Quasi-3D Plasmonic Nanowell Array for Molecular Enrichment and SERS-Based Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Gold Nanoparticle (Au-NP)-Decorated Nanowell Arrays
2.2. Characterization and Surface-Enhanced Raman Spectroscopy (SERS) Measurements
2.3. Numerical Simulations
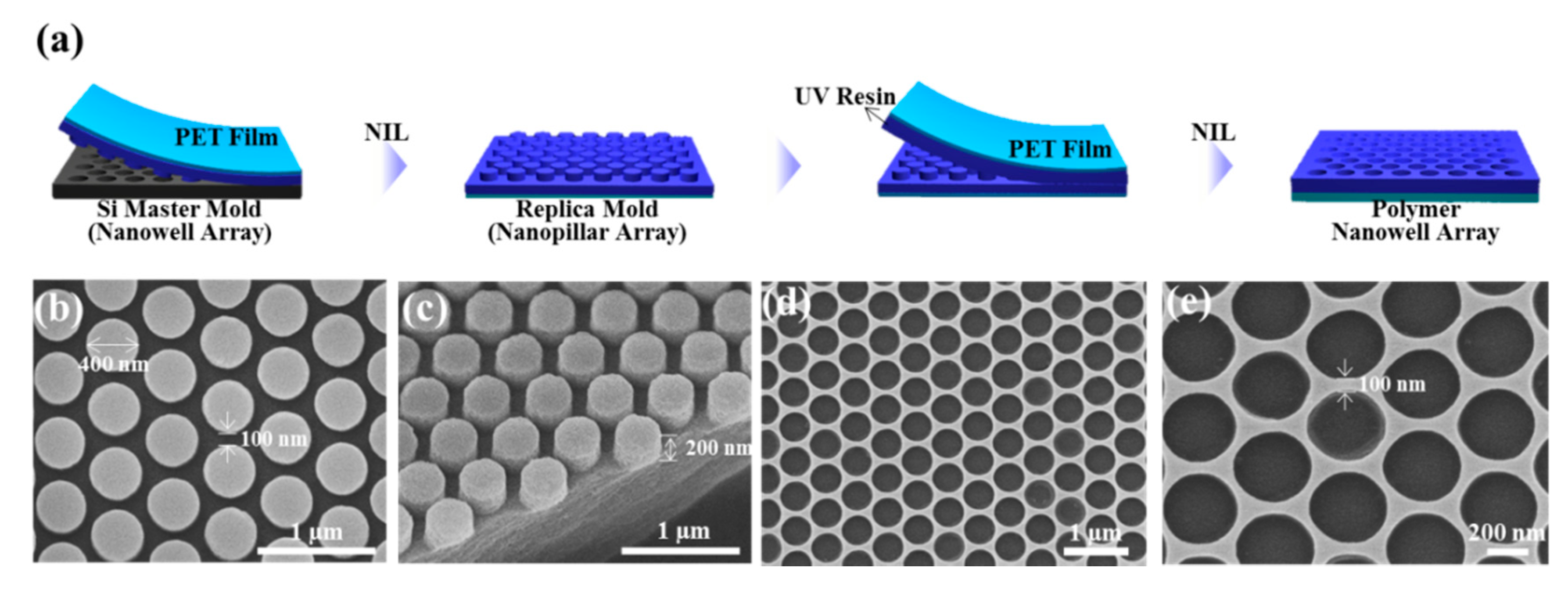
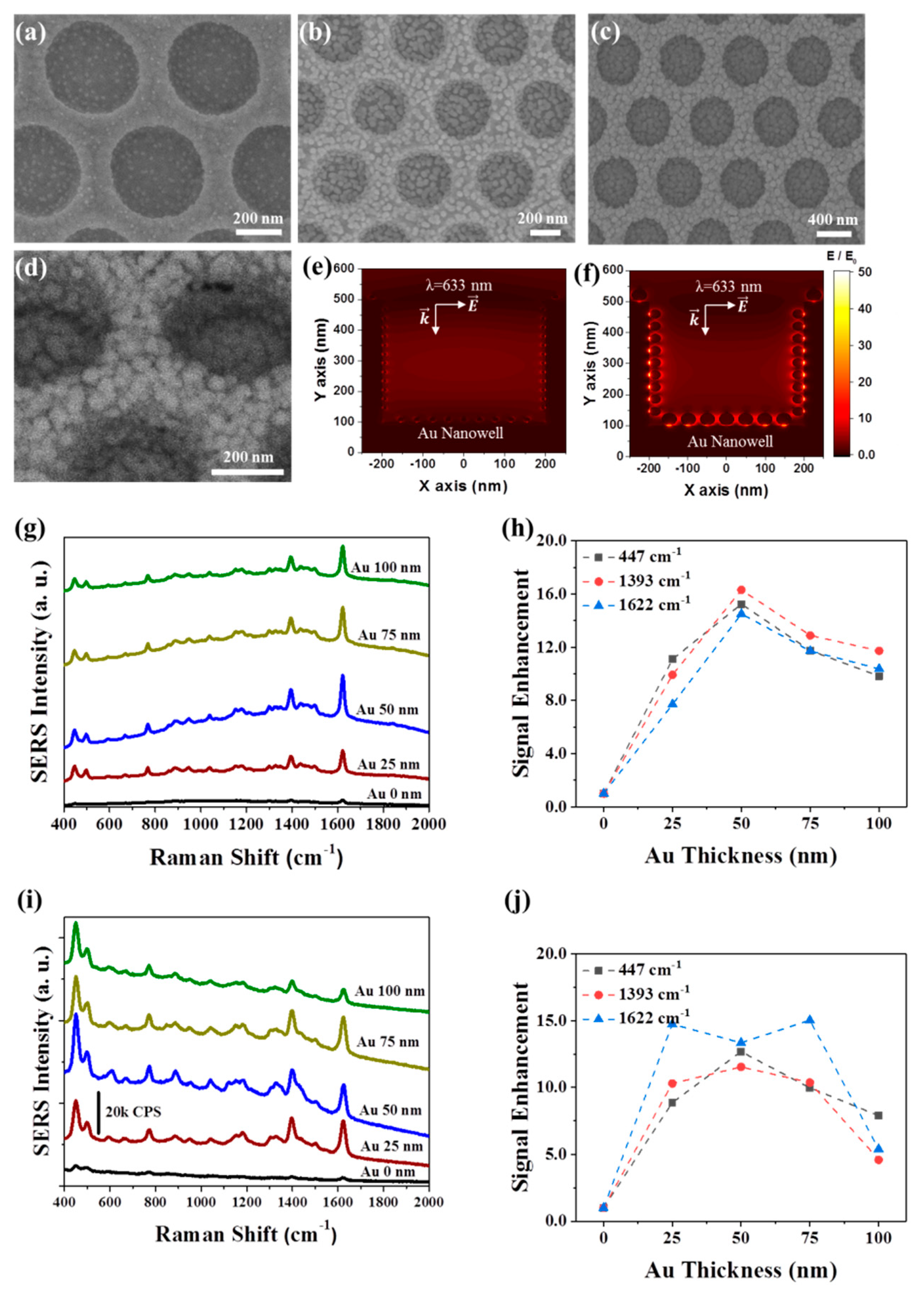
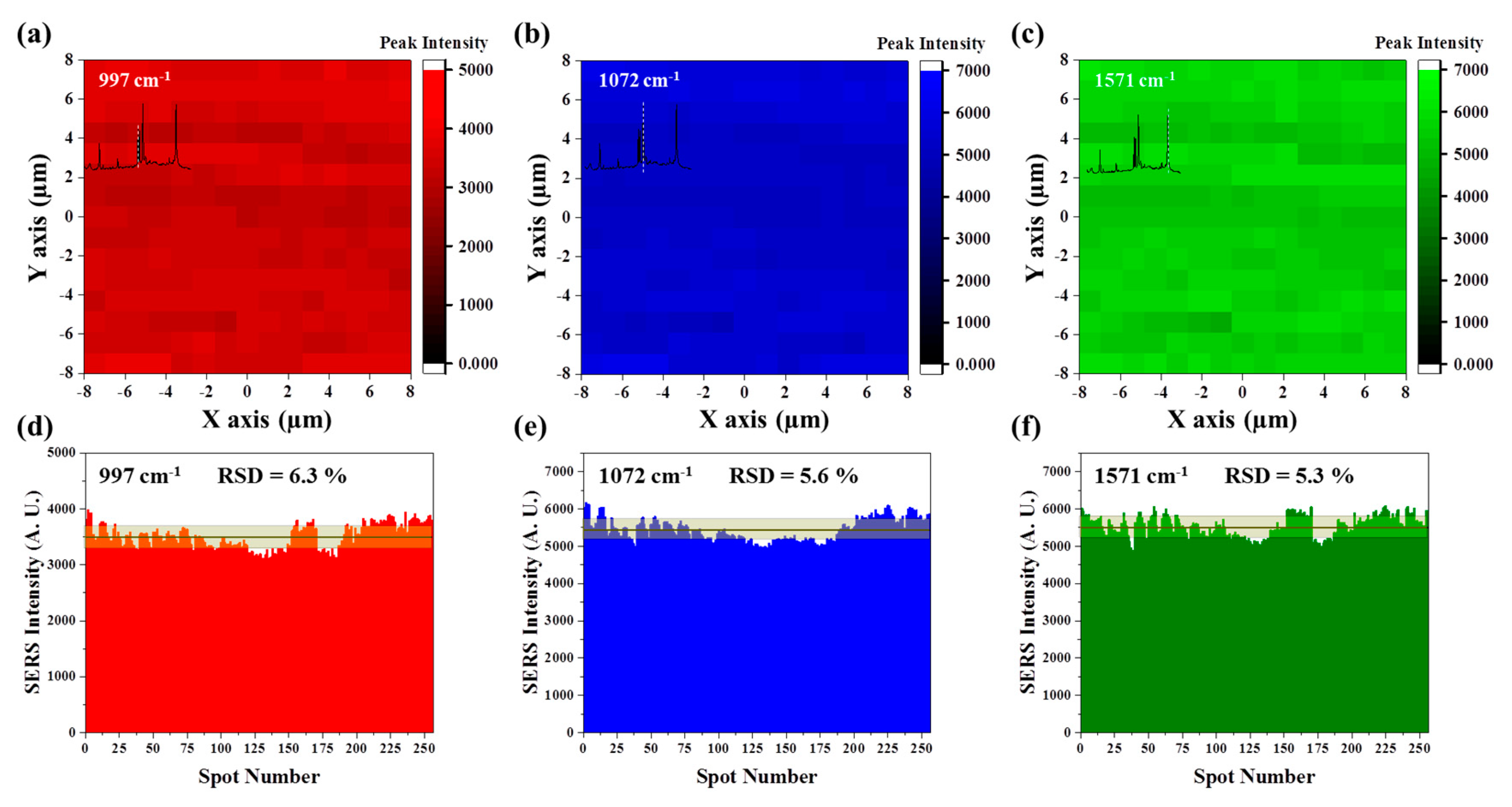
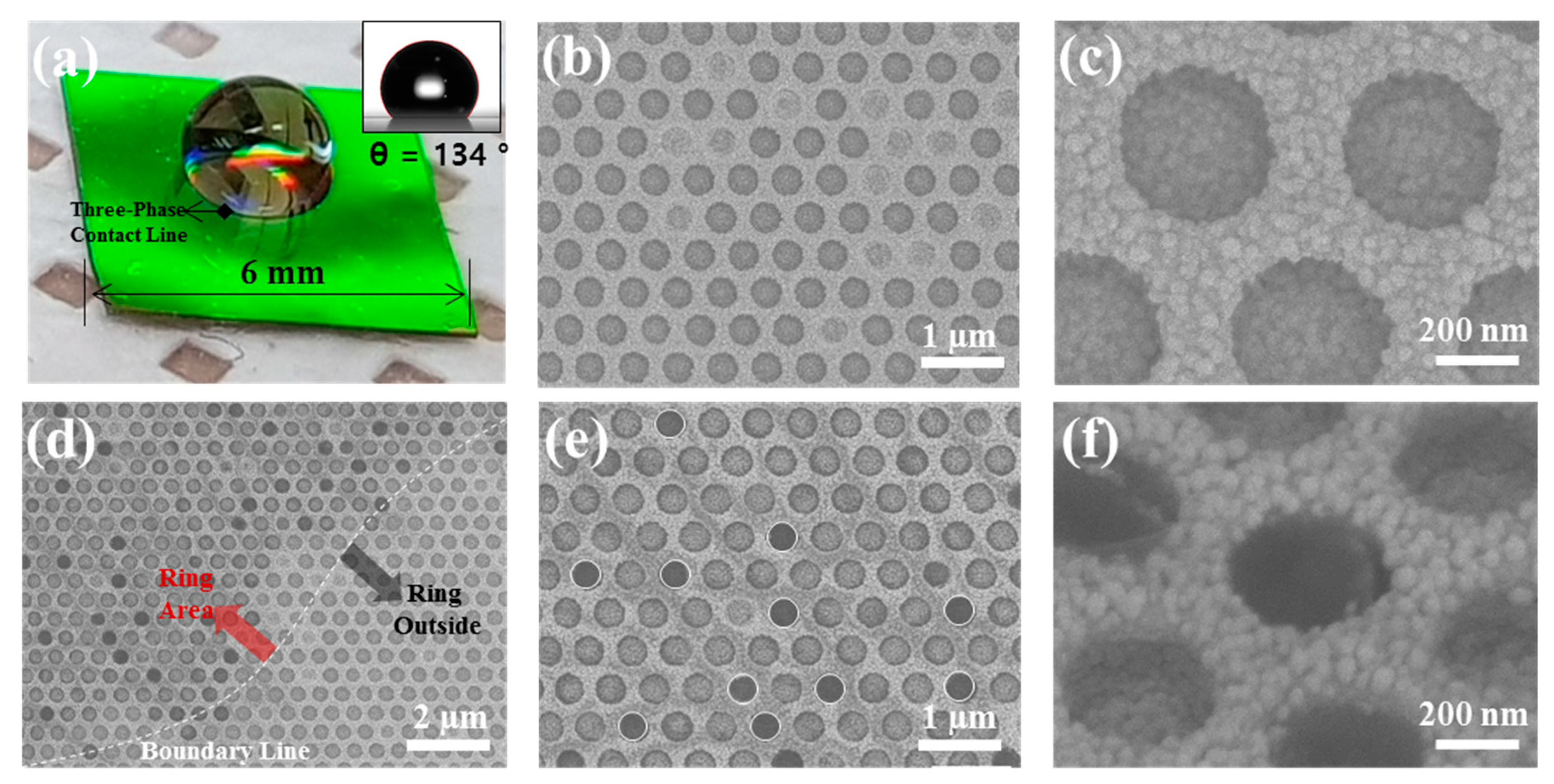
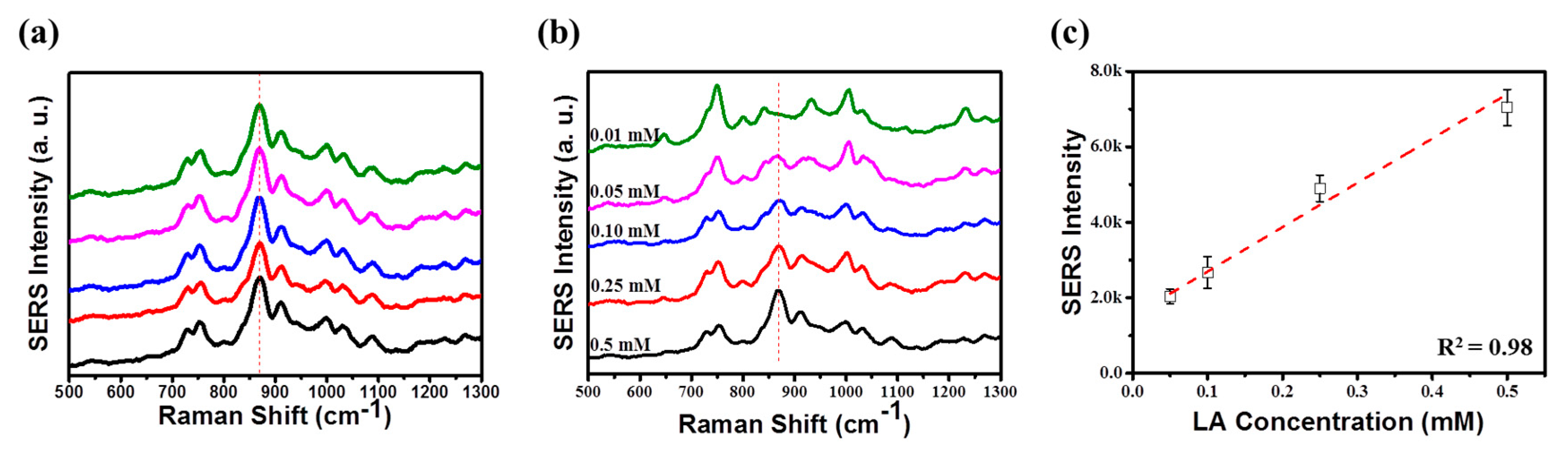
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mun, C.; Linh, V.T.N.; Kwon, J.-D.; Jung, H.S.; Kim, D.-H.; Park, S.-G. Highly Sensitive and Selective Nanogap-Enhanced SERS Sensing Platform. Nanomaterials 2019, 9, 619. [Google Scholar] [CrossRef]
- Park, S.-G.; Mun, C.; Xiao, X.; Braun, A.; Kim, S.; Giannini, V.; Maier, S.A.; Kim, D.-H. Surface Energy-Controlled SERS Substrates for Molecular Concentration at Plasmonic Nanogaps. Adv. Funct. Mater. 2017, 27, 1703376. [Google Scholar] [CrossRef]
- Lee, M.; Jeon, T.Y.; Mun, C.; Kwon, J.-D.; Yun, J.; Kim, S.-H.; Kim, D.-H.; Chang, S.-C.; Park, S.-G. 3D multilayered plasmonic nanostructures with high areal density for SERS. RSC Adv. 2017, 7, 17898–17905. [Google Scholar] [CrossRef]
