Efficient Hydrolysis of Chitin in a Deep Eutectic Solvent Synergism for Production of Chitin Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of Chitin with DES
2.3. Liberation of ChNCs
2.4. Characterization of ChNCs
2.5. ChNC-Stabilized Pickering Emulsions
3. Results and Discussion
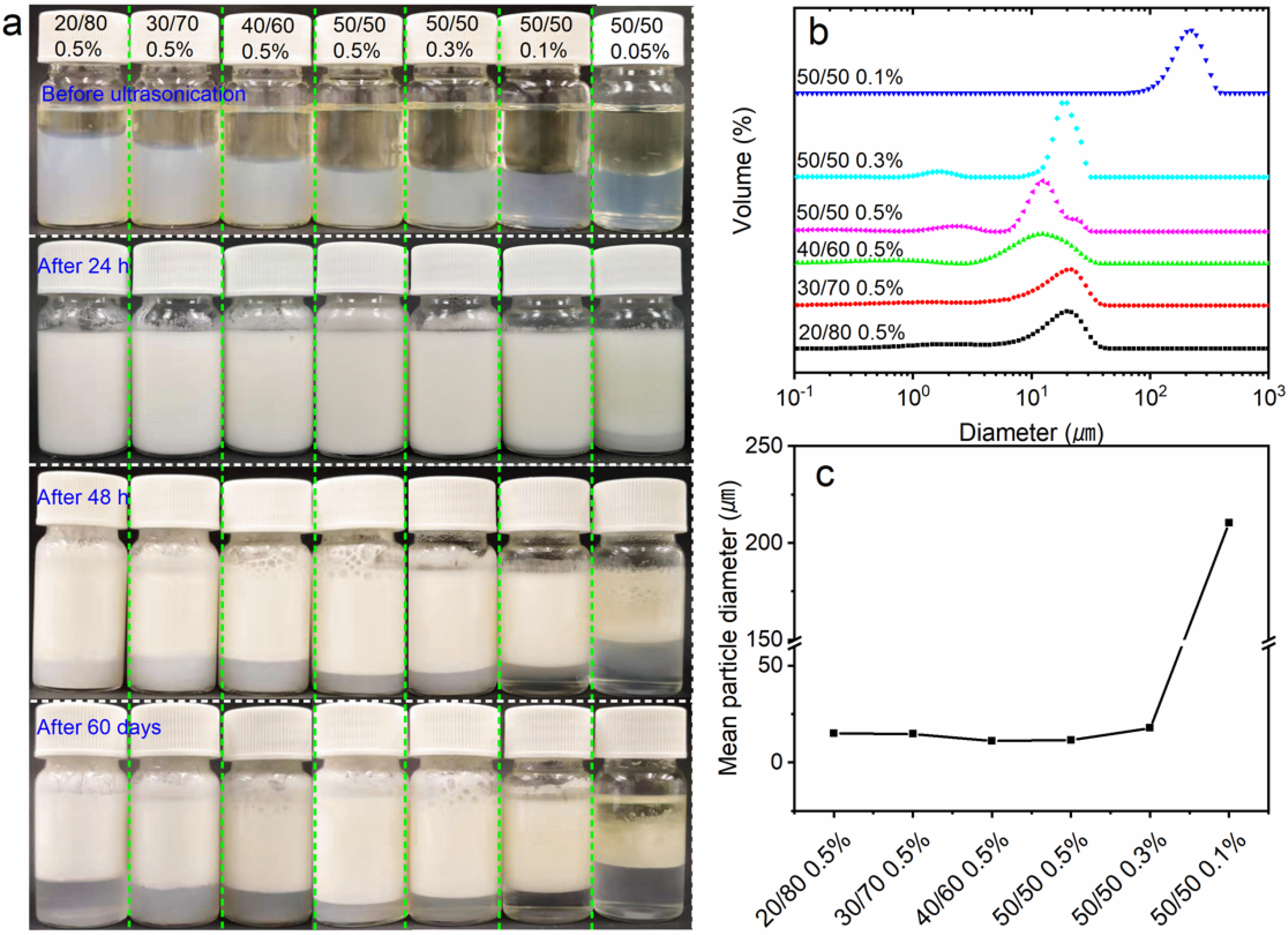
3.1. Effect of DES Treatment Conditions on the Appearance and Yield of ChNCs
3.2. Chemical Structure of ChNC
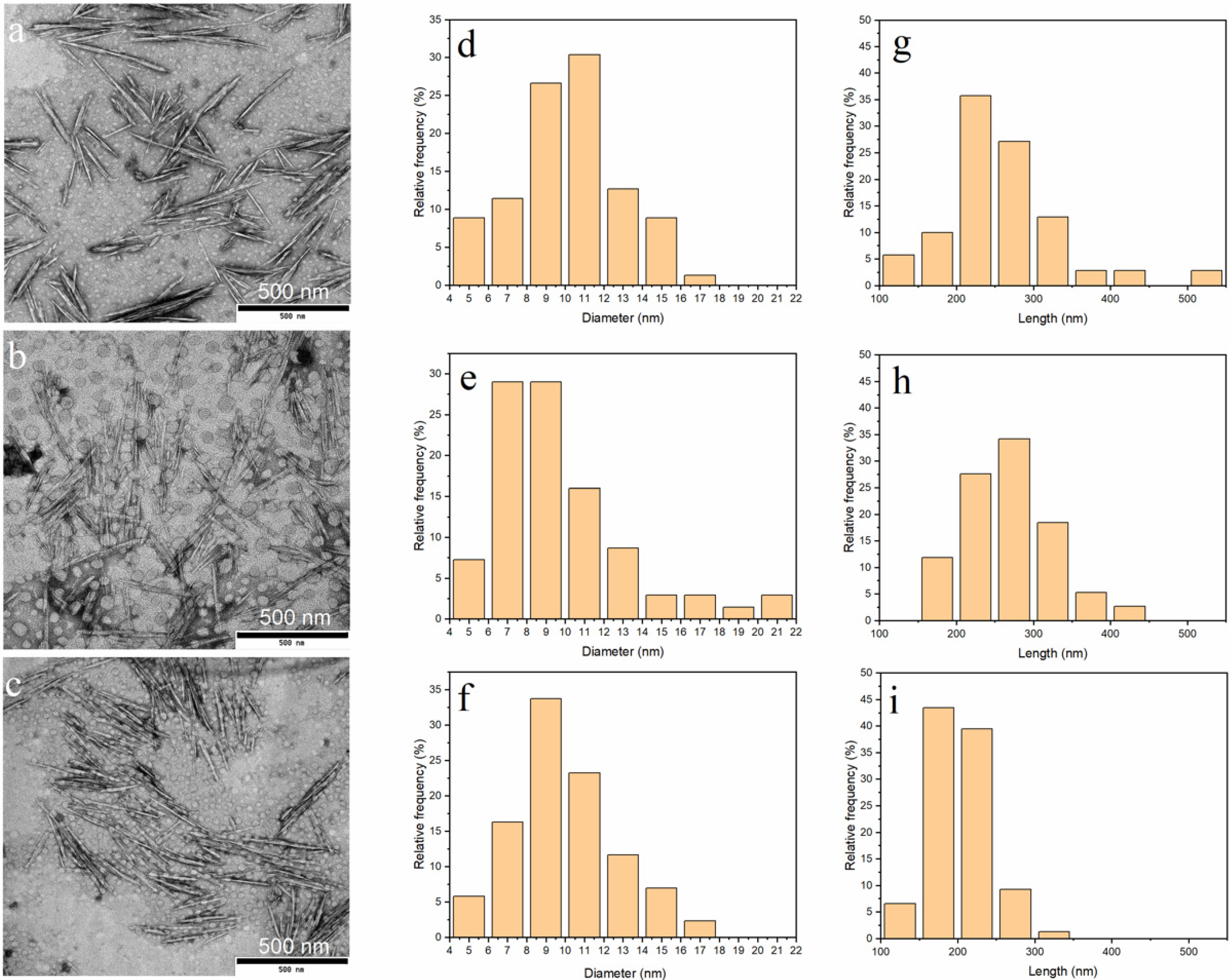
3.3. Morphology of ChNC
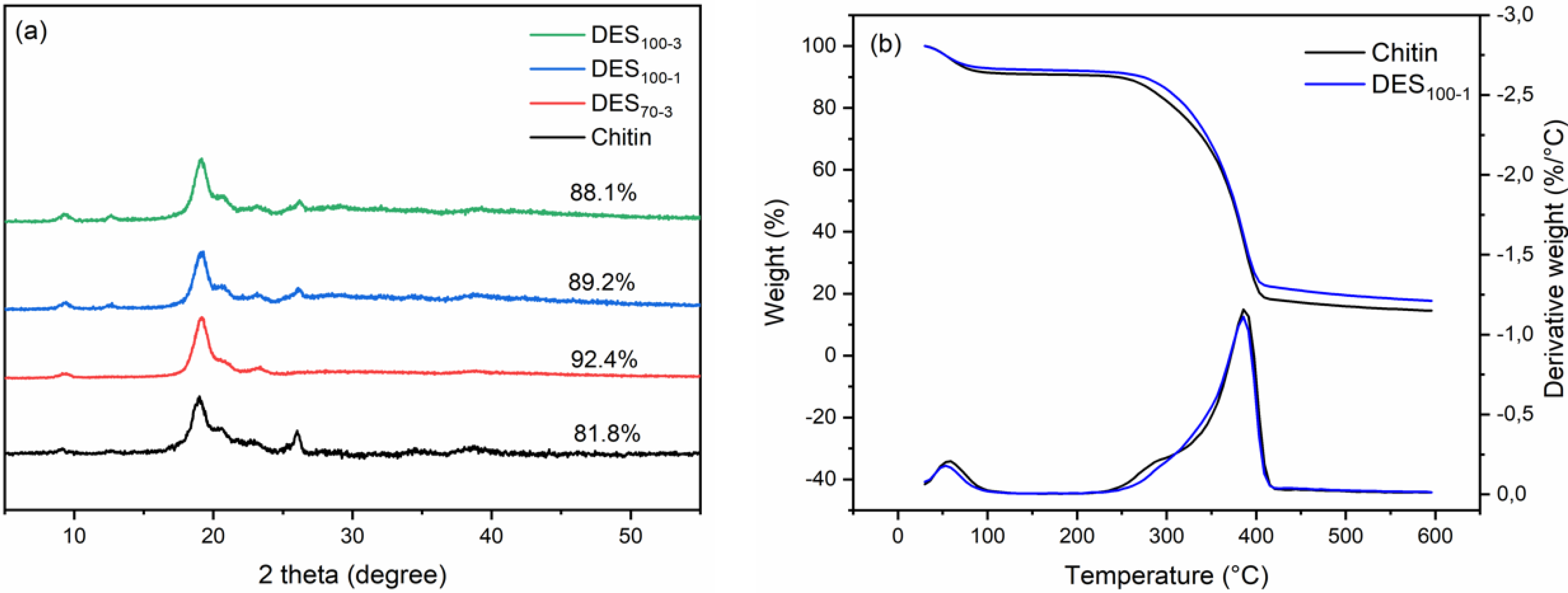
3.4. Crystalline Structure and Thermal Properties of ChNC
3.5. Pickering Emulsions Stabilized by ChNC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wysokowski, M.; Machalowski, T.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Galli, R.; Zietek, J.; Pantovic, S.; Voronkina, A.; Kovalchuk, V.; et al. 3D chitin scaffolds of marine demosponge origin for biomimetic mollusk hemolymph-sssociated biomineralization ex-vivo. Mar. Drugs 2020, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Hong, S.; Yang, Q.; Yuan, Y.; Chen, L.; Song, Y.; Lian, H. Sustainable co-solvent induced one step extraction of low molecular weight chitin with high purity from raw lobster shell. Carbohydr. Polym. 2019, 205, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile acid base sustainable solvent for fast extraction of various molecular weight chitin from lobster shell. Carbohydr. Polym. 2018, 201, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Karaarslan, M. A new method for fast chitin extraction from shells of crab, crayfish and shrimp. Nat. Prod. Res. 2015, 29, 1477–1480. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Machalowski, T.; Wysokowski, M.; Tsurkan, M.; Galli, R.; Schimpf, C.; Rafaja, D.; Brendler, E.; Viehweger, C.; Zoltowska-Aksamitowska, S.; Petrenko, I.; et al. Spider chitin: An ultrafast microwave-assisted method for chitin isolation from Caribena versicolor spider molt cuticle. Molecules 2019, 24, 3736. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Bechmann, N.; Binnewerg, B.; Schubert, M.; Guan, K.; Bornstein, S.R.; Czaczyk, K.; Pokrovsky, O.; et al. Spider Chitin. The biomimetic potential and applications of Caribena versicolor tubular chitin. Carbohydr. Polym. 2019, 226, 115301. [Google Scholar] [CrossRef]
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; van Pée, K.-H. Chitin-based organic networks: An integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew. Chem. Int. Ed. 2009, 48, 9724–9727. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yano, R.; Miyatake, K.; Tomohiro, I.; Shigemasa, Y.; Minami, S. Effects of chitin and chitosan on blood coagulation. Carbohydr. Polym. 2003, 53, 337–342. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.; Heras Caballero, A. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Kovalchuk, V.; Voronkina, A.; Binnewerg, B.; Schubert, M.; Muzychka, L.; Wysokowski, M.; Tsurkan, M.V.; Bechmann, N.; Petrenko, I.; Fursov, A.; et al. Naturally drug-loaded chitin: Isolation and applications. Mar. Drugs 2019, 17, 574. [Google Scholar] [CrossRef]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally prefabricated marine biomaterials: Isolation and applications of flat chitinous 3D scaffolds from Ianthella labyrinthus (demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine biomaterials: Biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater. Sci. Eng. C 2020, 109, 110566. [Google Scholar] [CrossRef]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express method for isolation of ready-to-use 3D chitin scaffolds from aplysina archeri (aplysineidae: Verongiida) demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Petrenko, I.; Fursov, A.; Rahimi-Nasrabadi, M.; Amro, M.M.; Meissner, H.; Joseph, Y.; Fazilov, B.; Ehrlich, H.; et al. Naturally pre-designed biomaterials: Spider molting cuticle as a functional crude oil sorbent. J. Environ. Manag. 2020, 261, 110218. [Google Scholar] [CrossRef]
