Noble Metal-Free TiO2-Coated Carbon Nitride Layers for Enhanced Visible Light-Driven Photocatalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Synthesis of TiO2 and g-C3N4/TiO2
2.2. Characterization
2.3. Measurement of Photocatalytic Activity
2.3.1. RhB degradation
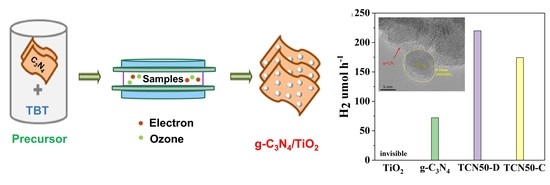
2.3.2. Hydrogen Generation
2.3.3. Photoelectrochemical Investigation
3. Results and Discussion
3.1. Physico-Chemical Properties
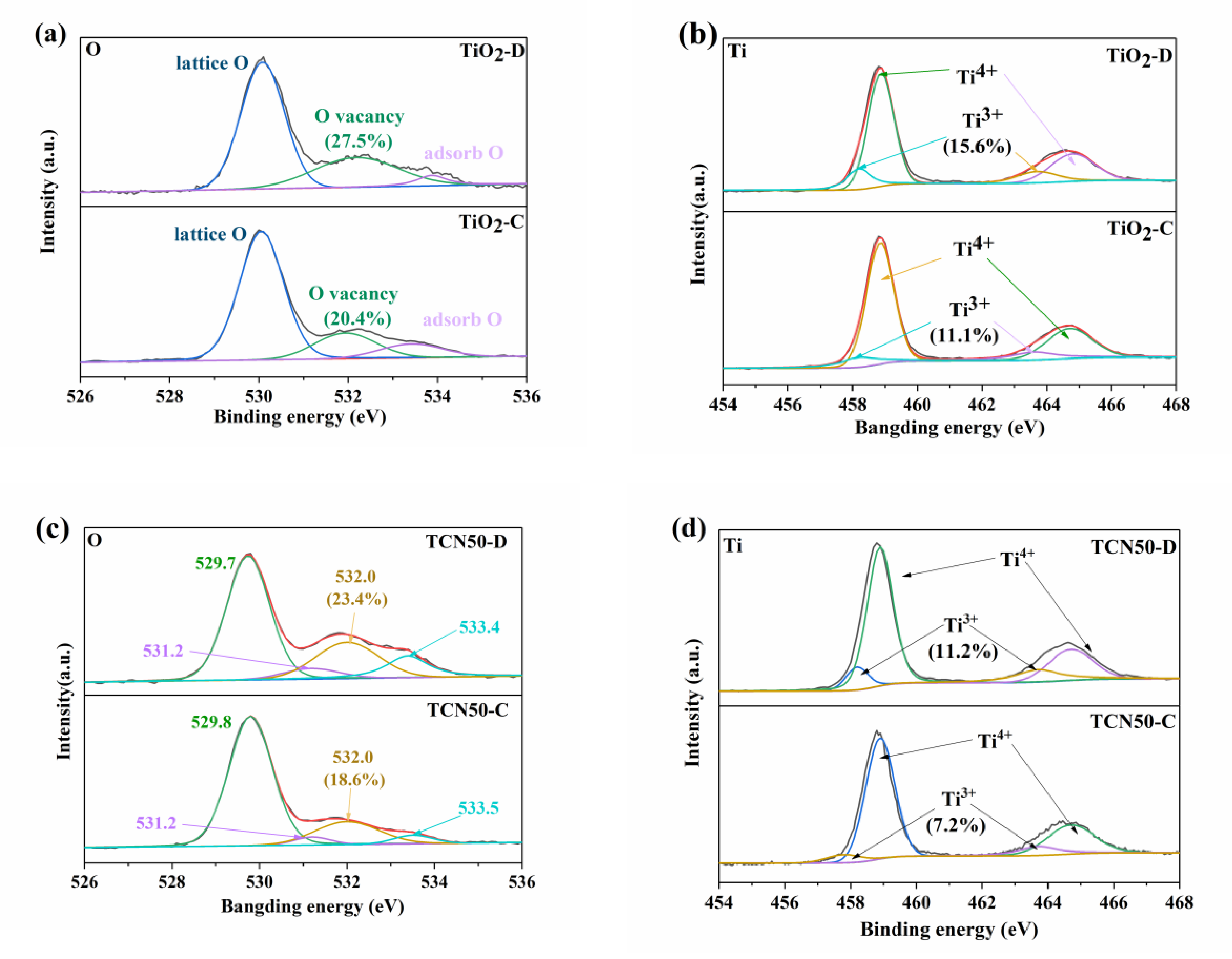
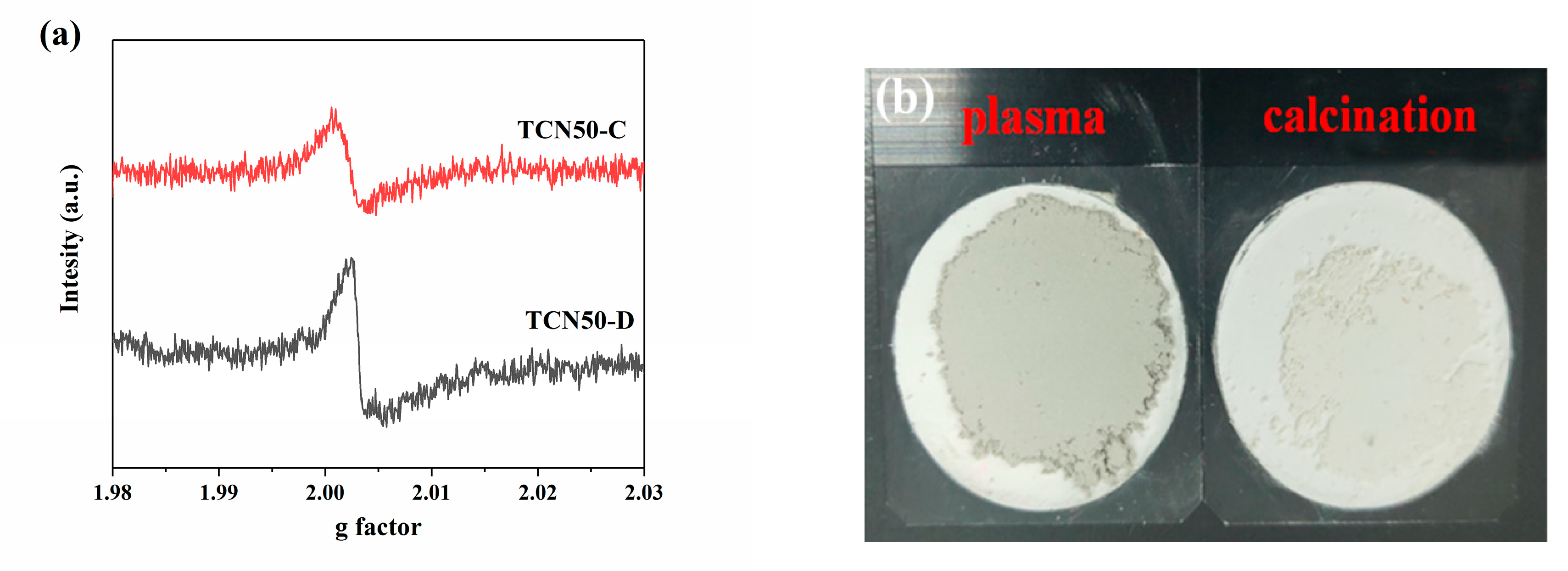
3.2. Characterizations of Oxygen Vacancies
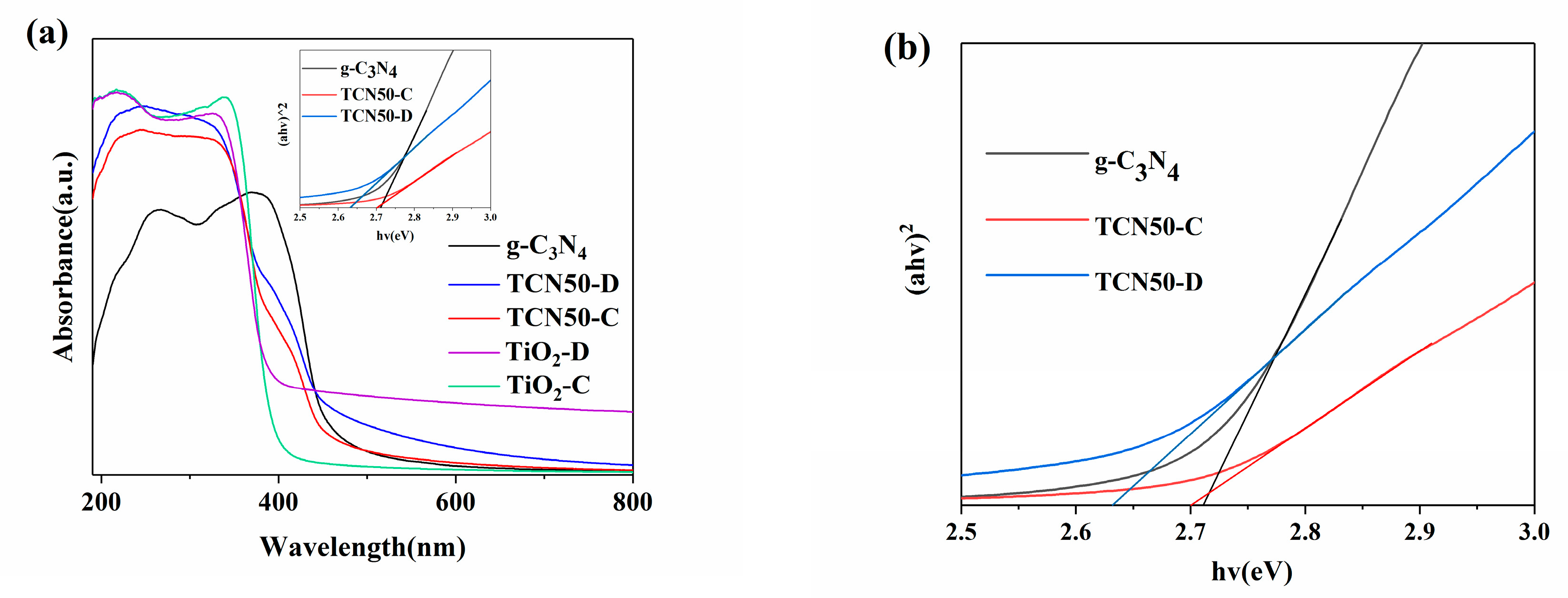
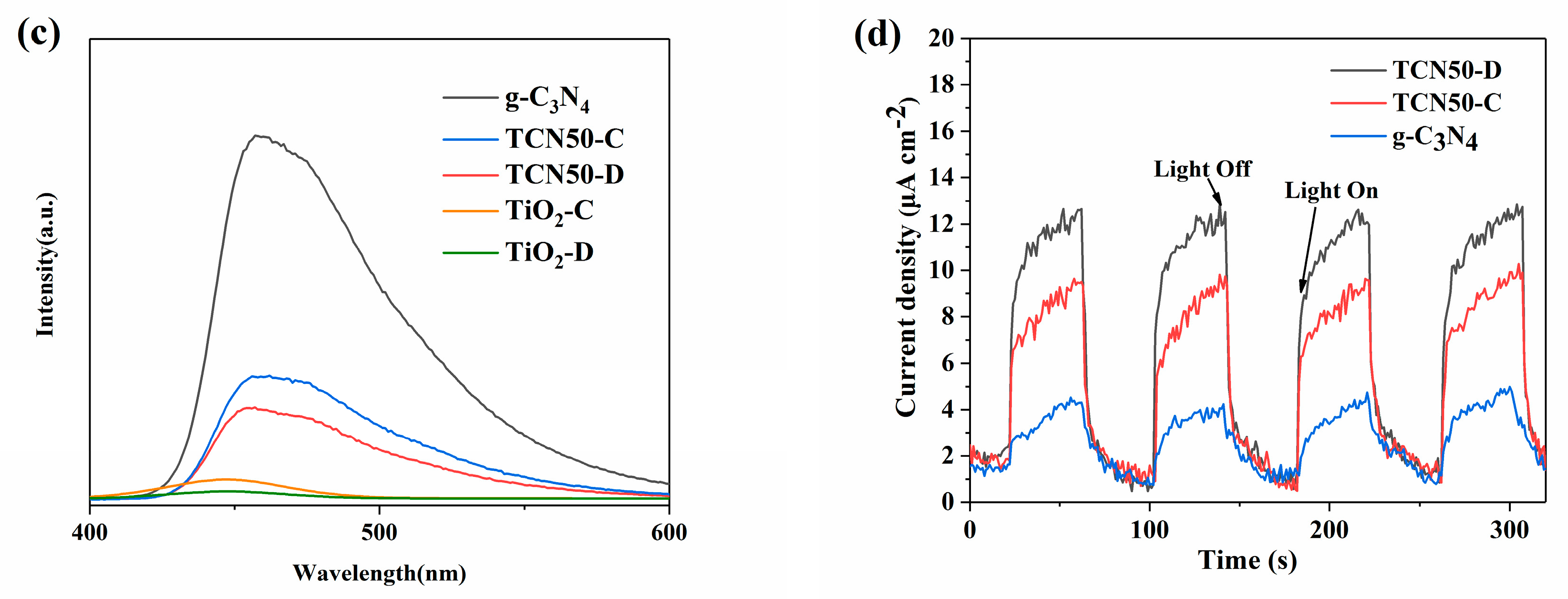
3.3. Optical Properties
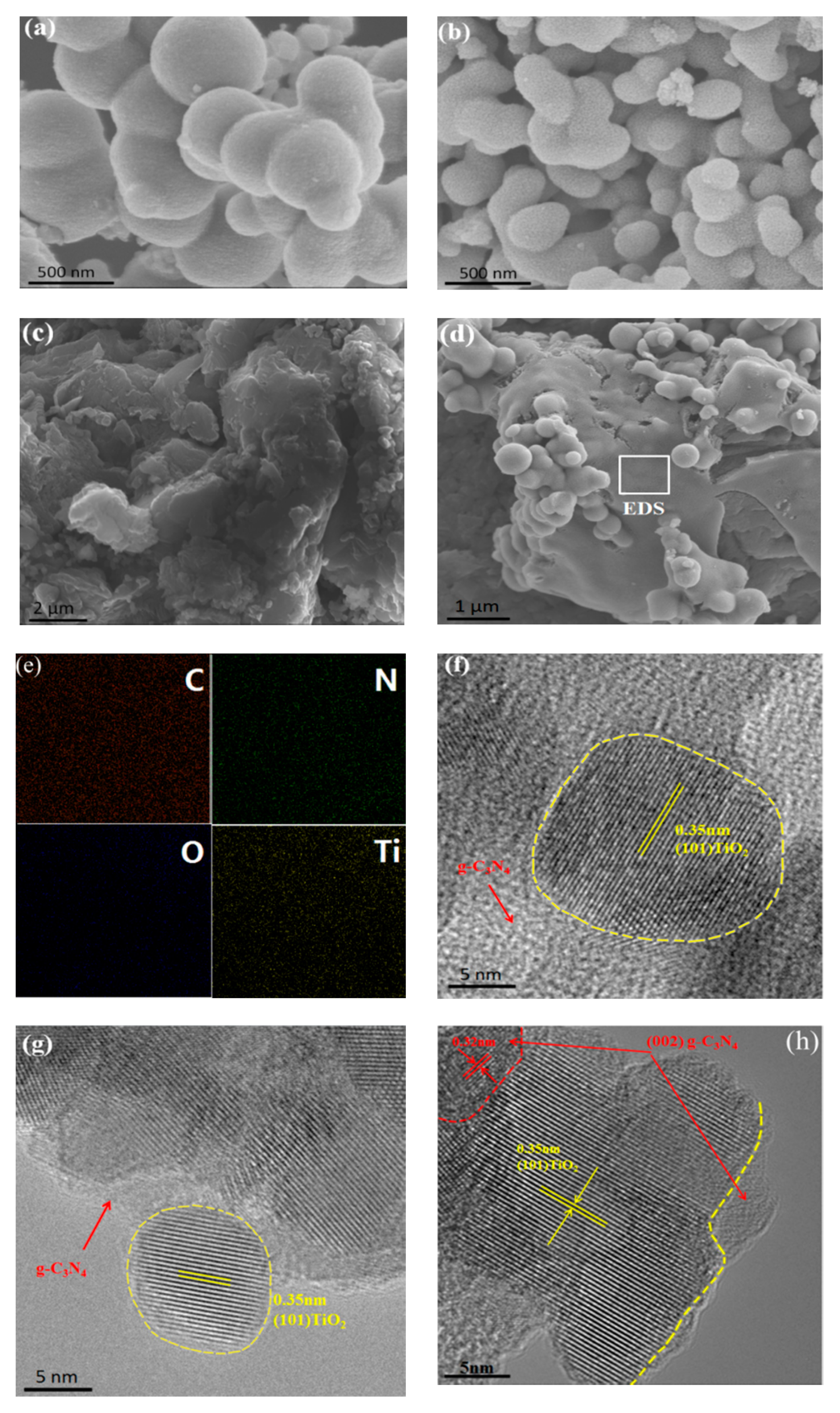
3.4. Morphologies
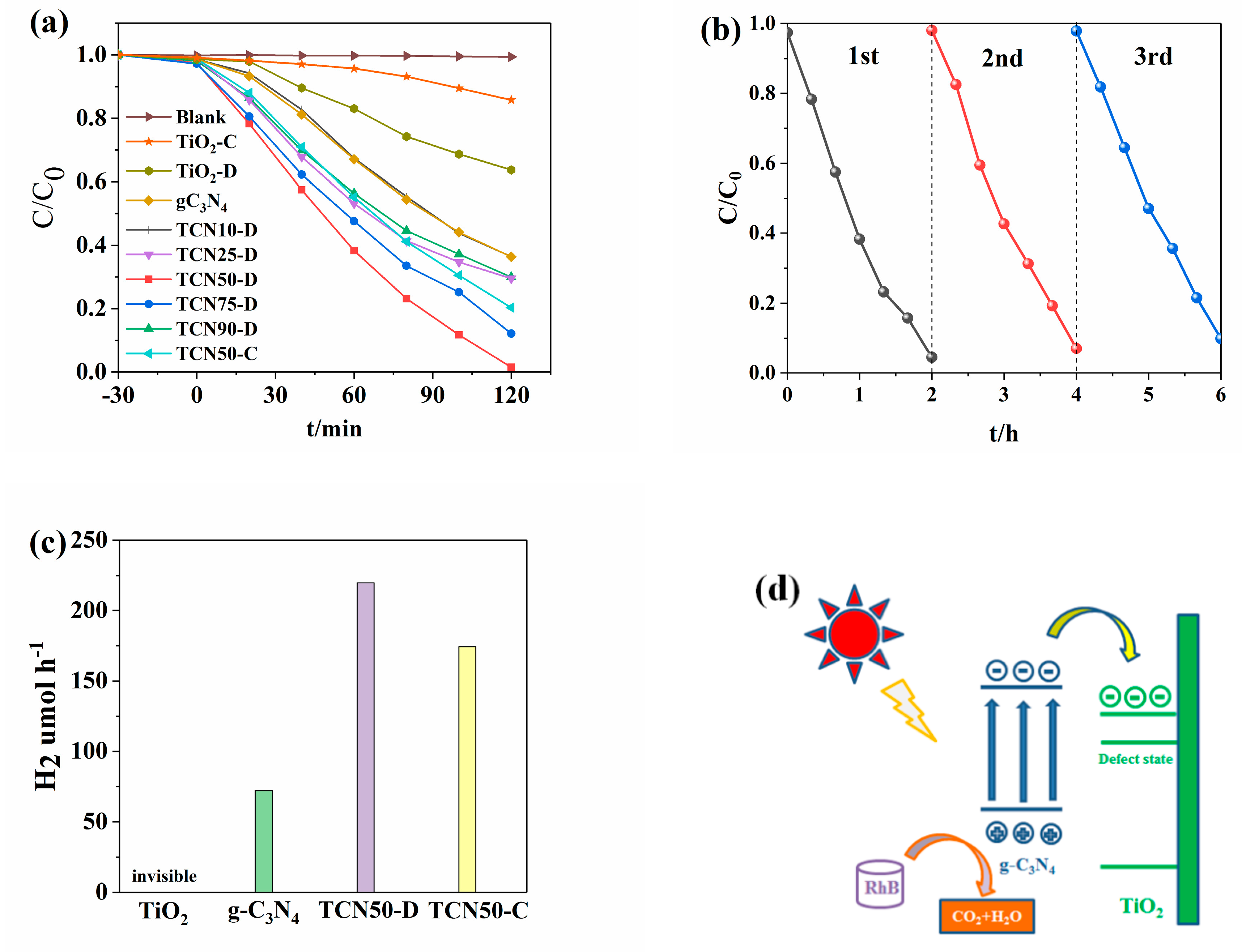
3.5. Photocatalytic Activity
3.6. Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chava, R.K.; Son, N.; Kim, Y.S.; Kang, M. Controlled Growth and Bandstructure Properties of One Dimensional Cadmium Sulfide Nanorods for Visible Photocatalytic Hydrogen Evolution Reaction. Nanomaterials 2020, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; You, Y.; Xin, S.; Li, Y.; Fu, G.; Cui, Z.; Men, Y.; Cao, F.; Yu, S.; Goodenough, J.B. Photocatalytic CO2 reduction by carbon-coated indium-oxide nanobelts. J. Am. Chem. Soc. 2017, 139, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yu, Z.; Wang, X. Crystalline carbon nitride semiconductors for photocatalytic water splitting. Angew. Chem. Int. Ed. 2019, 131, 6225–6236. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef]
- Nawaz, R.; Kait, C.F.; Chia, H.Y.; Isa, M.H.; Huei, L.W. Glycerol-mediated facile synthesis of colored titania nanoparticles for visible light photodegradation of phenolic compounds. Nanomaterials 2019, 9, 1586. [Google Scholar] [CrossRef]
