Gas-Phase Formation of Highly Luminescent 2D GaSe Nanoparticle Ensembles in a Nonequilibrium Laser Ablation Process
Abstract
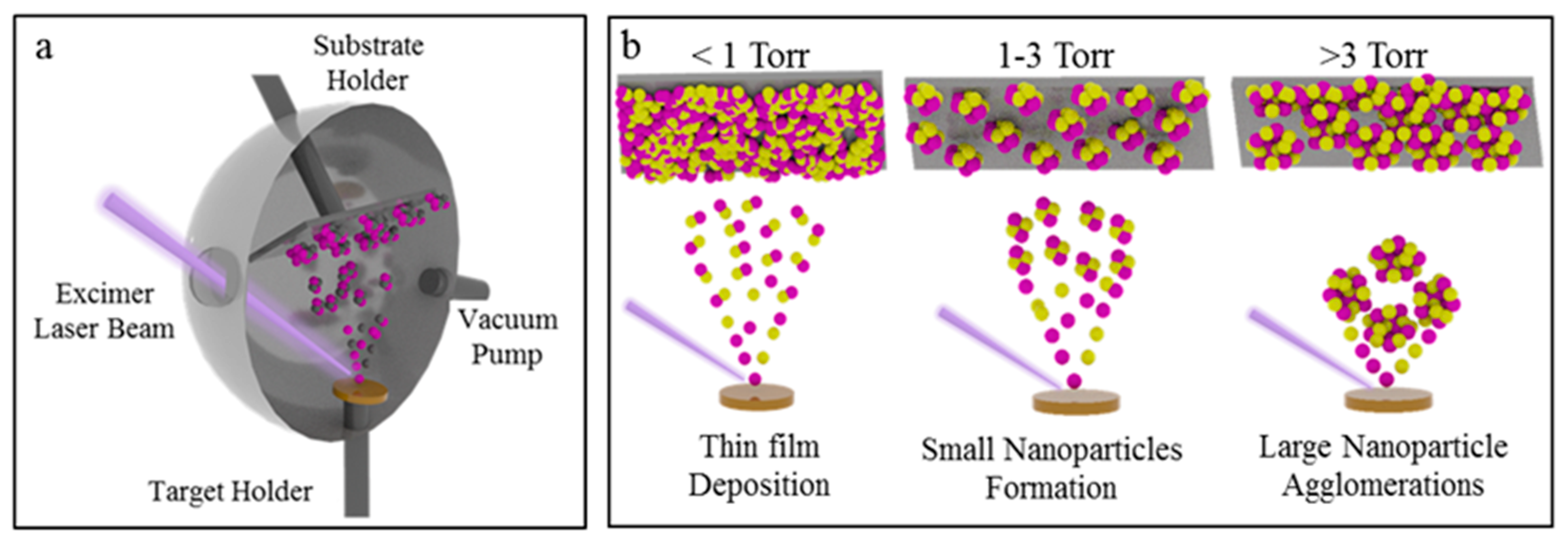
1. Introduction
2. Materials and Methods
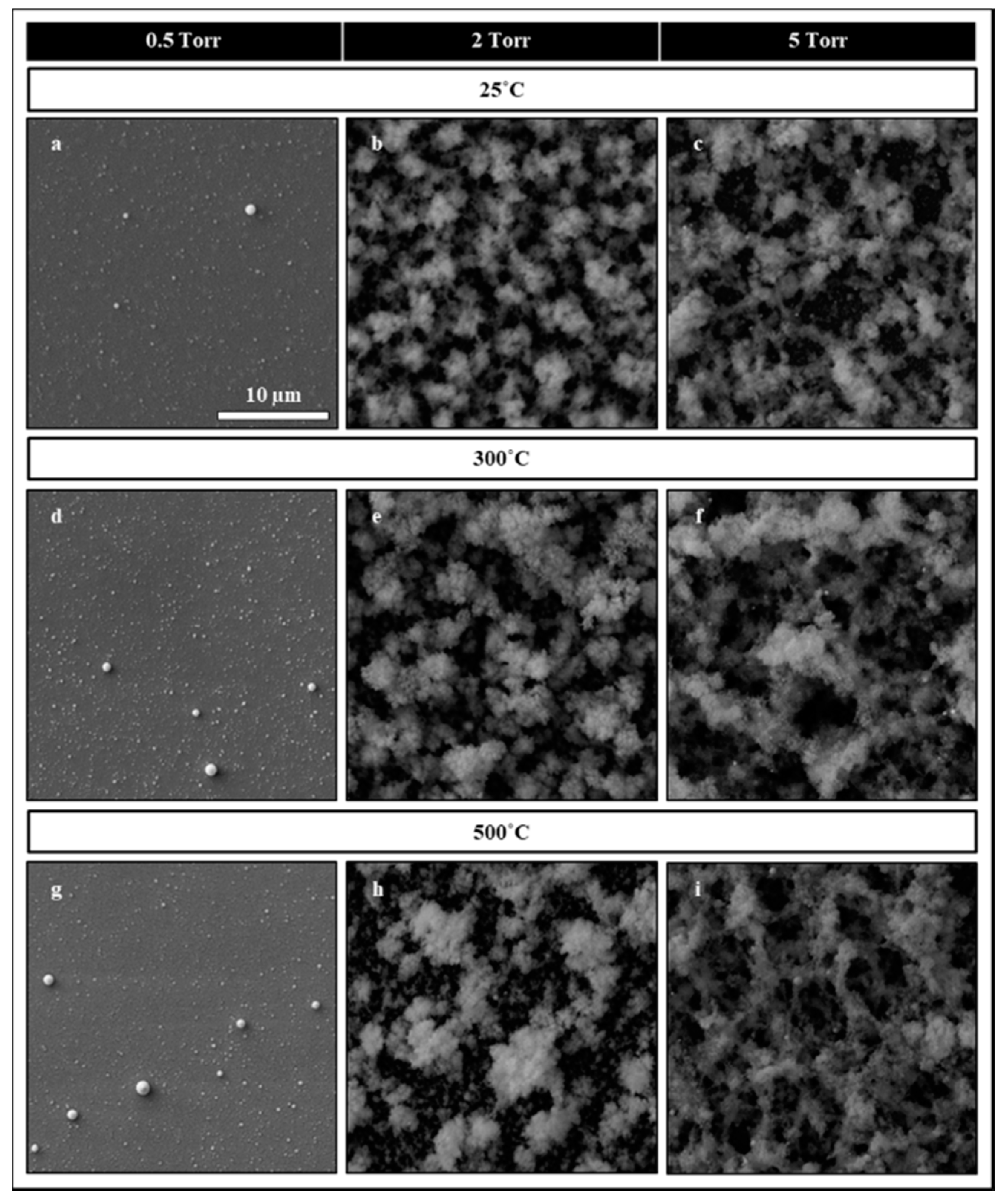
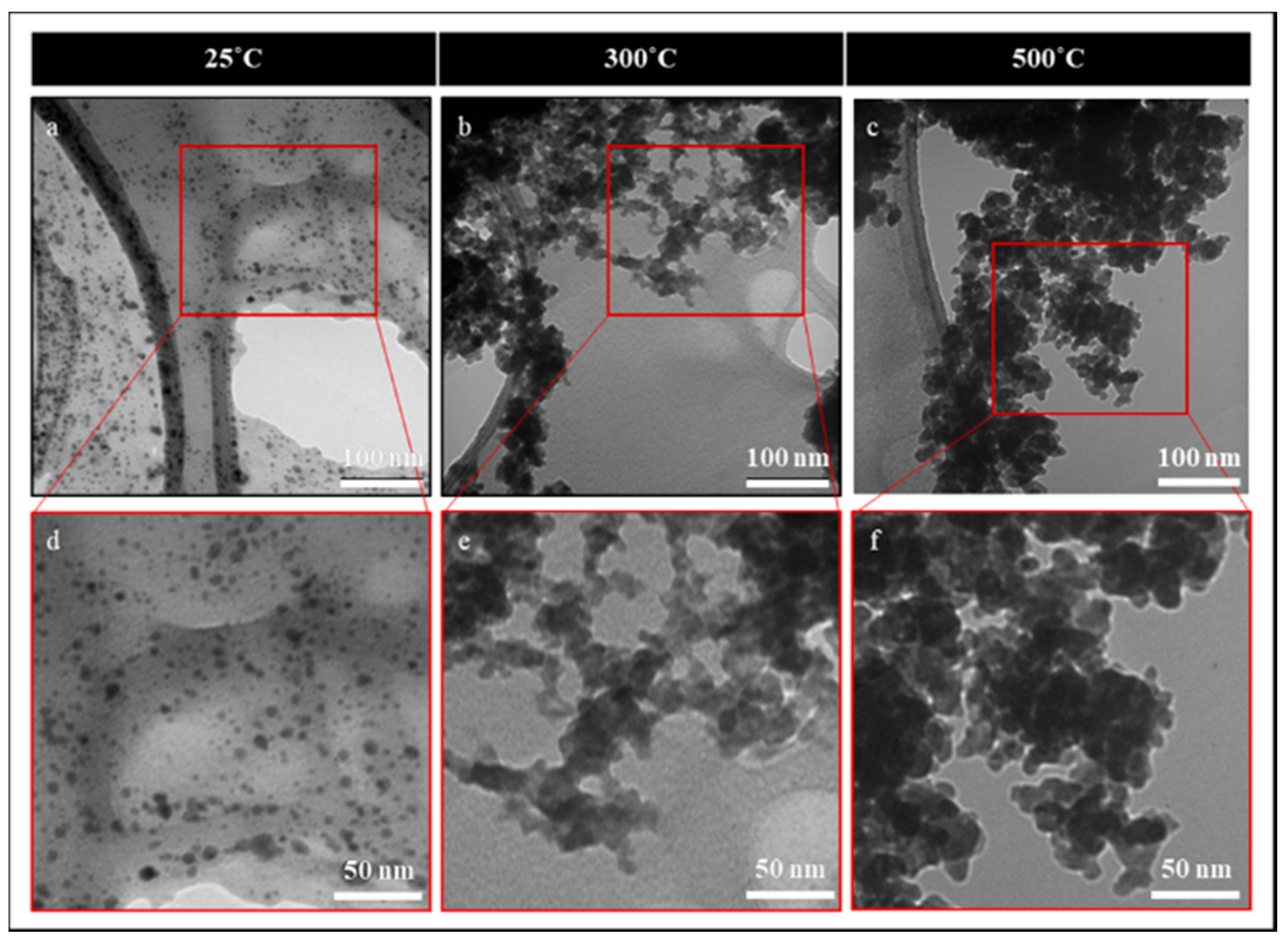
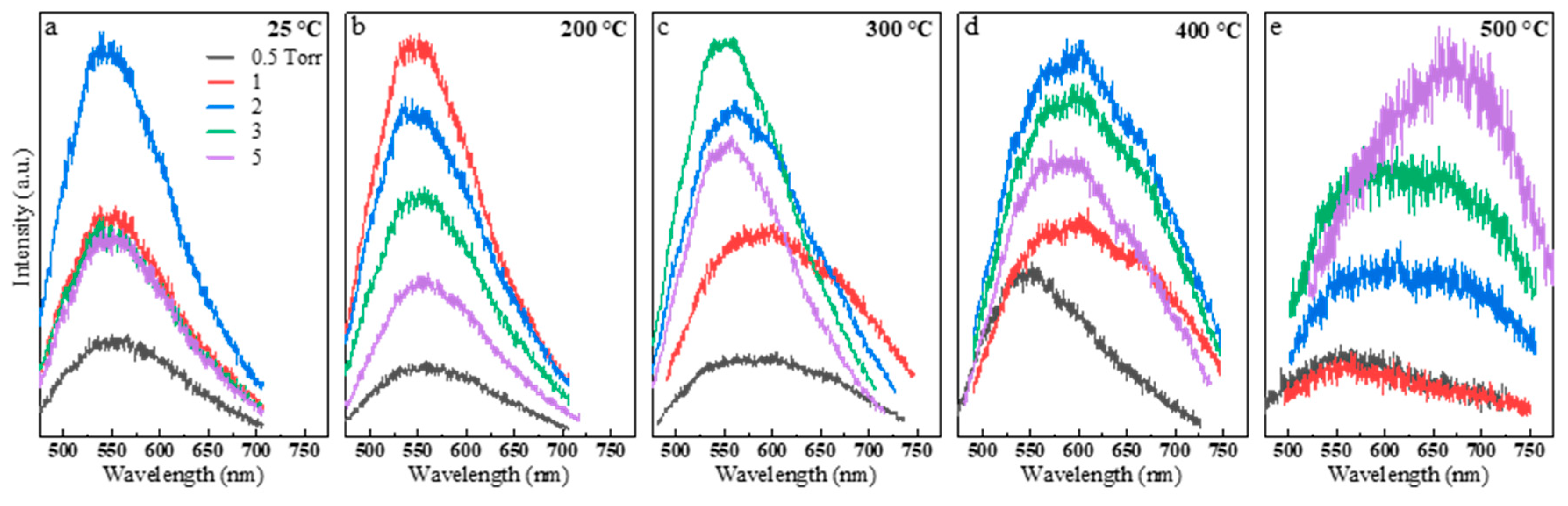
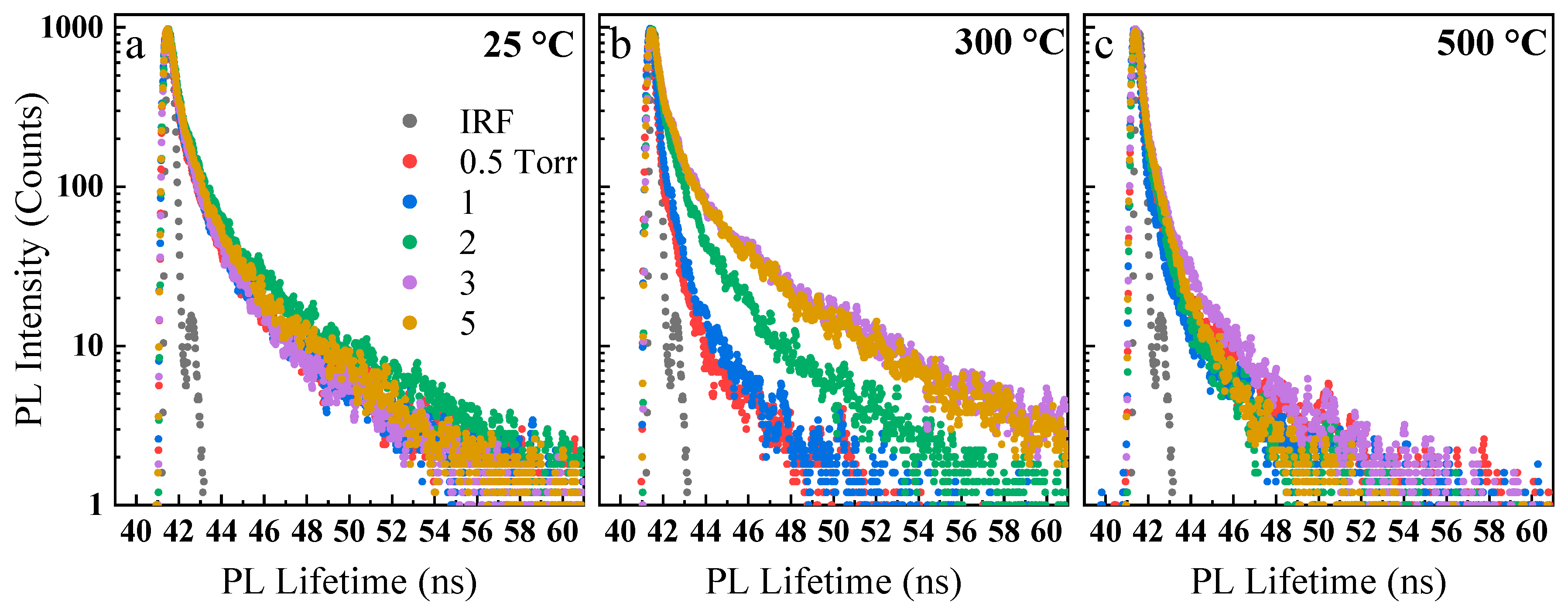
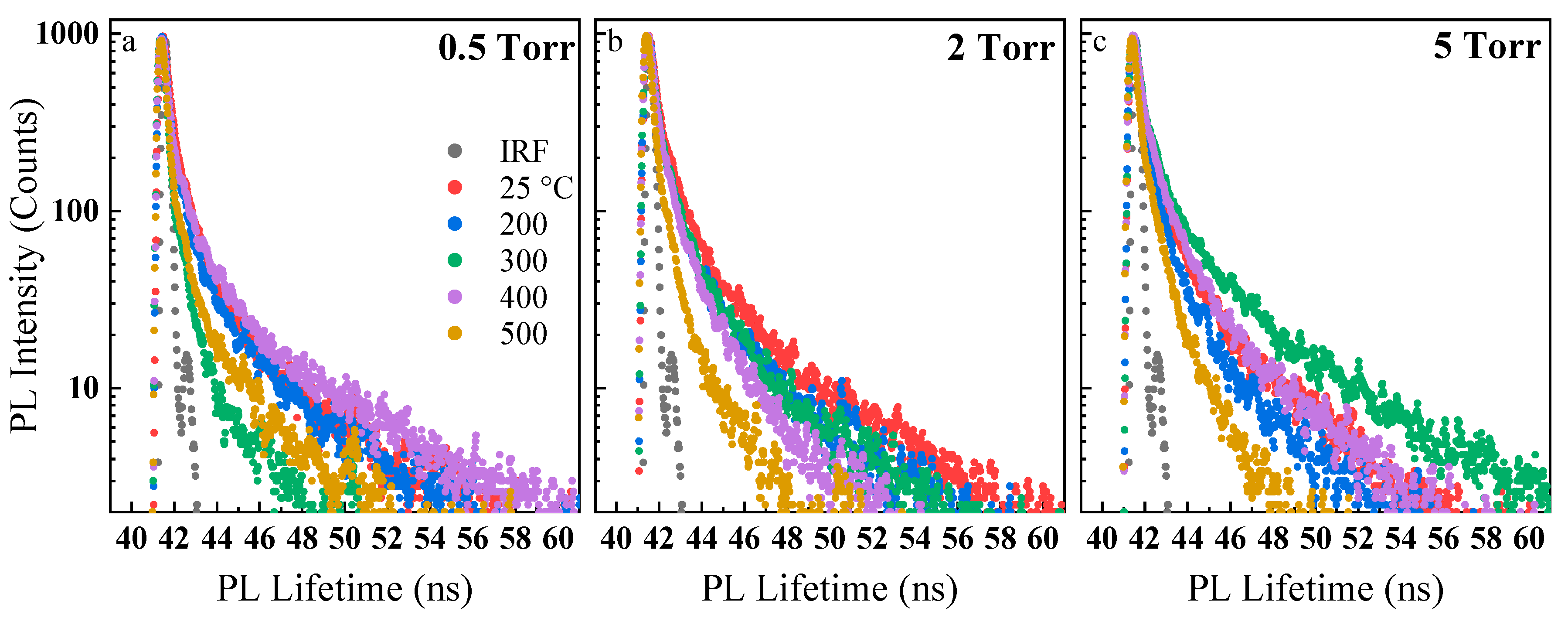
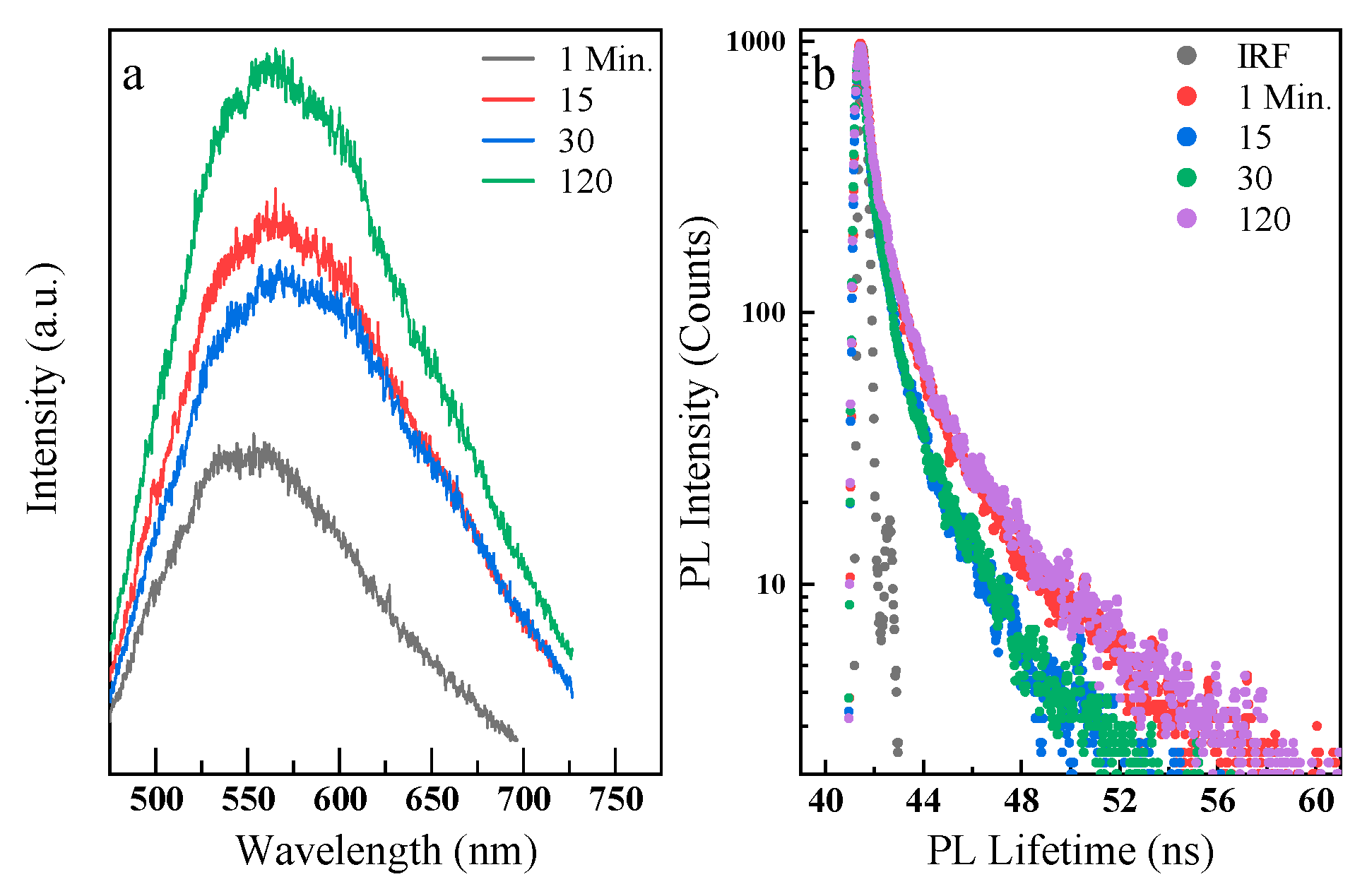
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmadi, Z.; Yakupoglu, B.; Azam, N.; Elafandi, S.; Mahjouri-Samani, M. Application of lasers in the synthesis and processing of two-dimensional quantum materials. J. Laser Appl. 2019, 31, 031202. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Lin, Z.; Meunier, V.; Jung, Y.; Cha, J.; Das, S.; Xiao, D.; Son, Y.; Strano, M.S.; Cooper, V.R.; et al. Recent Advances in Two-Dimensional Materials beyond Graphene. ACS Nano 2015, 9, 11509–11539. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Li, N.; Chen, P. Quantum dots derived from two-dimensional materials and their applications for catalysis and energy. Chem. Soc. Rev. 2016, 45, 2239–2262. [Google Scholar] [CrossRef]
- Gao, M.-R.; Xu, Y.-F.; Jiang, J.; Yu, S.-H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef]
- Azam, N.; Ahmadi, Z.; Yakupoglu, B.; Elafandi, S.; Tian, M.; Boulesbaa, A.; Mahjouri-Samani, M. Accelerated synthesis of atomically-thin 2D quantum materials by a novel laser-assisted synthesis technique. 2D Mater. 2019, 7, 015014. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Yakupoglu, B.; Azam, N.; Elafandi, S.; Mahjouri-Samani, M. Self-limiting laser crystallization and direct writing of 2D materials. Int. J. Extrem. Manuf. 2019, 1, 015001. [Google Scholar] [CrossRef]
