The Impact of Engineered Silver Nanomaterials on the Immune System
Abstract
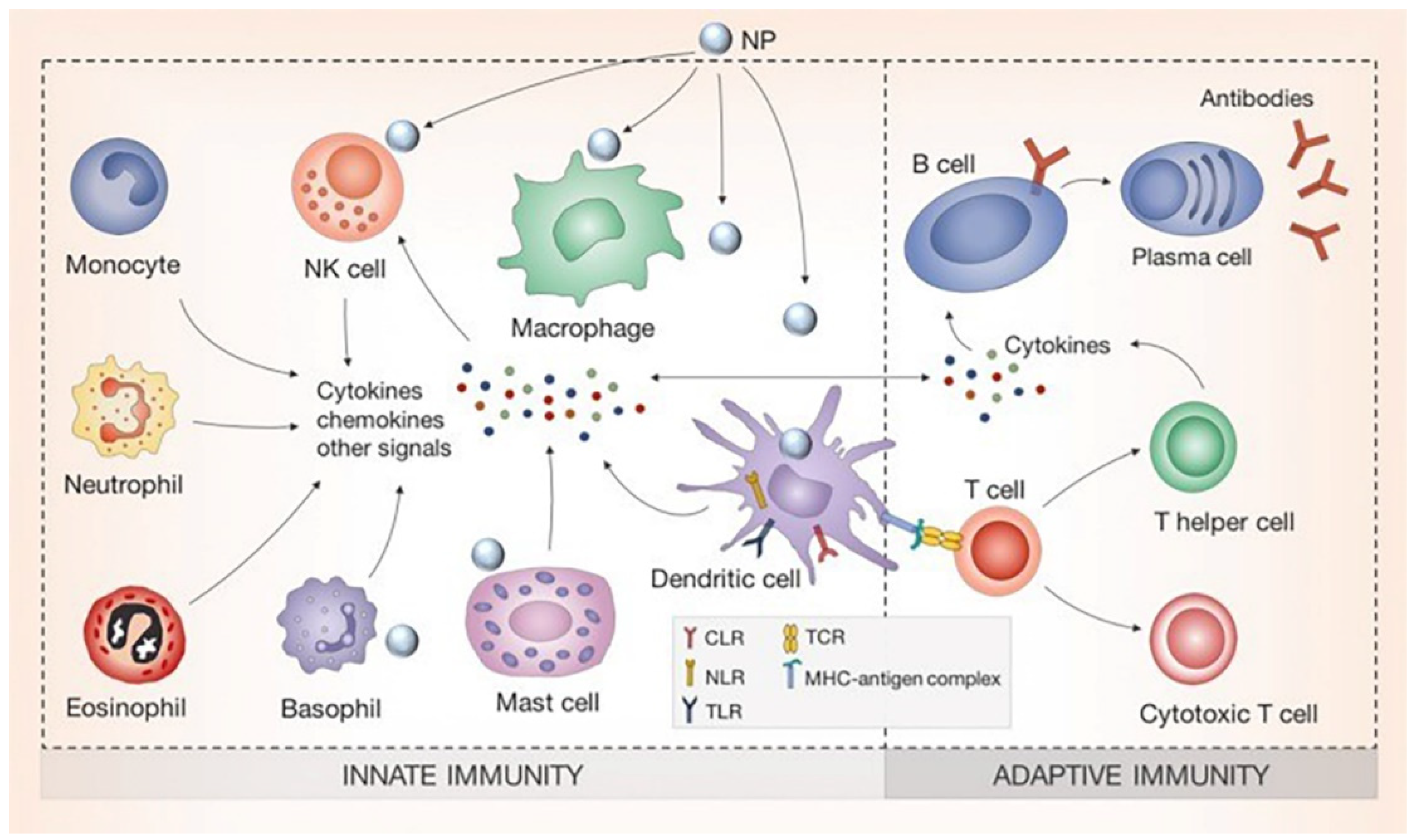
1. Introduction
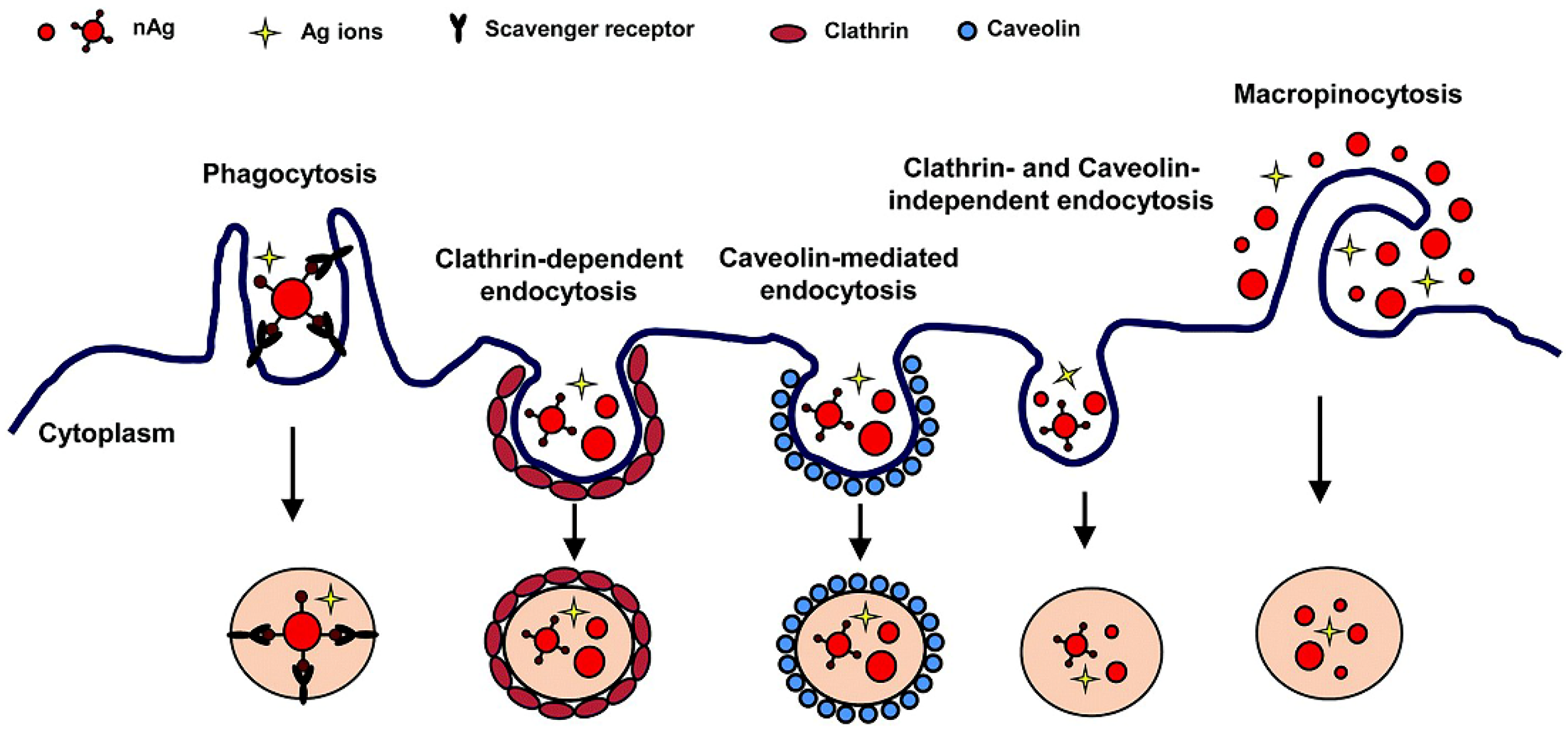
2. Uptake of Silver Nanomaterials by Immune Cells
3. Role of Physiochemical Properties of Silver Nanomaterials
3.1. Pro-Inflammatory Properties of Silver Nanomaterials
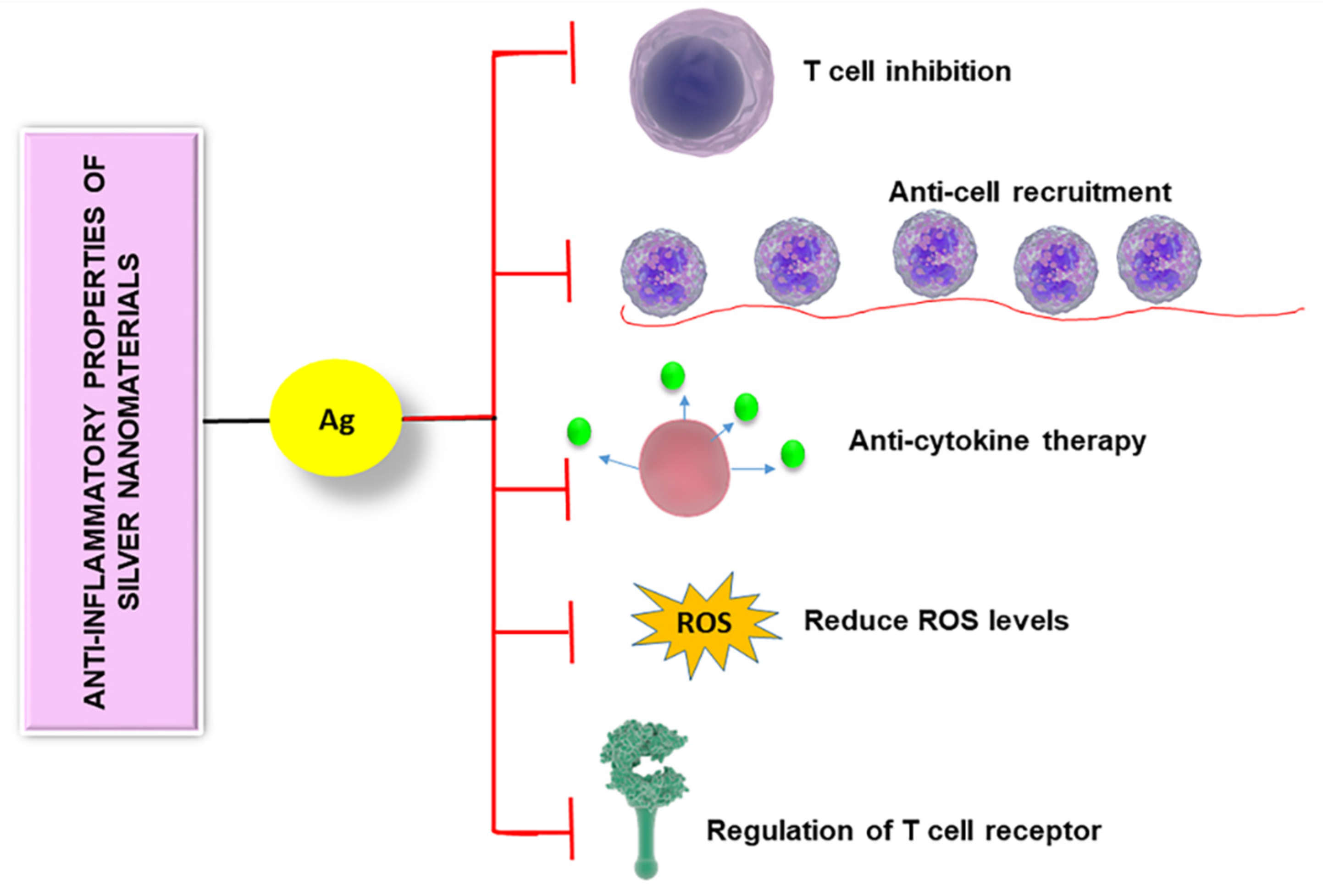
3.2. Anti-Inflammatory and Immunosuppressive Properties of Silver Nanomaterials
3.3. Adjuvant Properties of Silver Nanomaterials
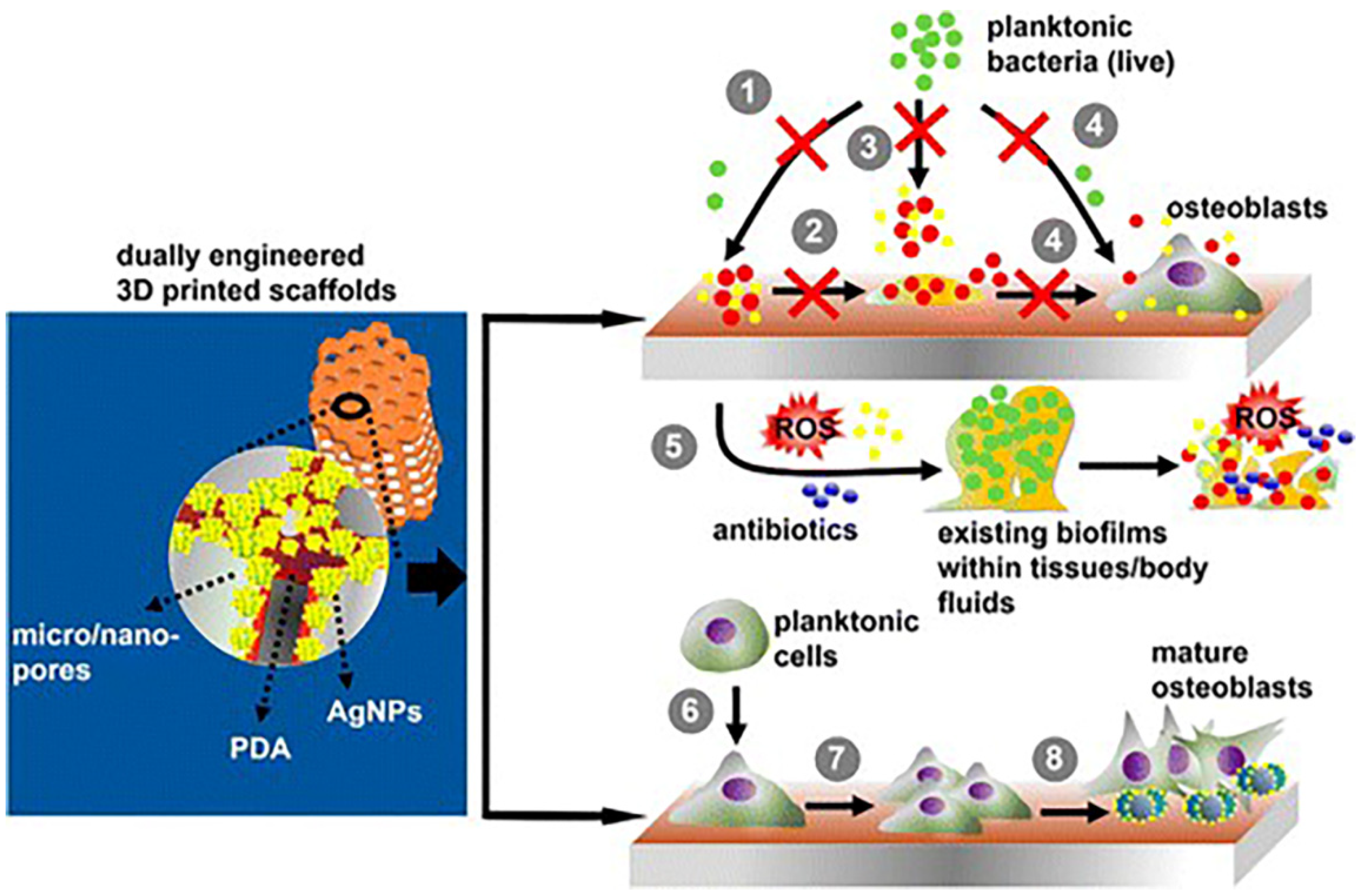
4. Immune Response of Silver-Coated Implants
4.1. Immune Response of Silver-Coated Dental Implants
4.2. Immune Response of Silver-Coated Bone Implants
4.3. Immune Response of Silver-Coated Wound Dressings
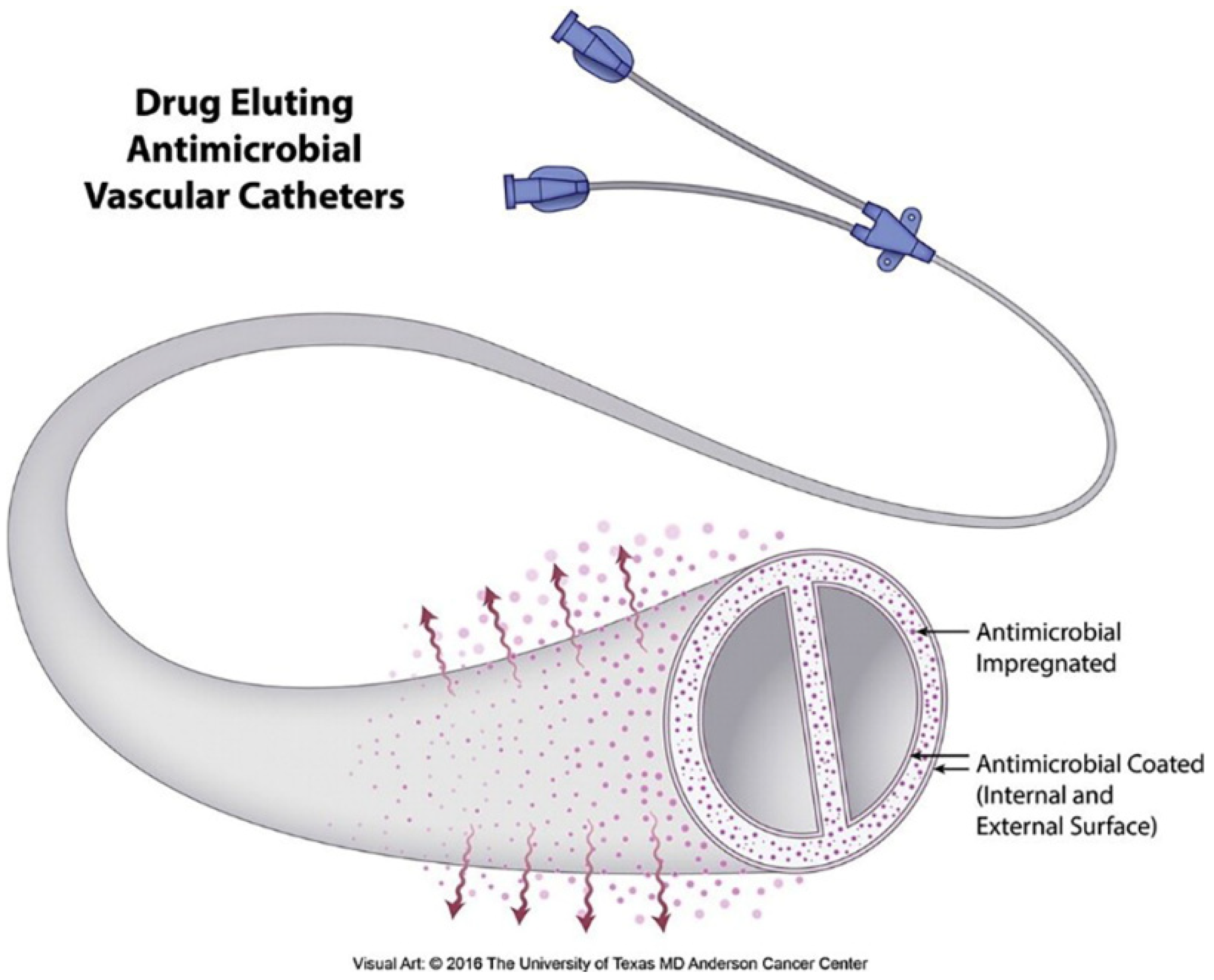
4.4. Immune Response of Silver-Coated Vascular Catheters
5. Bio-Modifications of AgNPs
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Geetha Bai, R.; Ninan, N.; Muthoosamy, K.; Manickam, S. Graphene: A versatile platform for nanotheranostics and tissue engineering. Prog. Mater. Sci. 2018, 91, 24–69. [Google Scholar] [CrossRef]
- Zhu, H.; Goswami, N.; Yao, Q.; Chen, T.; Liu, Y.; Xu, Q.; Chen, D.; Lu, J.; Xie, J. Cyclodextrin–gold nanocluster decorated TiO2 enhances photocatalytic decomposition of organic pollutants. J. Mater. Chem. A 2018, 6, 1102–1108. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Xia, T.; Rallo, R.; Zhao, Y.; Ji, Z.; Lin, S.; Wang, X.; Zhang, H.; France, B.; Schoenfeld, D.; et al. Use of a High-Throughput Screening Approach Coupled with In Vivo Zebrafish Embryo Screening To Develop Hazard Ranking for Engineered Nanomaterials. ACS Nano 2011, 5, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, B.; Li, Q.; Liu, B.; Mao, Y. Morphology-Tuned Phase Transitions of Horseshoe Shaped BaTiO3 Nanomaterials under High Pressure. J. Phys. Chem. C 2018, 122, 5188–5194. [Google Scholar] [CrossRef]
- Zhu, K.; Ju, Y.; Xu, J.; Yang, Z.; Gao, S.; Hou, Y. Magnetic Nanomaterials: Chemical Design, Synthesis, and Potential Applications. Acc. Chem. Res. 2018, 51, 404–413. [Google Scholar] [CrossRef]
- Wilms, M.; Conrad, J.; Vasilev, K.; Kreiter, M.; Wegner, G. Manipulation and conductivity measurements of gold nanowires. Appl. Surf. Sci. 2004, 238, 490–494. [Google Scholar] [CrossRef]
- Shi, S.; Chen, F.; Ehlerding, E.B.; Cai, W. Surface Engineering of Graphene-Based Nanomaterials for Biomedical Applications. Bioconjug. Chem. 2014, 25, 1609–1619. [Google Scholar] [CrossRef]
- Park, K.M.; Yang, J.-A.; Jung, H.; Yeom, J.; Park, J.S.; Park, K.-H.; Hoffman, A.S.; Hahn, S.K.; Kim, K. In Situ Supramolecular Assembly and Modular Modification of Hyaluronic Acid Hydrogels for 3D Cellular Engineering. ACS Nano 2012, 6, 2960–2968. [Google Scholar] [CrossRef]
- Gagner, J.E.; Shrivastava, S.; Qian, X.; Dordick, J.S.; Siegel, R.W. Engineering Nanomaterials for Biomedical Applications Requires Understanding the Nano-Bio Interface: A Perspective. J. Phys. Chem. Lett. 2012, 3, 3149–3158. [Google Scholar] [CrossRef]
- Björnmalm, M.; Thurecht, K.J.; Michael, M.; Scott, A.M.; Caruso, F. Bridging Bio–Nano Science and Cancer Nanomedicine. ACS Nano 2017, 11, 9594–9613. [Google Scholar] [CrossRef] [PubMed]
- Freund, R.; Lächelt, U.; Gruber, T.; Rühle, B.; Wuttke, S. Multifunctional Efficiency: Extending the Concept of Atom Economy to Functional Nanomaterials. ACS Nano 2018, 12, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Fu, J.; Li, H.; Zhu, L.; Hu, Y.; Xia, W.; Zhang, X.; Peng, Y.; Zhang, J. Direct Observation of Magnetocrystalline Anisotropy Tuning Magnetization Configurations in Uniaxial Magnetic Nanomaterials. ACS Nano 2018, 12, 3442–3448. [Google Scholar] [CrossRef] [PubMed]
- Stauber, R.H.; Siemer, S.; Becker, S.; Ding, G.-B.; Strieth, S.; Knauer, S.K. Small Meets Smaller: Effects of Nanomaterials on Microbial Biology, Pathology, and Ecology. ACS Nano 2018, 12, 6351–6359. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.-H.; Hill, D.J.; Park, H.-G.; Cahoon, J.F. Mie-coupled bound guided states in nanowire geometric superlattices. Nat. Commun. 2018, 9, 2781. [Google Scholar] [CrossRef] [PubMed]
- Carini, M.; Ruiz, M.P.; Usabiaga, I.; Fernández, J.A.; Cocinero, E.J.; Melle-Franco, M.; Diez-Perez, I.; Mateo-Alonso, A. High conductance values in π-folded molecular junctions. Nat. Commun. 2017, 8, 15195. [Google Scholar] [CrossRef]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K.-H. Clinical Applications of Carbon Nanomaterials in Diagnostics and Therapy. Adv. Mater. 2018, 30, 1802368. [Google Scholar] [CrossRef]
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Cassano, D.; Pocoví-Martínez, S.; Voliani, V. Ultrasmall-in-Nano Approach: Enabling the Translation of Metal Nanomaterials to Clinics. Bioconjug. Chem. 2018, 29, 4–16. [Google Scholar] [CrossRef]
- Dykas, M.M.; Desai, S.K.; Patra, A.; Motapothula, M.R.; Poddar, K.; Kenney, L.J.; Venkatesan, T. Identification of Biofilm Inhibitors by Screening Combinatorial Libraries of Metal Oxide Thin Films. ACS Appl. Mater. Interfaces 2018, 10, 12510–12517. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Cho, Y.; Byun, K.-E.; Shin, K.W.; Nam, S.-G.; Kim, C.; Kim, H.; Han, S.-A.; Kim, S.-W.; Shin, H.-J.; et al. Two-Dimensional Materials Inserted at the Metal/Semiconductor Interface: Attractive Candidates for Semiconductor Device Contacts. Nano Lett. 2018, 18, 4878–4884. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Dorobantu, L.S.; Goss, G.G.; Burrell, R.E. Effect of light on physicochemical and biological properties of nanocrystalline silver dressings. RSC Adv. 2015, 5, 14294–14304. [Google Scholar] [CrossRef]
- Caseli, L.; Soriano, G.B.; Oliveira, R.S.; Camilo, F.F. Physical Chemical Properties of Silver Nanoparticles Stabilized with Polyether-Block-Amide Interacting with Cellular Membrane Models at the Air-Water Interface. Biophys. J. 2017, 112, 376a. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, Z.; Lu, M.; Shao, D.; Yue, J.; Yang, D.; Li, M.; Dong, W.-F. Janus Silver/Silica Nanoplatforms for Light-Activated Liver Cancer Chemo/Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 30306–30317. [Google Scholar] [CrossRef]
