Scalable Fabrication of Modified Graphene Nanoplatelets as an Effective Additive for Engine Lubricant Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Nanoplatelets
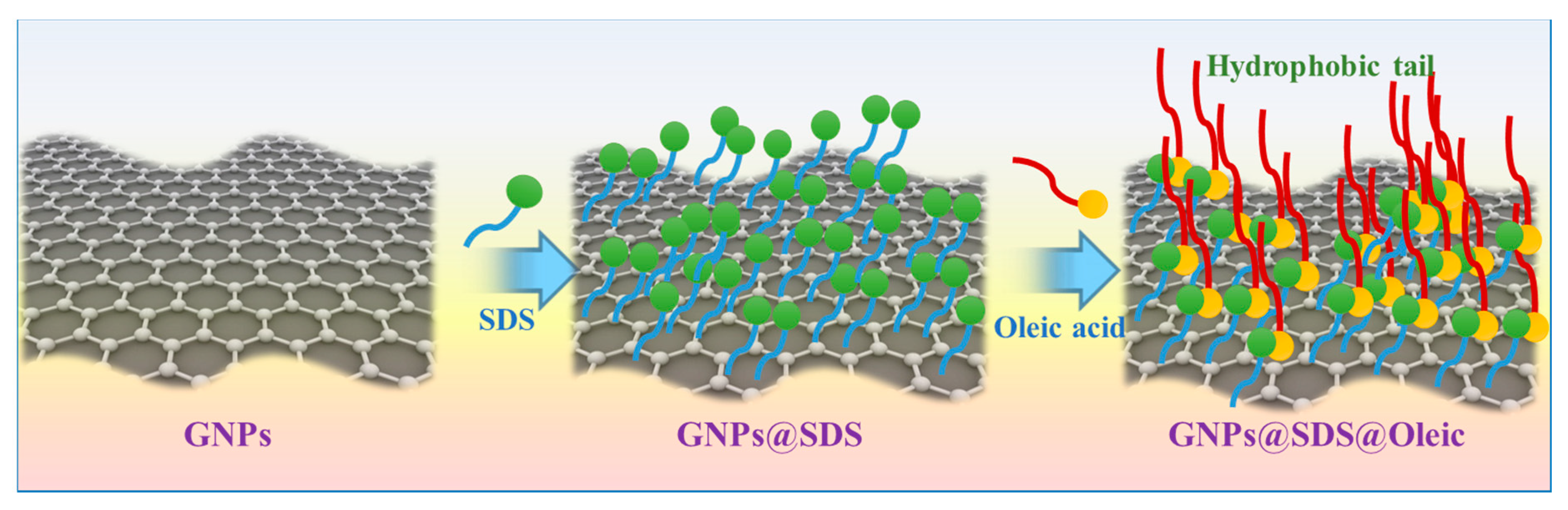
2.3. GNP Modification
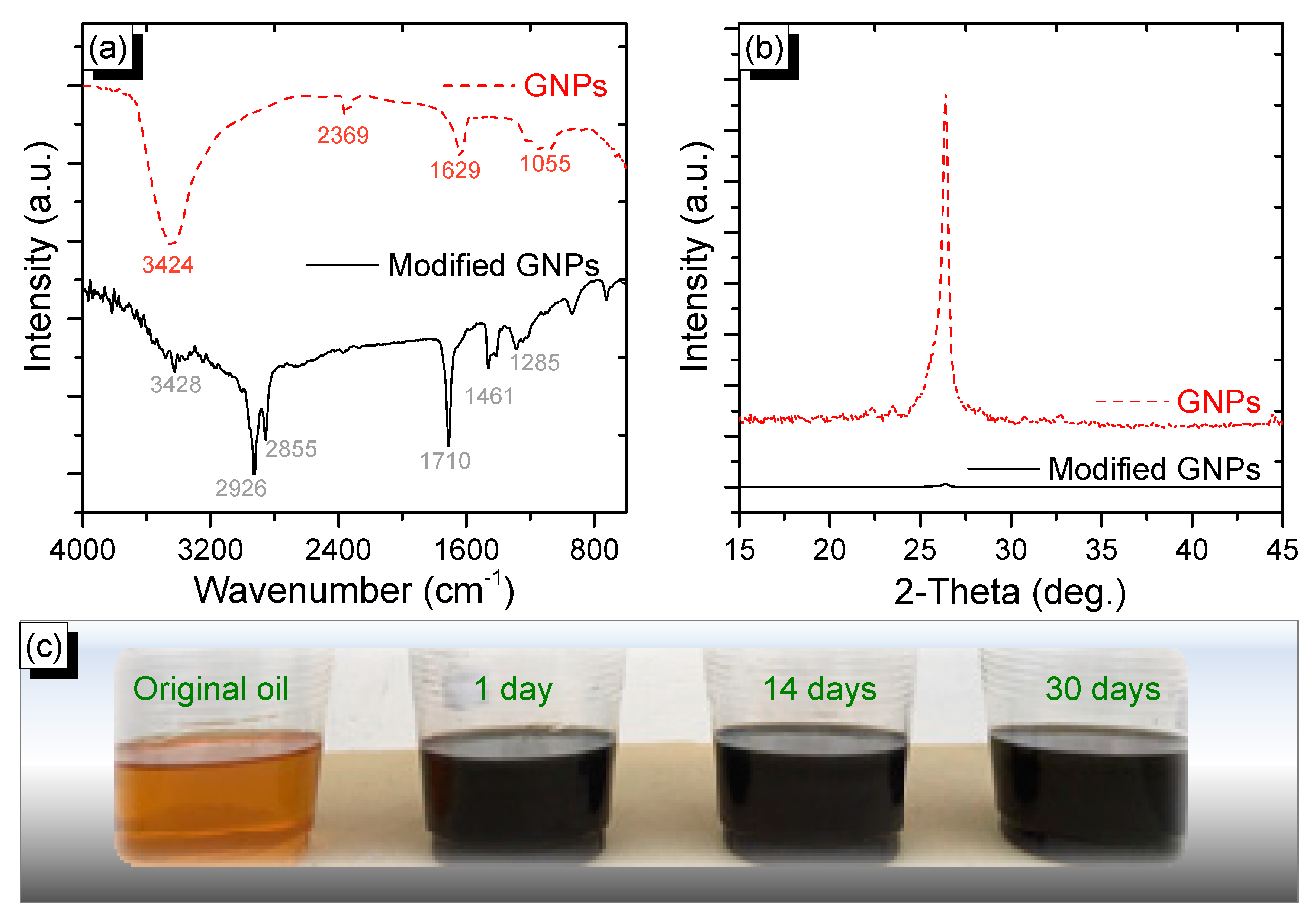
2.4. Characterization
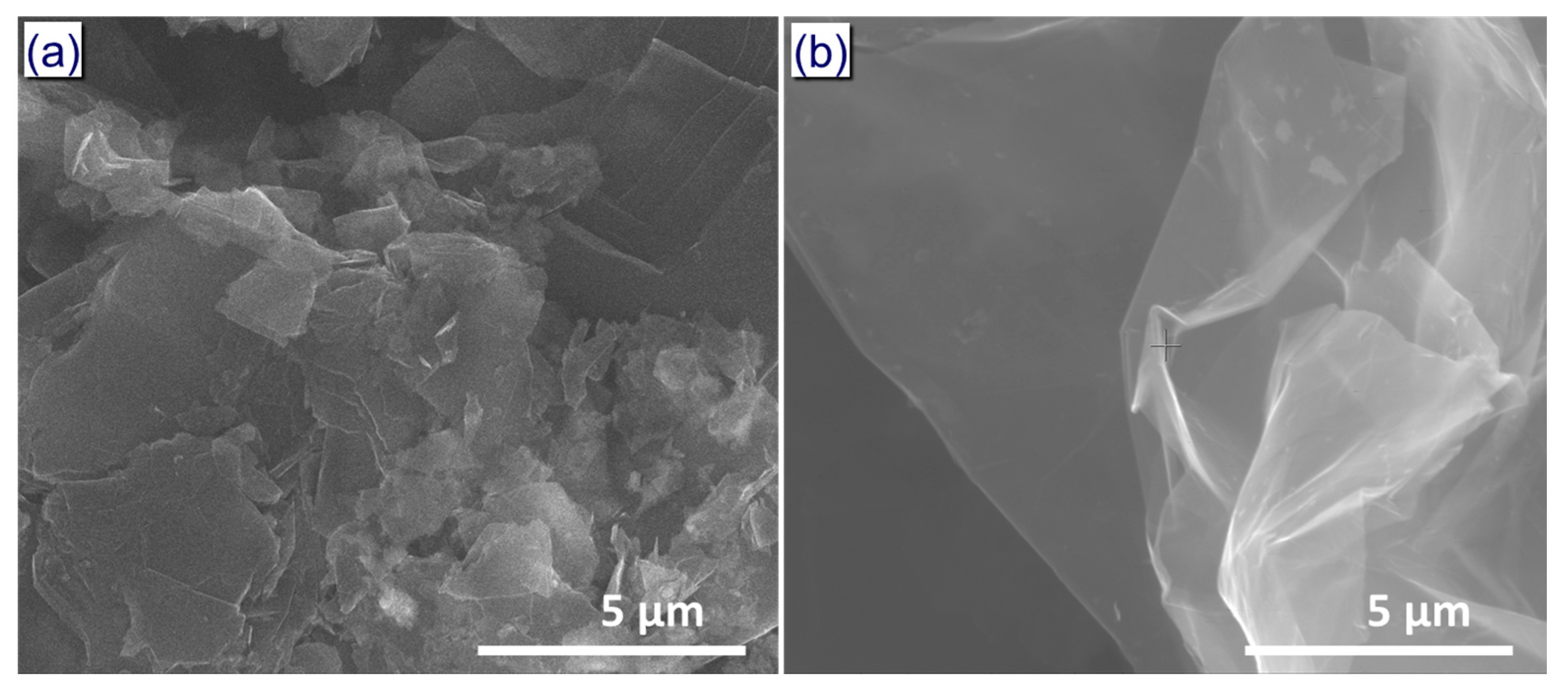
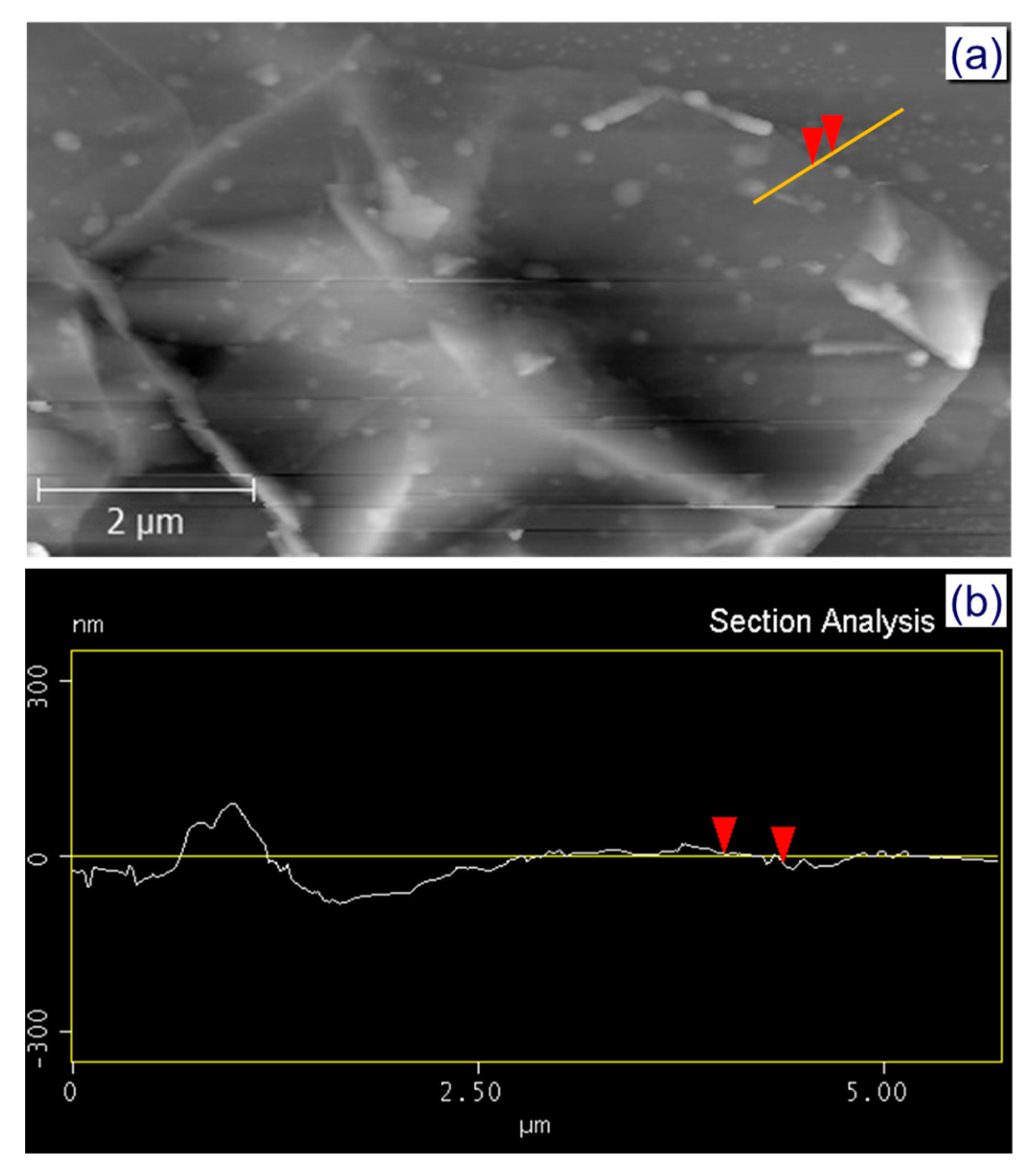
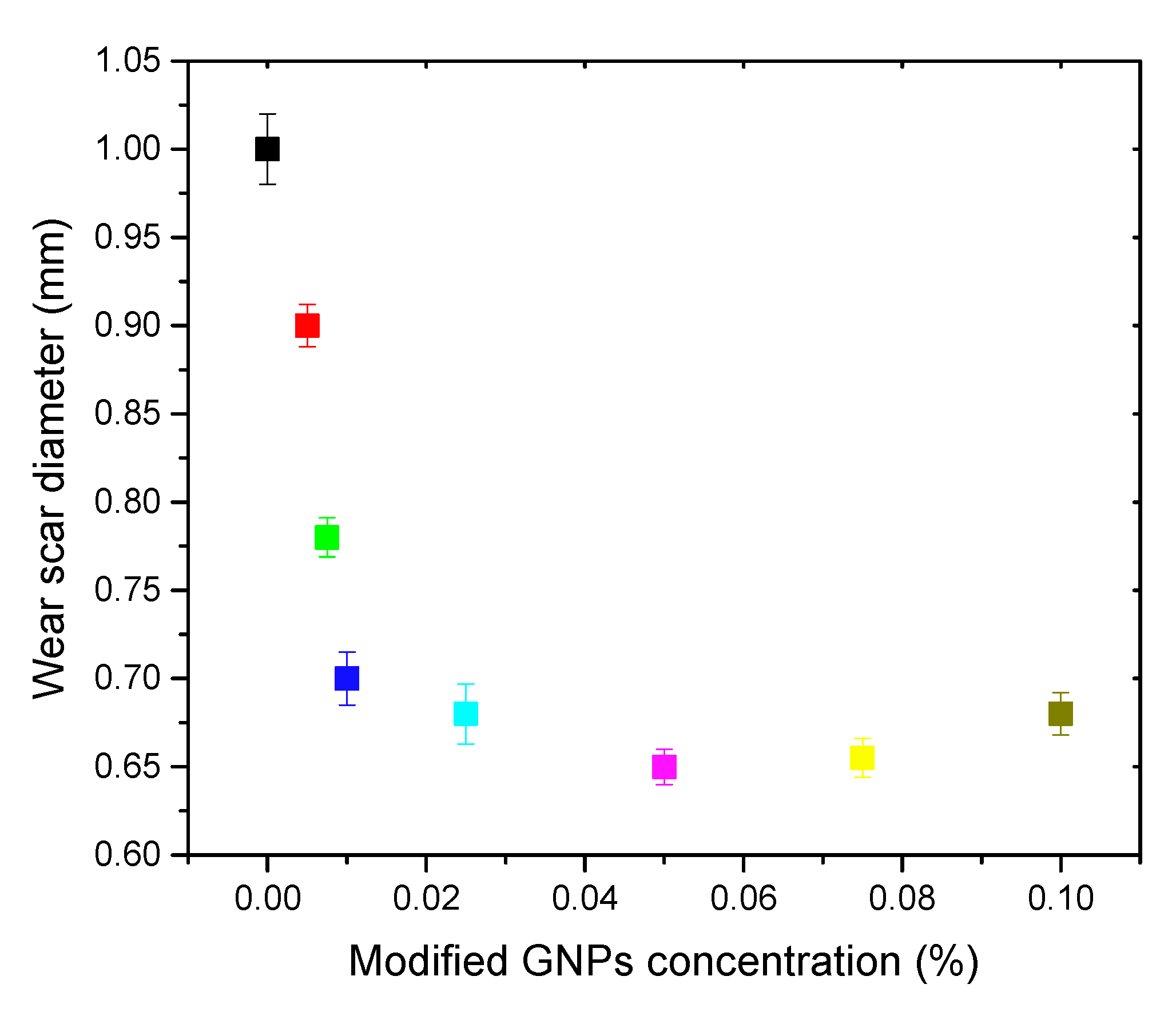
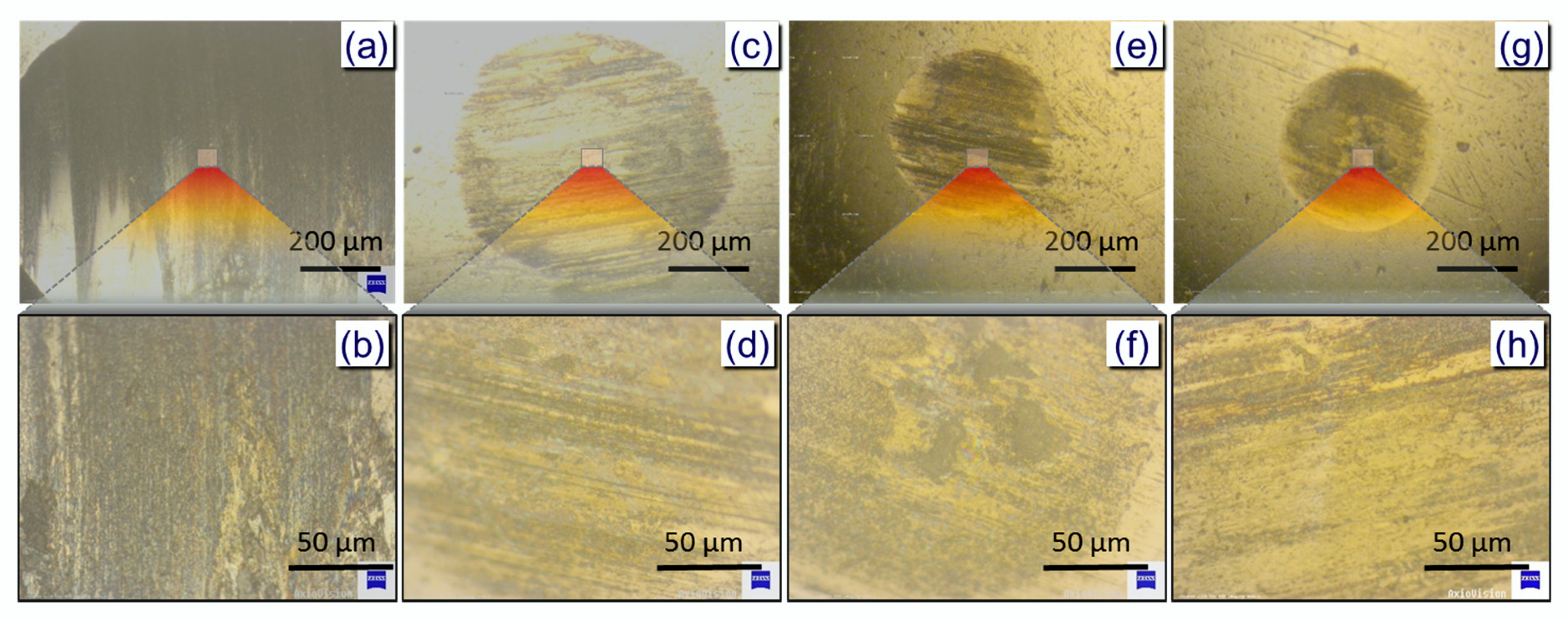
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, V.W.; Tung, S.C. Overview of automotive engine friction and reduction trends–Effects of surface, material, and lubricant-additive technologies. Friction 2016, 4, 1–28. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Talebi, H.; Silani, M.; Bordas, S.P.; Kerfriden, P.; Rabczuk, T. A computational library for multiscale modeling of material failure. Comput. Mech. 2014, 53, 1047–1071. [Google Scholar] [CrossRef]
- Abdalla, H.; Patel, S. The performance and oxidation stability of sustainable metalworking fluid derived from vegetable extracts. Proc. Inst. Mech. Eng. Part. B 2006, 220, 2027–2040. [Google Scholar] [CrossRef]
- Rizvi, S. Lubricant additives and their functions. Mater. Park OH ASM Int. 1992, 98–112. [Google Scholar]
- Guo, J.; Peng, R.; Du, H.; Shen, Y.; Li, Y.; Li, J.; Dong, G. The Application of Nano-MoS2 Quantum Dots as Liquid Lubricant Additive for Tribological Behavior Improvement. Nanomaterials 2020, 10, 200. [Google Scholar] [CrossRef]
- Li, C.; Li, M.; Wang, X.; Feng, W.; Zhang, Q.; Wu, B.; Hu, X. Novel Carbon Nanoparticles Derived from Biodiesel Soot as Lubricant Additives. Nanomaterials 2019, 9, 1115. [Google Scholar] [CrossRef]