- Kumar, A.; Ahluwalia, P.K. Electronic structure of transition metal dichalcogenides monolayers 1H-MX2 (M=Mo, W; X=S, Se, Te) from ab-initio theory: New direct band gap semiconductors. Eur. Phys. J. B 2012, 85, 186. [Google Scholar] [CrossRef]
- Stanford, M.G.; Pudasaini, P.R.; Gallmeier, E.T.; Cross, N.; Liang, L.; Oyedele, A.; Duscher, G.; Mahjouri-Samani, M.; Wang, K.; Xiao, K.; et al. High Conduction Hopping Behavior Induced in Transition Metal Dichalcogenides by Percolating Defect Networks: Toward Atomically Thin Circuits. Adv. Funct. Mater. 2017, 27, 1702829. [Google Scholar] [CrossRef]
- Ye, L.; Li, H.; Chen, Z.; Xu, J. Near-Infrared Photodetector Based on MoS2/Black Phosphorus Heterojunction. ACS Photonics 2016, 3, 692–699. [Google Scholar] [CrossRef]
- Fu, Q.; Yang, L.; Wang, W.; Han, A.; Huang, J.; Du, P.; Fan, Z.; Zhang, J.; Xiang, B. Synthesis and Enhanced Electrochemical Catalytic Performance of Monolayer WS2(1-x)Se2xwith a Tunable Band Gap. Adv. Mater. 2015, 27, 4732–4738. [Google Scholar] [CrossRef] [PubMed]
- Morell, N.; Reserbat-Plantey, A.; Tsioutsios, I.; Schädler, K.G.; Dubin, F.; Koppens, F.H.L.; Bachtold, A. High Quality Factor Mechanical Resonators Based on WSe2 Monolayers. Nano Lett. 2016, 16, 5102–5108. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; et al. Progress, Challenges, and Opportunities in Two-Dimensional Materials Beyond Graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Yi, J. Two-Dimensional Transition Metal Dichalcogenides and Their Charge Carrier Mobilities in Field-Effect Transistors. Nano-Micro Lett. 2017, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.B.; Madhvapathy, S.R.; Sachid, A.B.; Llinas, J.P.; Wang, Q.; Ahn, G.H.; Pitner, G.; Kim, M.J.; Bokor, J.; Hu, C.; et al. MoS2 transistors with 1-nanometer gate lengths. Science 2016, 354, 99–102. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-H.; Kum, H.; Kong, W.; Kim, Y.; Choi, C.; Lee, B.; Lin, P.; Park, Y.; Kim, J. Integration of bulk materials with two-dimensional materials for physical coupling and applications. Nat. Mater. 2019, 18, 550–560. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhang, J.; Wu, J.; Xu, H.; Wen, X.; Zhang, X.; Tiwary, C.S.; Yang, W.; Vajtai, R.; et al. Cryo-mediated exfoliation and fracturing of layered materials into 2D quantum dots. Sci. Adv. 2017, 3, e1701500. [Google Scholar] [CrossRef]
- Huang, H.; Du, C.; Shi, H.; Feng, X.; Li, J.; Tan, Y.; Song, W. Water-Soluble Monolayer Molybdenum Disulfide Quantum Dots with Upconversion Fluorescence. Part. Part. Syst. Charact. 2015, 32, 72–79. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, C.; Wu, J.; Varma, S.J.; Nakanishi, Y.; Aliyan, A.; Martí, A.A.; Wang, Y.; Xie, B.; Kumar, J.; et al. Reflux pretreatment-mediated sonication: A new universal route to obtain 2D quantum dots. Mater. Today 2019, 22, 17–24. [Google Scholar] [CrossRef]
- Xu, Q.; Cai, W.; Li, W.; Sreeprasad, T.S.; He, Z.; Ong, W.-J.; Li, N. Two-dimensional quantum dots: Fundamentals, photoluminescence mechanism and their energy and environmental applications. Mater. Today Energy 2018, 10, 222–240. [Google Scholar] [CrossRef]
- Li, B.L.; Setyawati, M.I.; Zou, H.L.; Dong, J.X.; Luo, H.Q.; Li, N.B.; Leong, D.T. Emerging 0D Transition-Metal Dichalcogenides for Sensors, Biomedicine, and Clean Energy. Small 2017, 13, 1700527. [Google Scholar] [CrossRef]