- Desireddy, A.; Conn, B.E.; Guo, J.; Yoon, B.; Barnett, R.N.; Monahan, B.M.; Kirschbaum, K.; Griffith, W.P.; Whetten, R.L.; Landman, U.; et al. Ultrastable silver nanoparticles. Nature 2013, 501, 399. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Huang, H.; Gell, L.; Lehtovaara, L.; Malola, S.; Häkkinen, H.; Zheng, N. All-thiol-stabilized Ag44 and Au12Ag32 nanoparticles with single-crystal structures. Nat. Commun. 2013, 4, 2422. [Google Scholar] [CrossRef]
- Harkness, K.M.; Tang, Y.; Dass, A.; Pan, J.; Kothalawala, N.; Reddy, V.J.; Cliffel, D.E.; Demeler, B.; Stellacci, F.; Bakr, O.M.; et al. Ag44(SR)30(4-): A silver-thiolate superatom complex. Nanoscale 2012, 4, 4269–4274. [Google Scholar] [CrossRef]
- Huang, L.; Wan, J.; Wei, X.; Liu, Y.; Huang, J.; Sun, X.; Zhang, R.; Gurav, D.D.; Vedarethinam, V.; Li, Y.; et al. Plasmonic silver nanoshells for drug and metabolite detection. Nat. Commun. 2017, 8, 220. [Google Scholar] [CrossRef]
- Fierascu, I.; Georgiev, M.I.; Ortan, A.; Fierascu, R.C.; Avramescu, S.M.; Ionescu, D.; Sutan, A.; Brinzan, A.; Ditu, L.M. Phyto-mediated metallic nano-architectures via Melissa officinalis L.: Synthesis, characterization and biological properties. Sci. Rep. 2017, 7, 12428. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Bright, R.; Visalakshan, R.M.; Biswas, B.; Zilm, P.; Vasilev, K. Core-in-cage structure regulated properties of ultra-small gold nanoparticles. Nanoscale Adv. 2019, 1, 2356–2364. [Google Scholar] [CrossRef]
- Ravindran Girija, A.; Balasubramanian, S.; Bright, R.; Cowin, A.J.; Goswami, N.; Vasilev, K. Ultrasmall Gold Nanocluster Based Antibacterial Nanoaggregates for Infectious Wound Healing. ChemNanoMat 2019, 5, 1176–1181. [Google Scholar] [CrossRef]
- Haidari, H.; Goswami, N.; Bright, R.; Kopecki, Z.; Cowin, A.J.; Garg, S.; Vasilev, K. The interplay between size and valence state on the antibacterial activity of sub-10 nm silver nanoparticles. Nanoscale Adv. 2019, 1, 2365–2371. [Google Scholar] [CrossRef]
- Gonzalez Garcia, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Ninan, N.; Cavallaro, A.A.; Trinidad, A.D.; Zhao, Y.; Hayball, A.J.D.; Vasilev, K. Self-sterilizing antibacterial silver-loaded microneedles. Chem. Commun. 2018, 55, 171–174. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.; Hussein, M.H.; El-Sawah, A.A. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci. Rep. 2017, 7, 10844. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. Engl. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver Enhances Antibiotic Activity against Gram-negative Bacteria. Sci. Transl. Med. 2013, 5, 190ra181. [Google Scholar] [CrossRef]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef]
- Singla, R.; Soni, S.; Patial, V.; Kulurkar, P.M.; Kumari, A.; Mahesh, S.M.; Padwad, Y.S.; Yadav, S.K. Cytocompatible Anti-microbial Dressings of Syzygium cumini Cellulose Nanocrystals Decorated with Silver Nanoparticles Accelerate Acute and Diabetic Wound Healing. Sci. Rep. 2017, 7, 10457. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; De Niederhausern, S.; Iseppi, R.; Bondi, M.; Sabia, C.; Toselli, M.; Pilati, F. Antibacterial Activity of Plastics Coated with Silver-Doped Organic−Inorganic Hybrid Coatings Prepared by Sol−Gel Processes. Biomacromolecules 2007, 8, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, M.; Hou, X.; Li, J.; Sun, L.; Wang, X. Coloration of Cotton Fibers with Anisotropic Silver Nanoparticles. Ind. Eng. Chem. Res. 2012, 51, 12807–12813. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Rimmele, E.; Wichser, A.; Erni, R.; Height, M.; Nowack, B. Presence of Nanoparticles in Wash Water from Conventional Silver and Nano-silver Textiles. ACS Nano 2014, 8, 7208–7219. [Google Scholar] [CrossRef]
- Cherrie, M.W.; Matteo Dalla, V.; Kevin, C.J.; Andy, J.S. Challenges in assessing the environmental fate and exposure of nano silver. J. Phys. Conf. Ser. 2011, 304, 012070. [Google Scholar]
- Fauss, E.; Gorman, M.; Swami, N. Case Study of an Emergent Nanotechnology: Identifying Environmental Risks from Silver Nanotechnology through an Expert Elicitation Methodology. In Biotechnology and Nanotechnology Risk Assessment: Minding and Managing the Potential Threats around Us; American Chemical Society: Washington, DC, USA, 2011; Volume 1079, pp. 17–40. [Google Scholar]
- Gunawan, C.; Marquis, C.P.; Amal, R.; Sotiriou, G.A.; Rice, S.A.; Harry, E.J. Widespread and Indiscriminate Nanosilver Use: Genuine Potential for Microbial Resistance. ACS Nano 2017, 11, 3438–3445. [Google Scholar] [CrossRef]
- Silva, A.L.; Peres, C.; Conniot, J.; Matos, A.I.; Moura, L.; Carreira, B.; Sainz, V.; Scomparin, A.; Satchi-Fainaro, R.; Préat, V.; et al. Nanoparticle impact on innate immune cell pattern-recognition receptors and inflammasomes activation. Semin. Immunol. 2017, 34, 3–24. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar]
- Wang, Z.; Xia, T.; Liu, S. Mechanisms of nanosilver-induced toxicological effects: More attention should be paid to its sublethal effects. Nanoscale 2015, 7, 7470–7481. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Srinivasan, S.; McGoron, A.J. Efficient intracellular delivery and improved biocompatibility of colloidal silver nanoparticles towards intracellular SERS immuno-sensing. Analyst 2015, 140, 3929–3934. [Google Scholar] [CrossRef] [PubMed]
- Benyettou, F.; Rezgui, R.; Ravaux, F.; Jaber, T.; Blumer, K.; Jouiad, M.; Motte, L.; Olsen, J.C.; Platas-Iglesias, C.; Magzoub, M.; et al. Synthesis of silver nanoparticles for the dual delivery of doxorubicin and alendronate to cancer cells. J. Mater. Chem. B 2015, 3, 7237–7245. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, K.; Banerjee, S.L.; Kundu, P.P.; Madras, G.; Chatterjee, K. Biofunctionalized surface-modified silver nanoparticles for gene delivery. J. Mater. Chem. B 2015, 3, 5266–5276. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Kuhlbusch, T.A.J. In vivo effects: Methodologies and biokinetics of inhaled nanomaterials. NanoImpact 2018, 10, 38–60. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef]
- Park, J.; Lim, D.-H.; Lim, H.-J.; Kwon, T.; Choi, J.-S.; Jeong, S.; Choi, I.-H.; Cheon, J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem. Commun. 2011, 47, 4382–4384. [Google Scholar] [CrossRef]