- Borda, F.L.G.; de Oliveira, S.J.R.; Lazaro, L.M.S.M.; Leiróz, A.J.K. Experimental investigation of the tribological behavior of lubricants with additive containing copper nanoparticles. Tribol. Int. 2018, 117, 52–58. [Google Scholar] [CrossRef]
- Grossiord, C.; Varlot, K.; Martin, J.-M.; Le Mogne, T.; Esnouf, C.; Inoue, K. MoS2 single sheet lubrication by molybdenum dithiocarbamate. Tribol. Int. 1998, 31, 737–743. [Google Scholar] [CrossRef]
- Chen, S.; Liu, W. Oleic acid capped PbS nanoparticles: Synthesis, characterization and tribological properties. Mater. Chem. Phys. 2006, 98, 183–189. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Xu, H.; Tan, H.; Ye, X. Preparation and Tribological Properties of WS2 Hexagonal Nanoplates and Nanoflowers. Nanomaterials 2019, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Guo, Y.; Jiang, M.; Sheng, Y.; Hari, B.; Zhang, G.; Jiang, Y.; Zhou, B.; Zhu, Y.; Wang, Z. Synthesis of hydrophobic zinc borate nanodiscs for lubrication. Mater. Lett. 2006, 60, 2511–2515. [Google Scholar] [CrossRef]
- Tang, W.; Yu, C.; Zhang, S.; Liu, S.; Wu, X.; Zhu, H. Antifriction and Antiwear Effect of Lamellar ZrS2 Nanobelts as Lubricant Additives. Nanomaterials 2019, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.; Lovell, M.; Sawyer, W.G.; Mobley, A. On the friction and wear performance of boric acid lubricant combinations in extended duration operations. Wear 2006, 260, 1295–1304. [Google Scholar] [CrossRef]
- Lovell, M.R.; Kabir, M.; Menezes, P.L.; Higgs, C.F., III. Influence of boric acid additive size on green lubricant performance. Philosoph. Trans. R. Soc. A 2010, 368, 4851–4868. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.X.; Chen, C.H.; Kang, Y.; Chang, Y.P.; Chang, S.Y. Size effects of SiO2 nanoparticles as oil additives on tribology of lubricant. Ind. Lubricat. Tribol. 2010, 62, 111–120. [Google Scholar] [CrossRef]
