Multigenerational Exposure to WCCo Nanomaterials—Epigenetics in the Soil Invertebrate Enchytraeus crypticus
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Organisms
2.2. Test Soil
2.3. Test Materials, Characterisation, and Spiking
2.4. Exposure Procedure and Characterization
2.5. Epigenetic Analysis
2.5.1. DNA Extraction
2.5.2. Global DNA Methylation
2.6. Data Analysis
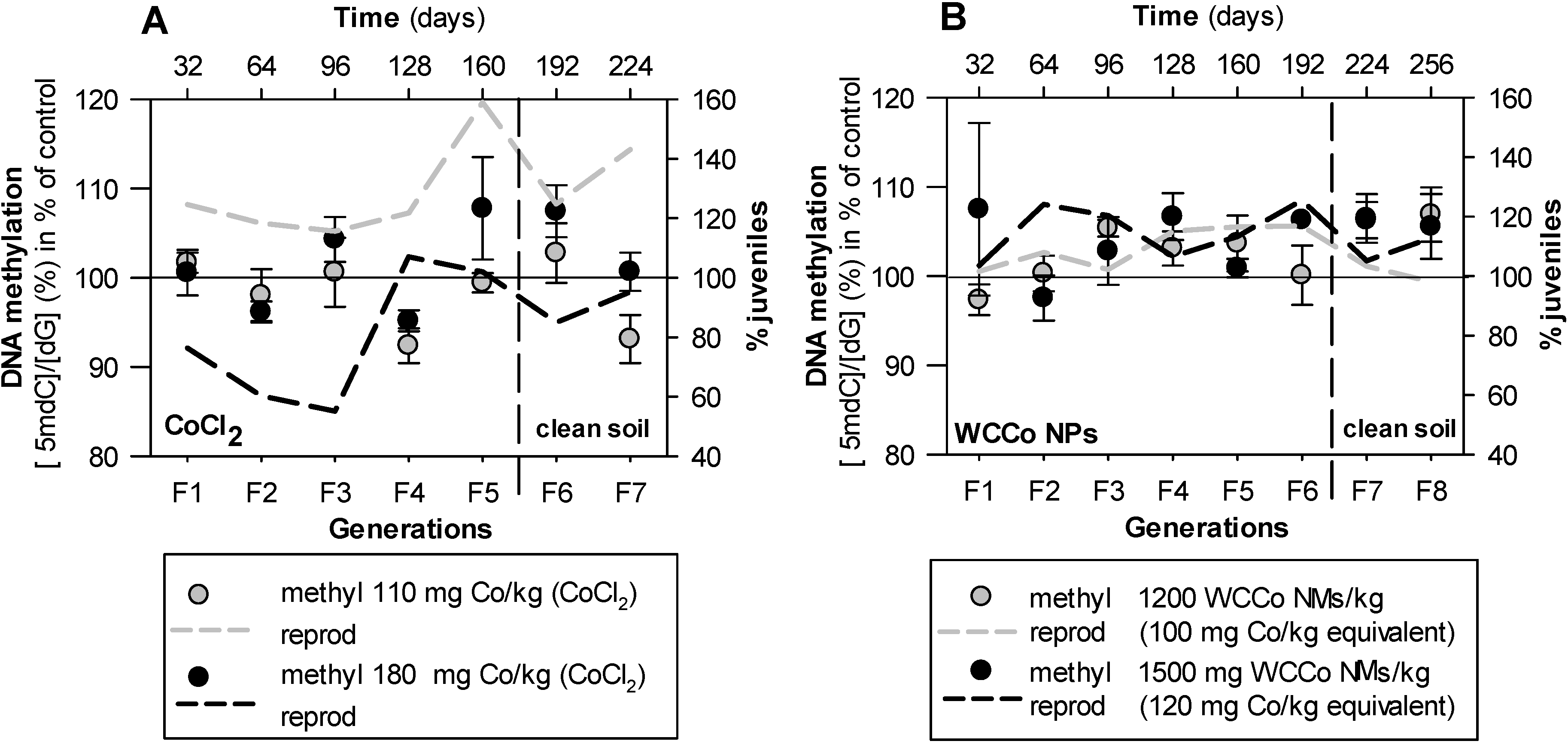
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cote, I.; Vandenberg, J.J.; Druwe, I.L.; Angrish, M.M. Incorporating Epigenetics Into a Risk Assessment Framework. In Toxicoepigenetics; McCullough, S.D., Dolinoy, D.C.B.T.-T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–310. [Google Scholar]
- Smolkova, B.; Dusinska, M.; Gabelova, A. Epigenetic Effects of Nanomaterials. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2019; pp. 678–685. [Google Scholar]
- Gedda, M.R.; Babele, P.K.; Zahra, K.; Madhukar, P. Epigenetic Aspects of Engineered Nanomaterials: Is the Collateral Damage Inevitable? Front. Bioeng. Biotechnol. 2019, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Gim, J.; Choi, J. Epigenetic profiling to environmental stressors in model and non-model organisms: Ecotoxicology perspective. Environ. Health Toxicol. 2018, 33, e2018015. [Google Scholar] [CrossRef] [PubMed]
- Stoccoro, A.; Karlsson, H.L.; Coppedè, F.; Migliore, L. Epigenetic effects of nano-sized materials. Toxicology 2013, 313, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gorrochategui, E.; Li, J.; Fullwood, N.J.; Ying, G.-G.; Tian, M.; Cui, L.; Shen, H.; Lacorte, S.; Tauler, R.; Martin, F.L. Diet-sourced carbon-based nanoparticles induce lipid alterations in tissues of zebrafish (Danio rerio) with genomic hypermethylation changes in brain. Mutagenesis 2017, 32, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-H.; Guan, P.; Zhang, T.; Lu, C.; Li, G.; Liu, J.-X. Silver nanoparticles impair zebrafish skeletal and cardiac myofibrillogenesis and sarcomere formation. Aquat. Toxicol. 2018, 200, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, Q.; Li, Y.; Nouara, A.; Jia, R.; Wang, D. In vivo translocation and toxicity of multi-walled carbon nanotubes are regulated by microRNAs. Nanoscale 2014, 6, 4275. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Gao, Y.; Mu, L.; Zhou, Q. Knowledge gaps between nanotoxicological research and nanomaterial safety. Environ. Int. 2016, 94, 8–23. [Google Scholar] [CrossRef]
- Oomen, A.G.; Steinhäuser, K.G.; Bleeker, E.A.; van Broekhuizen, F.; Sips, A.; Dekkers, S.; Wijnhoven, S.W.; Sayre, P.G. NanoImpact Risk assessment frameworks for nanomaterials: Scope, link to regulations, applicability, and outline for future directions in view of needed increase in efficiency. NanoImpact 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Scott-fordsmand, J.J.; Amorim, M.J.B.; Sørensen, P.B. Implementing the DF4 in a robust model, allowing for enhanced comparison, prioritisation and grouping of Nanomaterials. Regul. Toxicol. Pharmacol. 2018, 92, 207–212. [Google Scholar] [CrossRef]