- Wang, X.; Park, S.-G.; Ko, J.; Xiao, X.; Giannini, V.; Maier, S.A.; Kim, D.-H.; Choo, J. Sensitive and Reproducible Immunoassay of Multiple Mycotoxins Using Surface-Enhanced Raman Scattering Mapping on 3D Plasmonic Nanopillar Arrays. Small 2018, 14, e1801623. [Google Scholar] [CrossRef]
- Park, S.-G.; Xiao, X.; Min, J.; Mun, C.; Jung, H.S.; Giannini, V.; Weissleder, R.; Maier, S.A.; Im, H.; Kim, D. Self-Assembly of Nanoparticle-Spiked Pillar Arrays for Plasmonic Biosensing. Adv. Funct. Mater. 2019, 29, 29. [Google Scholar] [CrossRef]
- Jeon, T.Y.; Park, S.-G.; Kim, S.-H.; Kim, D.J.; Kim, D.-H. Nanostructured plasmonic substrates for use as SERS sensors. Nano Converg. 2016, 3, 2957. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Park, S.-G.; Lee, S.; Wang, X.; Mun, C.; Kim, S.; Kim, D.-H.; Choo, J. Culture-Free Detection of Bacterial Pathogens on Plasmonic Nanopillar Arrays Using Rapid Raman Mapping. ACS Appl. Mater. Interfaces 2018, 10, 6831–6840. [Google Scholar] [CrossRef] [PubMed]
- Linh, V.T.N.; Xiao, X.; Jung, H.S.; Giannini, V.; Maier, S.A.; Kim, D.-H.; Lee, Y.-I.; Park, S.