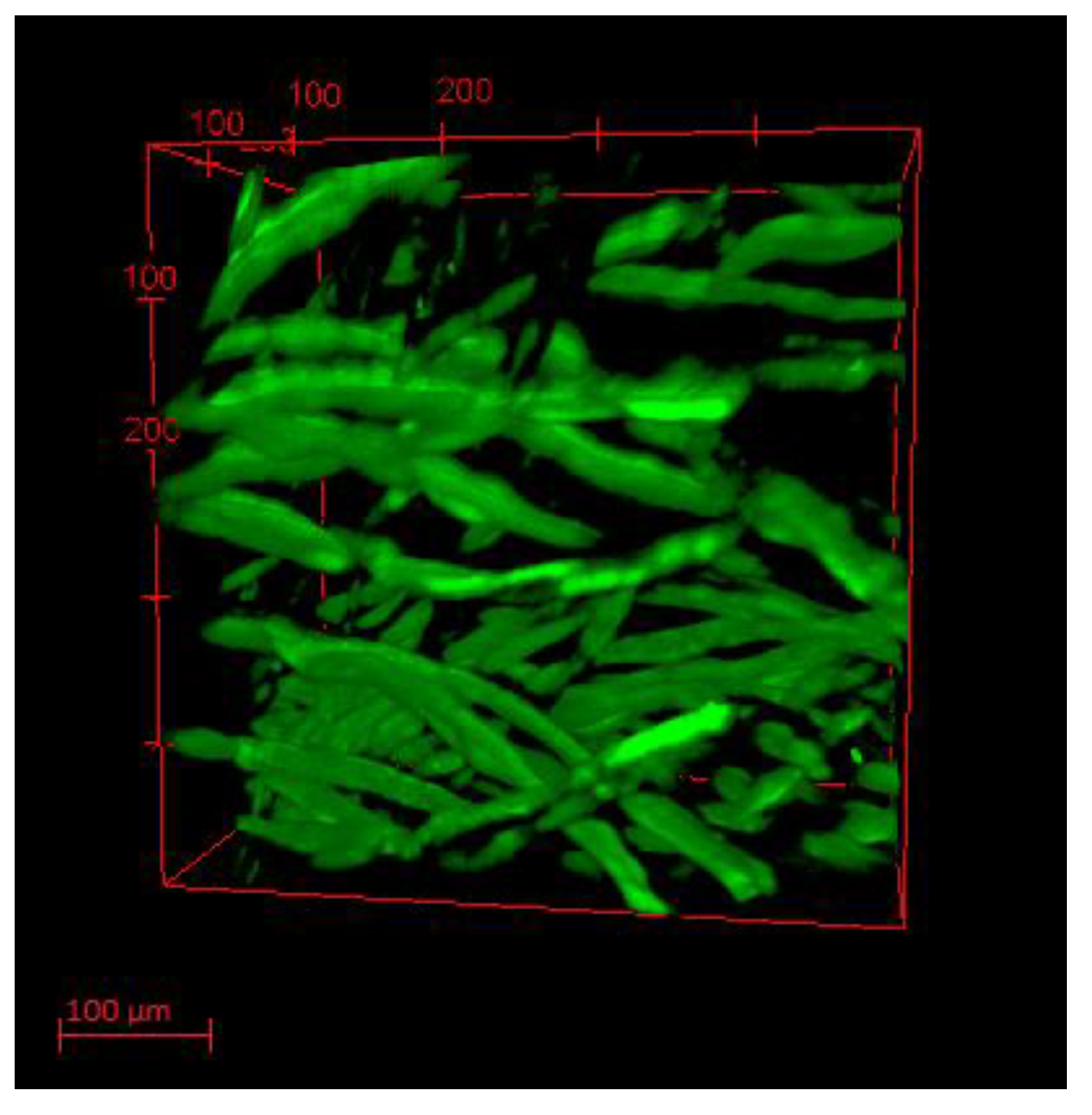
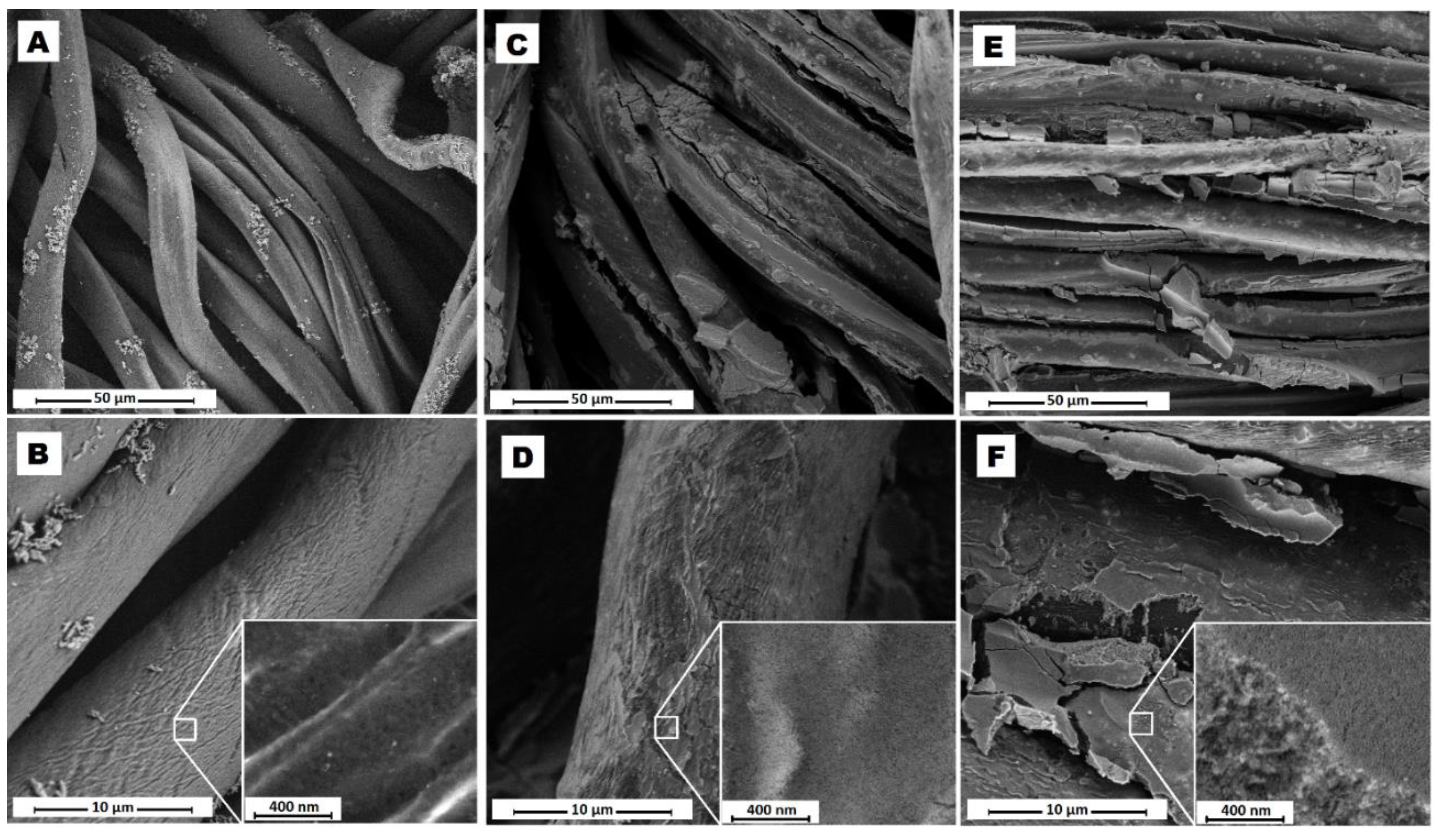
2.6. Morphological Characterization of Fabric Samples
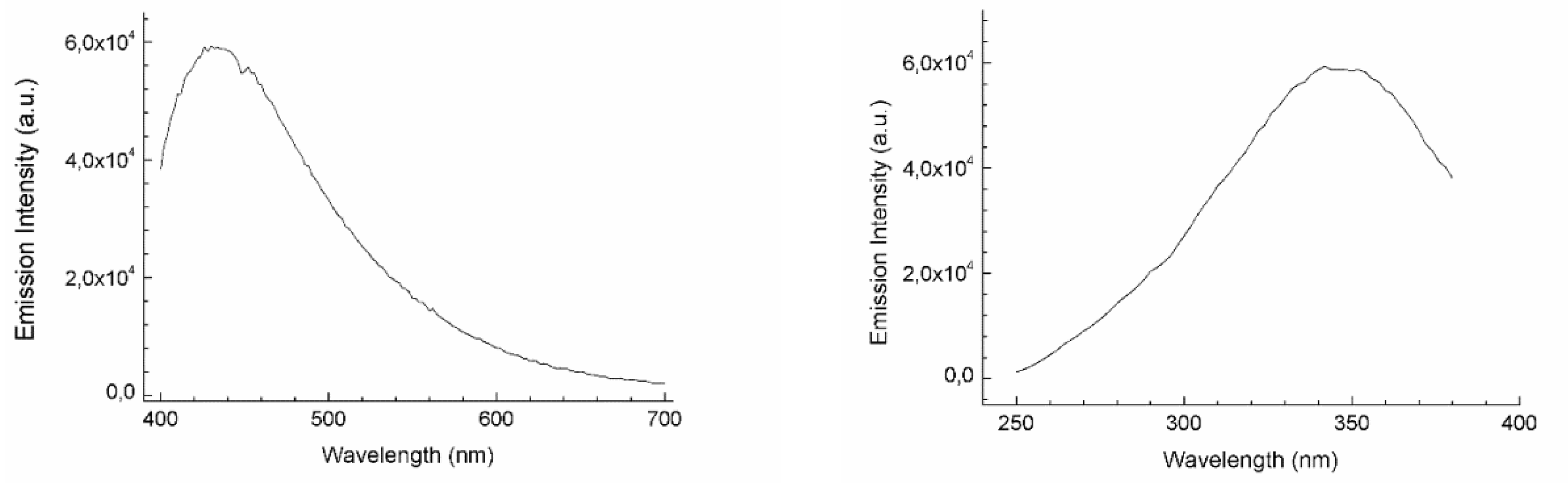
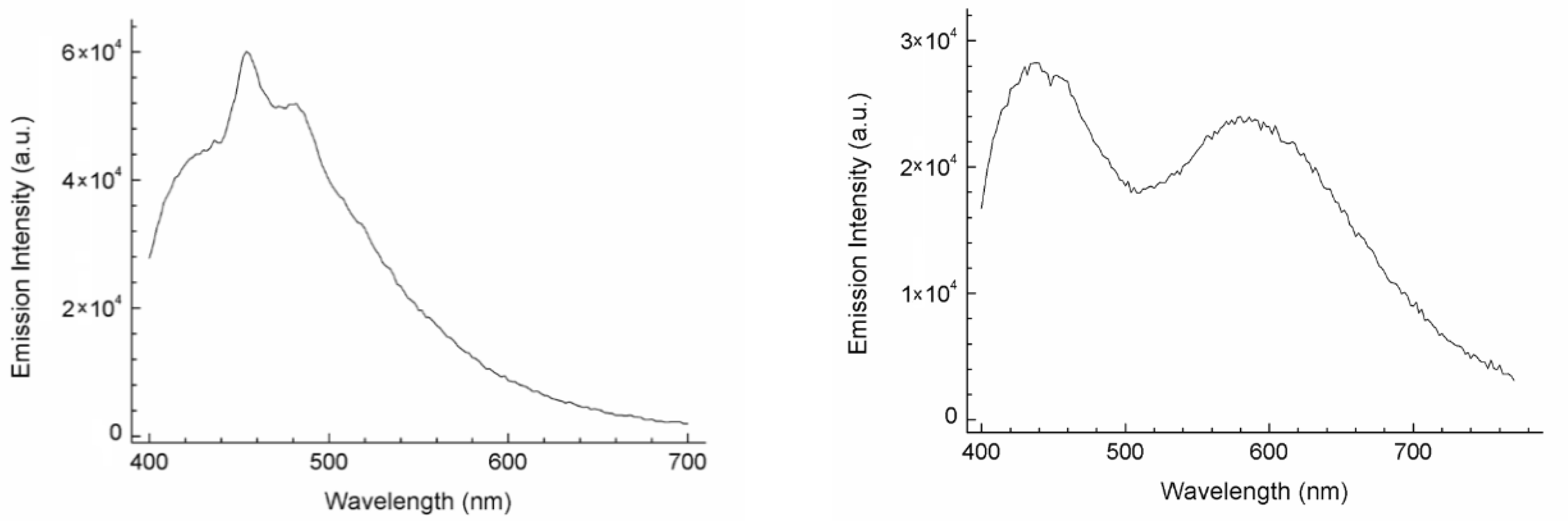
2.7. Photophysical Characterization
2.8. Washing Fastness Test
2.9. Bacterial Strains and Media
2.10. Antibacterial Evaluation
2.11. Preparation of Skin Membranes
2.12. In Vitro Diffusion System
3. Results and Discussion
3.1. Synthesis of Luminescent Ir@SiO2 Nanosols Systems
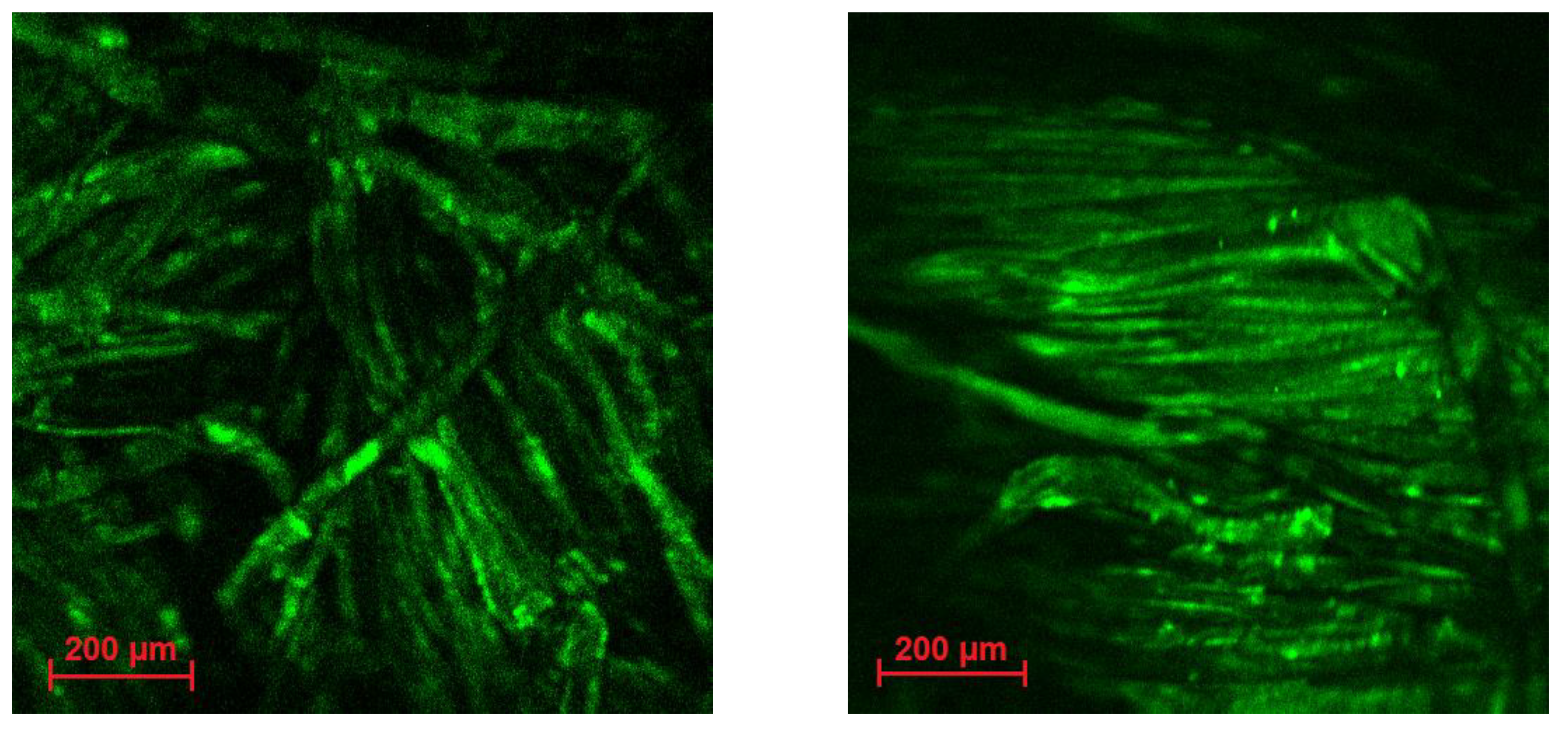
3.2. Ir@SiO2 Nano-Coated Textiles
3.3. Washing Fastness
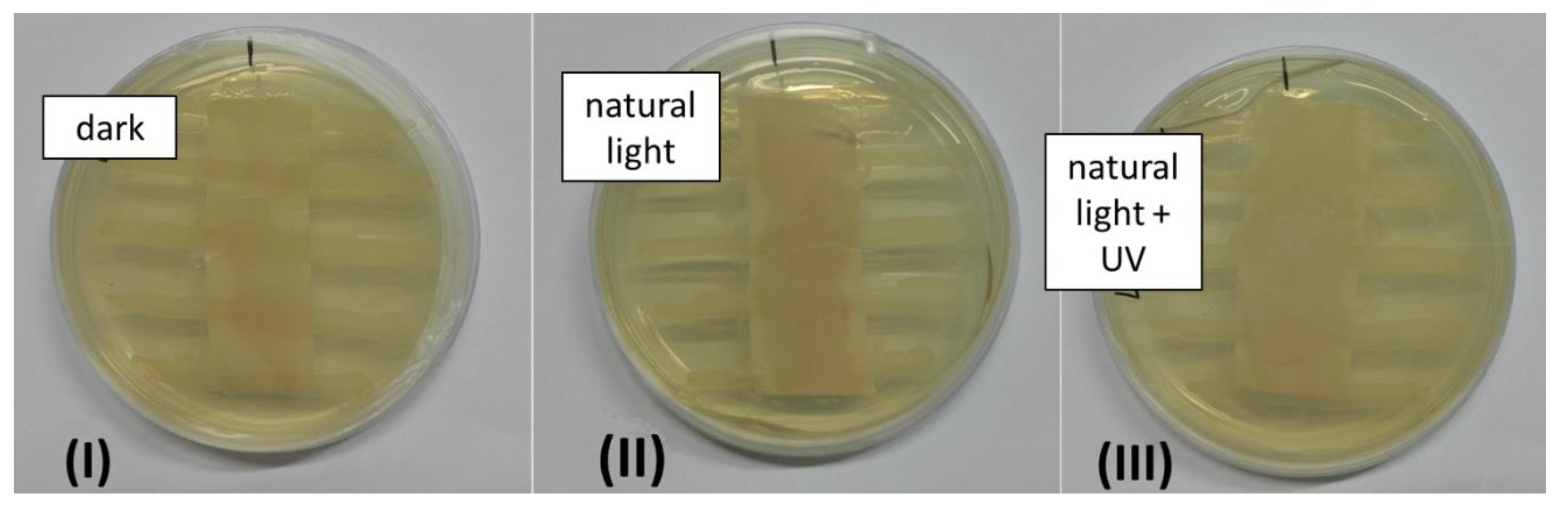
3.4. Antibacterial Properties
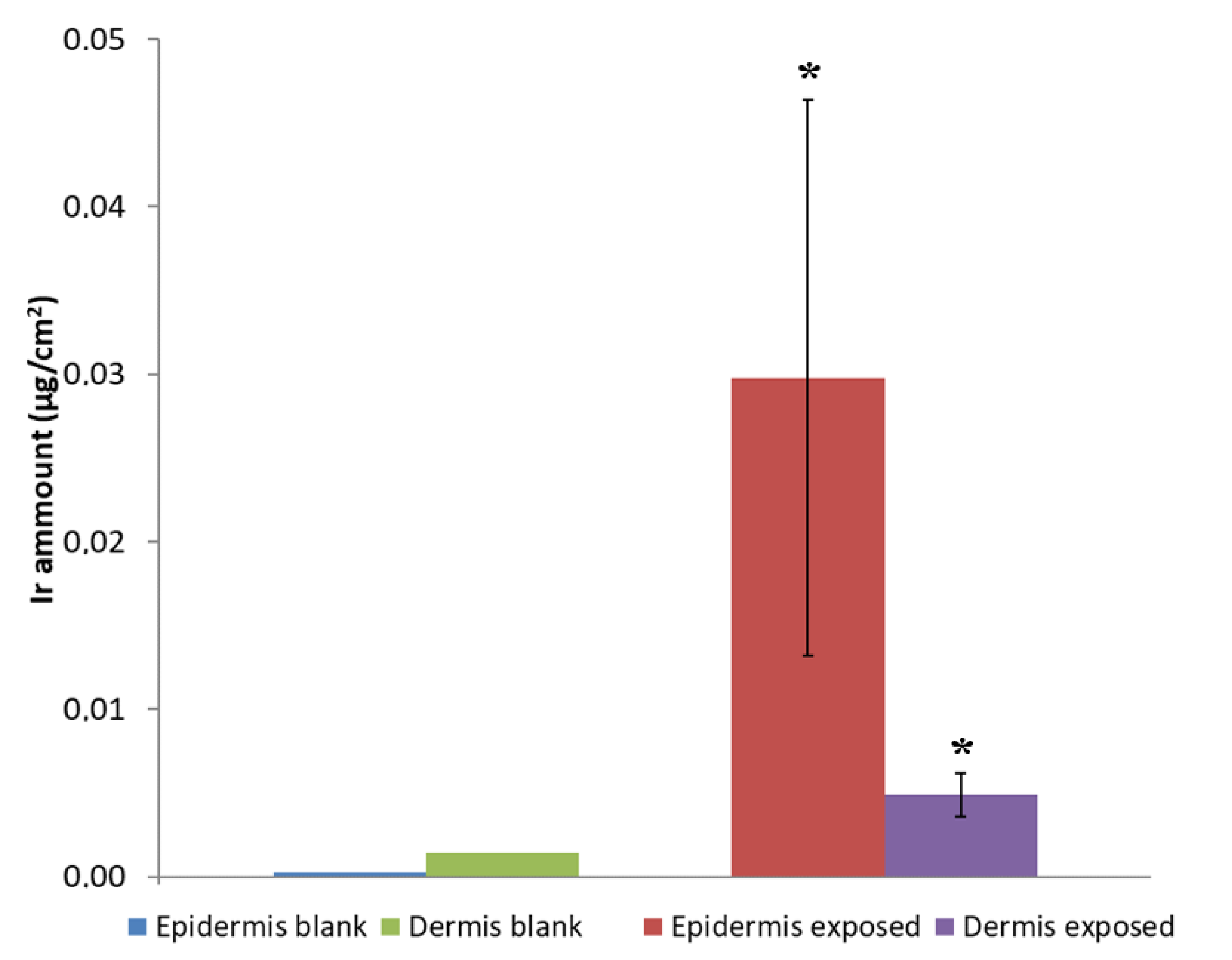
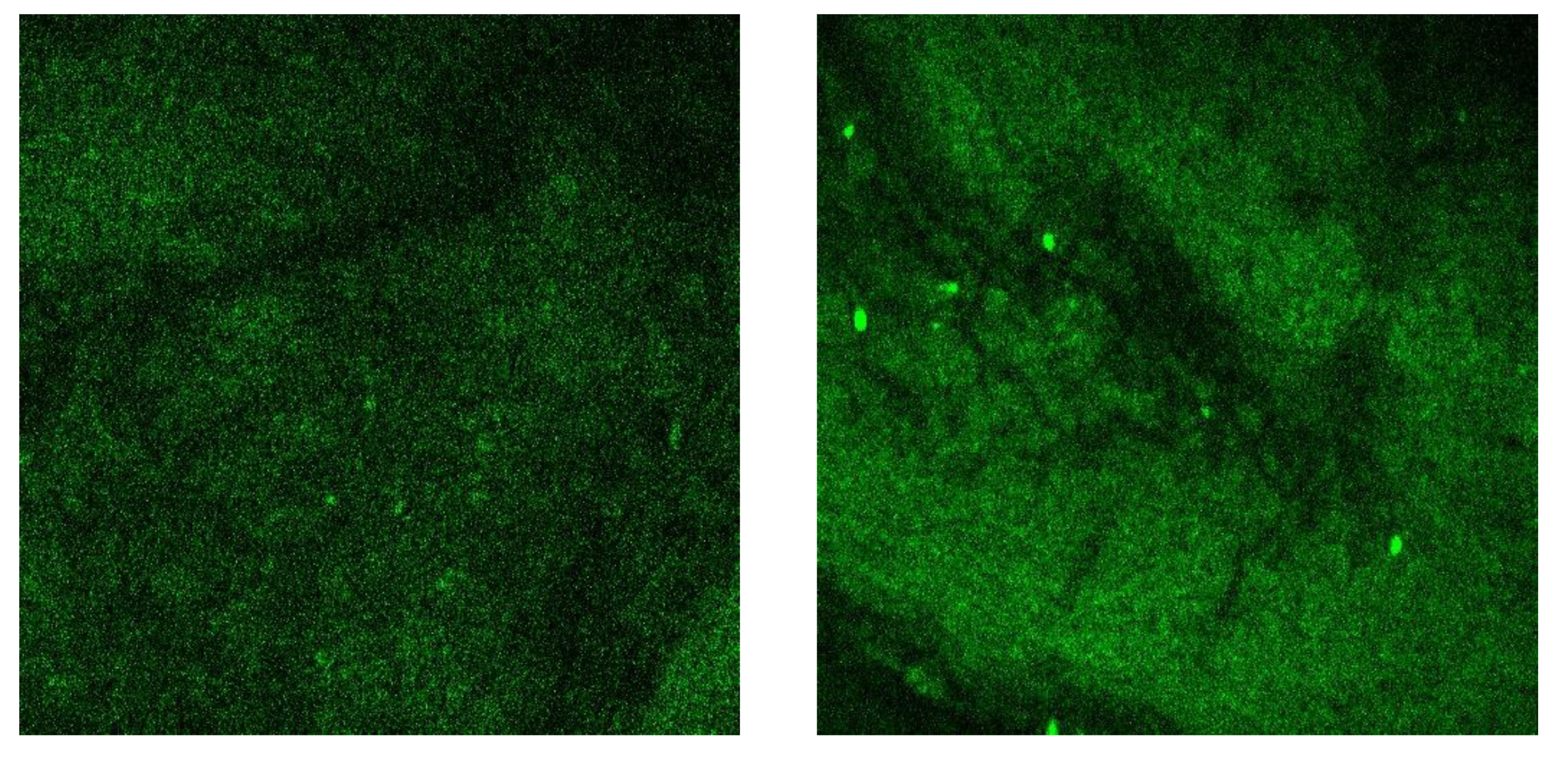
3.5. Wearable-Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ortelli, S.; Costa, A.L.; Dondi, M. TiO2 nanosols applied directly on textiles using different purification treatments. Materials 2015, 8, 7988–7996. [Google Scholar] [CrossRef] [PubMed]