- Moghadam, A.D.; Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Mechanical and tribological properties of self-lubricating metal matrix nanocomposites reinforced by carbon nanotubes (CNTs) and graphene—A review. Compos. Part B Eng. 2015, 77, 402–420. [Google Scholar] [CrossRef]
- Ahmadi, H.; Rashidi, A.; Nouralishahi, A.; Mohtasebi, S.S. Preparation and thermal properties of oil-based nanofluid from multi-walled carbon nanotubes and engine oil as nano-lubricant. Int. Commun. Heat Mass Transf. 2013, 46, 142–147. [Google Scholar]
- Hokkirigawa, K.; Okabe, T.; Saito, K. Friction properties of new porous carbon materials: Woodceramics. J. Porous Mater. 1996, 2, 237–243. [Google Scholar] [CrossRef]
- Rapoport, L.; Fleischer, N.; Tenne, R. Fullerene-like WS2 nanoparticles: Superior lubricants for harsh conditions. Adv. Mater. 2003, 15, 651–655. [Google Scholar] [CrossRef]
- Lee, K.; Hwang, Y.; Cheong, S.; Kwon, L.; Kim, S.; Lee, J. Performance evaluation of nano-lubricants of fullerene nanoparticles in refrigeration mineral oil. Curr. Appl. Phys. 2009, 9, e128–e131. [Google Scholar] [CrossRef]
- Kinoshita, H.; Nishina, Y.; Alias, A.A.; Fujii, M. Tribological properties of monolayer graphene oxide sheets as water-based lubricant additives. Carbon 2014, 66, 720–723. [Google Scholar] [CrossRef]
- Song, H.-J.; Li, N. Frictional behavior of oxide graphene nanosheets as water-base lubricant additive. Appl. Phys. A 2011, 105, 827–832. [Google Scholar] [CrossRef]