-G. Compact Integration of TiO₂ Nanoparticles into the Cross-Points of 3D Vertically Stacked Ag Nanowires for Plasmon-Enhanced Photocatalysis. Nanomaterials 2019, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zong, S.; Wu, L.; Zhu, D.; Cui, Y. SERS-Activated Platforms for Immunoassay: Probes, Encoding Methods, and Applications. Chem. Rev. 2017, 117, 7910–7963. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, R.E.; Aybeke, E.N.; Bourillot, E.; Lacroute, Y.; Lesniewska, E.; Adam, P.; Bijeon, J.-L. Fabrication of Annealed Gold Nanostructures on Pre-Treated Glow-Discharge Cleaned Glasses and Their Used for Localized Surface Plasmon Resonance (LSPR) and Surface Enhanced Raman Spectroscopy (SERS) Detection of Adsorbed (Bio)molecules. Sensors 2017, 17, 236. [Google Scholar] [CrossRef]
- Aybeke, E.N.; Belliot, G.; Lemaire-Ewing, S.; Estienney, M.; Lacroute, Y.; Pothier, P.; Bourillot, E.; Lesniewska, E. HS-AFM and SERS Analysis of Murine Norovirus Infection: Involvement of the Lipid Rafts. Small 2016, 13, 1600918. [Google Scholar] [CrossRef] [PubMed]
- Punj, D.; Mivelle, M.; Moparthi, S.B.; Van Zanten, T.; Rigneault, H.; Van Hulst, N.F.; Garcia-Parajo, M.F.; Wenger, J. A plasmonic ‘antenna-in-box’ platform for enhanced single-molecule analysis at micromolar concentrations. Nat. Nanotechnol. 2013, 8, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-F.; Li, C.