- Schultz, C.L.; Wamucho, A.; Tsyusko, O.V.; Unrine, J.M.; Crossley, A.; Svendsen, C.; Spurgeon, D.J. Multigenerational exposure to silver ions and silver nanoparticles reveals heightened sensitivity and epigenetic memory in Caenorhabditis elegans. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152911. [Google Scholar] [CrossRef]
- Arndt, D.A.; Chen, J.; Moua, M.; Klaper, R.D. Multigeneration impacts on Daphnia magna of carbon nanomaterials with differing core structures and functionalizations. Environ. Toxicol. Chem. 2014, 33, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Noordhoek, J.W.; Koning, J.T.; Mariën, J.; Kamstra, J.H.; Amorim, M.J.B.; van Gestel, C.A.M.; van Straalen, N.M.; Roelofs, D. Exploring DNA methylation patterns in copper exposed Folsomia candida and Enchytraeus crypticus. Pedobiologia (Jena) 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Bicho, R.C.; Roelofs, D.; Mariën, J.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Epigenetic effects of (nano)materials in environmental species—Cu case study in Enchytraeus crypticus. Environ. Int. 2020, 136, 105447. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Stiglich, J.J.; Sudarshan, T. Nanosized WCCo Holds Promise for the Future. Metal Powder Report 1998, 53, 26–33. [Google Scholar]
- Upadhyaya, G.S. Cemented Tungsten Carbides: Production, Properties and Testing; William Andrew: Westwood, NJ, USA, 1998. [Google Scholar]
- Lison, D.; Carbonnelle, P.; Mollo, L.; Lauwerys, R.; Fubini, B. Physicochemical Mechanism of the Interaction between Cobalt Metal and Carbide Particles to Generate Toxic Activated Oxygen Species. Chem. Res. Toxicol. 1995, 8, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.J.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Multigenerational exposure to cobalt (CoCl2) and WCCo nanoparticles in Enchytraeus crypticus. Nanotoxicology 2019, 13, 751–760. [Google Scholar] [CrossRef]
- Erturk, F.A.; Agar, G.; Nardemir, G.; Arslan, E. Cytogenetic and epigenetic alterations by cobalt and nickel on Zea mays L. Toxicol. Environ. Chem. 2015, 97, 1350–1362. [Google Scholar] [CrossRef]
- Rancelis, V.; Cesniene, T.; Kleizaite, V.; Zvingila, D.; Balciuniene, L. Influence of cobalt uptake by Vicia faba seeds on chlorophyll morphosis induction, SOD polymorphism, and DNA methylation. Environ. Toxicol. 2012, 27, 32–41. [Google Scholar] [CrossRef]
- Steinberg, J.; Shah, K.M.; Gartland, A.; Zeggini, E.; Wilkinson, J.M. Effects of chronic cobalt and chromium exposure after metal-on-metal hip resurfacing: An epigenome-wide association pilot study. J. Orthop. Res. 2017, 35, 2323–2328. [Google Scholar] [CrossRef]