- Yu, X.; Fan, X.; An, L.; Liu, G.; Li, Z.; Liu, J.; Hu, P. Mesocrystalline Ti3+-TiO2 hybridized g-C3N4 for efficient visible-light photocatalysis. Carbon 2018, 128, 21–30. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B-Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Chen, Y. Anatase TiO2 co-doped with silver and ceria for antibacterial application. Catal. Today 2018, 310, 68–74. [Google Scholar] [CrossRef]
- Ma, J.; Tan, X.; Jiang, F.; Yu, T. Graphitic C3N4 nanosheet-sensitized brookite TiO2 to achieve photocatalytic hydrogen evolution under visible light. Catal. Sci. Technol. 2017, 7, 3275–3282. [Google Scholar] [CrossRef]
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B-Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Zhu, S.; Yu, F.; Dai, B.; Yang, D. A review of recent advances of dielectric barrier discharge plasma in catalysis. Nanomaterials 2019, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.L.; Liu, C. Catalyst preparation with plasmas: How does it work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Dou, S.; Tao, L.; Wang, R.; El Hankari, S.; Chen, R.; Wang, S. Plasma-assisted synthesis and surface modification of electrode materials for renewable energy. Adv. Mater. 2018, 30, 1705850. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, B.; Liu, Y.; Li, Y. Dielectric barrier discharge plasma for preparation of Ni-based catalysts with enhanced coke resistance: Current status and perspective. Catal. Today 2015, 256, 29–40. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, B.; Liu, C. Characterization of ZnO nanotube fabricated by the plasma decomposition of Zn(OH)2 via dielectric barrier discharge. Plasma Chem. Plasma Process. 2012, 32, 201–209. [Google Scholar] [CrossRef]
- Zada, A.; Qu, Y.; Ali, S.; Sun, N.; Lu, H.; Yan, R.; Zhang, X.; Jing, L. Improved visible-light activities for degrading pollutants on TiO2/g-C3N4 nanocomposites by decorating SPR Au nanoparticles and 2,4-dichlorophenol decomposition path. J. Hazard. Mater. 2018, 342, 715–723. [Google Scholar] [CrossRef]
