Thickness Optimization of Highly Porous Flame-Aerosol Deposited WO3 Films for NO2 Sensing at ppb
Abstract
1. Introduction
2. Materials and Methods
2.1. Particle and Sensor Film Fabrication
2.2. Powder and Film Characterization
2.3. Gas Sensor Characterization
3. Results and Discussion
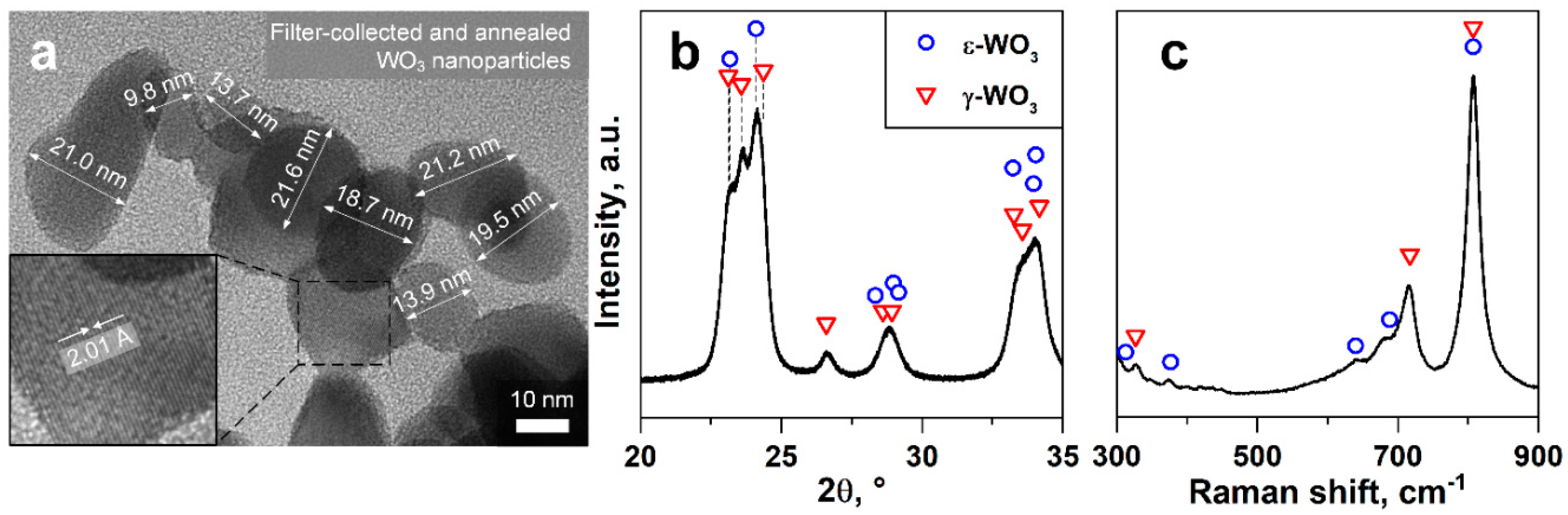
3.1. WO3 Nanoparticle Characterization
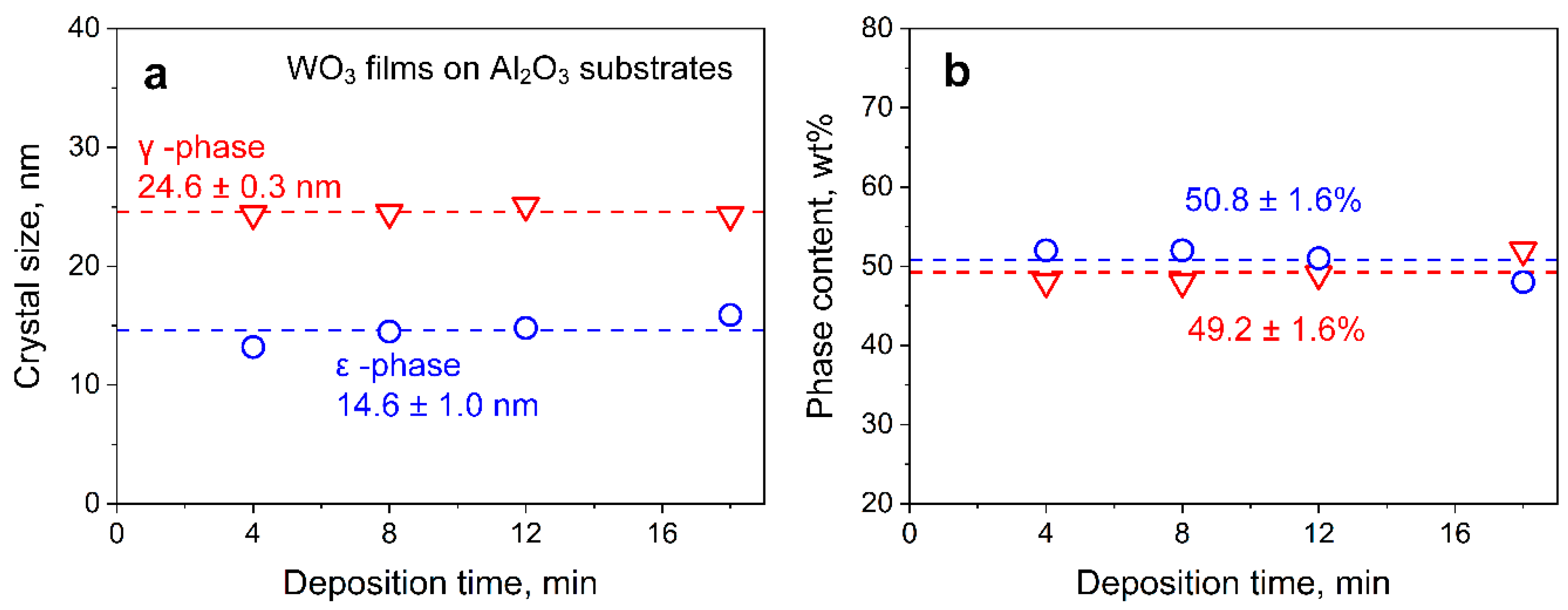
3.2. Film Characterization
3.3. Effects of Film Thickness on NO2 Sensing
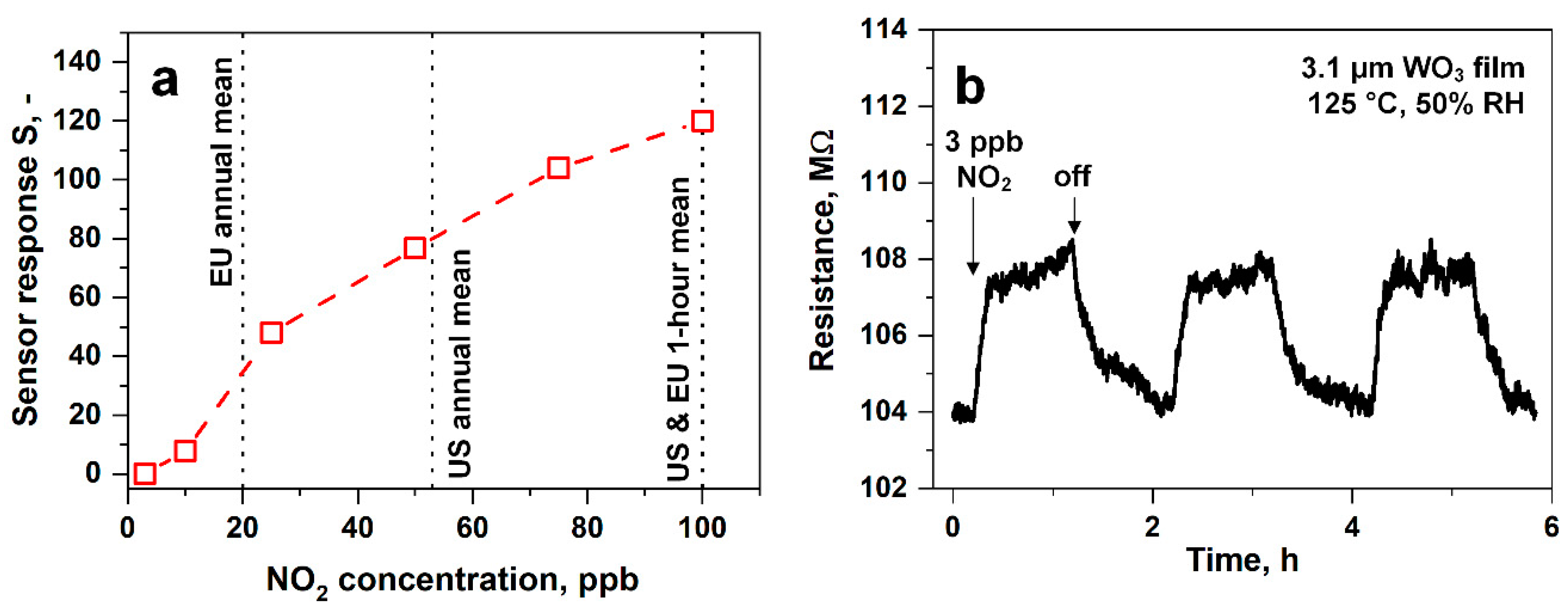
3.4. Low-ppb NO2 Sensing
3.5. NO2 Selectivity in Realistic Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beloconi, A.; Vounatsou, P. Bayesian geostatistical modelling of high-resolution NO2 exposure in Europe combining data from monitors, satellites and chemical transport models. Environ. Int. 2020, 138, 105578. [Google Scholar] [CrossRef]
- Robinson, E.; Robbins, R.C. Gaseous nitrogen compound pollutants from urban and natural sources. J. Air Pollut. Control Assoc. 1970, 20, 303–306. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Integrated Science Assessment for Oxides of Nitrogen—Health Criteria EPA/600/R-15/068; U.S. Environmental Protection Agency: Washington, DC, USA, 2016.
- Shima, M.; Adachi, M. Effect of outdoor and indoor nitrogen dioxide on respiratory symptoms in schoolchildren. Int. J. Epidemiol. 2000, 29, 862–870. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the Council of the European Union. Directive 2008/50/EC. Off. J. Eur. Union 2008, 51, 1–44. [Google Scholar]
- Rigby, M.; Park, S.; Saito, T.; Western, L.M.; Redington, A.L.; Fang, X.; Henne, S.; Manning, A.J.; Prinn, R.G.; Dutton, G.S.; et al. Increase in CFC-11 emissions from eastern China based on atmospheric observations. Nature 2019, 569, 546–550. [Google Scholar] [CrossRef]
