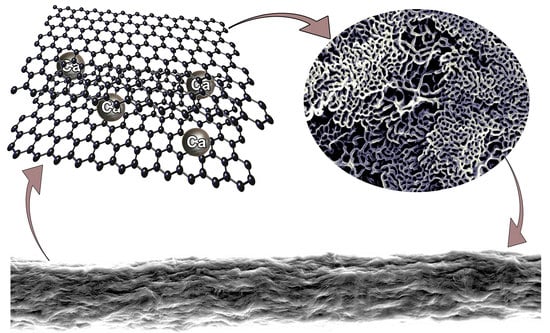
Tuning Properties of Partially Reduced Graphene Oxide Fibers upon Calcium Doping
Abstract
1. Introduction
2. Materials and Methods
2.1. Graphene Oxide Preparation
2.2. Reduced Graphene Oxide Fiber Preparation
2.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
2.4. Vibrating Sample Magnetometry (VSM)
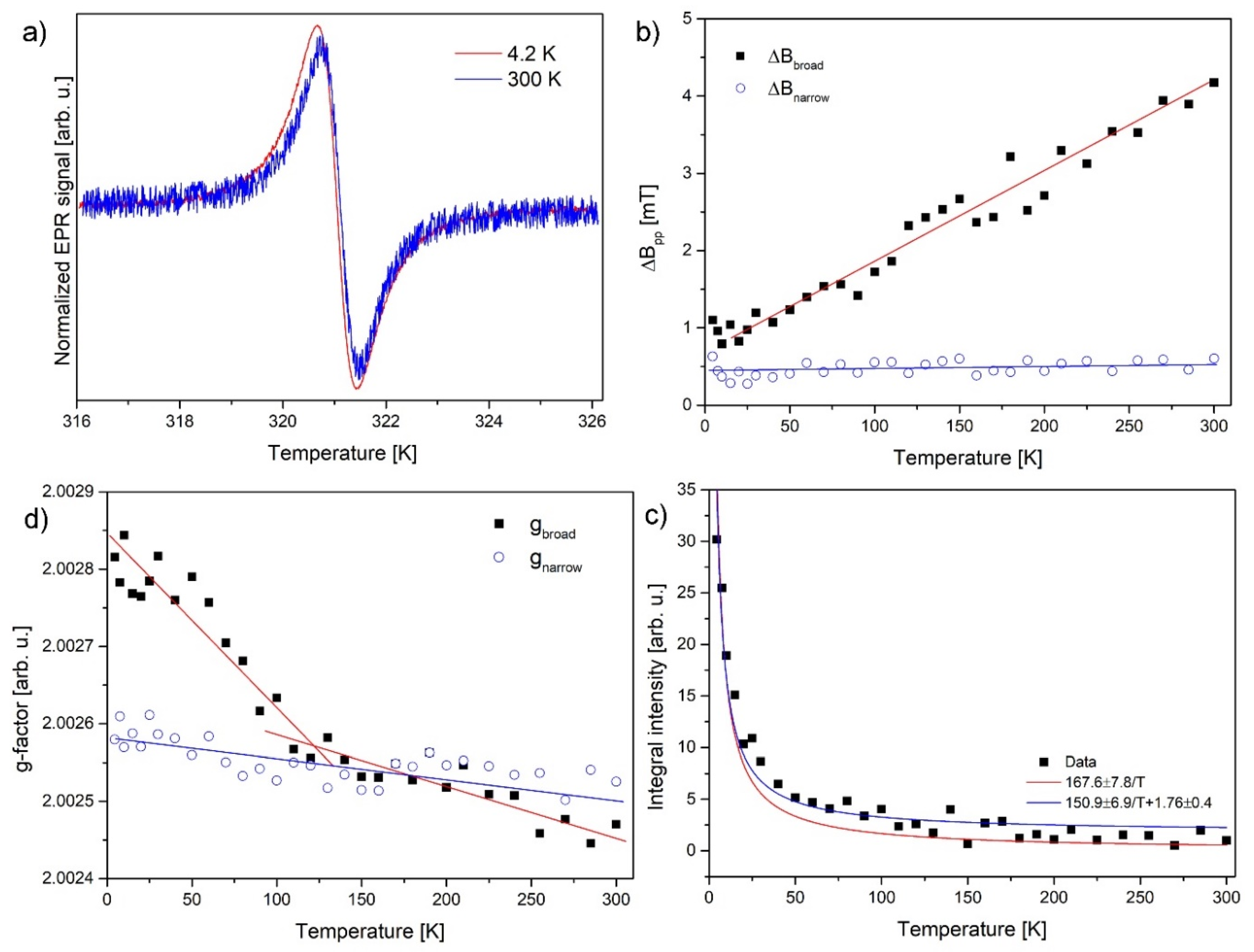
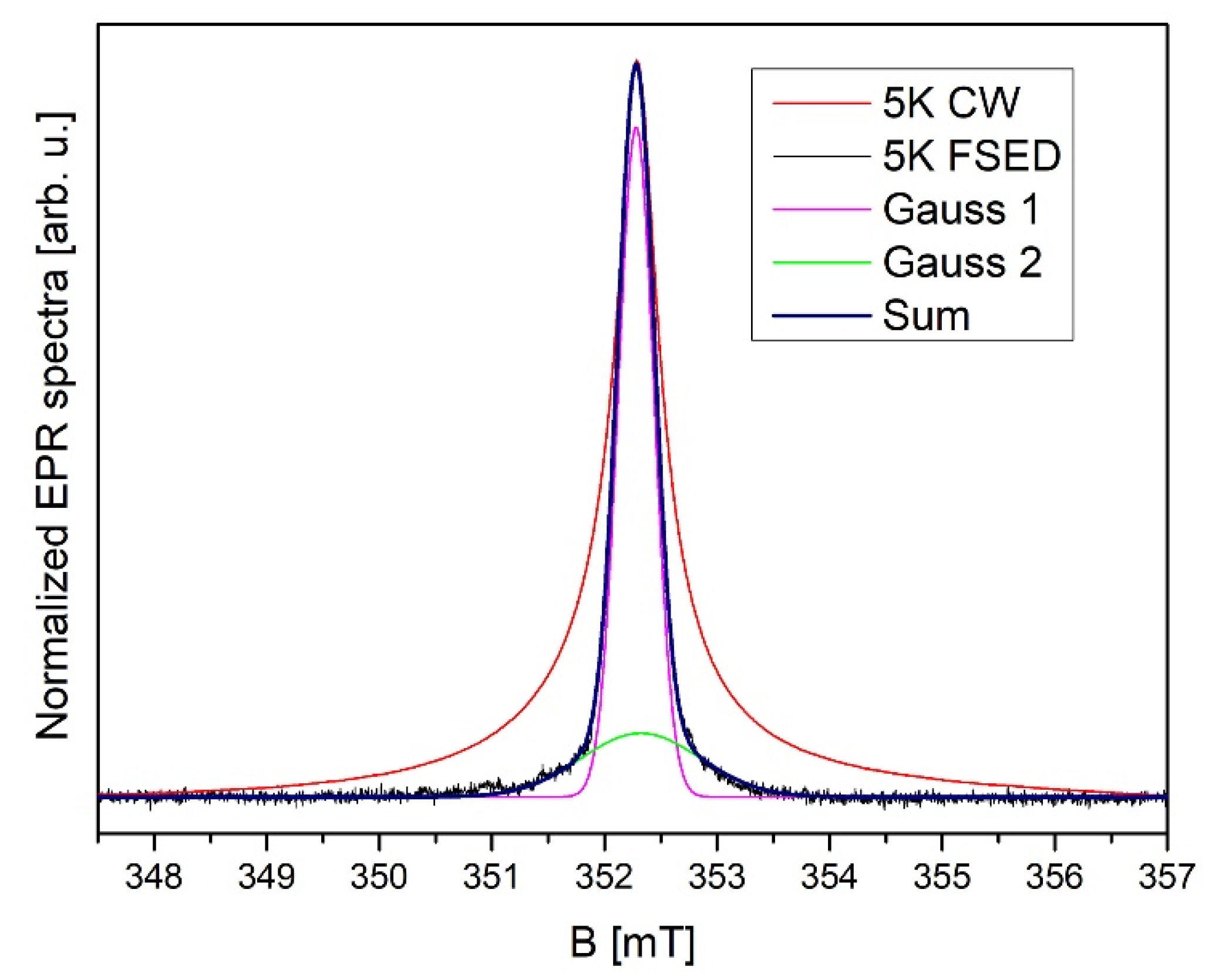
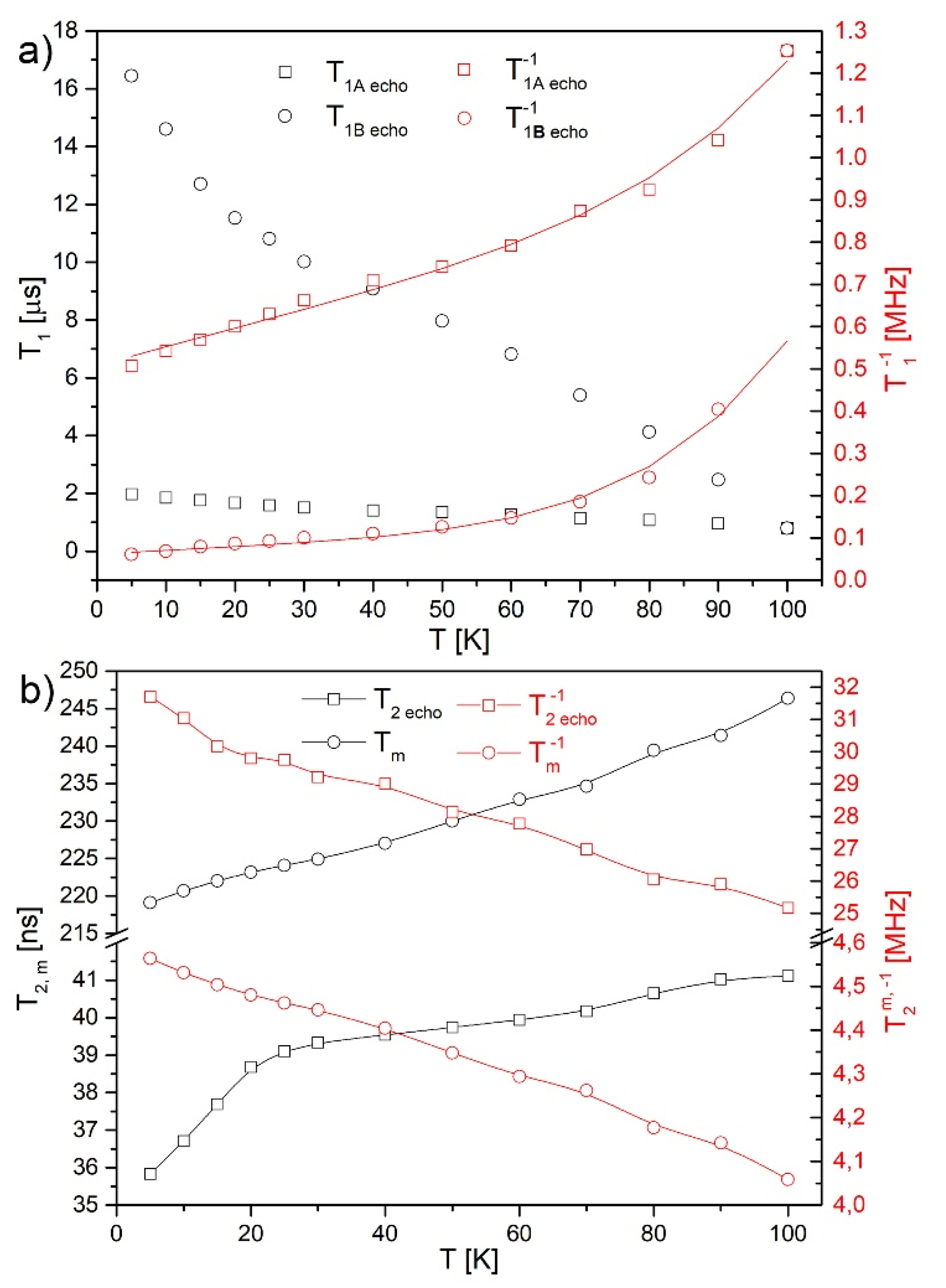
2.5. Electron Paramagnetic Resonance (EPR)
2.6. Electric Conductivity Measurements
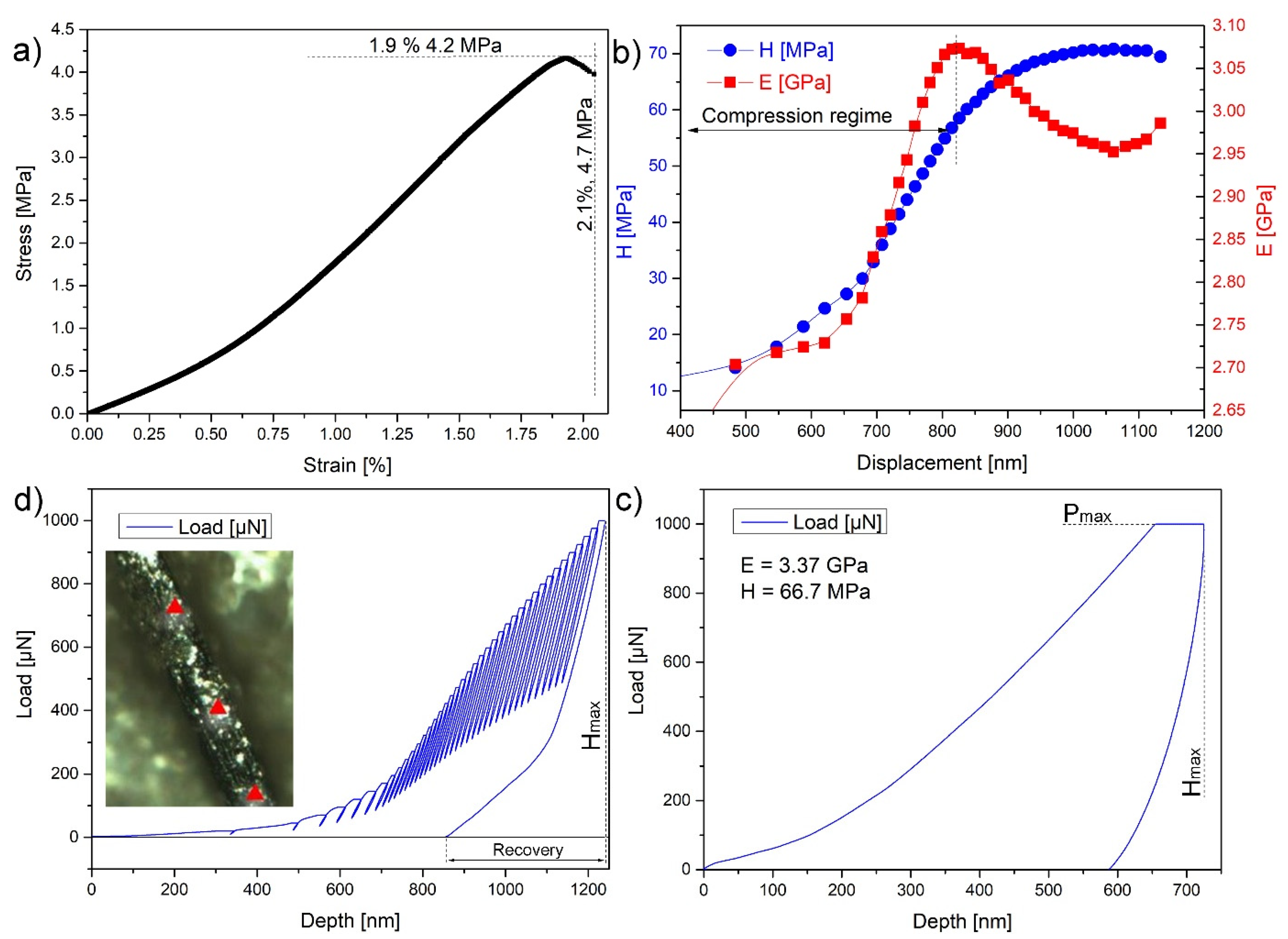
2.7. Nanoindentation
3. Results and Discussion
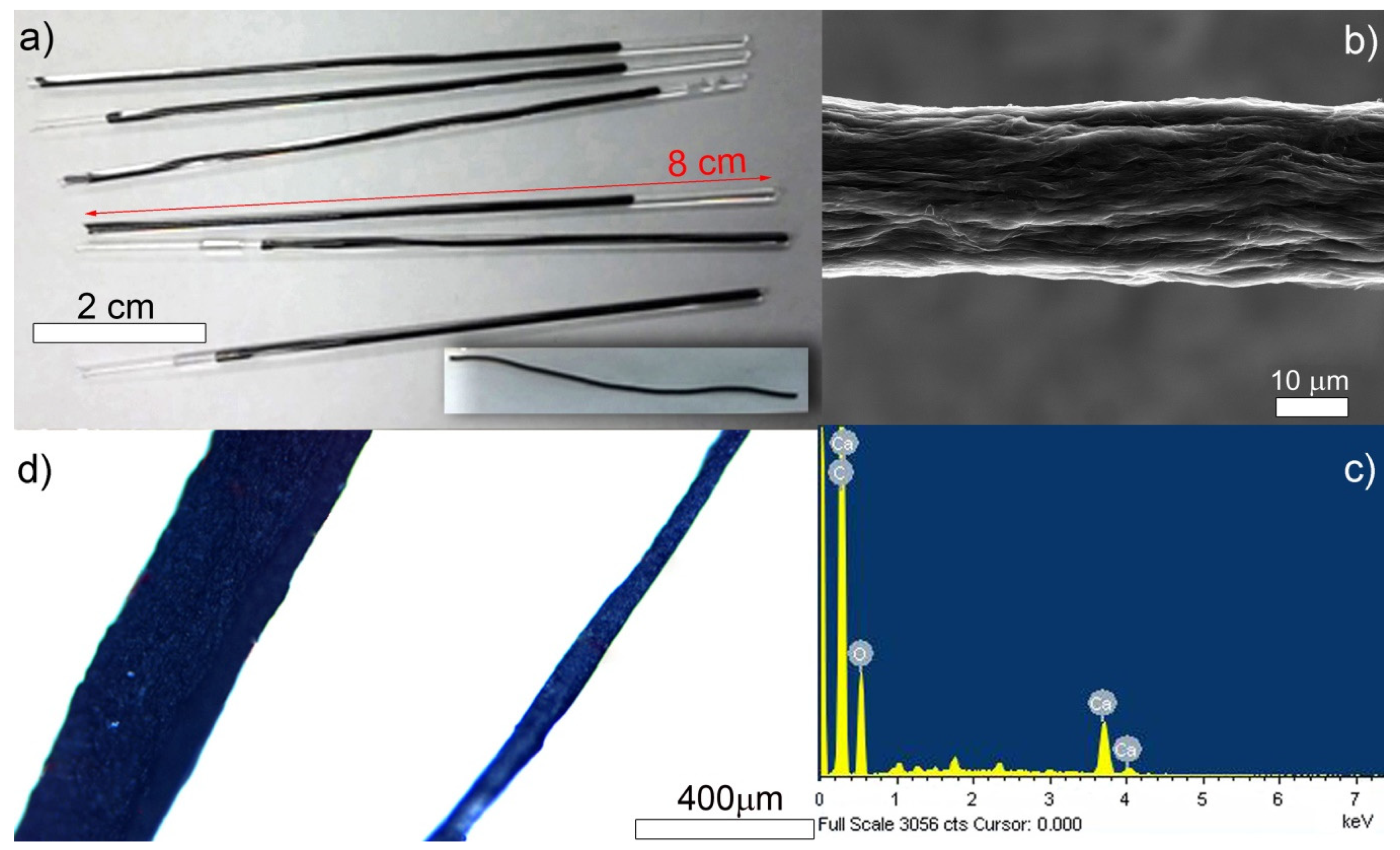
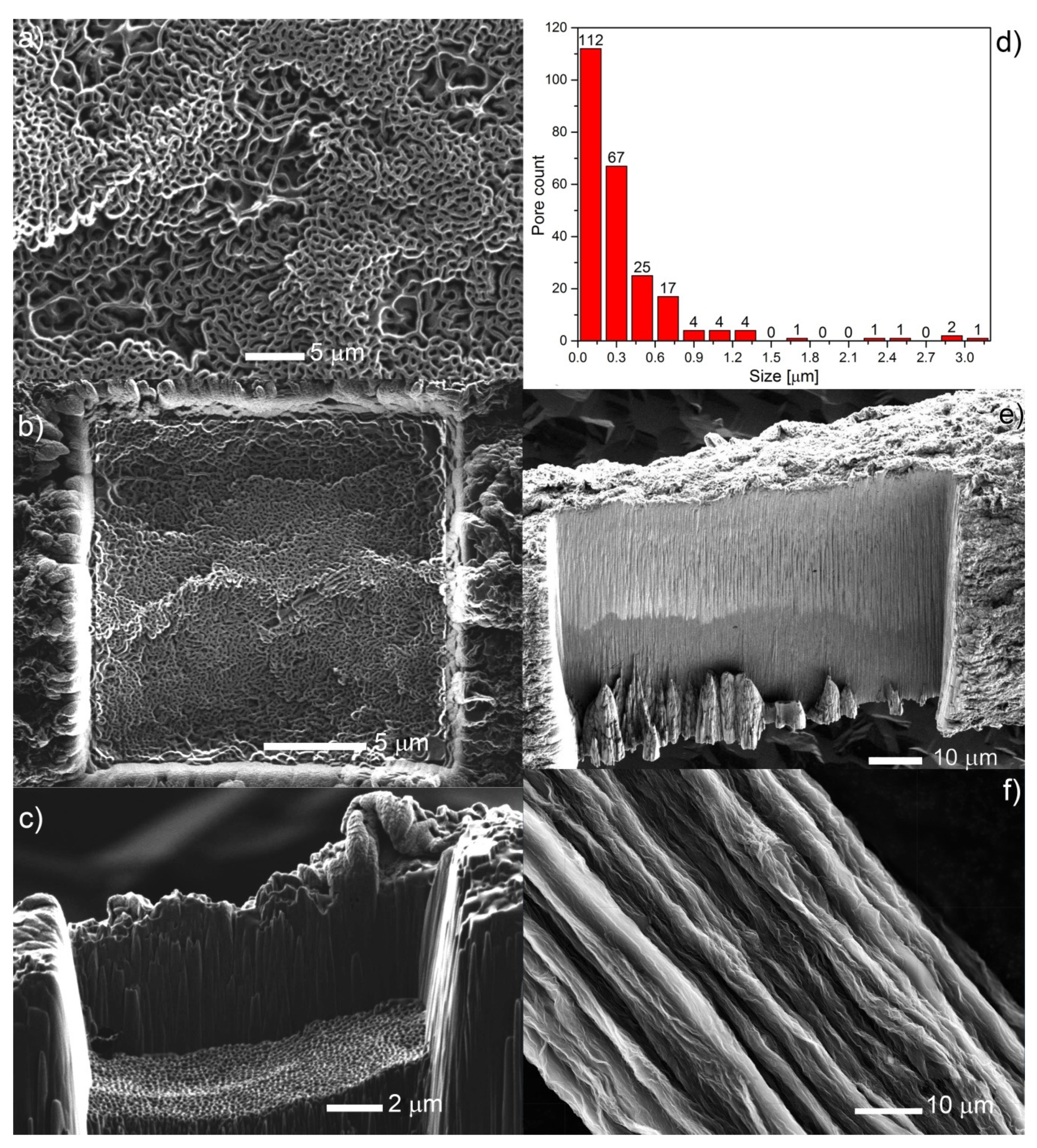
3.1. Fabrication of Reduced Graphene Oxide (rGO) Fibers
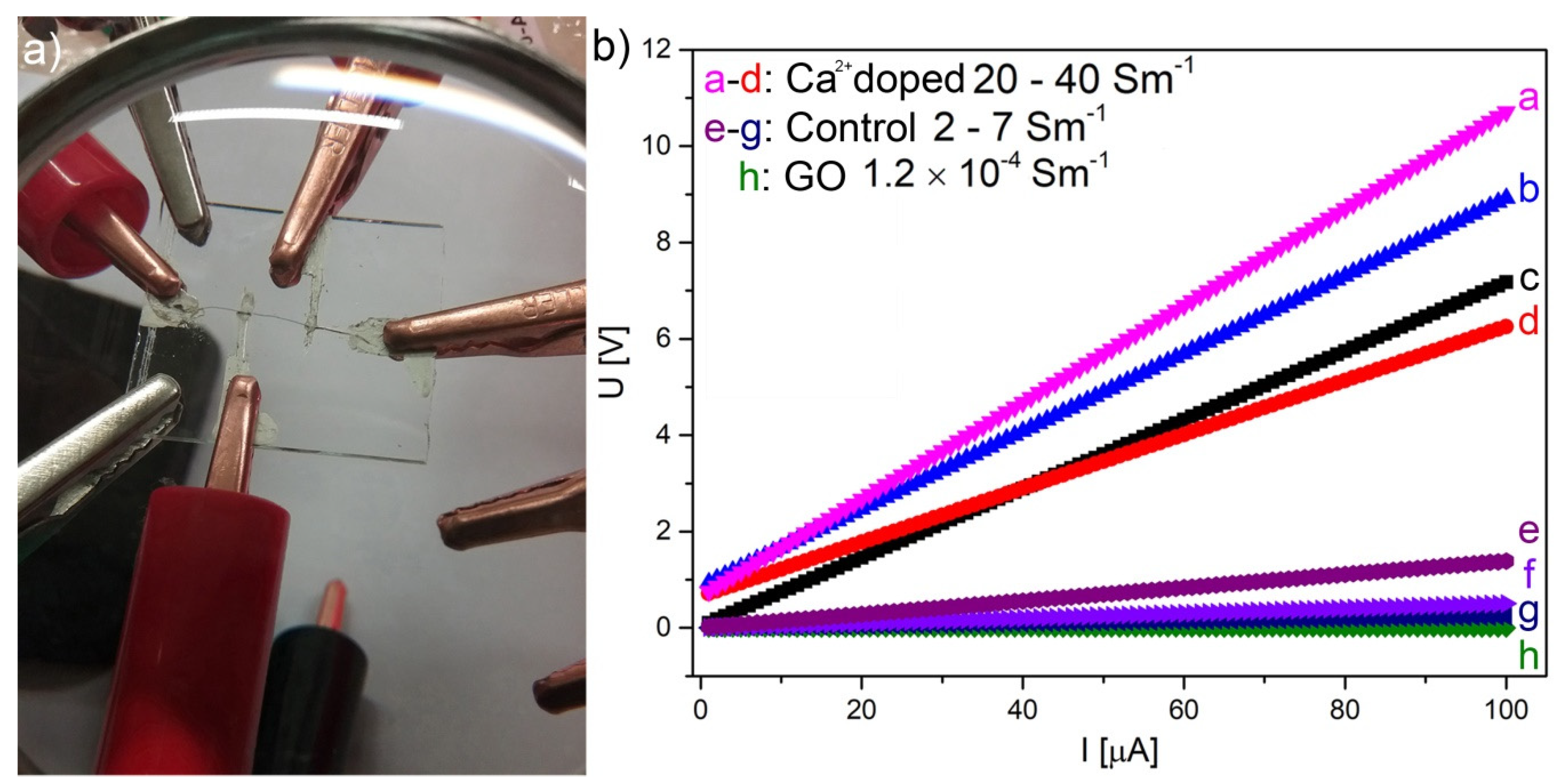
3.2. Electrical Properties
3.3. Mechanical Properties
3.4. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colburn, A.; Wanninayake, N.; Kim, D.Y.; Bhattacharyya, D. Cellulose-graphene quantum dot composite membranes using ionic liquid. J. Membr. Sci. 2018, 556, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Tadyszak, K.; Majchrzycki, Ł.; Szyller, Ł.; Scheibe, B. Preparation and characterization of partially reduced graphene oxide aerogels doped with transition metal ions. J. Mater. Sci. 2018, 53, 16086–16098. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrión, C.; Valcárcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Martínez, S.; Valcárcel, M. Graphene quantum dots in analytical science. TrAC Trends Anal. Chem. 2015, 72, 93–113. [Google Scholar] [CrossRef]
- De, S.; Patra, K.; Ghosh, D.; Dutta, K.; Dey, A.; Sarkar, G.; Maiti, J.; Basu, A.; Rana, D.; Chattopadhyay, D. Tailoring the Efficacy of Multifunctional Biopolymeric Graphene Oxide Quantum Dot-Based Nanomaterial as Nanocargo in Cancer Therapeutic Application. ACS Biomater. Sci. Eng. 2018, 4, 514–531. [Google Scholar] [CrossRef]