- Ding, X.; Peng, F.; Zhou, J.; Gong, W.; Slaven, G.; Loh, K.P.; Lim, C.T.; Leong, D.T. Defect engineered bioactive transition metals dichalcogenides quantum dots. Nat. Commun. 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Sarikhani, N.; Zirak, M.; Zhang, H.; Zhang, H.-L.; Moshfegh, A.Z. Group 6 transition metal dichalcogenide nanomaterials: Synthesis, applications and future perspectives. Nanoscale Horiz. 2018, 3, 90–204. [Google Scholar] [CrossRef]
- Yang, L.; Xie, C.; Jin, J.; Ali, R.; Feng, C.; Liu, P.; Xiang, B. Properties, Preparation and Applications of Low Dimensional Transition Metal Dichalcogenides. Nanomaterials 2018, 8, 463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ni, Y. Molybdenum Disulfide Quantum Dots as a Photoluminescence Sensing Platform for 2,4,6-Trinitrophenol Detection. Anal. Chem. 2014, 86, 7463–7470. [Google Scholar] [CrossRef]
- Gu, W.; Yan, Y.; Zhang, C.; Ding, C.; Xian, Y. One-Step Synthesis of Water-Soluble MoS2Quantum Dots via a Hydrothermal Method as a Fluorescent Probe for Hyaluronidase Detection. ACS Appl. Mater. Interfaces 2016, 8, 11272–11279. [Google Scholar] [CrossRef]
- Dong, H.; Tang, S.; Hao, Y.; Yu, H.; Dai, W.; Zhao, G.; Cao, Y.; Lu, H.; Zhang, X.; Ju, H. Fluorescent MoS2 Quantum Dots: Ultrasonic Preparation, Up-Conversion and Down-Conversion Bioimaging, and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2016, 8, 3107–3114. [Google Scholar] [CrossRef]
- Zhang, S.; Jia, X.; Wang, E. Facile synthesis of optical pH-sensitive molybdenum disulfide quantum dots. Nanoscale 2016, 8, 15152–15157. [Google Scholar] [CrossRef]
- Dhanabalan, S.C.; Dhanabalan, B.; Ponraj, J.S.; Bao, Q.; Zhang, H. 2D-Materials-Based Quantum Dots: Gateway Towards Next-Generation Optical Devices. Adv. Opt. Mater. 2017, 5, 1700257. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y.; et al. MoS2 Quantum Dot Growth Induced by S Vacancies in a ZnIn2S4 Monolayer: Atomic-Level Heterostructure for Photocatalytic Hydrogen Production. ACS Nano 2017, 12, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, A.; Bayan, S.; Gogurla, N.; Midya, A.; Ray, S.K. Highly Luminescent WS2 Quantum Dots/ZnO Heterojunctions for Light Emitting Devices. ACS Appl. Mater. Interfaces 2016, 9, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.H.; Irannejad, M.; Wales, B.; Sanderson, J.; Yavuz, M.; Musselman, K.P. Simultaneous Fabrication and Functionalization of Nanoparticles of 2D Materials with Hybrid Optical Properties. Adv. Opt. Mater. 2018, 6, 1701365. [Google Scholar] [CrossRef]
- Caigas, S.P.; Cheng, M.-C.; Lin, T.-N.; Santiago, S.R.M.S.; Yuan, C.-T.; Yang, C.-C.; Chou, W.-C.; Shen, J.-L. P-Type Doping of WS2 Quantum Dots via Pulsed Laser Ablation. ACS Photonics 2018, 5, 4828–4837. [Google Scholar] [CrossRef]
- Lin, L.; Xu, Y.; Zhang, S.; Ross, I.M.; Ong, A.C.M.; Allwood, D.A. Fabrication of Luminescent Monolayered Tungsten Dichalcogenides Quantum Dots with Giant Spin-Valley Coupling. ACS Nano 2013, 7, 8214–8223. [Google Scholar] [CrossRef]
- Vikraman, D.; Akbar, K.; Hussain, S.; Yoo, G.; Jang, J.-Y.; Chun, S.-H.; Jung, J.; Park, H.J. Direct synthesis of thickness-tunable MoS2 quantum dot thin layers: Optical, structural and electrical properties and their application to hydrogen evolution. Nano Energy 2017, 35, 101–114. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 Quantum Dot-Interspersed Exfoliated MoS2 Nanosheets. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef]
- Štengl, V.; Henych, J. Strongly luminescent monolayered MoS2 prepared by effective ultrasound exfoliation. Nanoscale 2013, 5, 3387–3394. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, C.; Gu, W.; Ding, C.; Li, X.; Xian, Y. Facile Synthesis of Water-Soluble WS2 Quantum Dots for Turn-On Fluorescent Measurement of Lipoic Acid. J. Phys. Chem. C 2016, 120, 12170–12177. [Google Scholar] [CrossRef]