- Popoola, O.A.; Carruthers, D.; Lad, C.; Bright, V.B.; Mead, M.I.; Stettler, M.E.; Saffell, J.R.; Jones, R.L. Use of networks of low cost air quality sensors to quantify air quality in urban settings. Atmos. Environ. 2018, 194, 58–70. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, L.; Li, Q.; Du, Y.; Ruan, S. Reduced graphene oxide/α-Fe2O3 hybrid nanocomposites for room temperature NO2 sensing. Sens. Actuators B 2017, 241, 109–115. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhang, Y.; Zhang, C.; Zhang, T. High performance room temperature NO2 sensors based on reduced graphene oxide-multiwalled carbon nanotubes-tin oxide nanoparticles hybrids. Sens. Actuators B 2015, 211, 318–324. [Google Scholar] [CrossRef]
- Koo, W.T.; Cha, J.H.; Jung, J.W.; Choi, S.J.; Jang, J.S.; Kim, D.H.; Kim, I.D. Few-layered WS2 nanoplates confined in Co, n-doped hollow carbon nanocages: Abundant WS2 edges for highly sensitive gas sensors. Adv. Funct. Mater. 2018, 28, 1802575. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, Z.; Ye, Z.; Zhu, L. Ultrasensitive ppb-level NO2 gas sensor based on WO3 hollow nanosphers doped with Fe. Appl. Surf. Sci. 2018, 434, 891–897. [Google Scholar] [CrossRef]
- Inyawilert, K.; Channei, D.; Tamaekong, N.; Liewhiran, C.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Pt-doped In2O3 nanoparticles prepared by flame spray pyrolysis for NO2 sensing. J. Nanopart. Res. 2016, 18. [Google Scholar] [CrossRef]
- Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. NO and NO2 sensing properties of WO3 and Co3O4 based gas sensors. Sensors 2013, 13, 12467–12481. [Google Scholar] [CrossRef] [PubMed]
- Casals, O.; Markiewicz, N.; Fabrega, C.; Gràcia, I.; Cané, C.; Wasisto, H.S.; Waag, A.; Prades, J.D. A parts per billion (ppb) sensor for NO2 with microwatt (μW) power requirements based on micro light plates. ACS Sens. 2019, 4, 822–826. [Google Scholar] [CrossRef]
- Mane, A.; Moholkar, A. Effect of film thickness on NO2 gas sensing properties of sprayed orthorhombic nanocrystalline V2O5 thin films. Appl. Surf. Sci. 2017, 416, 511–520. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Kong, F.; Wang, S.; Zhu, B.; Guo, X.; Zhang, J.; Wang, Y.; Wu, S. Au-doped WO3-based sensor for NO2 detection at low operating temperature. Sens. Actuators B 2008, 134, 133–139. [Google Scholar] [CrossRef]