- Tahir, M.B.; Sagir, M.; Shahzad, K. Removal of acetylsalicylate and methyl-theobromine from aqueous environment using nano-photocatalyst WO3-TiO2 @g-C3N4 composite. J. Hazard. Mater. 2019, 363, 205–213. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, X.; Liu, C.; Qian, X.; Wen, Y.; Yang, Q.; Sun, T.; Chang, W.; Liu, X.; Chen, Z. Facile construction of all-solid-state Z-scheme g-C3N4/TiO2 thin film for the efficient visible-light degradation of organic pollutant. Nanomaterials 2020, 10, 600. [Google Scholar] [CrossRef]
- Marques, J.; Gomes, T.D.; Forte, M.A.; Silva, R.F.; Tavares, C.J. A new route for the synthesis of highly-active N-doped TiO2 nanoparticles for visible light photocatalysis using urea as nitrogen precursor. Catal. Today 2019, 326, 36–45. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, L.; Song, W.; Qin, Z.; Zeng, D.; Xie, C. In situ synthesis of C-TiO2/g-C3N4 heterojunction nanocomposite as highly visible light active photocatalyst originated from effective interfacial charge transfer. Appl. Catal. B-Environ. 2017, 202, 489–499. [Google Scholar] [CrossRef]
- Sanabria-Arenas, B.E.; Mazare, A.; Yoo, J.; Nhat, T.N.; Hejazi, S.; Bian, H.; Diamanti, M.V.; Pedeferri, M.P.; Schmuki, P. Intrinsic AuPt-alloy particles decorated on TiO2 nanotubes provide enhanced photocatalytic degradation. Electrochim. Acta 2018, 292, 865–870. [Google Scholar] [CrossRef]
- Hampel, B.; Kovacs, G.; Czekes, Z.; Hernadi, K.; Danciu, V.; Ersen, O.; Girleanu, M.; Focsan, M.; Baia, L.; Pap, Z. Mapping the photocatalytic activity and ecotoxicology of Au, Pt/TiO2 composite photocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 12993–13006. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, Y.; Sun, F.; Wang, R.; Zhou, Y. Novel mpg-C3N4/TiO2 nanocomposite photocatalytic membrane reactor for sulfamethoxazole photodegradation. Chem. Eng. J. 2018, 337, 183–192. [Google Scholar] [CrossRef]