- Sharonova, A.; Loza, K.; Surmeneva, M.; Surmenev, R.; Prymak, O.; Epple, M. Synthesis of positively and negatively charged silver nanoparticles and their deposition on the surface of titanium. IOP Conf. Ser. Mater. Sci. Eng. 2016, 116, 012009. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Nordmeyer, D.; Sengstock, C.; Ahlberg, S.; Diendorf, J.; Raabe, J.; Epple, M.; Köller, M.; Lademann, J.; Vogt, A.; et al. Shape-Dependent Dissolution and Cellular Uptake of Silver Nanoparticles. Langmuir 2018, 34, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013, 42, 5552–5576. [Google Scholar] [CrossRef] [PubMed]
- Potter, T.M.; Neun, B.W.; Rodriguez, J.C.; Ilinskaya, A.N.; Dobrovolskaia, M.A. Analysis of Pro-inflammatory Cytokine and Type II Interferon Induction by Nanoparticles. Methods Mol. Biol. 2018, 1682, 173–187. [Google Scholar] [PubMed]
- Ninan, N.; Albrecht, H.; Blencowe, A. Chapter 5—Mammalian Cell-Based Assays for Studying Bio-Nano Interactions. In Characterization of Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 129–166. [Google Scholar]
- Martirosyan, A.; Grintzalis, K.; Polet, M.; Laloux, L.; Schneider, Y.-J. Tuning the inflammatory response to silver nanoparticles via quercetin in Caco-2(co-)cultures as model of the human intestinal mucosa. Toxicol. Lett. 2016, 253, 36–45. [Google Scholar] [CrossRef]
- Theodorou, I.G.; Müller, K.H.; Chen, S.; Goode, A.E.; Yufit, V.; Ryan, M.P.; Porter, A.E. Silver Nanowire Particle Reactivity with Human Monocyte-Derived Macrophage Cells: Intracellular Availability of Silver Governs Their Cytotoxicity. ACS Biomater. Sci. Eng. 2017, 3, 2336–2347. [Google Scholar] [CrossRef]
- Tao, Y.; Li, Z.; Ju, E.; Ren, J.; Qu, X. One-step DNA-programmed growth of CpG conjugated silver nanoclusters: A potential platform for simultaneous enhanced immune response and cell imaging. Chem. Commun. 2013, 49, 6918–6920. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Cheung, S.O.F.; Huang, L.; Niu, J.; Tao, C.; Ho, C.-M.; Che, C.-M.; Tam, P.K.H. Further Evidence of the Anti-inflammatory Effects of Silver Nanoparticles. ChemMedChem 2009, 4, 1129–1135. [Google Scholar] [CrossRef]
- Moldovan, B.; David, L.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Baldea, I.; Clichici, S.; Filip, G.A. In vitro and in vivo anti-inflammatory properties of green synthesized silver nanoparticles using Viburnum opulus L. fruits extract. Mater. Sci. Eng. C 2017, 79, 720–727. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B Biointerfaces 2014, 122, 767–777. [Google Scholar] [CrossRef]
- Liu, X.; Hao, W.; Lok, C.-N.; Wang, Y.C.; Zhang, R.; Wong, K.K.Y. Dendrimer encapsulation enhances anti-inflammatory efficacy of silver nanoparticles. J. Pediatr. Surg. 2014, 49, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Manikandan, B.; Raman, T.; Arunagirinathan, K.; Prabhu, N.M.; Jothi Basu, M.; Perumal, M.; Palanisamy, S.; Munusamy, A. Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti-inflammatory activities. Spectrochim. Acta A 2015, 138, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Palanisamy, S.; Goutham, G.; Koodalingam, A.; Prabhu, N.M.; Kannapiran, E.; Basu, M.J.; Arulvasu, C.; et al. Biosynthesis of silver nanoparticles using aqueous extract of Phyllanthus acidus L. fruits and characterization of its anti-inflammatory effect against H2O2 exposed rat peritoneal macrophages. Process Biochem. 2017, 55, 172–181. [Google Scholar] [CrossRef]
- Taheri, S.; Cavallaro, A.; Christo, S.N.; Majewski, P.; Barton, M.; Hayball, J.D.; Vasilev, K. Antibacterial Plasma Polymer Films Conjugated with Phospholipid Encapsulated Silver Nanoparticles. ACS Biomater. Sci. Eng. 2015, 1, 1278–1286. [Google Scholar] [CrossRef]
- Chung, K.F.; Seiffert, J.; Chen, S.; Theodorou, I.G.; Goode, A.E.; Leo, B.F.; McGilvery, C.M.; Hussain, F.; Wiegman, C.; Rossios, C.; et al. Inactivation, Clearance, and Functional Effects of Lung-Instilled Short and Long Silver Nanowires in Rats. ACS Nano 2017, 11, 2652–2664. [Google Scholar] [CrossRef]
- Hanaa Mohamed, E.-R.; Manal Abdel-Aziz, H. Antioxidant and anti-inflammatory activities of silver nanoparticles biosynthesized from aqueous leaves extracts of four Terminalia species. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 035008. [Google Scholar] [CrossRef]
- Tian, J.; Wong, K.K.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Rafie, M.H.; El-Sheikh, M.A.; Seleem, A.A.; El-Naggar, M.E. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int. J. Biol. Macromol. 2014, 65, 509–515. [Google Scholar] [CrossRef]
- Nadworny, P.L.; Wang, J.; Tredget, E.E.; Burrell, R.E. Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomedicine 2008, 4, 241–251. [Google Scholar] [CrossRef]
- Siczek, K.; Zatorski, H.; Chmielowiec-Korzeniowska, A.; Kordek, R.; Tymczyna, L.; Fichna, J. Evaluation of anti-inflammatory effect of silver-coated glass beads in mice with experimentally induced colitis as a new type of treatment in inflammatory bowel disease. Pharmacol. Rep. 2017, 69, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.M.; Kumar, A.; Mousa, S.; Park, T.-J.; Ajayan, P.; Kubotera, N.; Mousa, S.A.; Linhardt, R.J. Synthesis of Gold and Silver Nanoparticles Stabilized with Glycosaminoglycans Having Distinctive Biological Activities. Biomacromolecules 2009, 10, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-L.; Hsiao, I.L.; Lin, H.-C.; Wang, C.-F.; Huang, Y.-J.; Chuang, C.-Y. Silver nanoparticles affect on gene expression of inflammatory and neurodegenerative responses in mouse brain neural cells. Environ. Res. 2015, 136, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Suliman, Y.A.; Ali, D.; Alarifi, S.; Harrath, A.H.; Mansour, L.; Alwasel, S.H. Evaluation of cytotoxic, oxidative stress, proinflammatory and genotoxic effect of silver nanoparticles in human lung epithelial cells. Environ. Toxicol. 2015, 30, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, M.; Yue, J.; Zhang, L.; Xie, J.; Ong, C.N.; Leong, D.T. Pro-inflammatory responses of RAW264.7 macrophages when treated with ultralow concentrations of silver, titanium dioxide, and zinc oxide nanoparticles. J. Hazard. Mater. 2015, 297, 146–152. [Google Scholar] [CrossRef]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharm. 2001, 175, 191–199. [Google Scholar] [CrossRef]