- Simon, I.; Barsan, N.; Bauer, M.; Weimar, U. Micromachined metal oxide gas sensors: Opportunities to improve sensor performance. Sens. Actuators B 2001, 73, 1–26. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Göpel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Cantalini, C.; Lozzi, L.; Passacantando, M.; Santucci, S. The comparative effect of two different annealing temperatures and times on the sensitivity and long-term stability of WO3 thin films for detecting NO2. IEEE Sens. J. 2003, 3, 171–179. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yoon, J.-W.; Hong, Y.J.; Kang, Y.C.; Abdel-Hady, F.; Wazzan, A.; Lee, J.-H. Highly sensitive and selective detection of ppb-level NO2 using multi-shelled WO3 yolk–shell spheres. Sens. Actuators B 2016, 229, 561–569. [Google Scholar] [CrossRef]
- You, L.; He, X.; Wang, D.; Sun, P.; Sun, Y.; Liang, X.; Du, Y.; Lu, G. Ultrasensitive and low operating temperature NO2 gas sensor using nanosheets assembled hierarchical WO3 hollow microspheres. Sens. Actuators B 2012, 173, 426–432. [Google Scholar] [CrossRef]
- Prajapati, C.S.; Bhat, N. ppb level detection of NO2 using a WO3 thin film-based sensor: Material optimization, device fabrication and packaging. RSC Adv. 2018, 8, 6590–6599. [Google Scholar] [CrossRef]
- Han, D.; Zhai, L.; Gu, F.; Wang, Z. Highly sensitive NO2 gas sensor of ppb-level detection based on In2O3 nanobricks at low temperature. Sens. Actuators B 2018, 262, 655–663. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Z.; Tian, M.; Miao, J.; Zhang, H.; Sun, J. UV excitation NO2 gas sensor sensitized by ZnO quantum dots at room temperature. Sens. Actuators B 2018, 259, 526–531. [Google Scholar] [CrossRef]
- Lu, G.; Xu, J.; Sun, J.; Yu, Y.; Zhang, Y.; Liu, F. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuators B 2012, 162, 82–88. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Han, T.; Fei, T.; Liu, S.; Lu, G. Oxygen vacancy engineering for enhanced sensing performances: A case of SnO2 nanoparticles-reduced graphene oxide hybrids for ultrasensitive ppb-level room-temperature NO2 sensing. Sens. Actuators B 2018, 266, 812–822. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R Rep. 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Kida, T.; Nishiyama, A.; Hua, Z.; Suematsu, K.; Yuasa, M.; Shimanoe, K. WO3 nanolamella gas sensor: Porosity control using SnO2 nanoparticles for enhanced NO2 sensing. Langmuir 2014, 30, 2571–2579. [Google Scholar] [CrossRef]
