Improved Photocatalyzed Degradation of Phenol, as a Model Pollutant, over Metal-Impregnated Nanosized TiO2
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Catalyst Synthesis
2.3. Characterization Techniques
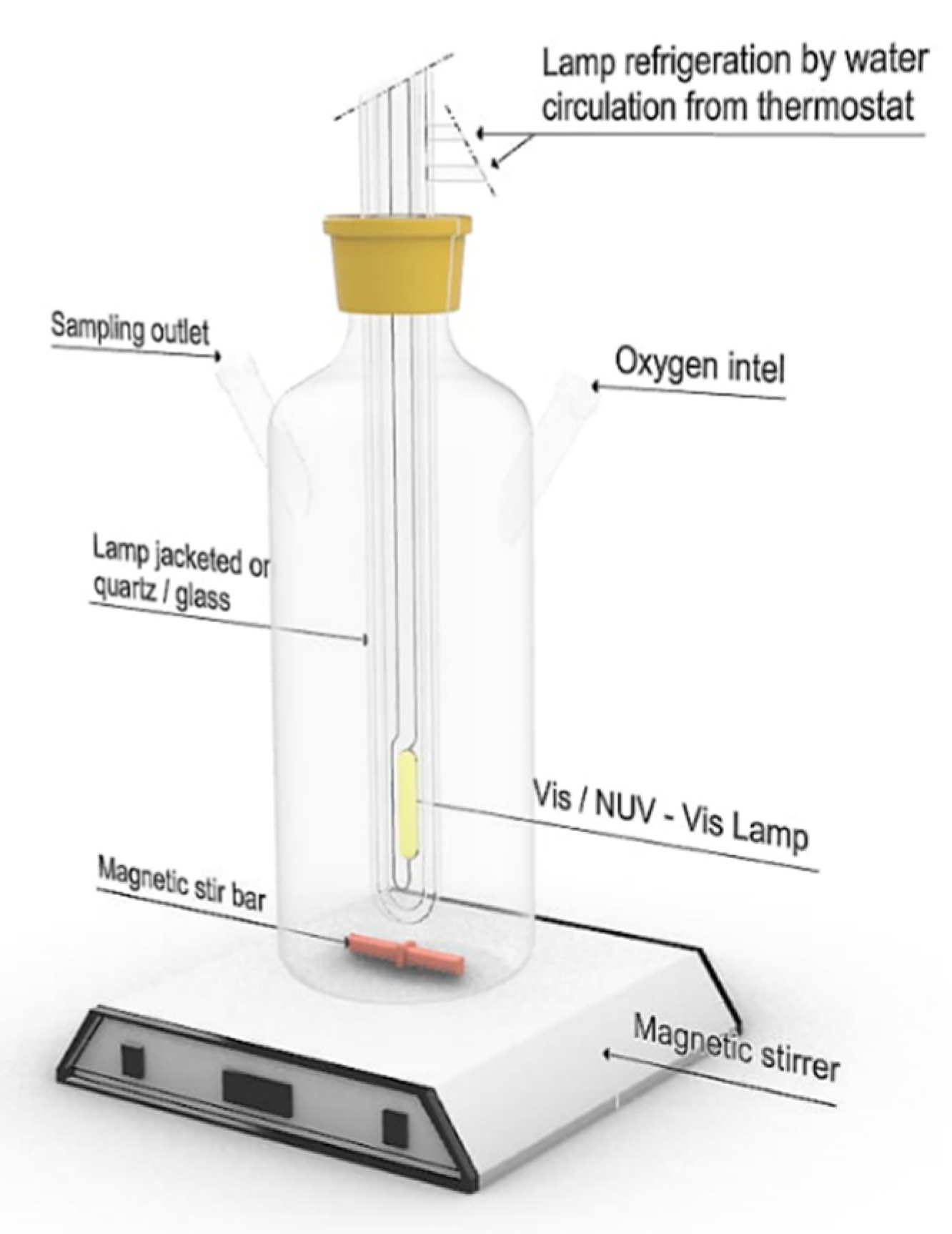
2.4. Photocatalytic Activity
3. Results and Discussion
3.1. Characterization of the Catalysts
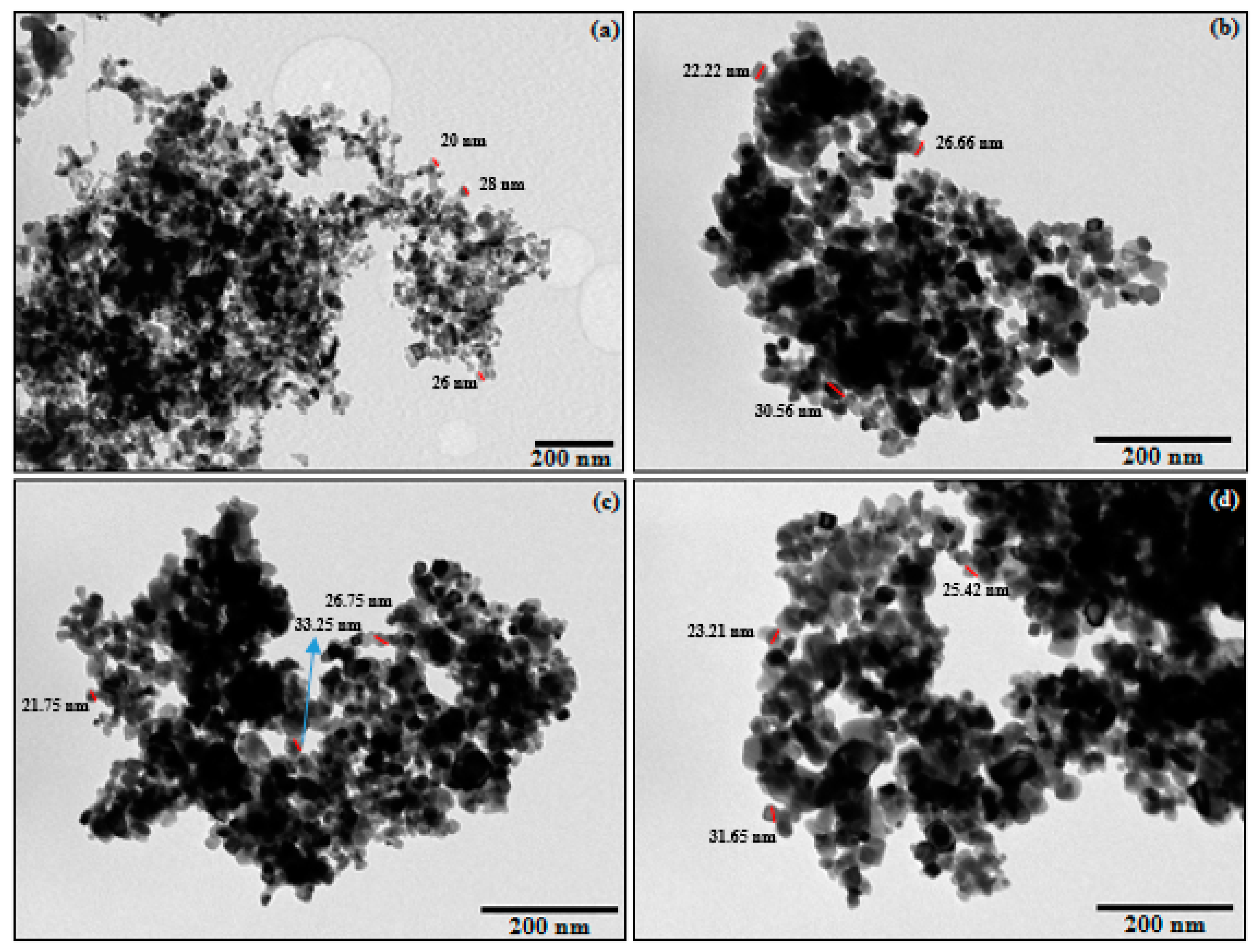
3.1.1. TEM
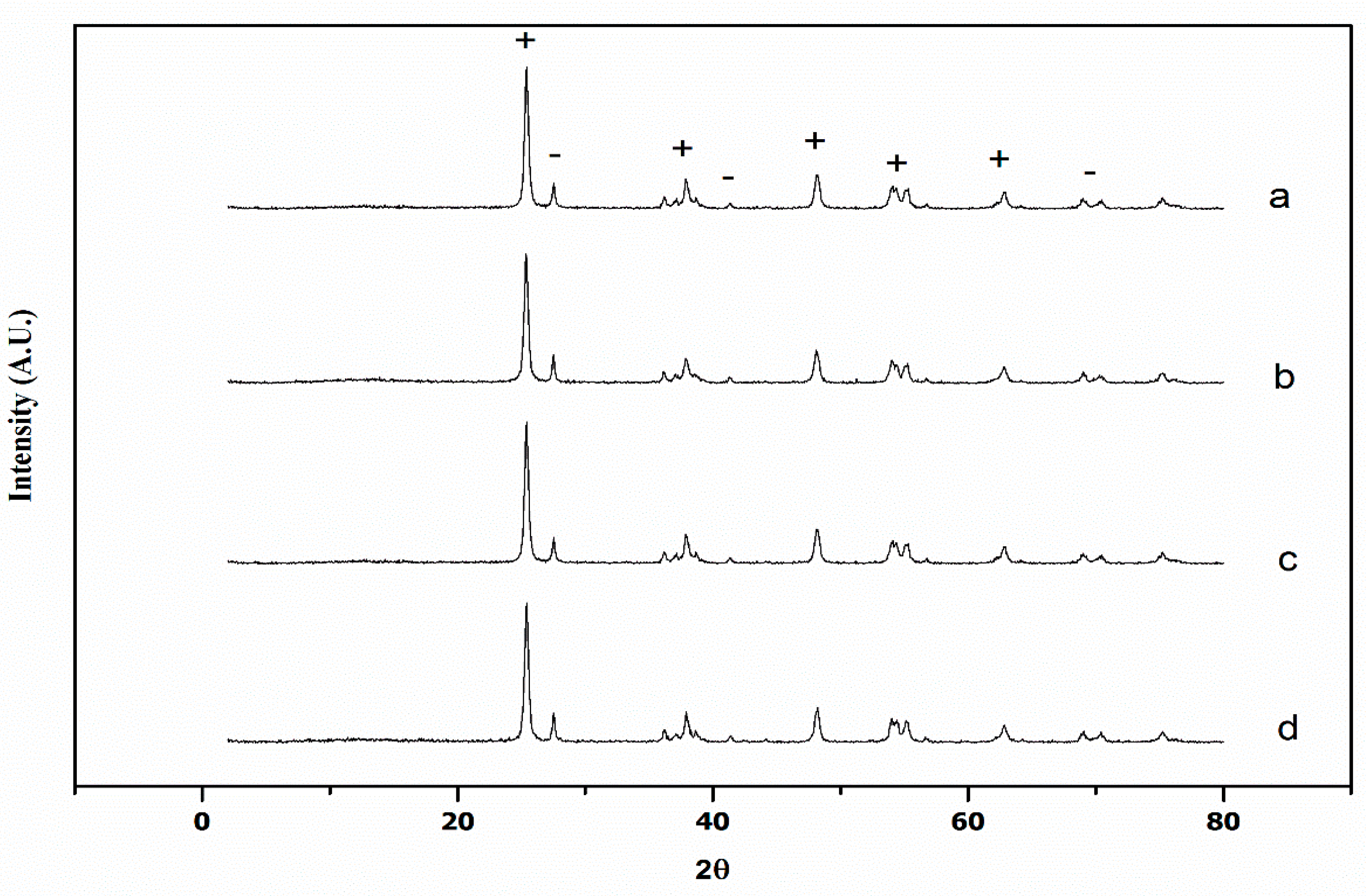
3.1.2. X-ray Diffraction
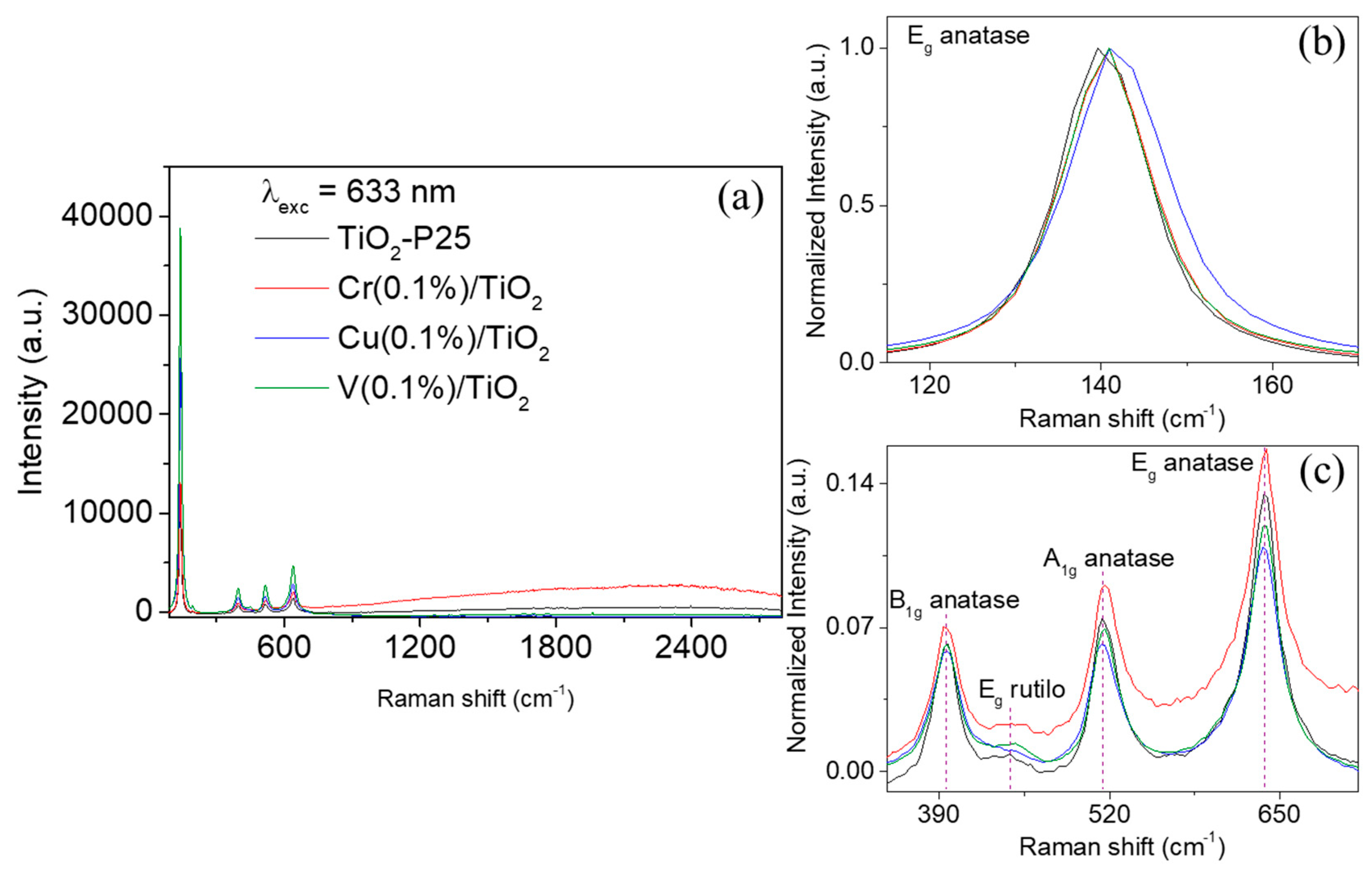
3.1.3. Raman Spectroscopy
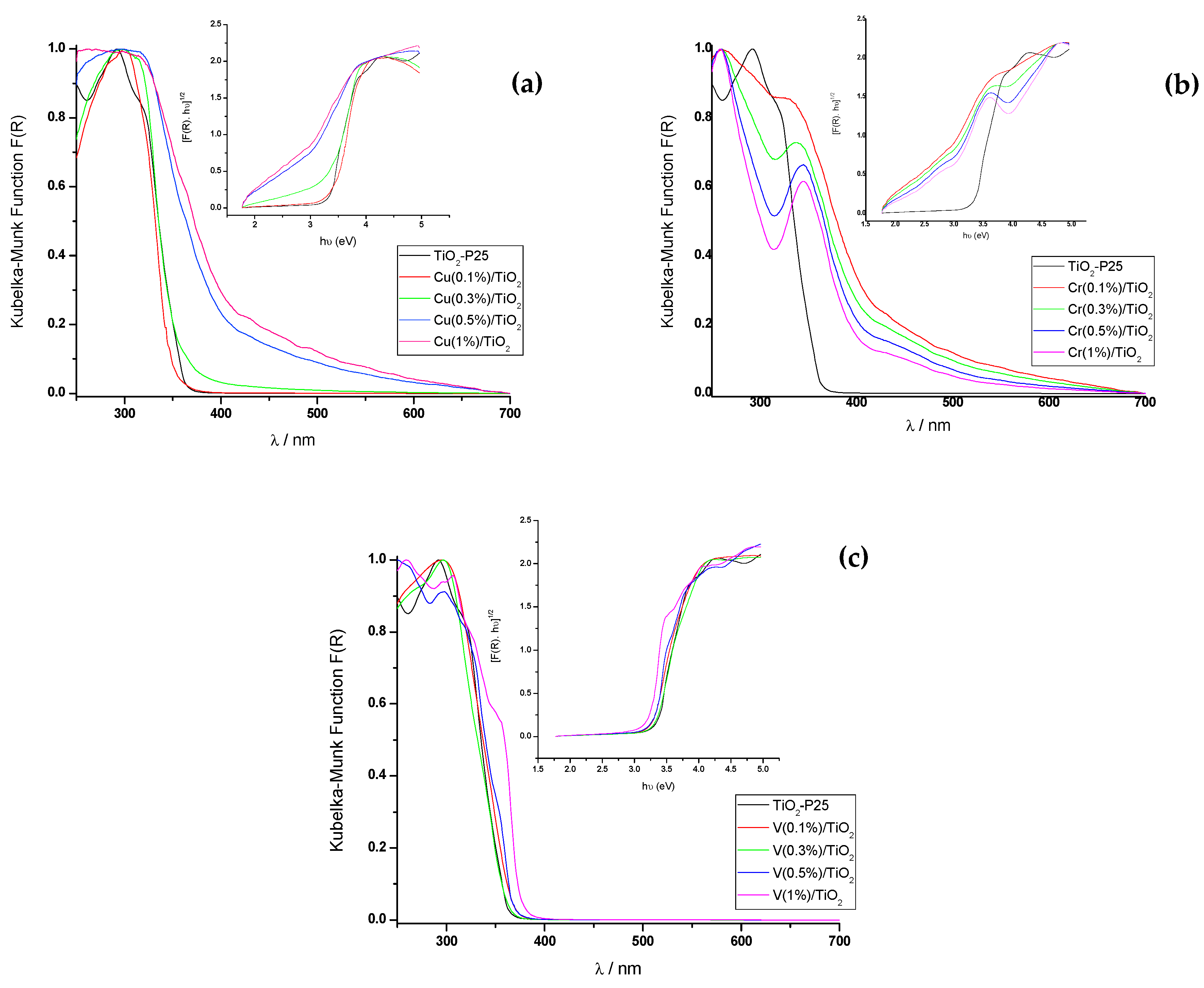
3.1.4. UV-Vis Diffuse Reflectance Spectroscopy
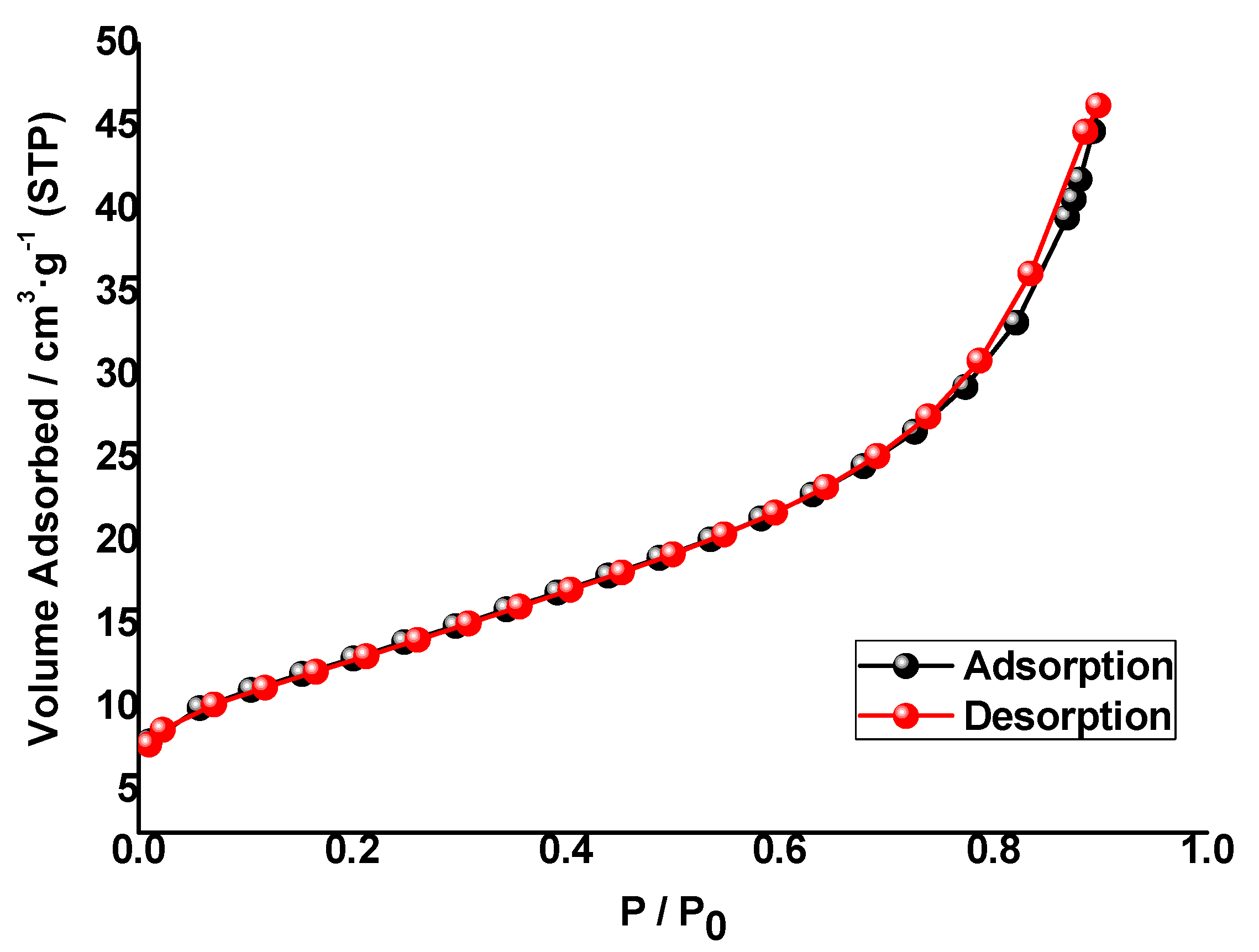
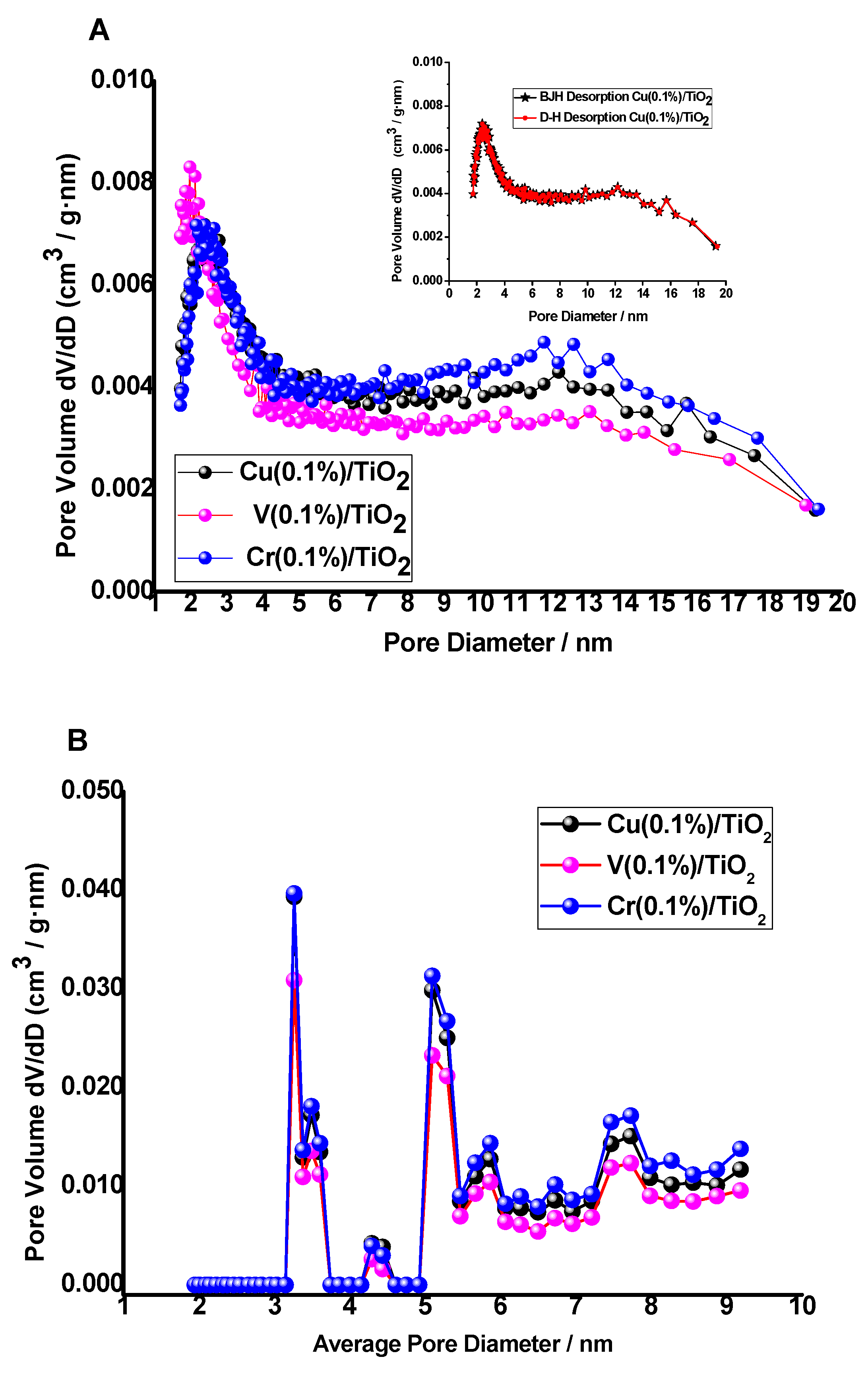
3.1.5. Textural Properties
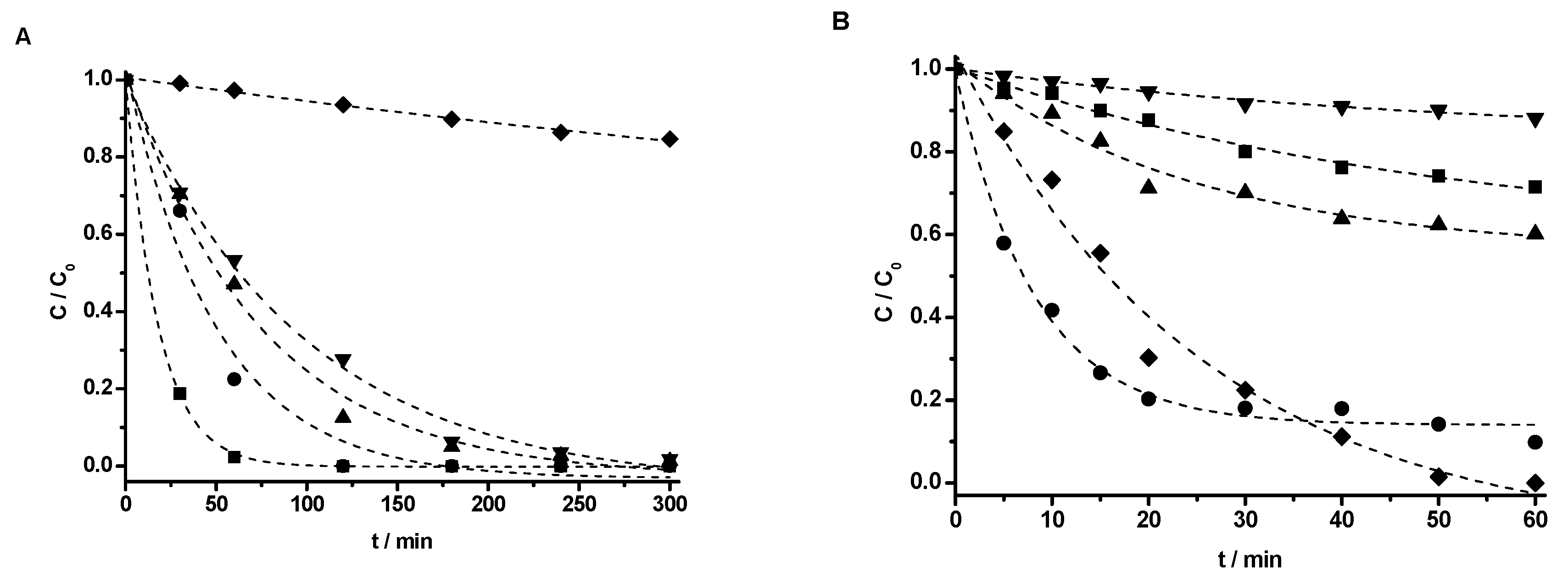
4. Photodegradation of Phenol under Vis and UV Light Irradiation
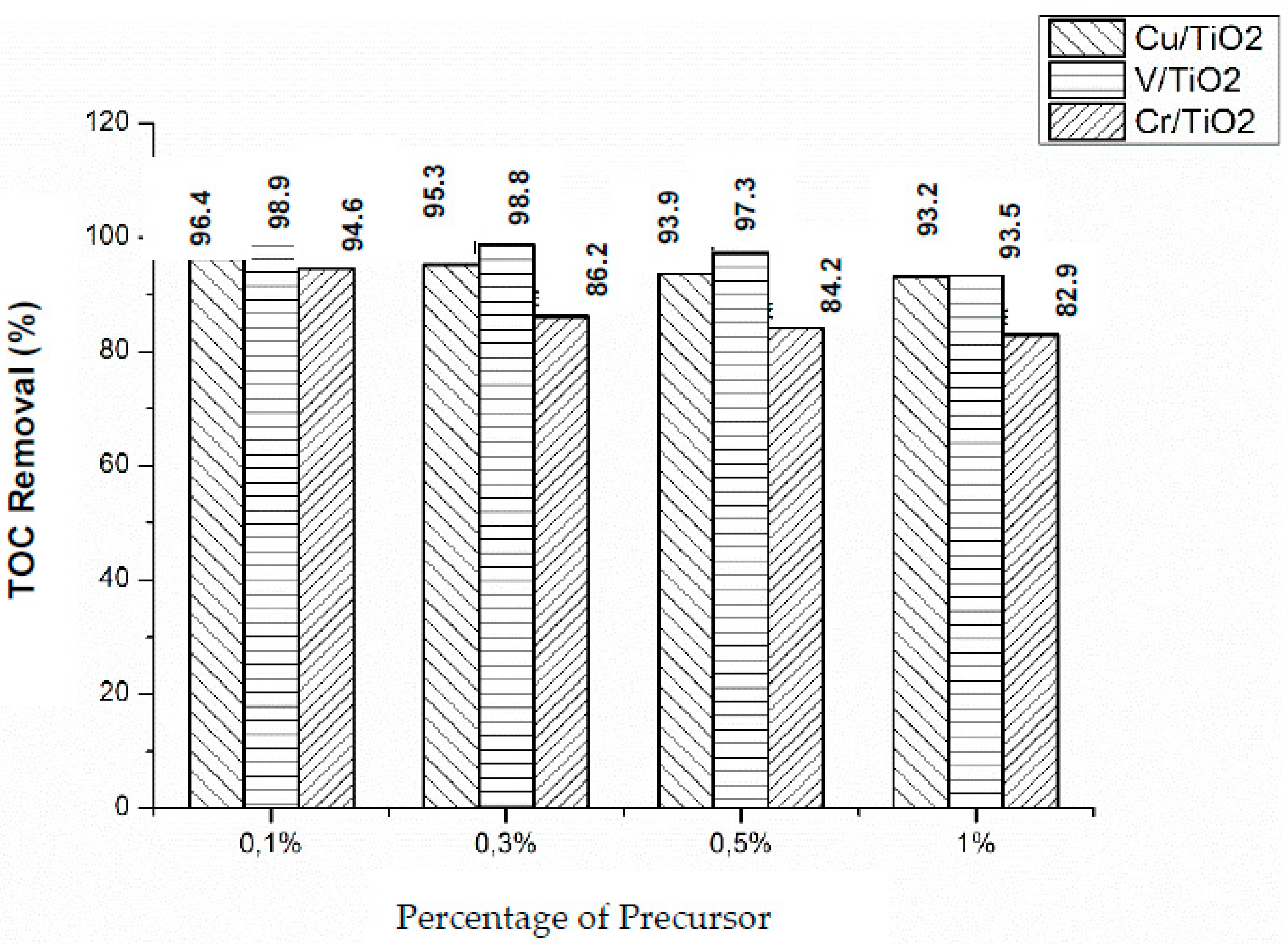
Total Organic Carbon
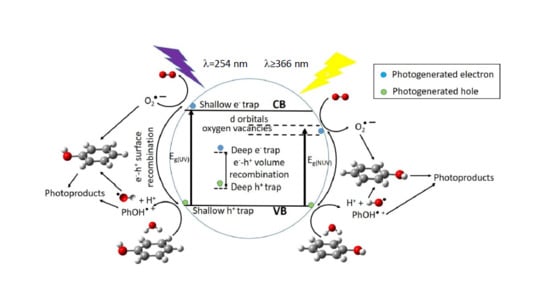
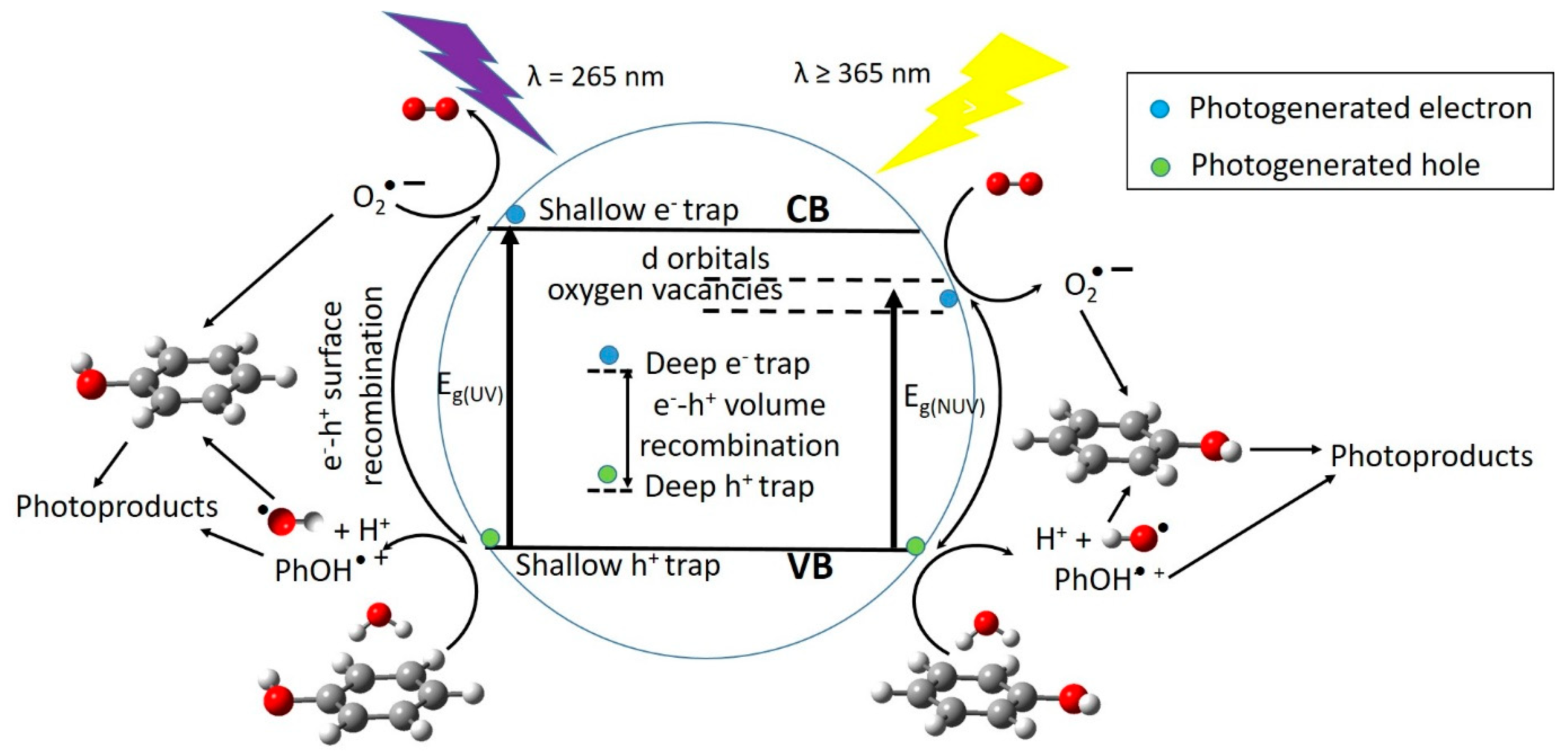
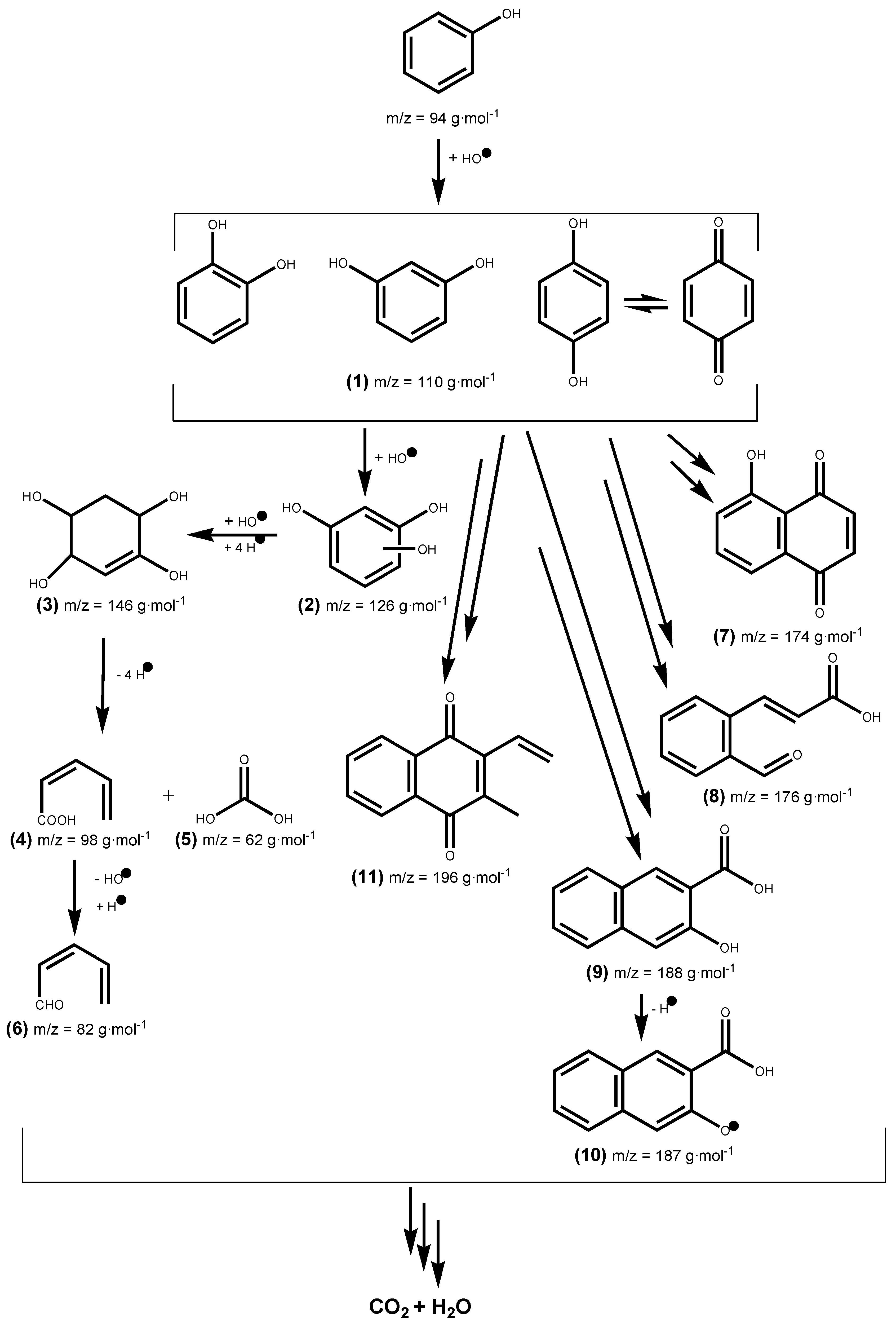
5. Reaction Pathways for Photocatalyzed Degradation
6. Photodegradation and Energetic Efficiency of the Process
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teh, C.M.; Mohamed, A.R. Roles of titanium dioxide and ion-doped titanium dioxide on photocatalytic degradation of organic pollutants (phenolic compounds and dyes) in aqueous solutions: A review. J. Alloys Compd. 2011, 509, 1648–1660. [Google Scholar] [CrossRef]
- Blanco, E.; Casais, M.C.; Mejuto, M.C.; Cela, R. Capillary Electrophoresis|Phenols; Wilson, I.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 1–9. [Google Scholar]
- Chimentão, R.J.; Medina, F.; Fierro, J.L.G.; Llorca, J.; Sueiras, J.E.; Cesteros, Y.; Salagre, P. Propene epoxidation by nitrous oxide over Au–Cu/TiO2 alloy catalysts. J. Mol. Catal. A Chem. 2007, 274, 159–168. [Google Scholar] [CrossRef]
- Fath, B.D. Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 2682–2689. [Google Scholar]
- Wang, F.; Hu, Y.; Guo, C.; Huang, W.; Liu, C. Enhanced phenol degradation in coking wastewater by immobilized laccase on magnetic mesoporous silica nanoparticles in a magnetically stabilized fluidized bed. Bioresour. Technol. 2012, 110, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Canle, L.M.; Santaballa, J.A.; Vulliet, E. On the mechanism of TiO2-photocatalyzed degradation of aniline derivatives. J. Photochem. Photobiol. A Chem. 2005, 175, 192–200. [Google Scholar] [CrossRef]
- Zhang, L.; Jaroniec, M. Toward designing semiconductor-semiconductor heterojunctions for photocatalytic applications. Appl. Surf. Sci. 2018, 430, 2–17. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Z.; Wang, X. Advances in photocatalysis in China. Chin. J. Catal. 2013, 34, 524–535. [Google Scholar] [CrossRef]