- Cao, J.; An, H.; Huang, X.; Fu, G.; Zhuang, R.; Zhu, L.; Xie, J.; Zhang, F. Monitoring of the tumor response to nano-graphene oxide-mediated photothermal/photodynamic therapy by diffusion-weighted and BOLD MRI. Nanoscale 2016, 8, 10152–10159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, B.; Huang, T.; Gao, C. Graphene-based single fiber supercapacitor with a coaxial structure. Nanoscale 2015, 7, 9399–9404. [Google Scholar] [CrossRef]
- Kim, B.J.; Jang, H.; Lee, S.-K.; Hong, B.H.; Ahn, J.-H.; Cho, J.H. High-Performance Flexible Graphene Field Effect Transistors with Ion Gel Gate Dielectrics. Nano Lett. 2010, 10, 3464–3466. [Google Scholar] [CrossRef]
- Yoon, S.S.; Lee, K.E.; Cha, H.-J.; Seong, D.G.; Um, M.-K.; Byun, J.-H.; Oh, Y.; Oh, J.H.; Lee, W.; Lee, J.U. Highly Conductive Graphene/Ag Hybrid Fibers for Flexible Fiber-Type Transistors. Sci. Rep. 2015, 5, 16366. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, H.; Zhao, X.; Gao, C. Ultrastrong Fibers Assembled from Giant Graphene Oxide Sheets. Adv. Mater. 2013, 25, 188–193. [Google Scholar] [CrossRef]
- Kim, S.H.; Haines, C.S.; Li, N.; Kim, K.J.; Mun, T.J.; Choi, C.; Di, J.; Oh, Y.J.; Oviedo, J.P.; Bykova, J.; et al. Harvesting electrical energy from carbon nanotube yarn twist. Science 2017, 357, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak-Jabłokow, M.A.; Fedaruk, R.; Strzelczyk, R.; Majchrzycki, Ł. Identification of a Slowly Relaxing Paramagnetic Center in Graphene Oxide. Appl. Magn. Reson. 2019, 50, 761–768. [Google Scholar] [CrossRef]
- Tadyszak, K.; Chybczyńska, K.; Ławniczak, P.; Zalewska, A.; Cieniek, B.; Gonet, M.; Murias, M. Magnetic and electric properties of partially reduced graphene oxide aerogels. J. Magn. Magn. Mater. 2019, 492, 165656. [Google Scholar] [CrossRef]
- Joung, D.; Khondaker, S.I. Efros-Shklovskii variable-range hopping in reduced graphene oxide sheets of varying carbon sp2 fraction. Phys. Rev. B 2012, 86, 235423. [Google Scholar] [CrossRef]
- Park, M.; Hong, S.J.; Kim, K.H.; Kang, H.; Lee, M.; Jeong, D.H.; Park, Y.W.; Kim, B.H. Electrical and thermoelectric transport by variable range hopping in reduced graphene oxide. Appl. Phys. Lett. 2017, 111, 173103. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Zhuo, Q.; Sun, J.; Qin, C.; Wang, J.; Dai, L. A facile continuous wet-spinning of graphene oxide fibers from aqueous solutions at high pH with the introduction of ammonia. Carbon 2018, 138, 292–299. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, Z.; Liu, Y.; Fang, B.; Peng, L.; Xi, J.; Li, Z.; Gao, C. Dry spinning approach to continuous graphene fibers with high toughness. Nanoscale 2017, 9, 12335–12342. [Google Scholar] [CrossRef]
- Feng, L.; Chang, Y.; Zhong, J.; Jia, D.-C. Dry Spin Graphene Oxide Fibers: Mechanical/Electrical Properties and Microstructure Evolution. Sci. Rep. 2018, 8, 10803. [Google Scholar] [CrossRef]
- Tadyszak, K.; Augustyniak-Jabłokow, M.A.; Więckowski, A.B.; Najder-Kozdrowska, L.; Strzelczyk, R.; Andrzejewski, B. Origin of electron paramagnetic resonance signal in anthracite. Carbon 2015, 94, 53–59. [Google Scholar] [CrossRef]
- Augustyniak-Jabłokow, M.A.; Tadyszak, K.; Strzelczyk, R.; Fedaruk, R.; Carmieli, R. Slow spin relaxation of paramagnetic centers in graphene oxide. Carbon 2019, 152, 98–105. [Google Scholar] [CrossRef]
- Augustyniak-Jabłokow, M.A.; Tadyszak, K.; MaćKowiak, M.; Lijewski, S. ESR study of spin relaxation in graphene. Chem. Phys. Lett. 2013, 557, 118–122. [Google Scholar] [CrossRef]
- Augustyniak-Jabłokow, M.A.; Maćkowiak, M.; Tadyszak, K.; Strzelczyk, R. FMR evidence of stable ferromagnetic correlations at zigzag edge states in graphene. Acta Phys. Pol. A 2015, 127, 537–539. [Google Scholar] [CrossRef]
- Tadyszak, K.; Maćkowiak, M.; Augustyniak-Jabłokow, M.A.; Roman, S. FMR evidence of ferromagnetic correlations at zigzag edge states in single-layer graphene. J. Mol. Struct. 2014, 1076, 31–34. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I. sp-Electron Magnetic Clusters with a Large Spin in Graphene. ACS Nano 2011, 5, 2440–2446. [Google Scholar] [CrossRef][Green Version]
- Santos, E.J.G.; Ayuela, A.; Sánchez-Portal, D. Universal magnetic properties of sp3-type defects in covalently functionalized graphene. New J. Phys. 2012, 14, 043022. [Google Scholar] [CrossRef]
- Augustyniak-Jabłokow, M.A.; Tadyszak, K.; Maćkowiak, M.; Yablokov, Y.V. EPR evidence of antiferromagnetic ordering in single-layer graphene. Phys. Status Solidi Rapid Res. Lett. 2011, 5, 271–273. [Google Scholar] [CrossRef]