- Gao, H.; Jia, H.; Bierer, B.; Wöllenstein, J.; Lu, Y.; Palzer, S. Scalable gas sensors fabrication to integrate metal oxide nanoparticles with well-defined shape and size. Sens. Actuators B 2017, 249, 639–646. [Google Scholar] [CrossRef]
- Ueda, T.; Maeda, T.; Huang, Z.; Higuchi, K.; Izawa, K.; Kamada, K.; Hyodo, T.; Shimizu, Y. Enhancement of methylmercaptan sensing response of WO3 semiconductor gas sensors by gas reactivity and gas diffusivity. Sens. Actuators B 2018, 273, 826–833. [Google Scholar] [CrossRef]
- Liewhiran, C.; Phanichphant, S. Influence of thickness on ethanol sensing characteristics of doctor-bladed thick film from flame-made ZnO nanoparticles. Sensors 2007, 7, 185–201. [Google Scholar] [CrossRef]
- Mädler, L.; Roessler, A.; Pratsinis, S.E.; Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles. Sens. Actuators B 2006, 114, 283–295. [Google Scholar] [CrossRef]
- Blattmann, C.O.; Güntner, A.T.; Pratsinis, S.E. In situ monitoring the deposition of flame-made chemoresistive gas sensing films. ACS Appl. Mater. Interfaces 2017, 9, 23926–23933. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Teleki, A.; Pratsinis, S.; Gouma, P. Ferroelectric WO3 nanoparticles for acetone selective detection. Chem. Mater. 2008, 20, 4794–4796. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Thermally stable, silica-doped epsilon-WO3 for sensing of acetone in the human breath. Chem. Mater. 2010, 22, 3152–3157. [Google Scholar] [CrossRef]
- Güntner, A.T.; Sievi, N.A.; Theodore, S.J.; Gulich, T.; Kohler, M.; Pratsinis, S.E. Noninvasive body fat burn monitoring from exhaled acetone with Si-doped WO3-sensing nanoparticles. Anal. Chem. 2017, 89, 10578–10584. [Google Scholar] [CrossRef]
- Güntner, A.T.; Kompalla, J.F.; Landis, H.; Theodore, S.; Geidl, B.; Sievi, N.; Kohler, M.; Pratsinis, S.E.; Gerber, P. Guiding ketogenic diet with breath acetone sensors. Sensors 2018, 18, 3655. [Google Scholar] [CrossRef]