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.J.; Stelling, A.L.; Niederschlag, E.; Stöker, H.; Kutsova, V.Z.; et al. Chitin of poriferan origin and the bioelectrometallurgy of copper/copper oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Rinaudo, M.; Chaabouni, M.; Jellouli, K.; Nasri, M. Optimization of proteins and minerals removal from shrimp shells to produce highly acetylated chitin. Int. J. Biol. Macromol. 2016, 84, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-pot production of chitin with high purity from lobster shells using choline chloride–malonic acid deep eutectic solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef]
- Shamshina, J.L. Chitin in ionic liquids: Historical insights into the polymer’s dissolution and isolation. A review. Green Chem. 2019, 21, 3974–3993. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Yang, Q.; Chen, L.; Deng, J.; Chen, W.; Lian, H.; Mota-Morales, J.D.; Liimatainen, H. Choline chloride-zinc chloride deep eutectic solvent mediated preparation of partial O-acetylation of chitin nanocrystal in one step reaction. Carbohydr. Polym. 2019, 220, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Morin, A.; Dufresne, A. Nanocomposites of chitin whiskers from Riftia Tubes and Poly(caprolactone). Macromolecules 2002, 35, 2190–2199. [Google Scholar] [CrossRef]
- Lertwattanaseri, T.; Ichikawa, N.; Mizoguchi, T.; Tanaka, Y.; Chirachanchai, S. Microwave technique for efficient deacetylation of chitin nanowhiskers to a chitosan nanoscaffold. Carbohydr. Res. 2009, 344, 331–335. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Morganti, A. Transforming nanostructured chitin from crustacean waste into beneficial health products: A must for our society. Nanotechnol. Sci. Appl. 2011, 123. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-Chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Sánchez-Leija, R.J.; Carranza, A.; Pojman, J.A.; del Monte, F.; Luna-Bárcenas, G. Free-radical polymerizations of and in deep eutectic solvents: Green synthesis of functional materials. Prog. Polym. Sci. 2018, 78, 139–153. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R. Ionic liquids based upon metal halide/substituted quaternary ammonium salt mixtures. Inorg. Chem. 2004, 43, 3447–3452. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Acidic Deep eutectic solvents as hydrolytic media for cellulose nanocrystal production. Biomacromolecules 2016, 17, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xie, H.; Du, H.; Zhang, X.; Zou, Z.; Zou, Y.; Liu, W.; Lan, H.; Zhang, X.; Si, C. Facile extraction of thermally stable and dispersible cellulose nanocrystals with high yield via a green and recyclable FeCl3-catalyzed deep eutectic solvent system. ACS Sustain. Chem. Eng. 2019, 7, 7200–7208. [Google Scholar] [CrossRef]
- Yuan, Y.; Hong, S.; Lian, H.; Zhang, K.; Liimatainen, H. Comparison of acidic deep eutectic solvents in production of chitin nanocrystals. Carbohydr. Polym. 2020, 236, 116095. [Google Scholar] [CrossRef] [PubMed]
- Perrin, E.; Bizot, H.; Cathala, B.; Capron, I. Chitin nanocrystals for Pickering high internal phase emulsions. Biomacromolecules 2014, 15, 3766–3771. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Xiang, W.; Liu, L.; Yang, Y.; Nugroho, R.W.N.; Fan, Y.; Rojas, O.J. Self-assembled networks of short and long chitin nanoparticles for oil/water interfacial superstabilization. ACS Sustain. Chem. Eng. 2019, 7, 6497–6511. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, C.; Wang, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Sui, X.; Qu, S. In Vitro Digestion of oil-in-water emulsions stabilized by regenerated chitin. J. Agric. Food Chem. 2018, 66, 12344–12352. [Google Scholar] [CrossRef]