- Lee, G.; Lee, X.; Lyu, C.; Liu, N.; Andandan, S.; Wu, J.J. Sonochemical synthesis of copper-doped BiVO4/g-C3N4 nanocomposite materials for photocatalytic degradation of bisphenol a under simulated sunlight irradiation. Nanomaterials 2020, 10, 498. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, X.; Tian, S.; Wang, Z. Enhanced hydrogen production from water on Pt/g-C3N4 by room temperature electron reduction. Mater. Res. Bull. 2018, 104, 1–5. [Google Scholar] [CrossRef]
- Yan, J.; Wu, H.; Chen, H.; Zhang, Y.; Zhang, F.; Liu, S.F. Fabrication of TiO2/C3N4 heterostructure for enhanced photocatalytic Z-scheme overall water splitting. Appl. Catal. B-Environ. 2016, 191, 130–137. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; He, H.; Wang, X.; Zhang, J.; Zhang, Q.; Tong, Y.; Liu, H.; Ramakrishna, S.; Yan, S.; et al. One-step synthesis heterostructured g-C3N4/TiO2 composite for rapid degradation of pollutants in utilizing visible light. Nanomaterials 2018, 8, 842. [Google Scholar] [CrossRef]
- Smýkalová, A.; Sokolová, B.; Foniok, K.; Matějka, V.; Praus, P. Photocatalytic degradation of selected pharmaceuticals using g-C3N4 and TiO2 nanomaterials. Nanomaterials 2019, 9, 1194. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Yu, J.; Dimotikali, D.; Trapalis, C. Photocatalytic activity of modified g-C3N4 /TiO2 nanocomposites for NOx removal. Catal. Today 2017, 280, 37–44. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Peng, X.; Wang, Z.; Zhou, L.; Yin, Q. A novel route to manufacture 2D layer MoS2 and g-C3N4 by atmospheric plasma with enhanced visible-light-driven photocatalysis. Nanomaterials 2019, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, Z.; Wang, Z.; Gong, J.; Hao, H. Electron reduction for the preparation of rGO with high electrochemical activity. Catal. Today 2019, 337, 63–68. [Google Scholar] [CrossRef]