- Pineau, N.J.; Kompalla, J.F.; Güntner, A.T.; Pratsinis, S.E. Orthogonal gas sensor arrays by chemoresistive material design. Microchim. Acta 2018, 185, 563. [Google Scholar] [CrossRef]
- Güntner, A.T.; Pineau, N.J.; Mochalski, P.; Wiesenhofer, H.; Agapiou, A.; Mayhew, C.A.; Pratsinis, S.E. Sniffing entrapped humans with sensor arrays. Anal. Chem. 2018, 90, 4940–4945. [Google Scholar] [CrossRef]
- Mädler, L.; Sahm, T.; Gurlo, A.; Grunwaldt, J.-D.; Barsan, N.; Weimar, U.; Pratsinis, S.E. Sensing low concentrations of CO using flame-spray-made Pt/SnO2 nanoparticles. J. Nanopart. Res. 2006, 8, 783–796. [Google Scholar] [CrossRef]
- Abegg, S.; Magro, L.; van den Broek, J.; Pratsinis, S.E.; Güntner, A.T. A pocket-sized device enables detection of methanol adulteration in alcoholic beverages. Nat. Food 2020, accepted. [Google Scholar] [CrossRef]
- Huelser, T.; Lorke, A.; Ifeacho, P.; Wiggers, H.; Schulz, C. Core and grain boundary sensitivity of tungsten-oxide sensor devices by molecular beam assisted particle deposition. J. Appl. Phys. 2007, 102, 124305. [Google Scholar] [CrossRef]
- Güntner, A.T.; Koren, V.; Chikkadi, K.; Righettoni, M.; Pratsinis, S.E. E-nose sensing of low-ppb formaldehyde in gas mixtures at high relative humidity for breath screening of lung cancer? ACS Sens. 2016, 1, 528–535. [Google Scholar] [CrossRef]
- Pineau, N.J.; Keller, S.D.; Güntner, A.T.; Pratsinis, S.E. Palladium embedded in SnO2 enhances the sensitivity of flame-made chemoresistive gas sensors. Microchim. Acta 2020, 187, 96. [Google Scholar] [CrossRef]
- You, L.; Sun, Y.; Ma, J.; Guan, Y.; Sun, J.; Du, Y.; Lu, G. Highly sensitive NO2 sensor based on square-like tungsten oxide prepared with hydrothermal treatment. Sens. Actuators B 2011, 157, 401–407. [Google Scholar] [CrossRef]
- Koppmann, R. (Ed.) Volatile Organic Compounds in the Atmosphere; Blackwell Publishing Ltd: Oxford, UK, 2007. [Google Scholar]
- Datasheet MSGS 5000i. Available online: http://microsens.ch/products/pdf/MSGS_5000i_Datasheet.pdf (accessed on 19 May 2020).