- Svadlakova, T.; Hubatka, F.; Turanek Knotigova, P.; Kulich, P.; Masek, J.; Kotoucek, J.; Macak, J.; Motola, M.; Kalbac, M.; Kolackova, M.; et al. Proinflammatory Effect of Carbon-Based Nanomaterials: In Vitro Study on Stimulation of Inflammasome NLRP3 via Destabilisation of Lysosomes. Nanomaterials 2020, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, M.; Zhang, L.; Xu, X.; Liu, Y.; Yan, J.; Wang, W.; Gao, J. Three-dimensional graphene-based aerogels prepared by a self-assembly process and its excellent catalytic and absorbing performance. J. Mater. Chem. A 2013, 1, 7612–7621. [Google Scholar] [CrossRef]
- Kopelevich, Y.; Esquinazi, P. Graphene physics in graphite. Adv. Mater. 2007, 19, 4559–4563. [Google Scholar] [CrossRef]
- Yang, K.; Huang, L.-J.; Wang, Y.-X.; Du, Y.-C.; Zhang, Z.-J.; Wang, Y.; Kipper, M.J.; Belfiore, L.A.; Tang, J.-G. Graphene Oxide Nanofiltration Membranes Containing Silver Nanoparticles: Tuning Separation Efficiency via Nanoparticle Size. Nanomaterials 2020, 10, 454. [Google Scholar] [CrossRef]
- La, D.D.; Hangarge, R.V.; Bhosale, S.; Ninh, H.D.; Jones, L.A.; Bhosale, S.V. Arginine-mediated self-assembly of porphyrin on graphene: A photocatalyst for degradation of dyes. Appl. Sci. 2017, 7, 643. [Google Scholar] [CrossRef]
- La, D.D.; Nguyen, T.A.; Nguyen, T.T.; Ninh, H.D.; Thi, H.P.N.; Nguyen, T.T.; Nguyen, D.A.; Dang, T.D.; Rene, E.R.; Chang, S.W. Absorption Behavior of Graphene Nanoplates toward Oils and Organic Solvents in Contaminated Water. Sustainability 2019, 11, 7228. [Google Scholar] [CrossRef]
- La, D.D.; Patwari, J.M.; Jones, L.A.; Antolasic, F.; Bhosale, S.V. Fabrication of a GNP/Fe–Mg binary oxide composite for effective removal of arsenic from aqueous solution. ACS Omega 2017, 2, 218–226. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Kiu, S.S.K.; Yusup, S.; Soon, C.V.; Arpin, T.; Samion, S.; Kamil, R.N.M. Tribological investigation of graphene as lubricant additive in vegetable oil. J. Phys. Sci. 2017, 28, 257. [Google Scholar]