- Murphy, A.; Casey, A.; Byrne, G.; Chambers, G.; Howe, O. Silver nanoparticles induce pro-inflammatory gene expression and inflammasome activation in human monocytes. J. Appl. Toxiciol. 2016, 36, 1311–1320. [Google Scholar] [CrossRef]
- Christo, S.; Bachhuka, A.; Diener, K.R.; Vasilev, K.; Hayball, J.D. The contribution of inflammasome components on macrophage response to surface nanotopography and chemistry. Sci. Rep. 2016, 6, 26207. [Google Scholar] [CrossRef]
- Christo, S.N.; Diener, K.R.; Manavis, J.; Grimbaldeston, M.A.; Bachhuka, A.; Vasilev, K.; Hayball, J.D. Inflammasome components ASC and AIM2 modulate the acute phase of biomaterial implant-induced foreign body responses. Sci. Rep. 2016, 6, 20635. [Google Scholar] [CrossRef]
- Li, J.; Zhong, X.; Cheng, F.; Zhang, J.-R.; Jiang, L.-P.; Zhu, J.-J. One-Pot Synthesis of Aptamer-Functionalized Silver Nanoclusters for Cell-Type-Specific Imaging. Anal. Chem. 2012, 84, 4140–4146. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.M.; Herrera, J.L.; Fernandez-Montesinos, R.; Caro, C.; Zaderenko, A.P.; Mejias, J.A.; Pozo, D. Tiopronin monolayer-protected silver nanoparticles modulate IL-6 secretion mediated by Toll-like receptor ligands. Nanomedicine 2008, 3, 627–635. [Google Scholar] [CrossRef]
- Rao, K.; Roome, T.; Aziz, S.; Razzak, A.; Abbas, G.; Imran, M.; Jabri, T.; Gul, J.; Hussain, M.; Sikandar, B.; et al. Bergenin loaded gum xanthan stabilized silver nanoparticles suppress synovial inflammation through modulation of the immune response and oxidative stress in adjuvant induced arthritic rats. J. Mater. Chem. B 2018, 6, 4486–4501. [Google Scholar] [CrossRef] [PubMed]
- Mugade, M.; Patole, M.; Pokharkar, V. Bioengineered mannan sulphate capped silver nanoparticles for accelerated and targeted wound healing: Physicochemical and biological investigations. Biomed. Pharmacother. 2017, 91, 95–110. [Google Scholar] [CrossRef]
- El-Feky, G.S.; Sharaf, S.S.; El Shafei, A.; Hegazy, A.A. Using chitosan nanoparticles as drug carriers for the development of a silver sulfadiazine wound dressing. Carbohydr. Polym. 2017, 158, 11–19. [Google Scholar] [CrossRef]
- Liu, X.; Gao, P.; Du, J.; Zhao, X.; Wong, K.K.Y. Long-term anti-inflammatory efficacy in intestinal anastomosis in mice using silver nanoparticle-coated suture. J. Pediatr. Surg. 2017, 52, 2083–2087. [Google Scholar] [CrossRef]
- Côté-Maurais, G.; Bernier, J. Silver and fullerene nanoparticles’ effect on interleukin-2-dependent proliferation of CD4 (+) T cells. Toxicol. In Vitro 2014, 28, 1474–1481. [Google Scholar] [CrossRef][Green Version]
- Chiarugi, P.; Pani, G.; Giannoni, E.; Taddei, L.; Colavitti, R.; Raugei, G.; Symons, M.; Borrello, S.; Galeotti, T.; Ramponi, G. Reactive oxygen species as essential mediators of cell adhesion: The oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 2003, 161, 933–944. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Gonzalez-Carter, D.A.; Leo, B.F.; Ruenraroengsak, P.; Chen, S.; Goode, A.E.; Theodorou, I.G.; Chung, K.F.; Carzaniga, R.; Shaffer, M.S.P.; Dexter, D.T.; et al. Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes. Sci. Rep. 2017, 7, 42871. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, H.; Liu, J.-H.; Wang, H.; Liu, Y. Evaluation of the adjuvant effect of silver nanoparticles both in vitro and in vivo. Toxicol. Lett. 2013, 219, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Asgary, V.; Shoari, A.; Baghbani-Arani, F.; Sadat Shandiz, S.A.; Khosravy, M.S.; Janani, A.; Bigdeli, R.; Bashar, R.; Cohan, R.A. Green synthesis and evaluation of silver nanoparticles as adjuvant in rabies veterinary vaccine. Int. J. Nanomed. 2016, 11, 3597–3605. [Google Scholar]
- Liu, Y.; Balachandran, Y.L.; Li, D.; Shao, Y.; Jiang, X. Polyvinylpyrrolidone–Poly(ethylene glycol) Modified Silver Nanorods Can Be a Safe, Noncarrier Adjuvant for HIV Vaccine. ACS Nano 2016, 10, 3589–3596. [Google Scholar] [CrossRef]
- Xue, P.; Li, Q.; Li, Y.; Sun, L.; Zhang, L.; Xu, Z.; Kang, Y. Surface Modification of Poly(dimethylsiloxane) with Polydopamine and Hyaluronic Acid To Enhance Hemocompatibility for Potential Applications in Medical Implants or Devices. ACS Appl. Mater. Interfaces 2017, 9, 33632–33644. [Google Scholar] [CrossRef]
- Min, J.; Choi, K.Y.; Dreaden, E.C.; Padera, R.F.; Braatz, R.D.; Spector, M.; Hammond, P.T. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano 2016, 10, 4441–4450. [Google Scholar] [CrossRef]
- Yanez, M.; Blanchette, J.; Jabbarzadeh, E. Modulation of Inflammatory Response to Implanted Biomaterials Using Natural Compounds. Curr. Pharm. Des. 2017, 23, 6347–6357. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhu, Y.; Yu, B.; Sun, Y.; Ding, X.; Xu, C.; Wu, Y.-W.; Tang, Z.; Xu, F.-J. Antimicrobial and Antifouling Polymeric Agents for Surface Functionalization of Medical Implants. Biomacromolecules 2018, 19, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, A.; Clarot, I.; Sobocinski, J.; Fattal, E.; Boudier, A. Nanotechnologies for Medical Devices: Potentialities and Risks. ACS Appl. Bio Mater. 2019, 2, 1–13. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2014, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K. Nanoengineered Antibacterial Coatings and Materials: A Perspective. Coatings 2019, 9, 654. [Google Scholar] [CrossRef]
- Taheri, S.; Cavallaro, A.; Christo, S.N.; Smith, L.E.; Majewski, P.; Barton, M.; Hayball, J.D.; Vasilev, K. Substrate independent silver nanoparticle based antibacterial coatings. Biomaterials 2014, 35, 4601–4609. [Google Scholar] [CrossRef]
- Taheri, S.; Baier, G.; Majewski, P.; Barton, M.; Forch, R.; Landfester, K.; Vasilev, K. Synthesis and antibacterial properties of a hybrid of silver-potato starch nanocapsules by miniemulsion/polyaddition polymerization. J. Mater. Chem. B 2014, 2, 1838–1845. [Google Scholar] [CrossRef]