- Augustyniak-Jabłokow, M.A.; Yablokov, Y.V.; Andrzejewski, B.; Kempiński, W.; Łoś, S.; Tadyszak, K.; Yablokov, M.Y.; Zhikharev, V.A. EPR and magnetism of the nanostructured natural carbonaceous material shungite. Phys. Chem. Miner. 2010, 37, 237–247. [Google Scholar] [CrossRef]
- Fedaruk, R.; Strzelczyk, R.; Tadyszak, K.; Markevich, S.A.; Augustyniak-Jabłokow, M.A. Effect of Rabi splitting on the low-temperature electron paramagnetic resonance signal of anthracite. J. Magn. Reson. 2017, 274, 73–79. [Google Scholar] [CrossRef]
- Tadyszak, K.; Strzelczyk, R.; Coy, E.; Mac’Kowiak, M.; Augustyniak-Jabłokow, M.A. Size effects in the conduction electron spin resonance of anthracite and higher anthraxolite. Magn. Reson. Chem. 2016, 54, 239–245. [Google Scholar] [CrossRef]
- Pharr, G.M.; Oliver, W.C. Measurement of Thin Film Mechanical Properties Using Nanoindentation. MRS Bull. 1992, 17, 28–33. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Litowczenko, J.; Tadyszak, K. Fabricating versatile cell supports from nano- and micro-sized graphene oxide flakes. J. Mech. Behav. Biomed. Mater. 2020, 103, 103594. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic Transport Properties of Individual Chemically Reduced Graphene Oxide Sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef] [PubMed]
- George, G.; Costas, G. Graphene aerogels: A review. 2D Mater. 2017, 4, 032001. [Google Scholar]
- Kaiser, A.B.; Gómez-Navarro, C.; Sundaram, R.S.; Burghard, M.; Kern, K. Electrical Conduction Mechanism in Chemically Derived Graphene Monolayers. Nano Lett. 2009, 9, 1787–1792. [Google Scholar] [CrossRef]
- Kempiński, M.; Florczak, P.; Jurga, S.; Śliwińska-Bartkowiak, M.; Kempiński, W. The impact of adsorption on the localization of spins in graphene oxide and reduced graphene oxide, observed with electron paramagnetic resonance. Appl. Phys. Lett. 2017, 111, 084102. [Google Scholar] [CrossRef]
- Rani, A.; Nam, S.; Oh, K.A.; Park, M. Electrical Conductivity of Chemically Reduced Graphene Powders under Compression. Carbon Lett. 2010, 11, 90–95. [Google Scholar] [CrossRef]