- Helali, S. Application de la Photocatalyse Pour la Dégradation des Polluants Chimiques et Bactériologiques Dans L’eau en Utilisant des Catalyseurs Irradiés Par des Photons de Lumière Naturelle ou Artificielle (UV-A/UV-B). Ph.D. Thesis, Claude Bernard-Lyon I, Villeurbanne, France, 17 December 2012. [Google Scholar]
- Kouamé, N.A.; Alaoui, O.T.; Herissan, A.; Larios, E.; José-Yacaman, M.; Etcheberry, A.; Colbeau-Justin, C.; Remita, H. Visible light-induced photocatalytic activity of modified titanium(iv) oxide with zero-valent bismuth clusters. New J. Chem. 2015, 39, 2316–2322. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the relationship between surface phases and photocatalytic activity of TiO. Angew. Chem. 2008, 47, 1766–1769. [Google Scholar] [CrossRef]
- Dobrosz-Gómez, I.; Gómez-García, M.Á.; López, Z.S.M.; GilPavas, E.; Bojarska, J.; Kozanecki, M.; Rynkowski, J.M. Transition metal loaded TiO2 for phenol photo-degradation. C. R. Chim. 2015, 18, 1170–1182. [Google Scholar] [CrossRef]
- Ata, R.; Sacco, O.; Vaiano, V.; Rizzo, L.; Tore, G.Y.; Sannino, D. Visible light active N-doped TiO2 immobilized on polystyrene as efficient system for wastewater treatment. J. Photochem. Photobiol. A Chem. 2017, 348, 255–262. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der grosse und der inneren struktur von kolloidteilchen mittels rontgenstrahlen. Math.-Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. ACTA Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals; Clarendon Press: Oxford, UK, 2008. [Google Scholar]
- Halsey, G. Physical adsorption on non-uniform surfaces. J. Chem. Phys. 1948, 16, 931–937. [Google Scholar] [CrossRef]
- Frenkel, J.I. Kinetic Theory of Liquids; Clarendon Press: Oxford, UK, 1946. [Google Scholar]
- Hill, T.L. Advances in Catalysis. In Theory of Physical Adsorption; Elsevier Science & Technology: Amsterdam, The Netherlands, 1952; Volume 4, pp. 211–258. [Google Scholar]
- López, R.; Gómez, R.; Oros-Ruiz, S. Photophysical and photocatalytic properties of TiO2-Cr sol–gel prepared semiconductors. Catal. Today 2011, 166, 159–165. [Google Scholar] [CrossRef]
- Kuhn, B.J.; Braslavsky, S.E.; Schmidt, R. Chemical actinometry (IUPAC Technical Report). Pure Appl. Chem. 2004, 76, 2105–2146. [Google Scholar] [CrossRef]