- Taheri, S.; Cavallaro, A.; Barton, M.; Whittle, J.D.; Majewski, P.; Smith, L.E.; Vasilev, K. Antibacterial Efficacy and Cytotoxicity of Silver Nanoparticle Based Coatings Facilitated by a Plasma Polymer Interlayer. Plasma Med. 2014, 4, 101–115. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Kmeth, R.; Obermeier, A.; Bauer, A.T.; Halter, N.; Kümpel, K.; Schneider, M.F.; Wixforth, A.; Gollwitzer, H.; Burgkart, R.; et al. Silver nanoparticle-enriched diamond-like carbon implant modification as a mammalian cell compatible surface with antimicrobial properties. Sci. Rep. 2016, 6, 22849. [Google Scholar] [CrossRef]
- Prasad, K.; Lekshmi, G.S.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M.; Vasilev, K.; Bottle, S.; Bazaka, K.; Ostrikov, K. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017, 7, 1591. [Google Scholar] [CrossRef]
- Alhmoud, H.; Delalat, B.; Ceto, X.; Elnathan, R.; Cavallaro, A.; Vasilev, K.; Voelcker, N.H. Antibacterial properties of silver dendrite decorated silicon nanowires. RSC Adv. 2016, 6, 65976–65987. [Google Scholar] [CrossRef]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Poulter, N.; Vasilev, K.; Griesser, S.S.; Griesser, H.J. Silver Containing Biomaterials. In Biomaterials Associated Infection: Immunological Aspects and Antimicrobial Strategies; Moriarty, T.F., Zaat, S.A.J., Busscher, H.J., Eds.; Springer: New York, NY, USA, 2013; pp. 355–378. [Google Scholar]
- Vasilev, K.; Sah, V.; Anselme, K.; Ndi, C.; Mateescu, M.; Dollmann, B.; Martinek, P.; Ys, H.; Ploux, L.; Griesser, H.J. Tunable Antibacterial Coatings That Support Mammalian Cell Growth. Nano Lett. 2010, 10, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, A.; Taheri, S.; Vasilev, K. Responsive and “smart” antibacterial surfaces: Common approaches and new developments (Review). Biointerphases 2014, 9, 029005. [Google Scholar] [CrossRef] [PubMed]
- Ploux, L.; Mateescu, M.; Anselme, K.; Vasilev, K. Antibacterial properties of silver-loaded plasma polymer coatings. J. Nanomater. 2012, 2012, 674145. [Google Scholar] [CrossRef]
- Lombi, E.; Donner, E.; Taheri, S.; Tavakkoli, E.; Jämting, Å.K.; McClure, S.; Naidu, R.; Miller, B.W.; Scheckel, K.G.; Vasilev, K. Transformation of four silver/silver chloride nanoparticles during anaerobic treatment of wastewater and post-processing of sewage sludge. Environ. Pollut. 2013, 176, 193–197. [Google Scholar] [CrossRef]
- Rochford, E.T.J.; Richards, R.G.; Moriarty, T.F. Influence of material on the development of device-associated infections. Clin. Microbiol. Infect. 2012, 18, 1162–1167. [Google Scholar] [CrossRef]
- Penkov, O.V.; Pukha, V.E.; Starikova, S.L.; Khadem, M.; Starikov, V.V.; Maleev, M.V.; Kim, D.-E. Highly wear-resistant and biocompatible carbon nanocomposite coatings for dental implants. Biomaterials 2016, 102, 130–136. [Google Scholar] [CrossRef]
- Piattelli, A.; Cosci, F.; Scarano, A.; Trisi, P. Localized chronic suppurative bone infection as a sequel of peri-implantitis in a hydroxyapatite-coated dental implant. Biomaterials 1995, 16, 917–920. [Google Scholar] [CrossRef]
- Saita, M.; Kaneko, J.; Sato, T.; Takahashi, S.-S.; Wada-Takahashi, S.; Kawamata, R.; Sakurai, T.; Lee, M.-C.-I.; Hamada, N.; Kimoto, K.; et al. Novel antioxidative nanotherapeutics in a rat periodontitis model: Reactive oxygen species scavenging by redox injectable gel suppresses alveolar bone resorption. Biomaterials 2016, 76, 292–301. [Google Scholar] [CrossRef]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef]
- Thiel, K.; Grunwald, I.; Marx, D.; Wildemann, B.; Bormann, N.; Borcherding, K.; Gaetjen, L.; Specht, U.; Salz, D. Burst Release of Antibiotics Combined with Long-Term Release of Silver Targeting Implant-Associated Infections: Design, Characterization and in vitro Evaluation of Novel Implant Hybrid Surface. Materials 2019, 12, 3838. [Google Scholar]
- Ryu, H.S.; Bae, I.H.; Lee, K.G.; Hwang, H.S.; Lee, K.H.; Koh, J.T.; Cho, J.H. Antibacterial effect of silver-platinum coating for orthodontic appliances. Angle Orthod. 2012, 82, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Arash, V.; Keikhaee, F.; Rabiee, S.M.; Rajabnia, R.; Khafri, S.; Tavanafar, S. Evaluation of Antibacterial Effects of Silver-Coated Stainless Steel Orthodontic Brackets. J. Dent. 2016, 13, 49–54. [Google Scholar]
- Metin-Gursoy, G.; Taner, L.; Baris, E. Biocompatibility of nanosilver-coated orthodontic brackets: An in vivo study. Prog. Orthod. 2016, 17, 39. [Google Scholar] [CrossRef]
- Gosau, M.; Haupt, M.; Thude, S.; Strowitzki, M.; Schminke, B.; Buergers, R. Antimicrobial effect and biocompatibility of novel metallic nanocrystalline implant coatings. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1571–1579. [Google Scholar] [CrossRef]
- Schwass, D.R.; Lyons, K.M.; Love, R.; Tompkins, G.R.; Meledandri, C.J. Antimicrobial Activity of a Colloidal AgNP Suspension Demonstrated In Vitro against Monoculture Biofilms: Toward a Novel Tooth Disinfectant for Treating Dental Caries. Adv. Dent. Res. 2018, 29, 117–123. [Google Scholar] [CrossRef]
- Goncalves, T.S.; Menezes, L.M.; Trindade, C.; Machado Mda, S.; Thomas, P.; Fenech, M.; Henriques, J.A. Cytotoxicity and genotoxicity of orthodontic bands with or without silver soldered joints. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2014, 762, 1–8. [Google Scholar] [CrossRef]
- Takamiya, A.S.; Monteiro, D.R.; Bernabe, D.G.; Gorup, L.F.; Camargo, E.R.; Gomes-Filho, J.E.; Oliveira, S.H.; Barbosa, D.B. In Vitro and In Vivo Toxicity Evaluation of Colloidal Silver Nanoparticles Used in Endodontic Treatments. J. Endod. 2016, 42, 953–960. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef]
- Gogia, J.S.; Meehan, J.P.; Di Cesare, P.E.; Jamali, A.A. Local antibiotic therapy in osteomyelitis. Semin. Plast. Surg. 2009, 23, 100–107. [Google Scholar] [CrossRef]
- Lu, M.; Liao, J.; Dong, J.; Wu, J.; Qiu, H.; Zhou, X.; Li, J.; Jiang, D.; He, T.-C.; Quan, Z. An effective treatment of experimental osteomyelitis using the antimicrobial titanium/silver-containing nHP66 (nano-hydroxyapatite/polyamide-66) nanoscaffold biomaterials. Sci. Rep. 2016, 6, 39174. [Google Scholar] [CrossRef] [PubMed]