- Yang, H.; Jin, Z.; Hu, H.; Bi, Y.; Lu, G. Ni-Mo-S nanoparticles modified graphitic C3N4 for efficient hydrogen evolution. Appl. Surf. Sci. 2018, 427, 587–597. [Google Scholar] [CrossRef]
- Du, X.; Bai, X.; Xu, L.; Yang, L.; Jin, P. Visible-light activation of persulfate by TiO2/g-C3N4 photocatalyst toward efficient degradation of micropollutants. Chem. Eng. J. 2020, 384, 123245. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Jiang, C.; Tang, H.; Xu, Q. In situ hydrothermal synthesis of g-C3N4/TiO2 heterojunction photocatalysts with high specific surface area for Rhodamine B degradation. Appl. Surf. Sci. 2017, 411, 400–410. [Google Scholar] [CrossRef]
- Di, T.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. A direct Z-scheme g-C3N4/SnS2 photocatalyst with superior visible-light CO2 reduction performance. J. Catal. 2017, 352, 532–541. [Google Scholar] [CrossRef]
- Lei, J.; Chen, Y.; Shen, F.; Wang, L.; Liu, Y.; Zhang, J. Surface modification of TiO2 with g-C3N4 for enhanced UV and visible photocatalytic activity. J. Alloys Compd. 2015, 631, 328–334. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Liu, L.; Hu, J.; Cui, W. Highly ordered TiO2 nanotube arrays wrapped with g-C3N4 nanoparticles for efficient charge separation and increased photoelectrocatalytic degradation of phenol. J. Hazard. Mater. 2018, 344, 369–380. [Google Scholar] [CrossRef]
- Ma, J.; Tan, X.; Yu, T.; Li, X. Fabrication of g-C3N4/TiO2 hierarchical spheres with reactive {001} TiO2 crystal facets and its visible-light photocatalytic activity. Int. J. Hydrogen Energy 2016, 41, 3877–3887. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Xiao, T.; Tian, Y.; Jiang, Z. Biomimetic fabrication of g-C3N4/TiO2 nanosheets with enhanced photocatalytic activity toward organic pollutant degradation. Chem. Eng. J. 2015, 260, 117–125. [Google Scholar] [CrossRef]