- van den Broek, J.; Abegg, S.; Pratsinis, S.E.; Güntner, A.T. Highly selective detection of methanol over ethanol by a handheld gas sensor. Nat. Commun. 2019, 10, 4220. [Google Scholar] [CrossRef]
- Tricoli, A.; Graf, M.; Mayer, F.; Kühne, S.; Hierlemann, A.; Pratsinis, S.E. Micropatterning layers by flame aerosol deposition-annealing. Adv. Mater. 2008, 20, 3005–3010. [Google Scholar] [CrossRef]
- Güntner, A.T.; Pineau, N.J.; Chie, D.; Krumeich, F.; Pratsinis, S.E. Selective sensing of isoprene by Ti-doped ZnO for breath diagnostics. J. Mater. Chem. B 2016, 4, 5358–5366. [Google Scholar] [CrossRef]
- Hubbell, J. Photon mass attenuation and energy-absorption coefficients. Int. J. Appl. Radiat. Isot. 1982, 33, 1269–1290. [Google Scholar] [CrossRef]
- Berger, M.; Hubbell, J.; Seltzer, S.; Chang, J.; Coursey, J.; Sukumar, R.; Zucker, D.; Olsen, K. XCOM: Photon cross Sections Database, NIST Standard Reference Database 8 (XGAM). Available online: http://physics.nist.gov/PhysRefData/Xcom/Text/XCOM.html (accessed on 30 April 2019).
- Güntner, A.T.; Righettoni, M.; Pratsinis, S.E. Selective sensing of NH3 by Si-doped alpha-MoO3 for breath analysis. Sens. Actuators B 2016, 223, 266–273. [Google Scholar] [CrossRef]
- Güntner, A.T.; Wied, M.; Pineau, N.J.; Pratsinis, S.E. Rapid and selective NH3 sensing by porous CuBr. Adv. Sci. 2020, 7, 1903390. [Google Scholar] [CrossRef]
- Sari, W.P.; Leigh, S.; Covington, J. Tungsten oxide based sensor for oxygen detection. Proceedings 2018, 2, 952. [Google Scholar] [CrossRef]
- Arai, M.; Hayashi, S.; Yamamoto, K.; Kim, S.S. Raman studies of phase-transitions in gas-evaporated WO3 microcrystals. Solid State Commun. 1990, 75, 613–616. [Google Scholar] [CrossRef]
- Strobel, R.; Maciejewski, M.; Pratsinis, S.E.; Baiker, A. Unprecedented formation of metastable monoclinic BaCO3 nanoparticles. Thermochim. Acta 2006, 445, 23–26. [Google Scholar] [CrossRef]
- Righettoni, M.; Pratsinis, S.E. Annealing dynamics of WO3 by in situ XRD. Mater. Res. Bull. 2014, 59, 199–204. [Google Scholar] [CrossRef]
- Labidi, A.; Lambert-Mauriat, C.; Jacolin, C.; Bendahan, M.; Maaref, M.; Aguir, K. DC and AC characterizations of WO3 sensors under ethanol vapors. Sens. Actuators B 2006, 119, 374–379. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C.; Gerand, B.; Figlarz, M. Infrared and raman-study of WO3 tungsten trioxides and WO3, xH2O tungsten trioxide hydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Salje, E. Lattice dynamics of WO3. Acta Crystallogr. A 1975, 31, 360–363. [Google Scholar] [CrossRef]
- Viera, G.; Huet, S.; Boufendi, L. Crystal size and temperature measurements in nanostructured silicon using Raman spectroscopy. J. Appl. Phys. 2001, 90, 4175–4183. [Google Scholar] [CrossRef]
- Sun, L.; Westerdahl, D.; Ning, Z. Development and evaluation of a novel and cost-effective approach for low-cost NO2 sensor drift correction. Sensors 2017, 17, 1916. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor gas sensors: Dry synthesis and application. Angew. Chem. Int. Edit. 2010, 49, 7632–7659. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Pratsinis, S.E. Dispersed nanoelectrode devices. Nat. Nanotechnol. 2010, 5, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Diffusion equation-based study of thin film semiconductor gas sensor-response transient. Sens. Actuators B 2002, 83, 216–221. [Google Scholar] [CrossRef]