- Boukhatem, H.; Khalaf, H.; Djouadi, L.; González, F.V.; Navarro, R.M.; Santaballa, J.A.; Canle, M. Photocatalytic activity of mont-La (6%)-Cu0.6Cd0.4S catalyst for phenol degradation under near UV Visible light irradiation. Appl. Catal. B Environ. 2017, 211, 114–125. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Zakaria, R.; Ying, J.Y. Role of particle size in nanocrystalline TiO2-Based photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Zhu, S.; Wang, F. Copper doping in titanium oxide catalyst film prepared by dc reactive magnetron sputtering. Catal. Today 2004, 93, 589–594. [Google Scholar] [CrossRef]
- Chiang, K.; Amal, R.; Tran, T. Photocatalytic degradation of cyanide using titanium dioxide modified with copper oxide. Adv. Environ. Res. 2002, 6, 471–485. [Google Scholar] [CrossRef]
- Doong, R.; Chang, P.; Huang, C. Microstructural and photocatalytic properties of sol–gel-derived vanadium-doped mesoporous titanium dioxide nanoparticles. J. Non-Cryst. Solids 2009, 355, 2302–2308. [Google Scholar] [CrossRef]
- Tian, B.; Li, C.; Gu, F.; Jiang, H.; Hu, Y.; Zhang, J. Flame sprayed V-doped TiO2 nanoparticles with enhanced photocatalytic activity under visible light irradiation. Chem. Eng. J. 2009, 151, 220–227. [Google Scholar] [CrossRef]
- Wang, L.; Egerton, T.A. The influence of chromium on photocatalysis of propan-2-ol and octadecanoic acid oxidation by rutile TiO. J. Photochem. Photobiol. A Chem. 2013, 252, 211–215. [Google Scholar] [CrossRef]
- Devi, L.G.; Murthy, B.N.; Kumar, S.G. Photocatalytic activity of TiO2 doped with Zn2+ and V5+ transition metal ions: Influence of crystallite size and dopant electronic configuration on photocatalytic activity. Mater. Sci. Eng. B 2010, 166, 1–6. [Google Scholar] [CrossRef]
- Devi, L.G.; Kumar, S.G. Influence of physicochemical–electronic properties of transition metal ion doped polycrystalline titania on the photocatalytic degradation of Indigo Carmine and 4-nitrophenol under UV/solar light. Appl. Surf. Sci. 2011, 257, 2779–2790. [Google Scholar] [CrossRef]
- Ahlawat, A.; Sathe, V.G.; Reddy, V.R.; Gupta, A. Mossbauer, Raman and X-ray diffraction studies of superparamagnetic NiFe2O4 nanoparticles prepared by sol–gel auto-combustion method. J. Magn. Magn. Mater. 2011, 323, 2049–2054. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys.: PCCP 2012, 14, 14567–14572. [Google Scholar] [CrossRef]
- Abdulrazzak, F.H.; Hussein, F.H. Effects of nanoparticle size on catalytic and photocatalytic activity of carbon nanotubes-titanium dioxide composites. J. Environ. Anal. Chem. 2015, 2, 2. [Google Scholar] [CrossRef]
- Mougin, J.; Le Bihan, T.; Lucazeau, G. High-pressure study of Cr2O3 obtained by high-temperature oxidation by X-ray diffraction and Raman spectroscopy. J. Phys. Chem. Solids 2001, 62, 553–563. [Google Scholar] [CrossRef]