- Ma, L.; Wang, G.; Jiang, C.; Bao, H.; Xu, Q. Synthesis of core-shell TiO2@g-C3N4 hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl. Surf. Sci. 2018, 430, 263–272. [Google Scholar] [CrossRef]
- Li, F.T.; Liu, S.J.; Xue, Y.B.; Wang, X.J.; Hao, Y.J.; Zhao, J.; Liu, R.H.; Zhao, D.S. Structure modification function of g-C3N4 for Al2O3 in the in situ hydrothermal process for enhanced photocatalytic activity. Chem. A Eur. J. 2015, 21, 10149–10159. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Liu, W.; Zhang, S. Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4-RGO-TiO2 nanoheterojunctions. Appl. Surf. Sci. 2017, 405, 60–70. [Google Scholar] [CrossRef]
- Shi, L.; Yang, L.; Zhou, W.; Liu, Y.; Yin, L.; Hai, X.; Song, H.; Ye, J. Photoassisted construction of holey defective g-C3N4 photocatalysts for efficient visible-light-driven H2O2 production. Small 2018, 14, 1703142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Feng, G.; Hu, M.; Huang, Y.; Zeng, H. Liquid-plasma hydrogenated synthesis of gray titania with engineered surface defects and superior photocatalytic activity. Nanomaterials 2020, 10, 342. [Google Scholar] [CrossRef]
- Shi, H.; Long, S.; Hu, S.; Hou, J.; Ni, W.; Song, C.; Li, K.; Gurzadyan, G.G.; Guo, X. Interfacial charge transfer in 0D/2D defect-rich heterostructures for efficient solar-driven CO2 reduction. Appl. Catal. B-Environ. 2019, 245, 760–769. [Google Scholar] [CrossRef]
- Feng, X.; Wang, P.; Hou, J.; Qian, J.; Ao, Y.; Wang, C. Significantly enhanced visible light photocatalytic efficiency of phosphorus doped TiO2 with surface oxygen vacancies for ciprofloxacin degradation: Synergistic effect and intermediates analysis. J. Hazard. Mater. 2018, 351, 196–205. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Afzal, N.; Pan, L.; Zhang, X.; Zou, J.J. Structure-activity relationship of defective metal-based photocatalysts for water splitting: Experimental and theoretical perspectives. Adv. Sci. 2019, 6, 1900053. [Google Scholar] [CrossRef]