- Park, W.; Hu, J.; Jauregui, L.A.; Ruan, X.; Chen, Y.P. Electrical and thermal conductivities of reduced graphene oxide/polystyrene composites. Appl. Phys. Lett. 2014, 104, 113101. [Google Scholar] [CrossRef]
- Haque, A.; Abdullah-Al Mamun, M.; Taufique, M.F.N.; Karnati, P.; Ghosh, K. Large Magnetoresistance and Electrical Transport Properties in Reduced Graphene Oxide Thin Film. IEEE Trans. Magn. 2018, 54, 1–9. [Google Scholar] [CrossRef]
- Lin, X.; Liu, X.; Jia, J.; Shen, X.; Kim, J.-K. Electrical and mechanical properties of carbon nanofiber/graphene oxide hybrid papers. Compos. Sci. Technol. 2014, 100, 166–173. [Google Scholar] [CrossRef]
- Chartarrayawadee, W.; Molloy, R.; Ratchawet, A.; Janmee, N.; Butsamran, M.; Panpai, K. Fabrication of poly(lactic acid)/graphene oxide/stearic acid composites with improved tensile strength. Polym. Compos. 2015, 38, 2272–2282. [Google Scholar] [CrossRef]
- Uçar, N.; Ölmez, M.; Kayaoğlu, B.K.; Önen, A.; Karatepe Yavuz, N.; Eksik, O. Structural properties of graphene oxide fibers: From graphene oxide dispersion until continuous graphene oxide fiber. J. Text. Inst. 2018, 109, 1642–1652. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Y.; Han, D.; Yu, B.; Deng, S.; Chen, F.; Fu, Q. Mechanical property enhancement of high conductive reduced graphene oxide fiber by a small loading of polydopamine. Mater. Res. Express 2018, 5, 045602. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. Graphene fiber: A new trend in carbon fibers. Mater. Today 2015, 18, 480–492. [Google Scholar] [CrossRef]
- Dutta, S.; Lakshmi, S.; Pati, S.K. Electron-electron interactions on the edge states of graphene: A many-body configuration interaction study. Phys. Rev. B 2008, 77, 073412. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Song, Y.; Zhang, X.; Ma, Y.; Liang, J.; Chen, Y. Room-Temperature Ferromagnetism of Graphene. Nano Lett. 2009, 9, 220–224. [Google Scholar] [CrossRef]
- Yazyev, O.V. Emergence of magnetism in graphene materials and nanostructures. Rep. Prog. Phys. 2010, 73, 056501. [Google Scholar] [CrossRef]