- Penza, M.; Martucci, C.; Cassano, G. NOx gas sensing characteristics of WO3 thin films activated by noble metals (Pd, Pt, Au) layers. Sens. Actuators B 1998, 50, 52–59. [Google Scholar] [CrossRef]
- Tosun, B.S.; Feist, R.K.; Gunawan, A.; Mkhoyan, K.A.; Campbell, S.A.; Aydil, E.S. Sputter deposition of semicrystalline tin dioxide films. Thin Solid Films 2012, 520, 2554–2561. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamazaki, T.; Yoshioka, H.; Hikichi, K. Thickness dependence of H2 gas sensor in amorphous SnOx films prepared by ion-beam sputtering. J. Mater. Sci. 1988, 23, 145–149. [Google Scholar] [CrossRef]
- Rosental, A.; Tarre, A.; Gerst, A.; Sundqvist, J.; Hårsta, A.; Aidla, A.; Aarik, J.; Sammelselg, V.; Uustare, T. Gas sensing properties of epitaxial SnO2 thin films prepared by atomic layer deposition. Sens. Actuators B 2003, 93, 552–555. [Google Scholar] [CrossRef]
- Du, X.; George, S. Thickness dependence of sensor response for CO gas sensing by tin oxide films grown using atomic layer deposition. Sens. Actuators B 2008, 135, 152–160. [Google Scholar] [CrossRef]
- Tamaki, J.; Zhang, Z.; Fujimori, K.; Akiyama, M.; Harada, T.; Miura, N.; Yamazoe, N. Grain-size effects in tungsten oxide-based sensor for nitrogen oxides. J. Electrochem. Soc. 1994, 141, 2207–2210. [Google Scholar] [CrossRef]
- Kozuka, H.; Takenaka, S.; Tokita, H.; Hirano, T.; Higashi, Y.; Hamatani, T. Stress and cracks in gel-derived ceramic coatings and thick film formation. J. Sol Gel. Sci. Techn. 2003, 26, 681–686. [Google Scholar] [CrossRef]
- Wu, J.; Feng, S.; Wei, X.; Shen, J.; Lu, W.; Shi, H.; Tao, K.; Lu, S.; Sun, T.; Yu, L.; et al. Facile synthesis of 3D graphene flowers for ultrasensitive and highly reversible gas sensing. Adv. Funct. Mater. 2016, 26, 7462–7469. [Google Scholar] [CrossRef]
- Hoa, N.D.; El-Safty, S.A. Gas nanosensor design packages based on tungsten oxide: Mesocages, hollow spheres, and nanowires. Nanotechnology 2011, 22, 485503. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.W. A non-linear diffusion-reaction model of electrical conduction in semiconductor gas sensors. Sens. Actuators B 1990, 1, 166–170. [Google Scholar] [CrossRef]
- Staerz, A.; Berthold, C.; Russ, T.; Wicker, S.; Weimar, U.; Barsan, N. The oxidizing effect of humidity on WO3 based sensors. Sens. Actuators B 2016, 237, 54–58. [Google Scholar] [CrossRef]
- Xiao, B.; Song, S.; Wang, P.; Zhao, Q.; Chuai, M.; Zhang, M. Promoting effects of Ag on In2O3 nanospheres of sub-ppb NO2 detection. Sens. Actuators B 2017, 241, 489–497. [Google Scholar] [CrossRef]
- NO2-A43F Nitrogen Dioxide Sensor 4-Electrode (Alphasense). Available online: http://www.alphasense.com/WEB1213/wp-content/uploads/2019/09/NO2-A43F.pdf (accessed on 16 December 2019).