- Chen, X.; Wei, J.; Hou, R.; Liang, Y.; Xie, Z.; Zhu, Y.; Zhang, X.; Wang, H. Growth of g-C3N4 on mesoporous TiO2 spheres with high photocatalytic activity under visible light irradiation. Appl. Catal. B-Environ. 2016, 188, 342–350. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Wang, F.; Di, L.; Yang, S.; Zhu, S.; Yao, Y.; Ma, C.; Dai, B.; Yu, F. Enhanced photocatalytic degradation of organic dyes via defect-rich TiO2 prepared by dielectric barrier discharge plasma. Nanomaterials 2019, 9, 720. [Google Scholar] [CrossRef]
- Lv, Y.; Pan, C.; Ma, X.; Zong, R.; Bai, X.; Zhu, Y. Production of visible activity and UV performance enhancement of ZnO photocatalyst via vacuum deoxidation. Appl. Catal. B-Environ. 2013, 138–139, 26–32. [Google Scholar] [CrossRef]
- Xue, K.; He, R.; Yang, T.; Wang, J.; Sun, R.; Wang, L.; Yu, X.; Omeoga, U.; Pi, S.; Yang, T.; et al. MOF-based In2S3-X2S3 (X=Bi; Sb)@TFPT-COFs hybrid materials for enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2019, 493, 41–54. [Google Scholar] [CrossRef]
- Jiang, X.; Xing, Q.; Luo, X.; Li, F.; Zou, J.; Liu, S.; Li, X.; Wang, X. Simultaneous photoreduction of Uranium(VI) and photooxidation of Arsenic(III) in aqueous solution over g-C3N4/TiO2 heterostructured catalysts under simulated sunlight irradiation. Appl. Catal. B-Environ. 2018, 228, 29–38. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Wu, M.; Chen, J.; Zhao, W.; Tian, Y.; Ding, T.; Zhang, J.; Jiang, Z.; Li, X. Rational construction of oxygen vacancies onto tungsten trioxide to improve visible light photocatalytic water oxidation reaction. Appl. Catal. B-Environ. 2018, 239, 398–407. [Google Scholar] [CrossRef]