- Radetic, M. Functionalization of textile materials with TiO2 nanoparticles. J. Photochem. Photobiol. C Photochem. Rev. 2013, 16, 62–76. [Google Scholar] [CrossRef]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Nanomaterials for functional textiles and fibers. Nanoscale Res. Lett. 2015, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.L.; Comero, S.; Stahlmecke, B.; Romazanov, J.; Kuhlbusch, T.A.J.; Van Doren, E.; Mast, P.J.D.T.J.; Wick, P.; Krug, H.; Locoro, G.; et al. NM-Series of Representative Manufactured Nanomaterials, NM-300 Silver Characterisation, Stability, Homogeneity; Publications Office of the European Union: Luxembourg, 2011; ISBN 9789279190681. [Google Scholar]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Wehrschuetz-Sigl, E.; Hasmann, A.; Guebitz, G.; Gedanken, A. CuO-cotton nanocomposite: Formation, morphology, and antibacterial activity. Surf. Coat. Technol. 2009, 204, 54–57. [Google Scholar] [CrossRef]
- Pinto, T.V.; Costa, P.; Sousa, C.M.; Sousa, C.A.D.; Pereira, C.; Silva, C.J.S.M.; Pereira, M.F.R.; Coelho, P.J.; Freire, C. Screen-printed photochromic textiles through new inks based on SiO2@naphthopyran nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 28935–28945. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Carosio, F.; Malucelli, G. Current emerging techniques to impart flame retardancy to fabrics: An overview. Polym. Degrad. Stab. 2014, 106, 138–149. [Google Scholar] [CrossRef]
- Ortelli, S.; Malucelli, G.; Cuttica, F.; Blosi, M.; Zanoni, I.; Costa, A.L. Coatings made of proteins adsorbed on TiO2 nanoparticles: A new flame retardant approach for cotton fabrics. Cellulose 2018. [Google Scholar] [CrossRef]
- Ortelli, S.; Malucelli, G.; Blosi, M.; Zanoni, I.; Costa, A.L. NanoTiO2@DNA complex: A novel eco, durable, fire retardant design strategy for cotton textiles. J. Colloid Interface Sci. 2019, 546, 174–183. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Ge, M.; Fu, S.; Lin, T. Superhydrophobic and luminescent cotton fabrics prepared by dip-coating of APTMS modified SrAl2O4:Eu2+, Dy3+ particles in the presence of SU8 and fluorinated alkyl silane. J. Rare Earths 2016, 34, 653–660. [Google Scholar] [CrossRef]
- Titos-Padilla, S.; Colacio, E.; Pope, S.J.A.; Delgado, J.J.; Melgosa, M.; Herrera, J.M. Photophysical properties of [Ir(tpy)2]3+-doped silica nanoparticles and synthesis of a colour-tunable material based on an Ir(core)–Eu(shell) derivative. J. Mater. Chem. C 2013, 1, 3808. [Google Scholar] [CrossRef]
- Fiorini, V.; Zanoni, I.; Zacchini, S.; Costa, A.L.; Hochkoeppler, A.; Zanotti, V.; Ranieri, A.; Massi, M.; Stefan, A.; Stagni, S. Methylation of Ir(III)-tetrazolato complexes: An effective route to modulate the emission outputs and to switch to antimicrobial properties. Dalt. Trans. 2017, 46, 12328. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Fiorini, V.; Rosado, M.; Ballesteros, B.; Androulidaki, M.; Blosi, M.; Ortelli, S.; Stagni, S.; Dondi, M.; Costa, A.L. Encapsulation of cationic iridium(III) tetrazole complexes into a silica matrix: Synthesis, characterization and optical properties. New J. Chem. 2018, 42, 9635–9644. [Google Scholar] [CrossRef]
- Ow, H.; Larson, D.R.; Srivastava, M.; Baird, B.A.; Webb, W.W.; Wiesnert, U. Bright and stable core-shell fluorescent silica nanoparticles. Nano Lett. 2005, 5, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Zhang, P.; Wang, K.; Tapec, R.; Tan, W. Conjugation of biomolecules with luminophore-doped silica nanoparticles for photostable biomarkers. Anal. Chem. 2001, 73, 4988–4993. [Google Scholar] [CrossRef] [PubMed]
- Grace, W.R.; Conn, C. LUDOX® Colloidal Silica in Coatings. Grace Davison Eng. Mater. Available online: https://grace.com/coatings-and-inks/en-us/Documents/LUDOX%20Coatings%20TI.pdf (accessed on 1 January 2020).