- Bai, S.; Ma, Y.; Shu, X.; Sun, J.; Feng, Y.; Luo, R.; Li, D.; Chen, A. Doping metal elements of WO3 for enhancement of NO2-sensing performance at room temperature. Ind. Eng. Chem. Res. 2017, 56, 2616–2623. [Google Scholar] [CrossRef]
- Yang, L.; Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.; Khmelevsky, N.; Gaskov, A. Quasi similar routes of NO2 and NO sensing by nanocrystalline WO3: Evidence by in situ DRIFT spectroscopy. Sensors 2019, 19, 3405. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Hu, M.; Wang, W.; Chen, H.; Qin, Y. NO2-sensing properties of porous WO3 gas sensor based on anodized sputtered tungsten thin film. Sens. Actuators B 2012, 161, 447–452. [Google Scholar] [CrossRef]
- Lampe, U.; Gerblinger, J.; Meixner, H. Comparison of transient response of exhaust-gas sensors based on thin films of selected metal oxides. Sens. Actuators B 1992, 7, 787–791. [Google Scholar] [CrossRef]
- Bevan, M.A.; Proctor, C.J.; Baker-Rogers, J.; Warren, N.D. Exposure to carbon monoxide, respirable suspended particulates and volatile organic compounds while commuting by bicycle. Environ. Sci. Technol. 1991, 25, 788–791. [Google Scholar] [CrossRef]
- Güntner, A.T.; Abegg, S.; Wegner, K.; Pratsinis, S.E. Zeolite membranes for highly selective formaldehyde sensors. Sens. Actuators B 2018, 257, 916–923. [Google Scholar] [CrossRef]
- van den Broek, J.; Güntner, A.T.; Pratsinis, S.E. Highly selective and rapid breath isoprene sensing enabled by activated alumina filter. ACS Sens. 2018, 3, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Güntner, A.T.; Abegg, S.; Königstein, K.; Gerber, P.A.; Schmidt-Trucksäss, A.; Pratsinis, S.E. Breath sensors for health monitoring. ACS Sens. 2019, 4, 268–280. [Google Scholar] [CrossRef] [PubMed]



| Material | RH (%) (TRH (°C)) | LOD (ppb) | Tsensor (°C) | NO2 Selectivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | NH3 | CH4 | MeOH | EtOH | Ace | CO | H2S | FA | |||||
| WO3 | 45 (n/a) | 16 | 150 | 510 | 600 | [23] | |||||||
| WO3 | 80 (25) | 50 | 200 | >105 | 3000 | >104 | [21] | ||||||
| WO3 | 40 (25)c | 40 | 75 | >105 | 7130 | 6240 | [22] | ||||||
| Fe:WO3 | 45 (n/a) | 10 | 120 | 185 | 30 | 20 | 185 | [12] | |||||
| In2O3 | 25 (n/a) | 100 | 50 | 105 | >105 | >105 | >105 | >105 | [24] | ||||
| SnO2/ZnO | 10 (20) | 50 | 40 | 5000 | 4300 | 104 | >104 | 4700 | [25] | ||||
| SnO2/ZnO | 30 (20) | 200 | RT | >105 | >104 | >105 | 6600 | [26] | |||||
| rGO-SnO2 | 25 (n/a) | 50 | RT | 50 | 100 | 110 | [27] | ||||||
| rGO-Fe2O3 | 25 (RT) | 100 | RT | 12 | 10 | 16 | 9 | [8] | |||||
| rGO-CNT-SnO2 | 25 (n/a) | 1000 | RT | 38 | 77 | [9] | |||||||
| WO3 | 50 (23) | 3 | 125 | >104 | >105 | >104 | >105 | >105 | >104 | >105 | 835 | >103 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abegg, S.; Klein Cerrejon, D.; Güntner, A.T.; Pratsinis, S.E. Thickness Optimization of Highly Porous Flame-Aerosol Deposited WO3 Films for NO2 Sensing at ppb. Nanomaterials 2020, 10, 1170. https://doi.org/10.3390/nano10061170
Abegg S, Klein Cerrejon D, Güntner AT, Pratsinis SE. Thickness Optimization of Highly Porous Flame-Aerosol Deposited WO3 Films for NO2 Sensing at ppb. Nanomaterials. 2020; 10(6):1170. https://doi.org/10.3390/nano10061170
Chicago/Turabian StyleAbegg, Sebastian, David Klein Cerrejon, Andreas T. Güntner, and Sotiris E. Pratsinis. 2020. "Thickness Optimization of Highly Porous Flame-Aerosol Deposited WO3 Films for NO2 Sensing at ppb" Nanomaterials 10, no. 6: 1170. https://doi.org/10.3390/nano10061170
APA StyleAbegg, S., Klein Cerrejon, D., Güntner, A. T., & Pratsinis, S. E. (2020). Thickness Optimization of Highly Porous Flame-Aerosol Deposited WO3 Films for NO2 Sensing at ppb. Nanomaterials, 10(6), 1170. https://doi.org/10.3390/nano10061170