-Y.; Aroca, R.F. Plasmon-enhanced fluorescence spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.C.; Heo, C.-J.; Lee, S.Y.; Park, S.-G.; Yang, S.-M. Optically tunable arrayed structures for highly sensitive plasmonic detection via simplified holographic lithography. J. Mater. Chem. 2012, 22, 4603. [Google Scholar] [CrossRef]
- Jeon, H.C.; Park, S.-G.; Cho, S.; Yang, S.-M. Dual length-scale nanotip arrays with controllable morphological features for highly sensitive SERS applications. J. Mater. Chem. 2012, 22, 23650. [Google Scholar] [CrossRef]
- Park, S.-G.; Yang, S.-M. Multicolor patterning using holographic woodpile photonic crystals at visible wavelengths. Nanoscale 2013, 5, 4110. [Google Scholar] [CrossRef][Green Version]
- Park, S.-G.; Jeon, T.Y.; Jeon, H.C.; Yang, S.-M.; Kwon, J.-D.; Mun, C.-W.; Cho, B.; Kim, C.S.; Kim, D.-H. Fabrication of 3D ZnO hollow shell structures by prism holographic lithography and atomic layer deposition. J. Mater. Chem. C 2014, 2, 1957–1961. [Google Scholar] [CrossRef]
- Park, S.-G.; Lee, S.-K.; Moon, J.H.; Yang, S.-M. Holographic fabrication of three-dimensional nanostructures for microfluidic passive mixing. Lab Chip 2009, 9, 3144. [Google Scholar] [CrossRef]