- Fiorini, V.; Ignazio, A.D.; Magee, K.D.M.; Ogden, M.I.; Massi, M.; Stagni, S. Fully Ir(III) tetrazolate soft salts: The road to white-emitting ion pairs. Dalt. Trans. 2016, 45, 3256–3259. [Google Scholar] [CrossRef]
- Monti, F.; Baschieri, A.; Gualandi, I.; Serrano-Pérez, J.J.; Junquera-Hernández, J.M.; Tonelli, D.; Mazzanti, A.; Muzzioli, S.; Stagni, S.; Roldan-Carmona, C.; et al. Iridium(III) complexes with phenyl-tetrazoles as cyclometalating ligands. Inorg. Chem. 2014, 53, 7709–7721. [Google Scholar] [CrossRef]
- Caporale, C.; Bader, C.A.; Sorvina, A.; MaGee, K.D.M.; Skelton, B.W.; Gillam, T.A.; Wright, P.J.; Raiteri, P.; Stagni, S.; Morrison, J.L.; et al. Investigating intracellular localisation and cytotoxicity trends for neutral and cationic iridium tetrazolato complexes in live cells. Chem. A Eur. J. 2017, 23, 15666–15679. [Google Scholar] [CrossRef]
- Kumar, S.V.; Scottwell, S.O.; Waugh, E.; McAdam, C.J.; Hanton, L.R.; Brooks, H.J.L.; Crowley, J.D. Antimicrobial properties of tris(homoleptic) ruthenium(II) 2-Pyridyl-1,2,3-triazole “click” complexes against pathogenic bacteria, including methicillin-resistant staphylococcus aureus (MRSA). Inorg. Chem. 2016, 55, 9767–9777. [Google Scholar] [CrossRef]
- Lu, L.; Liu, L.J.; Chao, W.; Zhong, H.J.; Wang, M.; Chen, X.P.; Lu, J.J.; Li, R.; Ma, D.L.; Leung, C.H. Identification of an iridium(III) complex with anti-bacterial and anti-cancer activity. Sci. Rep. 2015, 5, 14544. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Tang, H.; Xie, C.; Zhang, J.; Wu, W.; Jiang, X. Tracking cancer metastasis in vivo by using an iridium-based hypoxia-activated optical oxygen nanosensor. Angew. Chem. Int. Ed. 2015, 54, 8094–8099. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, X.; Mao, H.; Wu, W.; Liu, B.; Jiang, X. Hypoxia-specific ultrasensitive detection of tumours and cancer cells in vivo. Nat. Commun. 2015, 6, 5834. [Google Scholar] [CrossRef] [PubMed]
- Yellol, J.; Pérez, S.A.; Yellol, G.; Zajac, J.; Donaire, A.; Vigueras, G.; Novohradsky, V.; Janiak, C.; Brabec, V.; Ruiz, J. Highly potent extranuclear-targeted luminescent iridium(iii) antitumor agents containing benzimidazole-based ligands with a handle for functionalization. Chem. Commun. 2016, 52, 14165–14168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Che, W.; Zhu, D.; Li, G.; Xie, Z.; Song, N.; Liu, S.; Tang, B.Z.; Liu, X.; et al. AIE Multinuclear Ir(III) Complexes for biocompatible organic nanoparticles with highly enhanced photodynamic performance. Adv. Sci. 2019, 6, 1802050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Wang, Y.J.; Du, F.; He, M.; Gu, Y.Y.; Bai, L.; Yang, L.L.; Liu, Y.J. Evaluation of anticancer effect in vitro and in vivo of iridium(III) complexes on gastric carcinoma SGC-7901 cells. Eur. J. Med. Chem. 2019, 178, 401–416. [Google Scholar] [CrossRef]
- Ma, D.L.; Wu, C.; Wu, K.J.; Leung, C.H. Iridium(III) Complexes targeting apoptotic cell death in cancer cells. Molecules 2019, 24, 2739. [Google Scholar] [CrossRef]
- Ma, D.L.; Wu, C.; Tang, W.; Gupta, A.R.; Lee, F.W.; Li, G.; Leung, C.H. Recent advances in iridium(III) complex-assisted nanomaterials for biological applications. J. Mater. Chem. B 2018, 6, 537–544. [Google Scholar] [CrossRef]
- Crosby, G.A.; Demas, J.N. Measurement of photoluminescence quantum yields. Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Nakamaru, K. Synthesis, Luminescence quantum yields, and lifetimes of trischelated ruthenium(II) mixed-ligand complexes including 3,3′-dimethyl-2,2′-bipyridyl. Bull. Chem. Soc. Jpn. 1982, 55, 2697–2705. [Google Scholar] [CrossRef]
- Psilodimitrakopoulos, S.; Mouchliadis, L.; Paradisanos, I.; Lemonis, A.; Kioseoglou, G.; Stratakis, E. Ultrahigh-resolution nonlinear optical imaging of the armchair orientation in 2D transition metal dichalcogenides. Light Sci. Appl. 2018, 7, 18005–18009. [Google Scholar] [CrossRef]
- Microchem Laboratory. Available online: https://microchemlab.com/test/aatcc-147-assessment-textile-materials-parallel-streak-method (accessed on 1 December 2018).