- Ney, A.; Papakonstantinou, P.; Kumar, A.; Shang, N.-G.; Peng, N. Irradiation enhanced paramagnetism on graphene nanoflakes. Appl. Phys. Lett. 2011, 99, 102504. [Google Scholar] [CrossRef][Green Version]
- Yazyev, O.V.; Helm, L. Defect-induced magnetism in graphene. Phys. Rev. B 2007, 75, 125408. [Google Scholar] [CrossRef]
- López-Sancho, M.P.; de Juan, F.; Vozmediano, M.A.H. Magnetic moments in the presence of topological defects in graphene. Phys. Rev. B 2009, 79, 075413. [Google Scholar] [CrossRef]
- Nair, R.R.; Sepioni, M.; Tsai, I.L.; Lehtinen, O.; Keinonen, J.; Krasheninnikov, A.V.; Thomson, T.; Geim, A.K.; Grigorieva, I.V. Spin-half paramagnetism in graphene induced by point defects. Nat. Phys. 2012, 8, 199–202. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Poh, H.L.; Šaněk, F.; Maryško, M.; Matějková, S.; Sofer, Z.; Pumera, M. Searching for Magnetism in Hydrogenated Graphene: Using Highly Hydrogenated Graphene Prepared via Birch Reduction of Graphite Oxides. ACS Nano 2013, 7, 5930–5939. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, X.; Lu, J.; Ni, Z.; Luo, Z.; Mao, H.; Wang, R.; Wang, Y.; Huang, H.; Qi, D.; et al. Room temperature ferromagnetism in partially hydrogenated epitaxial graphene. Appl. Phys. Lett. 2011, 98, 193113. [Google Scholar] [CrossRef]
- Feng, Q.; Tang, N.; Liu, F.; Cao, Q.; Zheng, W.; Ren, W.; Wan, X.; Du, Y. Obtaining High Localized Spin Magnetic Moments by Fluorination of Reduced Graphene Oxide. ACS Nano 2013, 7, 6729–6734. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Cho, J.-H. Fluorine-induced local magnetic moment in graphene: A hybrid DFT study. Phys. Rev. B 2013, 87, 174435. [Google Scholar] [CrossRef]
- Sepioni, M.; Nair, R.R.; Rablen, S.; Narayanan, J.; Tuna, F.; Winpenny, R.; Geim, A.K.; Grigorieva, I.V. Limits on Intrinsic Magnetism in Graphene. Phys. Rev. Lett. 2010, 105, 207205. [Google Scholar] [CrossRef]
- Lehtinen, P.O.; Foster, A.S.; Ayuela, A.; Krasheninnikov, A.; Nordlund, K.; Nieminen, R.M. Magnetic Properties and Diffusion of Adatoms on a Graphene Sheet. Phys. Rev. Lett. 2003, 91, 017202. [Google Scholar] [CrossRef]