- Navas, J.; Sánchez-Coronilla, A.; Aguilar, T.; Hernández, N.C.; de los Santos, D.M.; Sánchez-Márquez, J.; Zorrilla, D.; Fernández-Lorenzo, C.; Alcántara, R.; Martín-Calleja, J. Experimental and theoretical study of the electronic properties of Cu-doped anatase TiO. Phys. Chem. Chem. Phys.: PCCP 2014, 16, 3835–3845. [Google Scholar] [CrossRef] [PubMed]
- Ramana, C.V.; Smith, R.J.; Hussain, O.M.; Massot, M.; Julien, C.M. Surface analysis of pulsed laser-deposited V2O5 thin films and their lithium intercalated products studied by Raman spectroscopy. Surf. Interface Anal. 2005, 37, 406–411. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Varma, S.; Tripathi, A.K.; Bharadwaj, S.R.; Tyagi, A.K. Effect of vanadia doping and its oxidation state on the photocatalytic activity of TiO2 for gas-phase oxidation of ethene. J. Phys. Chem. C 2008, 112, 19102–19112. [Google Scholar] [CrossRef]
- Sahoo, S.; Arora, A.K.; Sridharan, V. Raman line shapes of optical phonons of different symmetries in anatase TiO2 nanocrystals. J. Phys. Chem. C 2009, 113, 16927–16933. [Google Scholar] [CrossRef]
- Zhang, Y.; Harris, C.X.; Wallenmeyer, P.; Murowchick, J.; Chen, X. Asymmetric lattice vibrational characteristics of rutile TiO2 as revealed by laser power dependent raman spectroscopy. J. Phys. Chem. C 2013, 117, 24015–24022. [Google Scholar] [CrossRef]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navío, J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B Environ. 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Coulter, J.B.; Dunbar, P.B. Assessing Tauc Plot Slope Quantification: ZnO Thin Films as a Model System. Phys. Status Solidi B 2018, 255, 1700393. [Google Scholar] [CrossRef]
- Khairy, M.; Zakaria, W. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef]
- Kamble, R.; Mahajan, S.; Puri, V.; Shinde, H.; Garadkar, K. Visible light-driven high photocatalytic activity of Cu-doped TiO2 nanoparticles synthesized by hydrothermal method. Mater. Sci. Res. India 2018, 15, 197–208. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Petranovskii, V.P.; Kryazhov, A.; Ozhereliev, O.; Pfänder, N.; Knop-Gericke, A. Study of copper nanoparticles formation on supports of different nature by UV–Vis diffuse reflectance spectroscopy. Chem. Phys. Lett. 2004, 385, 173–176. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid Cu(x)O/TiO2 nanocomposites as risk-reduction materials in indoor environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef]