- Crosera, M.; Mauro, M.; Bovenzi, M.; Adami, G.; Baracchini, E.; Maina, G.; Larese Filon, F. In vitro permeation of palladium powders through intact and damaged human skin. Toxicol. Lett. 2018, 287, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Crosera, M.; Ortelli, S.; Blosi, M.; Adami, G.; Filon, L.; Costa, A.L. CuO nanoparticle penetration through intact and damaged human skin. New J. Chem. 2019. [Google Scholar] [CrossRef]
- Franz, T.J. Percutaneous Absorption. On the Relevance of in Vitro Data. J. Invest. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef]
- Guth, K.; Schäfer-Korting, M.; Fabian, E.; Landsiedel, R.; van Ravenzwaay, B. Suitability of skin integrity tests for dermal absorption studies in vitro. Toxicol. Vitr. 2015, 29, 113–123. [Google Scholar] [CrossRef]
- Tiki, A.; Amin, A.; Kanwal, A. Chemistry of optical brighteners and uses in textile industries. Pakistan Text. J. 2010, 59, 42–43. [Google Scholar]
- Stagni, S.; Colella, S.; Palazzi, A.; Valenti, G.; Zacchini, S.; Paolucci, F.; Marcaccio, M.; Albuquerque, R.Q.; De Cola, L. Essential role of the ancillary ligand in the color tuning of iridium tetrazolate complexes. Inorg. Chem. 2008, 47, 10509–10521. [Google Scholar] [CrossRef] [PubMed]
- Flamigni, L.; Barbieri, A.; Sabatini, C.; Ventura, B.; Barigelletti, F. Topics in Current Chemistry, Biochemistry; Springer: Berlin, Germany, 2007; Volume 281, ISBN 9783642255281. [Google Scholar]
- Werrett, M.V.; Chartrand, D.; Gale, J.D.; Hanan, G.S.; MacLellan, J.G.; Massi, M.; Muzzioli, S.; Raiteri, P.; Skelton, B.W.; Silberstein, M.; et al. Synthesis, structural, and photophysical investigation of diimine triscarbonyl Re(I) tetrazolato complexes. Inorg. Chem. 2011, 50, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Kartini, I.; Ilmi, I.; Kunarti, E.S.; Kamariah. Wash fastness improvement of malachite green-dyed cotton fabrics coated with nanosol composites of silica—Titania. Bull. Mater. Sci. 2014, 37, 1419–1426. [Google Scholar] [CrossRef]
- Takizawa, S.; Aboshi, R.; Murata, S. Photooxidation of 1,5-dihydroxynaphthalene with iridium complexes as singlet oxygen sensitizers. Photochem. Photobiol. Sci. 2011, 10, 895. [Google Scholar] [CrossRef]
- Gao, R.; Ho, D.G.; Hernandez, B.; Selke, M.; Murphy, D.; Djurovich, P.I.; Thompson, M.E. Bis-cyclometalated Ir(III) complexes as efficient singlet oxygen sensitizers. J. Am. Chem. Soc. 2002, 124, 14828–14829. [Google Scholar] [CrossRef] [PubMed]
- Von Goetz, N.; Lorenz, C.; Windler, L.; Nowack, B.; Heuberger, M.; Hungerbühler, K. Migration of Ag- and TiO2-(nano)particles from textiles into artificial sweat under physical stress: Experiments and exposure modeling. Environ. Sci. Technol. 2013, 47, 9979–9987. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Riviere, N.A.; Baroli, B. Toxicology of the Skin; Monteiro-Riviere, N.A., Ed.; TARGET ORG; Taylor & Francis Group: Boca Raton, FL, USA, 2010; ISBN 9781420079180. [Google Scholar]