- Boukhvalov, D.W. Modeling of hydrogen and hydroxyl group migration on graphene. Phys. Chem. Chem. Phys. 2010, 12, 15367–15371. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the Thermal Deoxygenation of Graphene Oxide Using High-Resolution In Situ X-ray-Based Spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of Electrical, Chemical, and Structural Properties of Transparent and Conducting Chemically Derived Graphene Thin Films. Adv. Funct. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- Panich, A.M.; Shames, A.I.; Tsindlekht, M.I.; Osipov, V.Y.; Patel, M.; Savaram, K.; He, H. Structure and Magnetic Properties of Pristine and Fe-Doped Micro- and Nanographenes. J. Phys. Chem. C 2016, 120, 3042–3053. [Google Scholar] [CrossRef]
- Tadyszak, K.; Rudowicz, C.; Ohta, H.; Sakurai, T. Electron magnetic resonance data on high-spin Mn (III; S = 2) ions in porphyrinic and salen complexes modeled by microscopic spin Hamiltonian approach. J. Inorg. Biochem. 2017, 175, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Tadyszak, K.; Rudowicz, C. EMR data on Mn(III; S = 2) ions in MnTPPCl complex modelled by microscopic spin hamiltonian approach. Acta Phys. Pol. A 2017, 132, 15–18. [Google Scholar] [CrossRef]
- Rudowicz, C.; Tadyszak, K. Single magnetic 3dNadatoms on surfaces—Proper outlook on compatibility of orthorhombic zero-field splitting parameters and their relationships with magnetic anisotropy quantities. Polyhedron 2017, 127, 126–134. [Google Scholar] [CrossRef]
- Panich, A.M.; Shames, A.I.; Aleksenskii, A.E.; Dideikin, A. Magnetic resonance evidence of manganese–graphene complexes in reduced graphene oxide. Solid State Commun. 2012, 152, 466–468. [Google Scholar] [CrossRef]
- Panich, A.M.; Shames, A.I.; Sergeev, N.A. Paramagnetic Impurities in Graphene Oxide. Appl. Magn. Reson. 2013, 44, 107–116. [Google Scholar] [CrossRef]
- Ćirić, L.; Sienkiewicz, A.; Djokić, D.M.; Smajda, R.; Magrez, A.; Kaspar, T.; Nesper, R.; Forró, L. Size dependence of the magnetic response of graphite oxide and graphene flakes—An electron spin resonance study. Phys. Status Solidi B 2010, 247, 2958–2961. [Google Scholar] [CrossRef]