- González-Domínguez, J.M.; León, V.; Lucío, M.I.; Prato, M.; Vázquez, E. Production of ready-to-use few-layer graphene in aqueous suspensions. Nat. Protoc. 2018, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P. Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. D Appl. Phys. 2011, 44, 205303. [Google Scholar] [CrossRef]
- Azman, S.S.N.; Zulkifli, N.W.M.; Masjuki, H.; Gulzar, M.; Zahid, R. Study of tribological properties of lubricating oil blend added with graphene nanoplatelets. J. Mater. Res. 2016, 31, 1932–1938. [Google Scholar] [CrossRef]
- La, M.D.D.; Bhargava, S.; Bhosale, S.V. Improved and a simple approach for mass production of graphene nanoplatelets material. ChemistrySelect 2016, 1, 949–952. [Google Scholar] [CrossRef]
- Parvez, K.; Wu, Z.-S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Müllen, K. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef]
- Sheka, E.F.; Hołderna-Natkaniec, K.; Natkaniec, I.; Krawczyk, J.X.; Golubev, Y.A.; Rozhkova, N.N.; Kim, V.V.; Popova, N.A.; Popova, V.A. Computationally Supported Neutron Scattering Study of Natural and Synthetic Amorphous Carbons. J. Phys. Chem. C 2019, 123, 15841–15850. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef]
- Dimiev, A.; Kosynkin, D.V.; Sinitskii, A.; Slesarev, A.; Sun, Z.; Tour, J.M. Layer-by-layer removal of graphene for device patterning. Science 2011, 331, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Genorio, B.; Lu, W.; Dimiev, A.M.; Zhu, Y.; Raji, A.-R.O.; Novosel, B.; Alemany, L.B.; Tour, J.M. In situ intercalation replacement and selective functionalization of graphene nanoribbon stacks. ACS Nano 2012, 6, 4231–4240. [Google Scholar] [CrossRef] [PubMed]