- Park, S.-G.; Moon, J.H.; Lee, S.-K.; Shim, J.; Yang, S.-M. Bioinspired Holographically Featured Superhydrophobic and Supersticky Nanostructured Materials. Langmuir 2010, 26, 1468–1472. [Google Scholar] [CrossRef]
- Park, S.-G.; Miyake, M.; Yang, S.-M.; Braun, P.V. Cu2O Inverse Woodpile Photonic Crystals by Prism Holographic Lithography and Electrodeposition. Adv. Mater. 2011, 23, 2749–2752. [Google Scholar] [CrossRef]
- Kim, A.; Barcelo, S.J.; Li, Z. SERS-based pesticide detection by using nanofinger sensors. Nanotechnology 2014, 26, 15502. [Google Scholar] [CrossRef] [PubMed]
- Ou, F.S.; Hu, M.; Naumov, I.; Kim, A.; Wu, W.; Bratkovsky, A.M.; Li, X.; Williams, R.S.; Li, Z. Hot-Spot Engineering in Polygonal Nanofinger Assemblies for Surface Enhanced Raman Spectroscopy. Nano Lett. 2011, 11, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ou, F.S.; Ohlberg, D.A.A.; Hu, M.; Williams, R.S.; Li, Z. Study of Molecular Trapping Inside Gold Nanofinger Arrays on Surface-Enhanced Raman Substrates. J. Am. Chem. Soc. 2011, 133, 8234–8239. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Barcelo, S.J.; Williams, R.S.; Li, Z. Melamine Sensing in Milk Products by Using Surface Enhanced Raman Scattering. Anal. Chem. 2012, 84, 9303–9309. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, K.; Wang, P.; He, L.; Carter, K.; Nugen, S.R. Nanoimprinted Patterned Pillar Substrates for Surface-Enhanced Raman Scattering Applications. ACS Appl. Mater. Interfaces 2015, 7, 22106–22113. [Google Scholar] [CrossRef]
- Chou, S.Y.; Krauss, P.R.; Renstrom, P.J. Imprint of sub-25 nm vias and trenches in polymers. Appl. Phys. Lett. 1995, 67, 3114–3116. [Google Scholar] [CrossRef]
- Hsu, P.-H.; Chiang, H.K. Surface-enhanced Raman spectroscopy for quantitative measurement of lactic acid at physiological concentration in human serum. J. Raman Spectrosc. 2010, 41, 1610–1614. [Google Scholar] [CrossRef]
- Dalvi, V.; Rossky, P.J. Molecular origins of fluorocarbon hydrophobicity. Proc. Natl. Acad. Sci. USA 2010, 107, 13603–13607. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Campbell, C.T. Ultrathin metal films and particles on oxide surfaces: Structural, electronic and chemisorptive properties. Surf. Sci. Rep. 1997, 27, 1–111. [Google Scholar] [CrossRef]
- Lee, S.-K.; Park, S.-G.; Moon, J.H.; Yang, S.-M. Holographic fabrication of photonic nanostructures for optofluidic integration. Lab Chip 2008, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Mun, C.; Kim, D.-H.; Chang, S.-C.; Park, S.-G. Analyte-concentrating 3D hybrid plasmonic nanostructures for use in highly sensitive chemical sensors. RSC Adv. 2016, 6, 92120–92126. [Google Scholar] [CrossRef]