- Xu, S.; Li, D.; Wu, P. One-Pot, Facile, and Versatile Synthesis of Monolayer MoS2/WS2Quantum Dots as Bioimaging Probes and Efficient Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Zhang, W.L.; Lv, F.; Guo, S. Recent progress in two-dimensional inorganic quantum dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Fan, P.; Ke, X.; Xu, Y.; Huang, K.; Wei, H.; Yu, W.; Zhang, H.; Zhong, M.; Wu, H.; et al. Defective molybdenum sulfide quantum dots as highly active hydrogen evolution electrocatalysts. Nano Res. 2017, 11, 751–761. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, L.; Li, X.; Xu, H. Fabrication of transition metal dichalcogenides quantum dots based on femtosecond laser ablation. Sci. Rep. 2019, 9, 2931. [Google Scholar] [CrossRef]
- Li, B.; Jiang, L.; Li, X.; Ran, P.; Zuo, P.; Wang, A.; Qu, L.; Zhao, Y.; Cheng, Z.; Lu, Y. Preparation of Monolayer MoS2 Quantum Dots using Temporally Shaped Femtosecond Laser Ablation of Bulk MoS2 Targets in Water. Sci. Rep. 2017, 7, 11182. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; He, X.; Zhong, W.; Du, Y. Luminescent monolayer MoS2 quantum dots produced by multi-exfoliation based on lithium intercalation. Appl. Surf. Sci. 2015, 359, 130–136. [Google Scholar] [CrossRef]
- Cao, X.; Ding, C.; Zhang, C.; Gu, W.; Yan, Y.; Shi, X.; Xian, Y. Transition metal dichalcogenide quantum dots: Synthesis, photoluminescence and biological applications. J. Mater. Chem. B 2018, 6, 8011–8036. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, H.; Zhang, H. Recent Progress in the Preparation, Assembly, Transformation, and Applications of Layer-Structured Nanodisks beyond Graphene. Adv. Mater. 2017, 29, 1701704. [Google Scholar] [CrossRef]