- Laitinen, O.; Ojala, J.; Sirviö, J.A.; Liimatainen, H. Sustainable stabilization of oil in water emulsions by cellulose nanocrystals synthesized from deep eutectic solvents. Cellulose 2017, 24, 1679–1689. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.-L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Focher, B.; Beltrame, P.L.; Naggi, A.; Torri, G. Alkaline N-deacetylation of chitin enhanced by flash treatments. Reaction kinetics and structure modifications. Carbohydr. Polym. 1990, 12, 405–418. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Mu, X.; Gong, W.; Lv, D.; Hong, Y.; Si, C.; Li, B. Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis. Cellulose 2016, 23, 2389–2407. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nishimura, T.; Saito, T.; Kato, T. CaCO3/chitin-whisker hybrids: Formation of CaCO3 crystals in chitin-based liquid-crystalline suspension. Polym. J. 2010, 42, 583. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, X.; Chang, Y.; Ren, L.; Zhou, J. Fabrication and characterization of chitin nanofibers through esterification and ultrasound treatment. Carbohydr. Polym. 2018, 180, 81–87. [Google Scholar] [CrossRef]
- Araki, J.; Yamanaka, Y.; Ohkawa, K. Chitin-chitosan nanocomposite gels: Reinforcement of chitosan hydrogels with rod-like chitin nanowhiskers. Polym. J. 2012, 44, 713–717. [Google Scholar] [CrossRef]
- Ma, B.; Qin, A.; Li, X.; Zhao, X.; He, C. Structure and properties of chitin whisker reinforced chitosan membranes. Int. J. Biol. Macromol. 2014, 64, 341–346. [Google Scholar] [CrossRef]
- Phongying, S.; Aiba, S.; Chirachanchai, S. Direct chitosan nanoscaffold formation via chitin whiskers. Polymer 2007, 48, 393–400. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Different routes to turn chitin into stunning nano-objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Ang-atikarnkul, P.; Watthanaphanit, A.; Rujiravanit, R. Fabrication of cellulose nanofiber/chitin whisker/silk sericin bionanocomposite sponges and characterizations of their physical and biological properties. Compos. Sci. Technol. 2014, 96, 88–96. [Google Scholar] [CrossRef]