- Funao, H.; Nagai, S.; Sasaki, A.; Hoshikawa, T.; Tsuji, T.; Okada, Y.; Koyasu, S.; Toyama, Y.; Nakamura, M.; Aizawa, M.; et al. A novel hydroxyapatite film coated with ionic silver via inositol hexaphosphate chelation prevents implant-associated infection. Sci. Rep. 2016, 6, 23238. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.M.; Lu, X.; Wang, K.F.; Meng, F.Z.; Jiang, O.; Zhang, H.P.; Zhi, W.; Fang, L.M. Silver nanoparticles and growth factors incorporated hydroxyapatite coatings on metallic implant surfaces for enhancement of osteoinductivity and antibacterial properties. ACS Appl. Mater. Interfaces 2014, 6, 8580–8589. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Poologasundarampillai, G.; Todd, N.; Devlin-Mullin, A.; Moore, K.L.; Golrokhi, Z.; Gilchrist, J.B.; Jones, E.; Potter, R.J.; Sutcliffe, C.; et al. Biotransformation of Silver Released from Nanoparticle Coated Titanium Implants Revealed in Regenerating Bone. ACS Appl. Mater. Interfaces 2017, 9, 21169–21180. [Google Scholar] [CrossRef]
- Jia, Z.; Xiu, P.; Xiong, P.; Zhou, W.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; Liu, Z.; et al. Additively Manufactured Macroporous Titanium with Silver-Releasing Micro-/Nanoporous Surface for Multipurpose Infection Control and Bone Repair—A Proof of Concept. ACS Appl. Mater. Interfaces 2016, 8, 28495–28510. [Google Scholar] [CrossRef]
- Ehrensberger, M.T.; Tobias, M.E.; Nodzo, S.R.; Hansen, L.A.; Luke-Marshall, N.R.; Cole, R.F.; Wild, L.M.; Campagnari, A.A. Cathodic voltage-controlled electrical stimulation of titanium implants as treatment for methicillin-resistant Staphylococcus aureus periprosthetic infections. Biomaterials 2015, 41, 97–105. [Google Scholar] [CrossRef]
- Hardes, J.; Henrichs, M.P.; Hauschild, G.; Nottrott, M.; Guder, W.; Streitbuerger, A. Silver-Coated Megaprosthesis of the Proximal Tibia in Patients with Sarcoma. J. Arthroplast. 2017, 32, 2208–2213. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Park, I.-K.; Elain, A.; Wong, T.W.; Thomas, S.; Grohens, Y. Faujasites Incorporated Tissue Engineering Scaffolds for Wound Healing: In Vitro and In Vivo Analysis. ACS Appl. Mater. Interfaces 2013, 5, 11194–11206. [Google Scholar] [CrossRef]
- Ninan, N.; Thomas, S.; Grohens, Y. Wound healing in urology. Adv. Drug Deliv. Rev. 2015, 82–83, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Parsons, D. Combatting wound biofilm and recalcitrance with a novel anti-biofilm Hydrofiber® wound dressing. Wound Med. 2016, 14, 6–11. [Google Scholar] [CrossRef]
- DeBoer, T.R.; Chakraborty, I.; Mascharak, P.K. Design and construction of a silver (I)-loaded cellulose-based wound dressing: Trackable and sustained release of silver for controlled therapeutic delivery to wound sites. J. Mater. Sci. Mater. Med. 2015, 26, 243. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.C.; Martin, K.R.; Taylor, C.; Spear, A.M.; Whiting, R.; Macildowie, S.; Clasper, J.C.; Watts, S.A. A pre-clinical evaluation of silver, iodine and Manuka honey based dressings in a model of traumatic extremity wounds contaminated with Staphylococcus aureus. Injury 2014, 45, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Li, Q.; Wang, X.; Wu, P.; Ho, J.K.; Jin, R.; Zhang, L.; Shao, H.; Han, C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7, 10489. [Google Scholar] [CrossRef]
- Seo, S.Y.; Lee, G.H.; Lee, S.G.; Jung, S.Y.; Lim, J.O.; Choi, J.H. Alginate-based composite sponge containing silver nanoparticles synthesized in situ. Carbohydr. Polym. 2012, 90, 109–115. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Jancar, J.; Abdel-Rahman, R.M.; Vojtek, L.; Hyrsl, P.; Duskova, M.; Nejezchlebova, H. A novel in situ silver/hyaluronan bio-nanocomposite fabrics for wound and chronic ulcer dressing: In vitro and in vivo evaluations. Int. J. Pharm. 2017, 520, 241–253. [Google Scholar] [CrossRef]
- Rupp, M.E.; Majorant, D. Prevention of Vascular Catheter-Related Bloodstream Infections. Infect. Dis. Clin. N. Am. 2016, 30, 853–868. [Google Scholar] [CrossRef]
- Bleyer, A.J. Use of Antimicrobial Catheter Lock Solutions to Prevent Catheter-Related Bacteremia. Clin. J. Am. Soc. Nephrol. 2007, 2, 1073–1078. [Google Scholar] [CrossRef]
- Viola, G.M.; Rosenblatt, J.; Raad, I.I. Drug eluting antimicrobial vascular catheters: Progress and promise. Adv. Drug Deliv. Rev. 2017, 112, 35–47. [Google Scholar] [CrossRef]
- Schuerer, D.J.; Zack, J.E.; Thomas, J.; Borecki, I.B.; Sona, C.S.; Schallom, M.E.; Venker, M.; Nemeth, J.L.; Ward, M.R.; Verjan, L.; et al. Effect of chlorhexidine/silver sulfadiazine-impregnated central venous catheters in an intensive care unit with a low blood stream infection rate after implementation of an educational program: A before-after trial. Surg. Infect. 2007, 8, 445–454. [Google Scholar] [CrossRef]
- Fraenkel, D.; Rickard, C.; Thomas, P.; Faoagali, J.; George, N.; Ware, R. A prospective, randomized trial of rifampicin-minocycline-coated and silver-platinum-carbon-impregnated central venous catheters. Crit. Care Med. 2006, 34, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Khare, M.D.; Bukhari, S.S.; Swann, A.; Spiers, P.; McLaren, I.; Myers, J. Reduction of catheter-related colonisation by the use of a silver zeolite-impregnated central vascular catheter in adult critical care. J. Infect. 2007, 54, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Bertini, G.; Elia, S.; Ceciarini, F.; Dani, C. Reduction of catheter-related bloodstream infections in preterm infants by the use of catheters with the AgION antimicrobial system. Early Hum. Dev. 2013, 89, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; O’Connor, S.; Becquemin, J.P. Efficacy of collagen silver-coated polyester and rifampin-soaked vascular grafts to resist infection from MRSA and Escherichia coli in a dog model. Ann. Vasc. Surg. 2008, 22, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, Y.; Zhang, Y.; Deng, J.; Lin, C. Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int. J. Nanomed. 2015, 10, 7241–7252. [Google Scholar]
- Stevens, K.N.; Croes, S.; Boersma, R.S.; Stobberingh, E.E.; van der Marel, C.; van der Veen, F.H.; Knetsch, M.L.; Koole, L.H. Hydrophilic surface coatings with embedded biocidal silver nanoparticles and sodium heparin for central venous catheters. Biomaterials 2011, 32, 1264–1269. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Lai, X.; Ke, P.C.; Podila, R.; Brown, J.M.; Witzmann, F.A. Silver Nanoparticle Protein Corona Composition in Cell Culture Media. PLoS ONE 2013, 8, e74001. [Google Scholar] [CrossRef]