- Li, J.; Weng, B.; Cai, S.; Chen, J.; Jia, H.; Xu, Y. Efficient promotion of charge transfer and separation in hydrogenated TiO2/WO3 with rich surface-oxygen-vacancies for photodecomposition of gaseous toluene. J. Hazard. Mater. 2018, 342, 661–669. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Wang, J.; Zhou, Y.; Wang, J.; Tang, J.; Wang, L.; Feng, C. Facile fabrication of mediator-free Z-scheme photocatalyst of phosphorous-doped ultrathin graphitic carbon nitride nanosheets and bismuth vanadate composites with enhanced tetracycline degradation under visible light. J. Colloid Interface Sci. 2018, 509, 219–234. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, J.; Xie, Y.; Chen, J.; Li, C.; Wang, J.; Luo, J.; Deng, B.; Hu, X. Fabrication, characterization, and photocatalytic performance of exfoliated g-C3N4-TiO2 hybrids. Appl. Surf. Sci. 2014, 311, 574–581. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]

| Sample | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Radius (nm) | Crystallite Size (nm) |
|---|---|---|---|---|
| TiO2-D | 64.5649 | 0.1566 | 2.99 | 14.3 |
| TiO2-C | 28.1876 | 0.1023 | 6.79 | 17.8 |
| TCN50-D | 72.8473 | 0.1451 | 2.86 | 12.3 |
| TCN50-C | 29.2735 | 0.1117 | 6.02 | 14.4 |
| g-C3N4 | 7.1313 | 0.0604 | 11.34 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Peng, X.; Wang, Z. Noble Metal-Free TiO2-Coated Carbon Nitride Layers for Enhanced Visible Light-Driven Photocatalysis. Nanomaterials 2020, 10, 805. https://doi.org/10.3390/nano10040805
Zhang B, Peng X, Wang Z. Noble Metal-Free TiO2-Coated Carbon Nitride Layers for Enhanced Visible Light-Driven Photocatalysis. Nanomaterials. 2020; 10(4):805. https://doi.org/10.3390/nano10040805
Chicago/Turabian StyleZhang, Bo, Xiangfeng Peng, and Zhao Wang. 2020. "Noble Metal-Free TiO2-Coated Carbon Nitride Layers for Enhanced Visible Light-Driven Photocatalysis" Nanomaterials 10, no. 4: 805. https://doi.org/10.3390/nano10040805
APA StyleZhang, B., Peng, X., & Wang, Z. (2020). Noble Metal-Free TiO2-Coated Carbon Nitride Layers for Enhanced Visible Light-Driven Photocatalysis. Nanomaterials, 10(4), 805. https://doi.org/10.3390/nano10040805