- Irie, H.; Kamiya, K.; Shibanuma, T.; Miura, S.; Tryk, D.A.; Yokoyama, T.; Hashimoto, K. Visible light-sensitive Cu(II)-grafted TiO2 photocatalysts: Activities and X-ray absorption fine structure analyses. J. Phys. Chem. C 2009, 113, 10761–10766. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y. Understanding the reaction mechanism of photocatalytic reduction of CO2 with H2O on TiO2-based photocatalysts: A review. Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Yoong, L.S.; Chong, F.K.; Dutta, B.K. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34, 1652–1661. [Google Scholar] [CrossRef]
- Shafei, A.; Sheibani, S. Visible light photocatalytic activity of Cu doped TiO2-CNT nanocomposite powder prepared by sol–gel method. Mater. Res. Bull. 2019, 110, 198–206. [Google Scholar] [CrossRef]
- Dvoranová, D.; Brezová, V.; Mazúr, M.; Malati, M.A. Investigations of metal-doped titanium dioxide photocatalysts. Appl. Catal. B Environ. 2002, 37, 91–105. [Google Scholar] [CrossRef]
- Jaimy, K.B.; Ghosh, S.; Sankar, S.; Warrier, K.G.K. An aqueous sol–gel synthesis of chromium(III) doped mesoporous titanium dioxide for visible light photocatalysis. Mater. Res. Bull. 2011, 46, 914–921. [Google Scholar] [CrossRef]
- Yan, H.; Wang, X.; Yao, M.; Yao, X. Band structure design of semiconductors for enhanced photocatalytic activity: The case of TiO. Prog. Nat. Sci. Mater. Int. 2013, 23, 402–407. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lastoskie, C.M.; Quirke, N.; Gubbins, K.E. Chapter Structure of porous adsorbents: Analysis using density functional theory and molecular simulation. Stud. Surf. Sci. Catal. 1997, 104, 745–775. [Google Scholar]
- Yang, X.; Wang, X.; Wang, S.; Sun, H.; Lian, J. Preparation and photocatalytic performance of Cu-doped TiO2 nanoparticles. Trans. Nonferrous Met. Soc. China 2015, 25, 504–509. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 1, 341–357. [Google Scholar] [CrossRef]
- Palmisano, L.; Augugliaro, V.; Sclafani, A.; Schiavello, M. Activity of chromium-ion-doped titania for the dinitrogen photoreduction to ammonia and for the phenol photodegradation. J. Phys. Chem. 1988, 92, 6710–6713. [Google Scholar] [CrossRef]
- Romeiro, A.; Azenha, M.E.; Canle, M.; Rodrigues, V.H.N.; Da Silva, J.P.; Burrows, H.D. Titanium dioxide nanoparticle photocatalysed degradation of ibuprofen and naproxen in water: Competing hydroxyl radical attack and oxidative decarboxylation by semiconductor holes. ChemSelect 2018, 3, 10915–10924. [Google Scholar] [CrossRef]
- Maruska, H.P.; Ghosh, A.K. Transition-metal dopants for extending the response of titanate photoelectrolysis anodes. Sol. Energy Mater. 1979, 1, 237–247. [Google Scholar] [CrossRef]