- Wongpanit, P.; Sanchavanakit, N.; Pavasant, P.; Bunaprasert, T.; Tabata, Y.; Rujiravanit, R. Preparation and characterization of chitin whisker-reinforced silk fibroin nanocomposite sponges. Eur. Polym. J. 2007, 43, 4123–4135. [Google Scholar] [CrossRef]
- Mukesh, C.; Mondal, D.; Sharma, M.; Prasad, K. Choline chloride–thiourea, a deep eutectic solvent for the production of chitin nanofibers. Carbohydr. Polym. 2014, 103, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Iwamoto, S.; Endo, T. 3 nm thick lignocellulose nanofibers obtained from esterified wood with maleic anhydride. ACS Macro Lett. 2015, 4, 80–83. [Google Scholar] [CrossRef]
- Sehaqui, H.; Kulasinski, K.; Pfenninger, N.; Zimmermann, T.; Tingaut, P. Highly carboxylated cellulose nanofibers via succinic anhydride esterification of wheat fibers and facile mechanical disintegration. Biomacromolecules 2017, 18, 242–248. [Google Scholar] [CrossRef]
- Sirviö, J.A. Cationization of lignocellulosic fibers with betaine in deep eutectic solvent: Facile route to charge stabilized cellulose and wood nanofibers. Carbohydr. Polym. 2018, 198, 34–40. [Google Scholar] [CrossRef]
- Journoux-Lapp, C.; Vigier, K.D.O.; Bachmann, C.; Marinkovic, S.; Estrine, B.; Frapper, G.; Jérôme, F. Elucidation of the role of betaine hydrochloride in glycerol esterification: Towards bio-based ionic building blocks. Green Chem. 2017, 19, 5647–5652. [Google Scholar] [CrossRef]
- Heath, L.; Zhu, L.; Thielemans, W. Chitin nanowhisker aerogels. ChemSusChem 2013, 6, 537–544. [Google Scholar] [CrossRef]