- Valappil, M.O.; Anil, A.; Shaijumon, M.; Pillai, V.K.; Alwarappan, S. A Single-Step Electrochemical Synthesis of Luminescent WS2 Quantum Dots. Chem. A Eur. J. 2017, 23, 9144–9148. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Damien, D.; Li, B.; Gullappalli, H.; Pillai, V.K.; Ajayan, P.M.; Shaijumon, M.M. Electrochemical synthesis of luminescent MoS2 quantum dots. Chem. Commun. 2015, 51, 6293–6296. [Google Scholar] [CrossRef]
- Mahjouri-Samani, M.; Gresback, R.; Tian, M.; Wang, K.; Puretzky, A.A.; Rouleau, C.M.; Eres, G.; Ivanov, I.N.; Xiao, K.; McGuire, M.A.; et al. Pulsed Laser Deposition of Photoresponsive Two-Dimensional GaSe Nanosheet Networks. Adv. Funct. Mater. 2014, 24, 6365–6371. [Google Scholar] [CrossRef]
- Stoll, S.L.; Gillan, E.G.; Barron, A.R. Chemical vapor deposition of Gallium selenide and indium selenide nanoparticles. Chem. Vap. Depos. 1996, 2, 182–184. [Google Scholar] [CrossRef]
- Allakhverdiev, K.; Hagen, J.; Salaeva, Z. On a Possibility to Form Small Crystallites of Layered Gallium Selenide via Ultrasonic Treatment. Phys. Status Solidi (a) 1997, 163, 121–127. [Google Scholar] [CrossRef]
- Chikan, V.; Kelley, D.F. Synthesis of Highly Luminescent GaSe Nanoparticles. Nano Lett. 2002, 2, 141–145. [Google Scholar] [CrossRef]
- Mahjouri-Samani, M.; Tian, M.; Puretzky, A.A.; Chi, M.; Wang, K.; Duscher, G.; Rouleau, C.M.; Eres, G.; Yoon, M.; Lasseter, J.; et al. Nonequilibrium Synthesis of TiO2 Nanoparticle “Building Blocks” for Crystal Growth by Sequential Attachment in Pulsed Laser Deposition. Nano Lett. 2017, 17, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Mahjouri-Samani, M.; Tian, M.; Wang, K.; Boulesbaa, A.; Rouleau, C.M.; Puretzky, A.A.; McGuire, M.A.; Srijanto, B.R.; Xiao, K.; Eres, G.; et al. Digital Transfer Growth of Patterned 2D Metal Chalcogenides by Confined Nanoparticle Evaporation. ACS Nano 2014, 8, 11567–11575. [Google Scholar] [CrossRef]