- Sayah, A.; Habelhames, F.; Bahloul, A.; Nessark, B.; Bonnassieux, Y.; Tendelier, D.; El Jouad, M. Electrochemical synthesis of polyaniline-exfoliated graphene composite films and their capacitance properties. J. Electroanal. Chem. 2018, 818, 26–34. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Meng, Q.; Jin, J.; Wang, R.; Kuan, H.-C.; Ma, J.; Kawashima, N.; Michelmore, A.; Zhu, S.; Wang, C.H. Processable 3-nm thick graphene platelets of high electrical conductivity and their epoxy composites. Nanotechnology 2014, 25, 125707. [Google Scholar] [CrossRef]
- Matthäus, C.; Chernenko, T.; Quintero, L.; Miljković, M.; Milane, L.; Kale, A.; Amiji, M.; Torchilin, V.; Diem, M. Raman micro-spectral imaging of cells and intracellular drug delivery using nanocarrier systems. In Confocal Raman Microscopy; Springer: Berlin/Heidelberg, Germany, 2010; pp. 137–163. [Google Scholar]
- Fan, H.-L.; Li, L.; Zhou, S.-F.; Liu, Y.-Z. Continuous preparation of Fe3O4 nanoparticles combined with surface modification by L-cysteine and their application in heavy metal adsorption. Ceram. Int. 2016, 42, 4228–4237. [Google Scholar] [CrossRef]
- Coenen, K.; Gallucci, F.; Mezari, B.; Hensen, E.; van Sint Annaland, M. An in-situ IR study on the adsorption of CO2 and H2O on hydrotalcites. J. CO2 Util. 2018, 24, 228–239. [Google Scholar] [CrossRef]
- Hong, R.-Y.; Li, J.-H.; Zhang, S.-Z.; Li, H.-Z.; Zheng, Y.; Ding, J.-M.; Wei, D.-G. Preparation and characterization of silica-coated Fe3O4 nanoparticles used as precursor of ferrofluids. Appl. Surf. Sci. 2009, 255, 3485–3492. [Google Scholar] [CrossRef]
- Kooter, I.M.; Pierik, A.J.; Merkx, M.; Averill, B.A.; Moguilevsky, N.; Bollen, A.; Wever, R. Difference Fourier transform infrared evidence for ester bonds linking the heme group in myeloperoxidase, lactoperoxidase, and eosinophil peroxidase. J. Am. Chem. Soc. 1997, 119, 11542–11543. [Google Scholar] [CrossRef]