- Larese Filon, F.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Crosera, M.; Bovenzi, M.; Maina, G.; Adami, G.; Zanette, C.; Florio, C.; Filon Larese, F. Nanoparticle dermal absorption and toxicity: A review of the literature. Int. Arch. Occup. Environ. Health 2009, 82, 1043–1055. [Google Scholar] [CrossRef]
- Lau, J.S.; Lee, P.; Tsang, K.H.; Ng, C.H.; Lam, Y.; Cheng, S.; Lo, K.K. Luminescent cyclometalated iridium (III) polypyridine indole properties, cytotoxicity, and cellular uptake. Inorg. Chem. 2009, 48, 708–718. [Google Scholar] [CrossRef]
- Lee, P.K.; Law, W.H.T.; Liu, H.W.; Lo, K.K.W. Luminescent cyclometalated iridium(III) polypyridine Di-2-picolylamine complexes: Synthesis, photophysics, electrochemistry, cation binding, cellular internalization, and cytotoxic activity. Inorg. Chem. 2011, 50, 8570–8579. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.K.; Liu, H.W.; Yiu, S.M.; Louie, M.W.; Kam-Wing Lo, K. Luminescent cyclometallated iridium(III) bis(quinolylbenzaldehyde) diimine complexes—Synthesis, photophysics, electrochemistry, protein cross-linking properties, cytotoxicity and cellular uptake. Dalt. Trans. 2011, 40, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
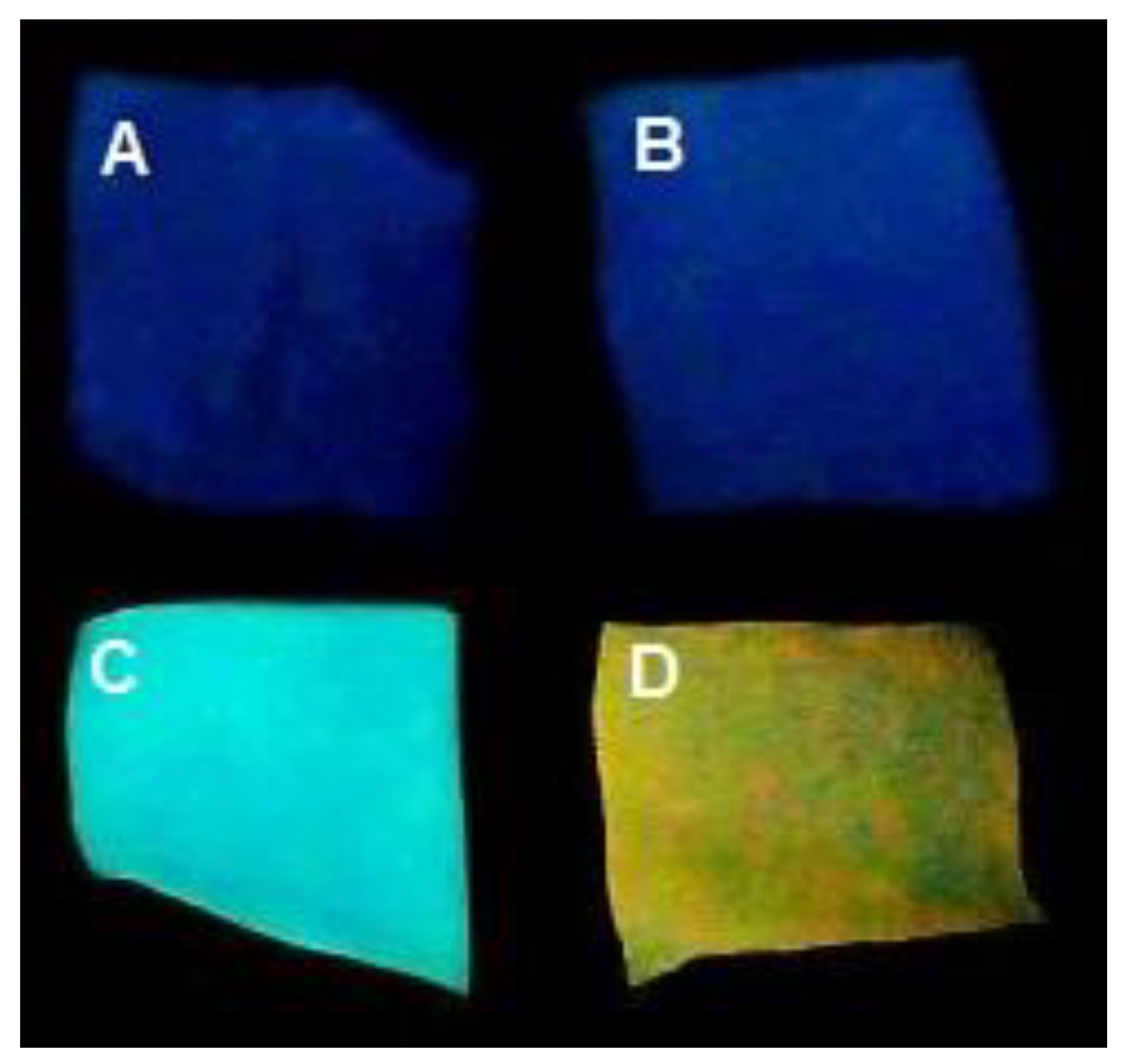


| Coated Textile | SiO2 * | Blue Ir@SiO2 | Red Ir@SiO2 |
|---|---|---|---|
| % add-on | 7.9 ± 0.5 | 8.0 ± 0.7 | 8.9 ± 1.0 |
| Release in Synthetic Sweat | Ir (mg·L−1) | Si (mg·L−1) | Weight Loss of SiO2% |
|---|---|---|---|
| Ir@SiO2 NPs | 0.01 ± 0.01 | 7.92 ± 0.16 | 0.95 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanoni, I.; Blosi, M.; Fiorini, V.; Crosera, M.; Ortelli, S.; Stagni, S.; Stefan, A.; Psilodimitrakopoulos, S.; Stratakis, E.; Larese Filon, F.; et al. Use of Cotton Textiles Coated by Ir(III) Tetrazole Complexes within Ceramic Silica Nanophases for Photo-Induced Self-Marker and Antibacterial Application. Nanomaterials 2020, 10, 1020. https://doi.org/10.3390/nano10061020
Zanoni I, Blosi M, Fiorini V, Crosera M, Ortelli S, Stagni S, Stefan A, Psilodimitrakopoulos S, Stratakis E, Larese Filon F, et al. Use of Cotton Textiles Coated by Ir(III) Tetrazole Complexes within Ceramic Silica Nanophases for Photo-Induced Self-Marker and Antibacterial Application. Nanomaterials. 2020; 10(6):1020. https://doi.org/10.3390/nano10061020
Chicago/Turabian StyleZanoni, Ilaria, Magda Blosi, Valentina Fiorini, Matteo Crosera, Simona Ortelli, Stefano Stagni, Alessandra Stefan, Sotiris Psilodimitrakopoulos, Emmanuel Stratakis, Francesca Larese Filon, and et al. 2020. "Use of Cotton Textiles Coated by Ir(III) Tetrazole Complexes within Ceramic Silica Nanophases for Photo-Induced Self-Marker and Antibacterial Application" Nanomaterials 10, no. 6: 1020. https://doi.org/10.3390/nano10061020
APA StyleZanoni, I., Blosi, M., Fiorini, V., Crosera, M., Ortelli, S., Stagni, S., Stefan, A., Psilodimitrakopoulos, S., Stratakis, E., Larese Filon, F., & Costa, A. L. (2020). Use of Cotton Textiles Coated by Ir(III) Tetrazole Complexes within Ceramic Silica Nanophases for Photo-Induced Self-Marker and Antibacterial Application. Nanomaterials, 10(6), 1020. https://doi.org/10.3390/nano10061020