- Li, Q.; Ke, Q.; Costa, M. Alterations of histone modifications by cobalt compounds. Carcinogenesis 2009, 30, 1243–1251. [Google Scholar] [CrossRef]
- Papageorgiou, I.; Brown, C.; Schins, R.; Singh, S.; Newson, R.; Davis, S.; Fisher, J.; Ingham, E.; Case, C.P. The effect of nano- and micron-sized particles of cobalt–chromium alloy on human fibroblasts in vitro. Biomaterials 2007, 28, 2946–2958. [Google Scholar] [CrossRef]
- Figgitt, M.; Newson, R.; Leslie, I.J.; Fisher, J.; Ingham, E.; Case, C.P. The genotoxicity of physiological concentrations of chromium (Cr(III) and Cr(VI)) and cobalt (Co(II)): An in vitro study. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 688, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Tsaousi, A.; Jones, E.; Case, C.P. The in vitro genotoxicity of orthopaedic ceramic (Al2O3) and metal (CoCr alloy) particles. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2010, 697, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Magaye, R.; Zhao, J.; Bowman, L.; Ding, M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp. Ther. Med. 2012, 4, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Kisin, E.R.; Zhao, J.; Bowman, L.; Lu, Y.; Jiang, B.; Leonard, S.; Vallyathan, V.; Castranova, V.; Murray, A.R.; et al. Size-dependent effects of tungsten carbide-cobalt particles on oxygen radical production and activation of cell signaling pathways in murine epidermal cells. Toxicol. Appl. Pharmacol. 2009, 241, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, F.; Lison, D.; Kirsch-Volders, M. Comparative evaluation of the in vitro micronucleus test and the alkaline single cell gel electrophoresis assay for the detection of DNA damaging agents: Genotoxic effects of cobalt powder, tungsten carbide and cobalt-tungsten carbide. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 1997, 392, 31–43. [Google Scholar] [CrossRef]
- De Boeck, M.; Kirsch-Volders, M.; Lison, D. Cobalt and antimony: Genotoxicity and carcinogenicity. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2003, 533, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Bicho, R.C.; Santos, F.C.F.; Gonçalves, M.F.M.; Soares, A.M.V.M.; Amorim, M.J.B. Enchytraeid Reproduction TestPLUS: Hatching, growth and full life cycle test—An optional multi-endpoint test with Enchytraeus crypticus. Ecotoxicology 2015, 24, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- OECD (Organization for Economic Cooperation and Development). Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials. In Safety on Manufactured Nanomaterials No. 36; OECD Publishing: Paris, France, 2012. [Google Scholar]