- Ćirić, L.; Sienkiewicz, A.; Gaál, R.; Jaćimović, J.; Vâju, C.; Magrez, A.; Forró, L. Defects and localization in chemically-derived graphene. Phys. Rev. B 2012, 86, 195139. [Google Scholar] [CrossRef]
- Shames, A.I.; Felner, I.; Osipov, V.Y.; Katz, E.A.; Mogilko, E.; Grinblat, J.; Panich, A.M.; Belousov, V.P.; Belousova, I.M.; Ponomarev, A.N. Closed pi-electron Network in Large Polyhedral Multi-shell Carbon Nanoparticles. Nanosci. Nanotechnol. Lett. 2011, 3, 41–43. [Google Scholar] [CrossRef]
- Rao, S.S.; Stesmans, A.; van Tol, J.; Kosynkin, D.V.; Higginbotham-Duque, A.; Lu, W.; Sinitskii, A.; Tour, J.M. Spin Dynamics and Relaxation in Graphene Nanoribbons: Electron Spin Resonance Probing. ACS Nano 2012, 6, 7615–7623. [Google Scholar] [CrossRef]

| T1A−1 | T1B−1 | T1GO−1 [20] |
|---|---|---|
| A0 = 0.51 s−1 | A0 = 0.06 s−1 | |
| A1 = 4.4 × 10−3 K−1·s−1 | A1 = 9.0 × 10−4 K−1·s−1 | A1 = 4.7 × 10−4 K−1·s−1 |
| A2 = 2.8 × 10−11 K−5·s−1 | A2 = 4.2 × 10−11 K−5·s−1 | A2 = 6.6 × 10−9 K−5·s−1 |
| R2 = 0.9995 | R2 = 0.9957 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadyszak, K.; Wychowaniec, J.K.; Załęski, K.; Coy, E.; Majchrzycki, Ł.; Carmieli, R. Tuning Properties of Partially Reduced Graphene Oxide Fibers upon Calcium Doping. Nanomaterials 2020, 10, 957. https://doi.org/10.3390/nano10050957
Tadyszak K, Wychowaniec JK, Załęski K, Coy E, Majchrzycki Ł, Carmieli R. Tuning Properties of Partially Reduced Graphene Oxide Fibers upon Calcium Doping. Nanomaterials. 2020; 10(5):957. https://doi.org/10.3390/nano10050957
Chicago/Turabian StyleTadyszak, Krzysztof, Jacek K. Wychowaniec, Karol Załęski, Emerson Coy, Łukasz Majchrzycki, and Raanan Carmieli. 2020. "Tuning Properties of Partially Reduced Graphene Oxide Fibers upon Calcium Doping" Nanomaterials 10, no. 5: 957. https://doi.org/10.3390/nano10050957
APA StyleTadyszak, K., Wychowaniec, J. K., Załęski, K., Coy, E., Majchrzycki, Ł., & Carmieli, R. (2020). Tuning Properties of Partially Reduced Graphene Oxide Fibers upon Calcium Doping. Nanomaterials, 10(5), 957. https://doi.org/10.3390/nano10050957