- Schaub, R.; Thostrup, P.; López, N.A.; Laegsgaard, E.; Stengsgaard, I.; Nørskov, J.K.; Besenbacher, F. Oxygen vacancies as active sites for water dissociation on rutile TiO2(110). Phys. Rev. Lett. 2001, 87, 266104. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Guo, X.; Wu, X.; Li, M.; Li, Q.; Ho, W.; Ye, H.; Du, D. Photocatalytic selective oxidation of phenol to produce dihydroxybenzenes in a TiO2/UV system: Hydroxyl radical versus hole. Appl. Catal. B Environ. 2016, 199, 405–411. [Google Scholar] [CrossRef]
- Turki, A.; Guillard, C.; Dappozze, F.; Ksibi, Z.; Berhault, G.; Kochkar, H. Phenol photocatalytic degradation over anisotropic TiO2 nanomaterials: Kinetic study, adsorption isotherms and formal mechanisms. Appl. Catal. B Environ. 2015, 163, 404–414. [Google Scholar] [CrossRef]
- Diak, M.; Klein, M.; Klimczuk, T.; Lisowski, W.; Remita, H.; Zaleska-Medynska, A.; Grabowska, E. Photoactivity of decahedral TiO2 loaded with bimetallic nanoparticles: Degradation pathway of phenol-1-13C and hydroxyl radical formation. Appl. Catal. B Environ. 2017, 200, 56–71. [Google Scholar] [CrossRef]
- Serpone, N. Relative photonic efficiencies and quantum yields in heterogeneous photocatalysis. J. PhotoChem. Photobiol. A Chem. 1997, 104, 1–12. [Google Scholar] [CrossRef]
- Serpone, N.; Salinaro, A. Terminology, relative photonic efficiencies and quantum yields in heterogeneous photocatalysis. Part I: Suggested protocol. Pure Appl. Chem. 1999, 71, 303–320. [Google Scholar] [CrossRef]
- Wong, C.C.; Chu, W. The direct photolysis and photocatalytic degradation of alachlor at different TiO2 and UV sources. Chemosphere 2003, 50, 981–987. [Google Scholar] [CrossRef]
- Chu, W.; Jafvert, C.T.; Diehl, C.A.; Marley, K.; Larson, R.A. Phototransformations of polychlorobiphenyls in Brij 58 micellar solutions. Environ. Sci. Technol. 1998, 32, 1989–1993. [Google Scholar] [CrossRef]
- Chu, W.; Jafvert, C.T. Photodechlorination of polychlorobenzene congeners in surfactant micelle solutio-ns. Environ. Sci. Technol. 1994, 28, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric and solar-driven systems. Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
| Cu(0.1%)/TiO2-P25 | Cr(0.1%)/TiO2-P25 | V(0.1%)/TiO2-P25 | |||||
|---|---|---|---|---|---|---|---|
| Phase | (h k l) | 2Ө/0 | Τ a/nm | 2Ө/0 | Τ a/nm | 2Ө/0 | Τ a/nm |
| Anatase | (1 0 1) | 25.399 | 22.4 | 25.362 | 22.4 | 25.404 | 22.4 |
| (0 0 4) | 37.923 | 21.9 | 37.894 | 24.4 | 37.92 | 24.4 | |
| (2 0 0) | 48.152 | 17.9 | 48.124 | 23.9 | 48.145 | 21.0 | |
| (2 1 5) | 62.834 | 20.0 | 62.824 | 27.1 | 62.819 | 26.3 | |
| W–H b | τ/nm | 27.4 | 20.2 | 20.8 | |||
| Strain (ε) | 8.5 × 10–4 | −8.0 × 10–4 c | −5.3 × 10–4 c | ||||