- Miclăuş, T.; Beer, C.; Chevallier, J.; Scavenius, C.; Bochenkov, V.E.; Enghild, J.J.; Sutherland, D.S. Dynamic protein coronas revealed as a modulator of silver nanoparticle sulphidation in vitro. Nat. Commun. 2016, 7, 11770. [Google Scholar] [CrossRef]
- Barbalinardo, M.; Caicci, F.; Cavallini, M.; Gentili, D. Protein Corona Mediated Uptake and Cytotoxicity of Silver Nanoparticles in Mouse Embryonic Fibroblast. Small 2018, 14, 1801219. [Google Scholar] [CrossRef]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, X.; Zheng, C.; Liu, Y.; Xu, T.; Liu, J. Preparation of different sized nano-silver loaded on functionalized graphene oxide with highly effective antibacterial properties. J. Mater. Chem. B 2015, 3, 7020–7029. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.O.; de Puig, H.; Yen, C.-W.; Bosch, I.; Gómez-Márquez, J.; Clavet, C.; Hamad-Schifferli, K.; Gehrke, L. A comparison of nanoparticle-antibody conjugation strategies in sandwich immunoassays. J. Immunoass. Immunochem. 2017, 38, 355–377. [Google Scholar] [CrossRef]
- Di Marco, M.; Shamsuddin, S.; Razak, K.A.; Aziz, A.A.; Devaux, C.; Borghi, E.; Levy, L.; Sadun, C. Overview of the main methods used to combine proteins with nanosystems: Absorption, bioconjugation, and encapsulation. Int. J. Nanomed. 2010, 5, 37–49. [Google Scholar] [CrossRef]
- Martin, M.N.; Allen, A.J.; MacCuspie, R.I.; Hackley, V.A. Dissolution, Agglomerate Morphology, and Stability Limits of Protein-Coated Silver Nanoparticles. Langmuir 2014, 30, 11442–11452. [Google Scholar] [CrossRef]
- Cruz, G.F.; Tofanello, A.; Araújo, J.N.; Nantes-Cardoso, I.L.; Ferreira, F.F.; Garcia, W. Fast One-Pot Photosynthesis of Plasmonic Protein-Coated Silver/Silver Bromide Nanoparticles with Efficient Photocatalytic Performance. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2056–2062. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Podila, R.; Aldossari, A.A.; Emerson, H.; Powell, B.A.; Ke, P.C.; Rao, A.M.; Brown, J.M. Formation of a Protein Corona on Silver Nanoparticles Mediates Cellular Toxicity via Scavenger Receptors. Toxicol. Sci. 2015, 143, 136–146. [Google Scholar] [CrossRef]
- Sprick, C.; Chede, S.; Oyanedel-Craver, V.; Escobar, I.C. Bio-inspired immobilization of casein-coated silver nanoparticles on cellulose acetate membranes for biofouling control. J. Environ. Chem. Eng. 2018, 6, 2480–2491. [Google Scholar] [CrossRef]
- Svirshchevskaya, E.V.; Alekseeva, L.; Marchenko, A.; Viskova, N.; Andronova, T.M.; Benevolenskii, S.V.; Kurup, V.P. Immune response modulation by recombinant peptides expressed in virus-like particles. Clin. Exp. Immunol. 2002, 127, 199–205. [Google Scholar] [CrossRef]
- Macgregor-Ramiasa, M.N.; Cavallaro, A.A.; Vasilev, K. Properties and reactivity of polyoxazoline plasma polymer films. J. Mater. Chem. B 2015, 3, 6327–6337. [Google Scholar] [CrossRef] [PubMed]
- Ramiasa, M.N.; Cavallaro, A.A.; Mierczynska, A.; Christo, S.N.; Gleadle, J.M.; Hayball, J.D.; Vasilev, K. Plasma polymerised polyoxazoline thin films for biomedical applications. Chem. Commun. 2015, 51, 4279–4282. [Google Scholar] [CrossRef] [PubMed]
- Goreham, R.V.; Mierczynska, A.; Pierce, M.; Short, R.D.; Taheri, S.; Bachhuka, A.; Cavallaro, A.; Smith, L.E.; Vasilev, K. A substrate independent approach for generation of surface gradients. Thin Solid Films 2013, 528, 106–110. [Google Scholar] [CrossRef]
- Hernandez-Lopez, J.L.; Bauer, R.E.; Chang, W.S.; Glasser, G.; Grebel-Koehler, D.; Klapper, M.; Kreiter, M.; Leclaire, J.; Majoral, J.P.; Mittler, S.; et al. Functional polymers as nanoscopic building blocks. Mater. Sci. Eng. C 2003, 23, 267–274. [Google Scholar] [CrossRef]
- Macgregor-Ramiasa, M.; McNicholas, K.; Ostrikov, K.; Li, J.; Michael, M.; Gleadle, J.M.; Vasilev, K. A platform for selective immuno-capture of cancer cells from urine. Biosens. Bioelectron. 2017, 96, 373–380. [Google Scholar] [CrossRef]
- Macgregor, M.; Vasilev, K. Perspective on Plasma Polymers for Applied Biomaterials Nanoengineering and the Recent Rise of Oxazolines. Materials 2019, 12, 191. [Google Scholar] [CrossRef]
- Chen, Z.; Visalakshan, R.M.; Guo, J.; Wei, F.; Zhang, L.; Chen, L.; Lin, Z.; Vasilev, K.; Xiao, Y. Plasma deposited poly-oxazoline nanotextured surfaces dictate osteoimmunomodulation towards ameliorative osteogenesis. Acta Biomater. 2019, 96, 568–581. [Google Scholar] [CrossRef]
- Walsh, G.; Jefferis, R. Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 2006, 24, 1241–1252. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.H.; Gringhuis, S.I. C-type lectin receptors in the control of T helper cell differentiation. Nat. Rev. Immunol. 2016, 16, 433–448. [Google Scholar] [CrossRef]
- De Oliveira, L.F.; Goncalves Jde, O.; Goncalves Kde, A.; Kobarg, J.; Cardoso, M.B. Sweeter but deadlier: Decoupling size, charge and capping effects in carbohydrate coated bactericidal silver nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 1817–1826. [Google Scholar] [CrossRef]
- Kennedy, D.C.; Orts-Gil, G.; Lai, C.-H.; Müller, L.; Haase, A.; Luch, A.; Seeberger, P.H. Carbohydrate functionalization of silver nanoparticles modulates cytotoxicity and cellular uptake. J. Nanobiotechnol. 2014, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Johannssen, T.; Lepenies, B. Glycan-Based Cell Targeting To Modulate Immune Responses. Trends Biotechnol. 2017, 35, 334–346. [Google Scholar] [CrossRef]
- Lautscham, L.A.; Lin, C.Y.; Auernheimer, V.; Naumann, C.A.; Goldmann, W.H.; Fabry, B. Biomembrane-mimicking lipid bilayer system as a mechanically tunable cell substrate. Biomaterials 2014, 35, 3198–3207. [Google Scholar] [CrossRef]
- Bothun, G.D. Hydrophobic silver nanoparticles trapped in lipid bilayers: Size distribution, bilayer phase behavior, and optical properties. J. Nanobiotechnol. 2008, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Ngobili, T.A.; Daniele, M.A. Nanoparticles and direct immunosuppression. Exp. Biol. Med. 2016, 241, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Mottram, P.L.; Leong, D.; Crimeen-Irwin, B.; Gloster, S.; Xiang, S.D.; Meanger, J.; Ghildyal, R.; Vardaxis, N.; Plebanski, M. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: Formulation of a model vaccine for respiratory syncytial virus. Mol. Pharm. 2007, 4, 73–84. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kunisawa, J.; Hayashi, A.; Tsutsumi, Y.; Kubo, K.; Nakagawa, S.; Nakanishi, M.; Tanaka, K.; Mayumi, T. Positively charged liposome functions as an efficient immunoadjuvant in inducing cell-mediated immune response to soluble proteins. J. Control. Release 1999, 61, 233–240. [Google Scholar] [CrossRef]