- Jang, M.-K.; Kong, B.-G.; Jeong, Y.-I.; Lee, C.H.; Nah, J.-W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Y.; Yu, L.; Zhang, C.; Xu, X.; Xue, Y.; Li, Z.; Xue, C. Crystalline structure and thermal property characterization of chitin from Antarctic krill (Euphausia superba). Carbohydr. Polym. 2013, 92, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Dhamodharan, R. Pretreatment in hot glycerol for facile and green separation of chitin from prawn shell waste. ACS Sustain. Chem. Eng. 2018, 6, 846–853. [Google Scholar] [CrossRef]
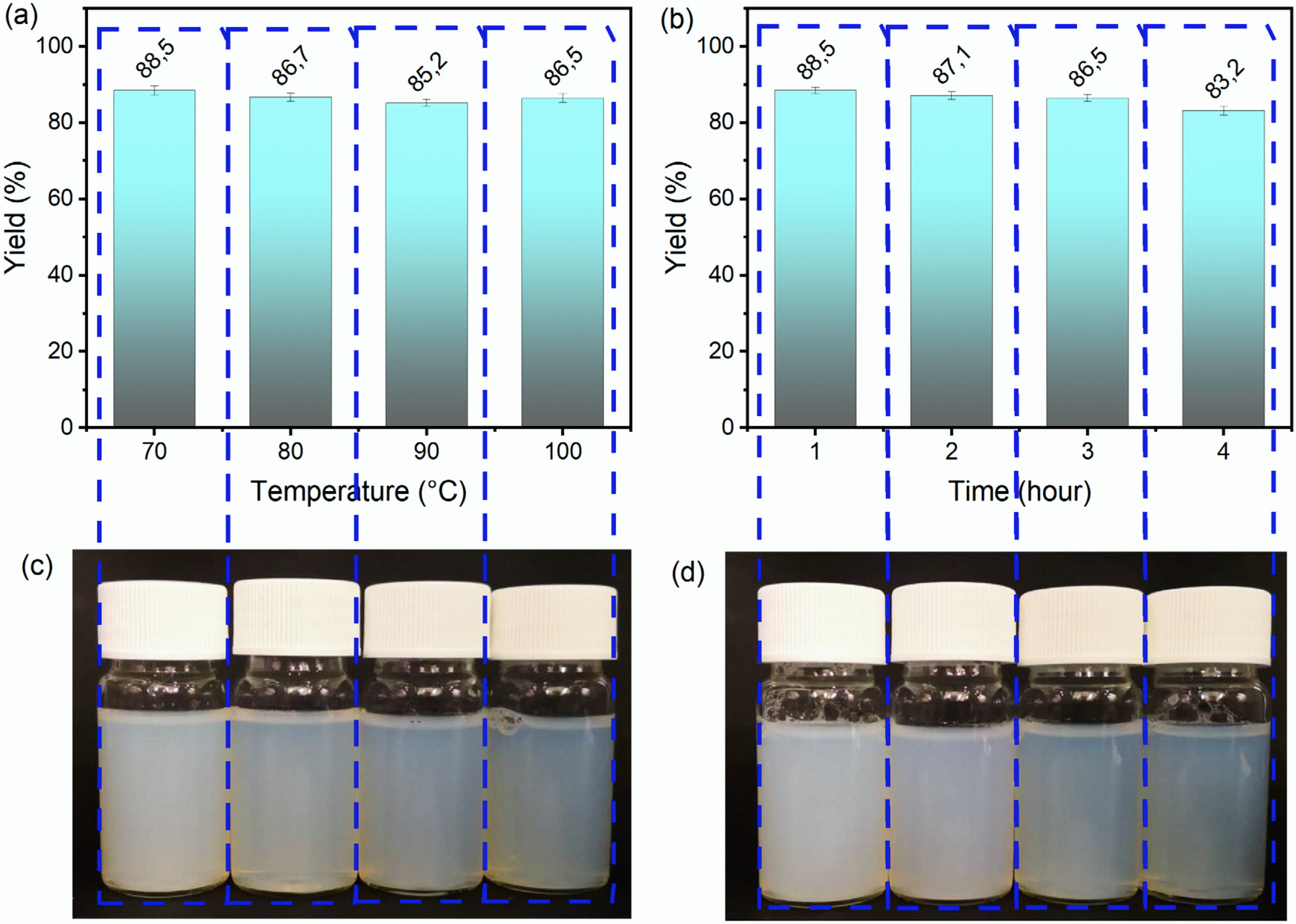

| Samples | Mass Ratio | Temperature (°C) | Time (h) |
|---|---|---|---|
| DES70-3 | 1:20 | 70 | 3 |
| DES80-3 | 1:20 | 80 | 3 |
| DES90-3 | 1:20 | 90 | 3 |
| DES100-3 | 1:20 | 100 | 3 |
| DES100-1 | 1:20 | 100 | 1 |
| DES100-2 | 1:20 | 100 | 2 |
| DES100-4 | 1:20 | 100 | 4 |
| Samples | Length (nm) | Diameter (nm) | Aspect Ratio |
|---|---|---|---|
| DES70-3 | 259 ± 75 | 10 ± 2.8 | 26 |
| DES100-1 | 268 ± 57 | 10 ± 3.4 | 27 |
| DES100-3 | 201 ± 39 | 10 ± 2.6 | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Yuan, Y.; Zhang, K.; Lian, H.; Liimatainen, H. Efficient Hydrolysis of Chitin in a Deep Eutectic Solvent Synergism for Production of Chitin Nanocrystals. Nanomaterials 2020, 10, 869. https://doi.org/10.3390/nano10050869
Hong S, Yuan Y, Zhang K, Lian H, Liimatainen H. Efficient Hydrolysis of Chitin in a Deep Eutectic Solvent Synergism for Production of Chitin Nanocrystals. Nanomaterials. 2020; 10(5):869. https://doi.org/10.3390/nano10050869
Chicago/Turabian StyleHong, Shu, Yang Yuan, Kaitao Zhang, Hailan Lian, and Henrikki Liimatainen. 2020. "Efficient Hydrolysis of Chitin in a Deep Eutectic Solvent Synergism for Production of Chitin Nanocrystals" Nanomaterials 10, no. 5: 869. https://doi.org/10.3390/nano10050869
APA StyleHong, S., Yuan, Y., Zhang, K., Lian, H., & Liimatainen, H. (2020). Efficient Hydrolysis of Chitin in a Deep Eutectic Solvent Synergism for Production of Chitin Nanocrystals. Nanomaterials, 10(5), 869. https://doi.org/10.3390/nano10050869