| SSP d | τ/nm | 12.9 | 12.9 | 12.1 | |||
| Strain (ε) c | 0.011 | 0.011 | −0.005 c | ||||
| Rutile | (1 0 1) | 27.511 | 36.8 | 27.524 | 32.4 | 27.516 | 40.4 |
| (1 0 1) | 36.185 | 30.6 | 36.162 | 39.4 | 36.163 | 31.8 | |
| (1 1 1) | 41.134 | 35.0 | 41.305 | 32.3 | 41.387 | 46.7 | |
| W–H b | τ/nm | 42.8 | 31.9 | 32.6 | |||
| Strain (ε) c | 6.8 × 10–4 | −2.8 × 10–4 c | −5.5 × 10–4 c | ||||
| SSP d | τ/nm | 21.4 | 16.3 | 19.7 | |||
| Strain (ε) | 0.009 | −0.006 c | −0.004 c | ||||
| Anatase mass fraction (%) e | 81.5 | 79.4 | 78.6 | ||||
| % M/TiO2 | V | Cr | Cu |
|---|---|---|---|
| 0 | 3.31; 3.26 [34,35] | 3.31; 3.18 [23] | 3.30 |
| 0.02 | 2.92 [35] | ||
| 0.06 | 2.72 [35] | ||
| 0.1 | 3.26; 2.78 [34,35] | 3.30; 3.16 [23] | 3.29 |
| 0.2 | 3.0 [48] | ||
| 0.3 | 3.29 | 2.05 | 3.50 |
| 0.5 | 3.26 | 2.30; 3.06 [23] | 2.44; 3.14 [23] |
| 0.88 | 2.72 [49] | ||
| 1.0 | 3.21 | 2.44, 3.04 [23] | 2.07; 3.22 [23] |
| Photocatalyst | V(0.1%)/TiO2 | Cu(0.1%)/TiO2 | Cr(0.1%)/TiO2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BET | SBET/m2·g−1 | 44.38 ± 0.07 | 46.92 ± 0.04 | 47.57 ± 0.05 | ||||||
| Constant C | 72 | 103 | 107 | |||||||
| Vm (monolayer adsorption volume)/cm3·g−1 | 10.2 | 10.8 | 10.9 | |||||||
| Parameter | Surface area (m2·g−1) | Pore volume (cm3·g−1) | Average pore width (4V/Å) | Surface area (m2·g−1) | Pore volume (cm3·g−1) | Average pore width (4V/Å) | Surface area (m2·g−1) | Pore volume (cm3·g−1) | Average pore width (4V/Å) | |
| t-plot external surface area | 46.84 | 46.58 | 47.04 | |||||||
| t-plot micropore volume | −0.001753 | -0.000222 | –0.000109 | |||||||
| BJH adsorption | 40.693 a | 0.061463 b | 60.416 | 43.234 | 0.068866 | 63.716 | 44.422 | 0.073953 | 66.592 | |
| BJH desorption | 40.754 a | 0.061655 b | 60.514 | 43.659 | 0.069223 | 63.422 | 44.922 | 0.074221 | 66.089 | |
| D–H adsorption | 40.584 a | 60.425 | 43.131 | 63.712 | 44.321 | 66.583 | ||||
| D–H desorption | 40.575 a | 60.543 | 43.555 | 63.405 | 44.820 | 66.067 | ||||
| Maximum pore volume at p/p°/cm³/g (STP) | 0.17713625 | Median pore width | 0.17706067 | Median pore width | 0.17714428 | Median pore width | ||||
| 0.01802 | 7.687 Å | 0.01936 | 7.759 Å | 0.01965 | 7.787 Å | |||||
| Average particle size/Å | 1352 | 1279 | 1261 | |||||||
| Fractal dimension (DS) | 2.523 | 2.542 | 2.535 | |||||||
| Catalyst | Detection a/Irradiation b | (k ± σk)·104/min−1 | (k±σk)·104/min−1 | ||
|---|---|---|---|---|---|
| TiO2-P25 | HPLC/UV | 765 ± 96 | HPLC/Vis | 6.0 ± 0.2 | |
| M(X%)/TiO2 | (k ± σk)·104/min−1 | ||||
| 0.1% | 0.3% | 0.5% | 1.0% | ||
| Cu | S/UV | 50 ± 3 | 328 ± 45 | 80 ± 6 | 16 ± 7 |