- Chanan-Khan, A.; Szebeni, J.; Savay, S.; Liebes, L.; Rafique, N.M.; Alving, C.R.; Muggia, F.M. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): Possible role in hypersensitivity reactions. Ann. Oncol. 2003, 14, 1430–1437. [Google Scholar] [CrossRef]
- Park, S.-H.; Oh, S.-G.; Mun, J.-Y.; Han, S.-S. Effects of silver nanoparticles on the fluidity of bilayer in phospholipid liposome. Colloids Surf. B Biointerfaces 2005, 44, 117–122. [Google Scholar] [CrossRef]
- Jeon, S.M.; Choi, S.; Lee, K.; Jung, H.-S.; Yu, J. Significantly improved stability of silver nanodots via nanoparticles encapsulation. J. Photochem. Photobiol. A. Chem. 2018, 355, 479–486. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Belyaev, A.A.; Sergeev, G.B. Silver/Thiocholesterol and Silver/Cholesterol Nanosized Aggregates Formation in Liquid Crystalline Mesophase. Mol. Cryst. Liq. Cryst. 2011, 540, 169–174. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N.; Pradhan, A.K.; Pradhan, P. Label free and high specific detection of mercury ions based on silver nano-liposome. Spectrochim. Acta A 2016, 163, 127–133. [Google Scholar] [CrossRef] [PubMed]

| Type of Ag | Size | Reagents Used | Type of Immune Cells | Cytokines Expression | In vitro Inflammatory Assays | Ref |
|---|---|---|---|---|---|---|
| NPs | <20 nm | AgNO3, Quercetin, Polyoxyethylene Glycerol trioleate, and Tween 20 | Caco-2 cells | Decreased IL-8 expression | qRT-PCR, ELISA, total protein content, Nitrate/Nitrite Colorimetric Assay | [68] |
| Nano wires | 10 µm | AgNO3, ethylene glycol, poly (vinylpyrrolidone) | Human monocyte-derived macrophages | Up taken by macrophages and transformed to silver chloride | High angle annular dark field scanning electron microscopy, Confocal analysis | [69] |
| Nanoclusters | 1.5 nm | NaBH4, AgNO3 | RAW264.7 cells | Release TNF-α, IL-6 | ELISA | [70] |
| NPs | 14 nm | NaBH4, AgNO3, Sodium citrate | RAW264.7 and J774.1 | Reduced TNF-α expression | ELISA | [71] |
| NPs | 10–50 nm | AgNO3, Extracts of Viburnum opulus | Hacat cells | Increased IL-1α and decreased IL-1α, IL-6 | ELISA | [72] |
| NPs | 20–80 nm | AgNO3, Extracts of Sambucus nigra | Hacat cells | Reduced IL-1α production | ELISA | [73] |
| NPs | 10 nm | Dendrimer, NaBH4, AgNO3, Sodium citrate | RAW264.7 and J774.1 | Decreased TNF-α, IL-6 | ELISA | [74] |
| NPs | 23.52–60.83 nm | AgNO3, Ethanolic petal extract of Rosa indica | Rat peritoneal macrophages | Attenuate production of NO and superoxide | Nitrate/Nitrite Colorimetric Assay, Estimate superoxide anion generation | [75] |
| NPs | 10.29–45.57 nm | AgNO3, Aqueous extracts of Phyllanthus acidus L. | Rat peritoneal macrophages | Attenuate production of IL-1α, NO and superoxide | ELISA, Immunoblotting, Nitrate/Nitrite Colorimetric Assay, Estimate superoxide anion generation | [76] |
| NPs | 4 nm | Chloroform, NaBH4, AgNO3, POPS | Bone marrow-derived macrophage cells | Decrease in IL-6 and IL-1β, no effect in TNF-α | ELISA | [77] |
| Nature of Ag | Size | Reducing Agent Used | Animal Strain | Model | Outcome | Ref |
|---|---|---|---|---|---|---|
| NPs | 9.3 ± 3.2 nm | NaBH4, AgNO3, Sodium citrate | Balb/c mice | Postoperative adhesion model | Decrease inflammation in peritoneal adhesion without toxic effects | [71] |
| Nano wires | 1.5 µm and 10 µm | AgNO3, ethylene glycol, polyvinyl pyrrolidone | Sprague Dawley rats | Intratracheal instillation, Lung model | Completely internalized by lung macrophages with toxic effects | [78] |
| NPs | 7–10 nm | AgNO3, Leaf extracts of Terminalia species | Wistar albino rats | Hind paw oedema model | Inhibition of oedema by 95% | [79] |
| NPs | 10–50 nm | AgNO3, Extracts of Viburnum opulus L. | Wistar rats | Carrageenan-induced inflammation models | Decreased inflammation | [72] |
| NPs | 14 ± 9.8 nm | NaBH4, AgNO3, Sodium citrate | Male Balb/c mice | Thermal injury animal models | Silver can modulate cytokine expression | [80] |
| NPs | 10 nm (5–15 nm) | Dendrimer, NaBH4, AgNO3, Sodium citrate | C57BL/6 N mice | Excisional and burn wound models | Enhanced anti-inflammatory efficacy | [74] |
| NPs | 20–80 nm | AgNO3, Extracts of Sambucus nigra | Male Wistar rats, | Carrageenan-induced inflammation models | AgNPs enhanced inflammation edema rate | [73] |
| NPs | 12–22 nm | Starch, NaOH, AgNO3, Absolute ethanol | Male and female rats | Grade II burn wound models | Reduce rat paw oedema | [81] |
| Nano crystalline silver | 10–15 nm | AgNO3, polyethene | Domestic White/Landrace swine | Porcine contact dermatitis model | Treated normal pigs have near-normal skin after 24 h | [82] |
| Silver-coated glass beads | 850–1400 µm and 5 µm | Borosilicate glass beads | Male Balb/c mice | Models mimicking Crohn’s disease and ulcerative colitis | Attenuated inflammation in colitis and Crohn’s disease models | [83] |
| NPs | 7 ± 3 nm | AgNO3, Diaminopyridiinyl Heparin, Glucose, | Male rats | Carrageenan-induced paw edema | Localization of anti-inflammatory effects | [84] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ninan, N.; Goswami, N.; Vasilev, K. The Impact of Engineered Silver Nanomaterials on the Immune System. Nanomaterials 2020, 10, 967. https://doi.org/10.3390/nano10050967
Ninan N, Goswami N, Vasilev K. The Impact of Engineered Silver Nanomaterials on the Immune System. Nanomaterials. 2020; 10(5):967. https://doi.org/10.3390/nano10050967
Chicago/Turabian StyleNinan, Neethu, Nirmal Goswami, and Krasimir Vasilev. 2020. "The Impact of Engineered Silver Nanomaterials on the Immune System" Nanomaterials 10, no. 5: 967. https://doi.org/10.3390/nano10050967
APA StyleNinan, N., Goswami, N., & Vasilev, K. (2020). The Impact of Engineered Silver Nanomaterials on the Immune System. Nanomaterials, 10(5), 967. https://doi.org/10.3390/nano10050967