- Dai, Q.; Chen, J.; Lu, L.; Tang, J.; Wang, W. Pulsed Laser Deposition of CdSe Quantum Dots on Zn2SnO4 Nanowires and Their Photovoltaic Applications. Nano Lett. 2012, 12, 4187–4193. [Google Scholar] [CrossRef]
- Fassl, P.; Zakharko, Y.; Falk, L.M.; Goetz, K.P.; Paulus, F.; Taylor, A.D.; Zaumseil, J.; Vaynzof, Y. Effect of density of surface defects on photoluminescence properties in MAPbI3 perovskite films. J. Mater. Chem. C 2019, 7, 5285–5292. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elafandi, S.; Ahmadi, Z.; Azam, N.; Mahjouri-Samani, M. Gas-Phase Formation of Highly Luminescent 2D GaSe Nanoparticle Ensembles in a Nonequilibrium Laser Ablation Process. Nanomaterials 2020, 10, 908. https://doi.org/10.3390/nano10050908
Elafandi S, Ahmadi Z, Azam N, Mahjouri-Samani M. Gas-Phase Formation of Highly Luminescent 2D GaSe Nanoparticle Ensembles in a Nonequilibrium Laser Ablation Process. Nanomaterials. 2020; 10(5):908. https://doi.org/10.3390/nano10050908
Chicago/Turabian StyleElafandi, Salah, Zabihollah Ahmadi, Nurul Azam, and Masoud Mahjouri-Samani. 2020. "Gas-Phase Formation of Highly Luminescent 2D GaSe Nanoparticle Ensembles in a Nonequilibrium Laser Ablation Process" Nanomaterials 10, no. 5: 908. https://doi.org/10.3390/nano10050908
APA StyleElafandi, S., Ahmadi, Z., Azam, N., & Mahjouri-Samani, M. (2020). Gas-Phase Formation of Highly Luminescent 2D GaSe Nanoparticle Ensembles in a Nonequilibrium Laser Ablation Process. Nanomaterials, 10(5), 908. https://doi.org/10.3390/nano10050908