- Ibarra, J.; Melendres, J.; Almada, M.; Burboa, M.G.; Taboada, P.; Juárez, J.; Valdez, M.A. Synthesis and characterization of magnetite/PLGA/chitosan nanoparticles. Mater. Res. Express 2015, 2, 095010. [Google Scholar] [CrossRef]
- Yang, D.; Gao, S.; Fang, Y.; Lin, X.; Jin, X.; Wang, X.; Ke, L.; Shi, K. The π–π stacking-guided supramolecular self-assembly of nanomedicine for effective delivery of antineoplastic therapies. Nanomed. 2018, 13, 3159–3177. [Google Scholar] [CrossRef] [PubMed]
- Raygoza, E.D.R.; Solorio, C.I.R.; Torres, E.G. Lubricating Oil for Automotive and Industrial Applications, Containing Decorated Graphene. U.S. Patent Application No. 9,679,674, 2019. [Google Scholar]
- Lee, G.-J.; Rhee, C.K. Enhanced thermal conductivity of nanofluids containing graphene nanoplatelets prepared by ultrasound irradiation. J. Mater. Sci. 2014, 49, 1506–1511. [Google Scholar] [CrossRef]
- Ma, W.; Yang, F.; Shi, J.; Wang, F.; Zhang, Z.; Wang, S. Silicone based nanofluids containing functionalized graphene nanosheets. Coll. Surf. A Phys. Chem. Eng. Asp. 2013, 431, 120–126. [Google Scholar] [CrossRef]
- Alves, S.; Barros, B.; Trajano, M.; Ribeiro, K.; Moura, E. Tribological behavior of vegetable oil-based lubricants with nanoparticles of oxides in boundary lubrication conditions. Tribol. Int. 2013, 65, 28–36. [Google Scholar] [CrossRef]
- Gulzar, M.; Masjuki, H.; Varman, M.; Kalam, M.; Mufti, R.; Zulkifli, N.; Yunus, R.; Zahid, R. Improving the AW/EP ability of chemically modified palm oil by adding CuO and MoS2 nanoparticles. Tribol. Int. 2015, 88, 271–279. [Google Scholar] [CrossRef]
- Viesca, J.; Battez, A.H.; González, R.; Chou, R.; Cabello, J. Antiwear properties of carbon-coated copper nanoparticles used as an additive to a polyalphaolefin. Tribol. Int. 2011, 44, 829–833. [Google Scholar] [CrossRef]