| HPLC/UV | 58 ± 4 | 324 ± 53 | 87 ± 10 | 21 ± 2 | |
| S/Vis | 435 ± 50 | 252 ± 40 | 257 ± 29 | 183 ± 15 | |
| HPLC/Vis | 493 ± 51 | 394 ± 103 | 151 ± 7 | 140 ± 8 | |
| V | S/UV | 44 ± 5 | 41 ± 4 | 35 ± 2 | 31 ± 3 |
| HPLC–UV | 50 ± 5 | 49 ± 3 | 45 ± 3 | 36 ± 3 | |
| S/Vis | 233 ± 13 | 211 ± 21 | 89 ±4 | 34 ± 2 | |
| HPLC/Vis | 244 ± 14 | 193 ± 13 | 131 ± 7 | 49 ± 3 | |
| Cr | S/UV | 27 ± 3 | 32 ± 2 | 52 ± 4 | 37 ± 3 |
| HPLC–UV | 24 ± 3 | 28 ± 1 | 46 ± 3 | 35 ± 2 | |
| S/Vis | 33 ± 5 | 25 ± 3 | 22 ± 1 | 19 ± 1 | |
| HPLC/Vis | 34 ± 5 | 23 ± 2 | 24 ± 1 | 21 ± 1 | |
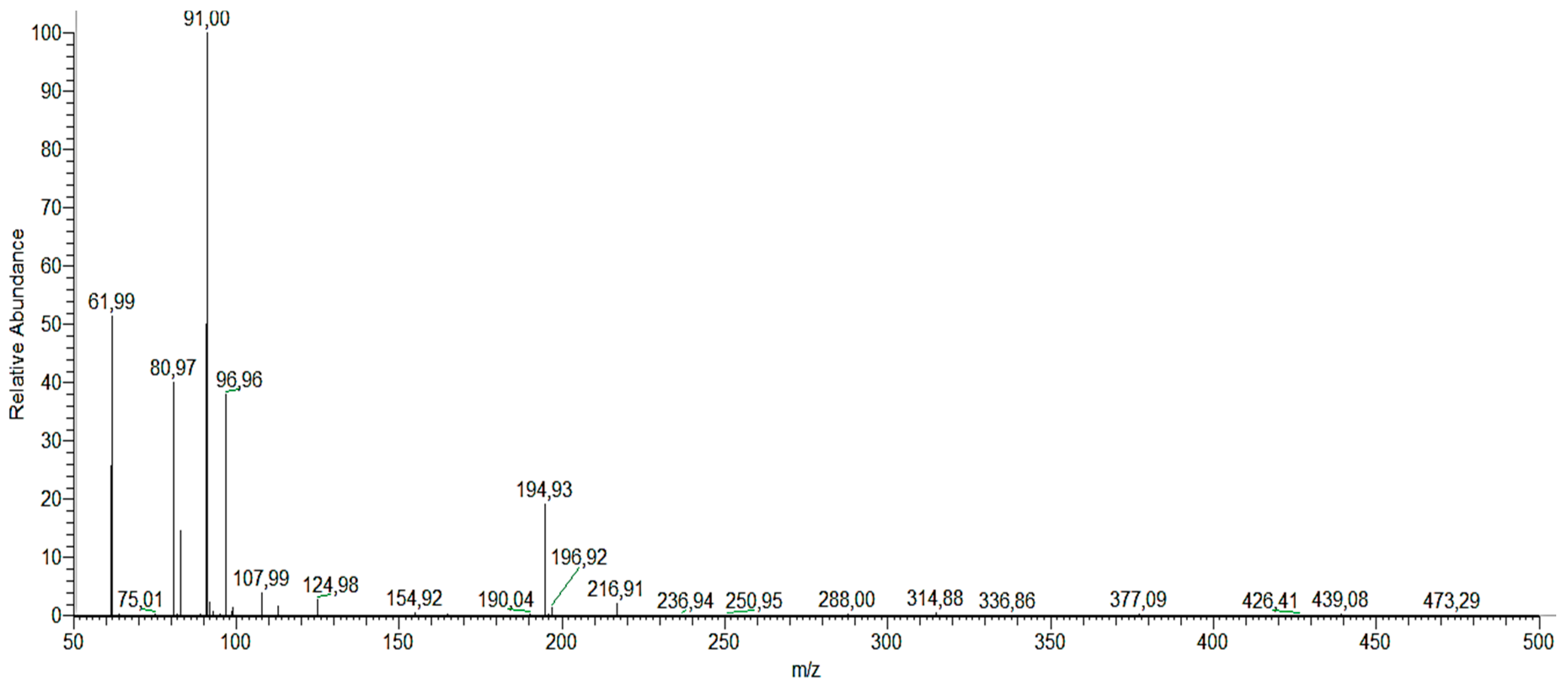
| Photoproducts | (M−H)− (m/z) | tR (min) |
|---|---|---|
| (1) catechol, resorcinol and/or hydroquinone | 109.967 | 1.7 |
| (2) phloroglucinol | 125.11 | 1.47 |
| (3) cyclohex-2-ene-1, 2, 4, 5-tetraol | 145.141 | 5.48 |
| (4) (Z)-penta-2,4-dienoic acid | 96.96 | 1.68 |
| (5) carbonic acid | 61.988 | 1.48 |
| (6) (Z)-penta-2,4-dienal | 80.974 | 1.38 |
| (7) juglone | 173.15 | 7.9 |
| (8) (2E)-3-(2-formylphenyl) acrylic acid | 194.927 | 1.51 |
| (9) 3-hydroxy-2-naphthoic acid | 187.101 | 7.9 |
| (10) 3-hydroxy-2-naphthoate | 186.172 | 6.95 |
| (11) 9H-xanthen-9-one | 174.96 | 0.97 |
| Lamp | (0.1% M)/TiO2 | Φphotodegradation | EEO/kW·L−1·s−1 |
|---|---|---|---|
| UV (254 nm) | Cu | 1.17 | 6400 |
| V | 1.01 | 7385 | |
| Cr | 0.56 | 13395 | |
| UVA-Vis (λexc >366 nm) | Cu | 2.81 | 37403 |
| V | 1.46 | 72000 | |
| Cr | 0.20 | 514286 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belekbir, S.; El Azzouzi, M.; El Hamidi, A.; Rodríguez-Lorenzo, L.; Santaballa, J.A.; Canle, M. Improved Photocatalyzed Degradation of Phenol, as a Model Pollutant, over Metal-Impregnated Nanosized TiO2. Nanomaterials 2020, 10, 996. https://doi.org/10.3390/nano10050996
Belekbir S, El Azzouzi M, El Hamidi A, Rodríguez-Lorenzo L, Santaballa JA, Canle M. Improved Photocatalyzed Degradation of Phenol, as a Model Pollutant, over Metal-Impregnated Nanosized TiO2. Nanomaterials. 2020; 10(5):996. https://doi.org/10.3390/nano10050996
Chicago/Turabian StyleBelekbir, S., M. El Azzouzi, A. El Hamidi, L. Rodríguez-Lorenzo, J. Arturo Santaballa, and M. Canle. 2020. "Improved Photocatalyzed Degradation of Phenol, as a Model Pollutant, over Metal-Impregnated Nanosized TiO2" Nanomaterials 10, no. 5: 996. https://doi.org/10.3390/nano10050996
APA StyleBelekbir, S., El Azzouzi, M., El Hamidi, A., Rodríguez-Lorenzo, L., Santaballa, J. A., & Canle, M. (2020). Improved Photocatalyzed Degradation of Phenol, as a Model Pollutant, over Metal-Impregnated Nanosized TiO2. Nanomaterials, 10(5), 996. https://doi.org/10.3390/nano10050996