- Fletcher, W.M.; Hopkins, F.G. Lactic acid in amphibian muscle1. J. Physiol. 1907, 35, 247–309. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Mun, C.; Choi, D.-G.; Jung, H.S.; Kim, D.-H.; Kim, S.-H.; Park, S.-G. Quasi-3D Plasmonic Nanowell Array for Molecular Enrichment and SERS-Based Detection. Nanomaterials 2020, 10, 939. https://doi.org/10.3390/nano10050939
Kim S, Mun C, Choi D-G, Jung HS, Kim D-H, Kim S-H, Park S-G. Quasi-3D Plasmonic Nanowell Array for Molecular Enrichment and SERS-Based Detection. Nanomaterials. 2020; 10(5):939. https://doi.org/10.3390/nano10050939
Chicago/Turabian StyleKim, Sunho, Chaewon Mun, Dae-Geun Choi, Ho Sang Jung, Dong-Ho Kim, Shin-Hyun Kim, and Sung-Gyu Park. 2020. "Quasi-3D Plasmonic Nanowell Array for Molecular Enrichment and SERS-Based Detection" Nanomaterials 10, no. 5: 939. https://doi.org/10.3390/nano10050939
APA StyleKim, S., Mun, C., Choi, D.-G., Jung, H. S., Kim, D.-H., Kim, S.-H., & Park, S.-G. (2020). Quasi-3D Plasmonic Nanowell Array for Molecular Enrichment and SERS-Based Detection. Nanomaterials, 10(5), 939. https://doi.org/10.3390/nano10050939