- Ma, S.; Zheng, S.; Cao, D.; Guo, H. Antiwear and friction performance of ZrO2 nanoparticles as lubricant additive. Particuol. 2010, 8, 468–472. [Google Scholar] [CrossRef]
- Laad, M.; Jatti, V.K.S. Titanium oxide nanoparticles as additives in engine oil. J. King Saud Univ. Eng. Sci. 2018, 30, 116–122. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, D.D.; Truong, T.N.; Pham, T.Q.; Vo, H.T.; Tran, N.T.; Nguyen, T.A.; Nadda, A.K.; Nguyen, T.T.; Chang, S.W.; Chung, W.J.; et al. Scalable Fabrication of Modified Graphene Nanoplatelets as an Effective Additive for Engine Lubricant Oil. Nanomaterials 2020, 10, 877. https://doi.org/10.3390/nano10050877
La DD, Truong TN, Pham TQ, Vo HT, Tran NT, Nguyen TA, Nadda AK, Nguyen TT, Chang SW, Chung WJ, et al. Scalable Fabrication of Modified Graphene Nanoplatelets as an Effective Additive for Engine Lubricant Oil. Nanomaterials. 2020; 10(5):877. https://doi.org/10.3390/nano10050877
Chicago/Turabian StyleLa, Duong Duc, Tuan Ngoc Truong, Thuan Q. Pham, Hoang Tung Vo, Nam The Tran, Tuan Anh Nguyen, Ashok Kumar Nadda, Thanh Tung Nguyen, S. Woong Chang, W. Jin Chung, and et al. 2020. "Scalable Fabrication of Modified Graphene Nanoplatelets as an Effective Additive for Engine Lubricant Oil" Nanomaterials 10, no. 5: 877. https://doi.org/10.3390/nano10050877
APA StyleLa, D. D., Truong, T. N., Pham, T. Q., Vo, H. T., Tran, N. T., Nguyen, T. A., Nadda, A. K., Nguyen, T. T., Chang, S. W., Chung, W. J., & Nguyen, D. D. (2020). Scalable Fabrication of Modified Graphene Nanoplatelets as an Effective Additive for Engine Lubricant Oil. Nanomaterials, 10(5), 877. https://doi.org/10.3390/nano10050877