- Ribeiro, M.J.; Maria, V.L.; Soares, A.M.V.M.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Fate and Effect of Nano Tungsten Carbide Cobalt (WCCo) in the Soil Environment: Observing a Nanoparticle Specific Toxicity in Enchytraeus crypticus. Environ. Sci. Technol. 2018, 52, 11394–11401. [Google Scholar] [CrossRef]
- SigmaPlot. Statistical Package for the Social Sciences e SigmaPlot for Windows. Systat Software: Santa Clara, CA, USA, 1997. [Google Scholar]
- Bahadori, T.; Bell, D.; Ceccatelli, S.; Corvi, R.; Hogstrand, C.; Munn, S.; Nilsson, E.; Spurgeon, D.; Vom Brocke, J.; Wray-Cahen, D.; et al. EFSA Scientific Colloquium 22—Epigenetics and Risk Assessment: Where do we stand? EFSA Supporting Publ. 2016, 13. [Google Scholar] [CrossRef]
- Vandegehuchte, M.B.; Janssen, C.R. Epigenetics in an ecotoxicological context. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2014, 764, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kresovich, J.K.; Harmon, Q.E.; Xu, Z.; Nichols, H.B.; Sandler, D.P.; Taylor, J.A. Reproduction, DNA methylation and biological age. Hum. Reprod. 2019, 34, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | WCCo NP | Technique |
|---|---|---|
| Source | NBM Nanomaterialia, Italy | |
| Composition (%) | Tungsten carbide (WC < 88% Wt., CAS 12070-12-1) Cobalt (Co = 8.32% Wt., CAS 744-48-4) | ICP-MS |
| Primary Size distribution [nm] Average (Min-Max) Mode [nm] (1st and 3rd quartile) | 170 (23-1446)48 (69; 280) | TEM |
| Crystalline size (Average) [nm] | 15.4 | XRD |
| Iso Electric Point (pH) | <2 | pH |
| Dispersability in water: D50 [nm]; Average Agglomeration Number (AAN) | 182.8 ± 21.5; 31 | DLS |
| Specific Surface Area [m2.g −1] | 6.6 ± 0.4 | BET |
| Z-potential [mV] | 7.1 ± 0.5 | ELS |
| Structure | O-W-O | FTIR and/or RAMAN |
| Pore size [nm] | Non-porous | BET |
| Surface Chemistry [atomic fraction] | Co = 0.08 ± 0.01 | XPS |
| W = 0.05 ± 0.01 | ||
| O = 0.31 ± 0.03 | ||
| C = 0.56 ± 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bicho, R.C.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Multigenerational Exposure to WCCo Nanomaterials—Epigenetics in the Soil Invertebrate Enchytraeus crypticus. Nanomaterials 2020, 10, 836. https://doi.org/10.3390/nano10050836
Bicho RC, Scott-Fordsmand JJ, Amorim MJB. Multigenerational Exposure to WCCo Nanomaterials—Epigenetics in the Soil Invertebrate Enchytraeus crypticus. Nanomaterials. 2020; 10(5):836. https://doi.org/10.3390/nano10050836
Chicago/Turabian StyleBicho, Rita C., Janeck J. Scott-Fordsmand, and Mónica J.B. Amorim. 2020. "Multigenerational Exposure to WCCo Nanomaterials—Epigenetics in the Soil Invertebrate Enchytraeus crypticus" Nanomaterials 10, no. 5: 836. https://doi.org/10.3390/nano10050836
APA StyleBicho, R. C., Scott-Fordsmand, J. J., & Amorim, M. J. B. (2020). Multigenerational Exposure to WCCo Nanomaterials—Epigenetics in the Soil Invertebrate Enchytraeus crypticus. Nanomaterials, 10(5), 836. https://doi.org/10.3390/nano10050836




