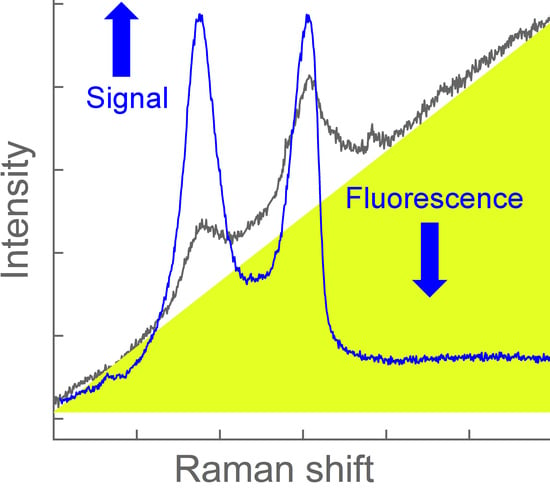
Fast Identification and Quantification of Graphene Oxide in Aqueous Environment by Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quantification of GO in Water
2.3. Analyses of Local Water Samples
2.4. Transport of GO in Quartz Sand Column
3. Results and Discussion
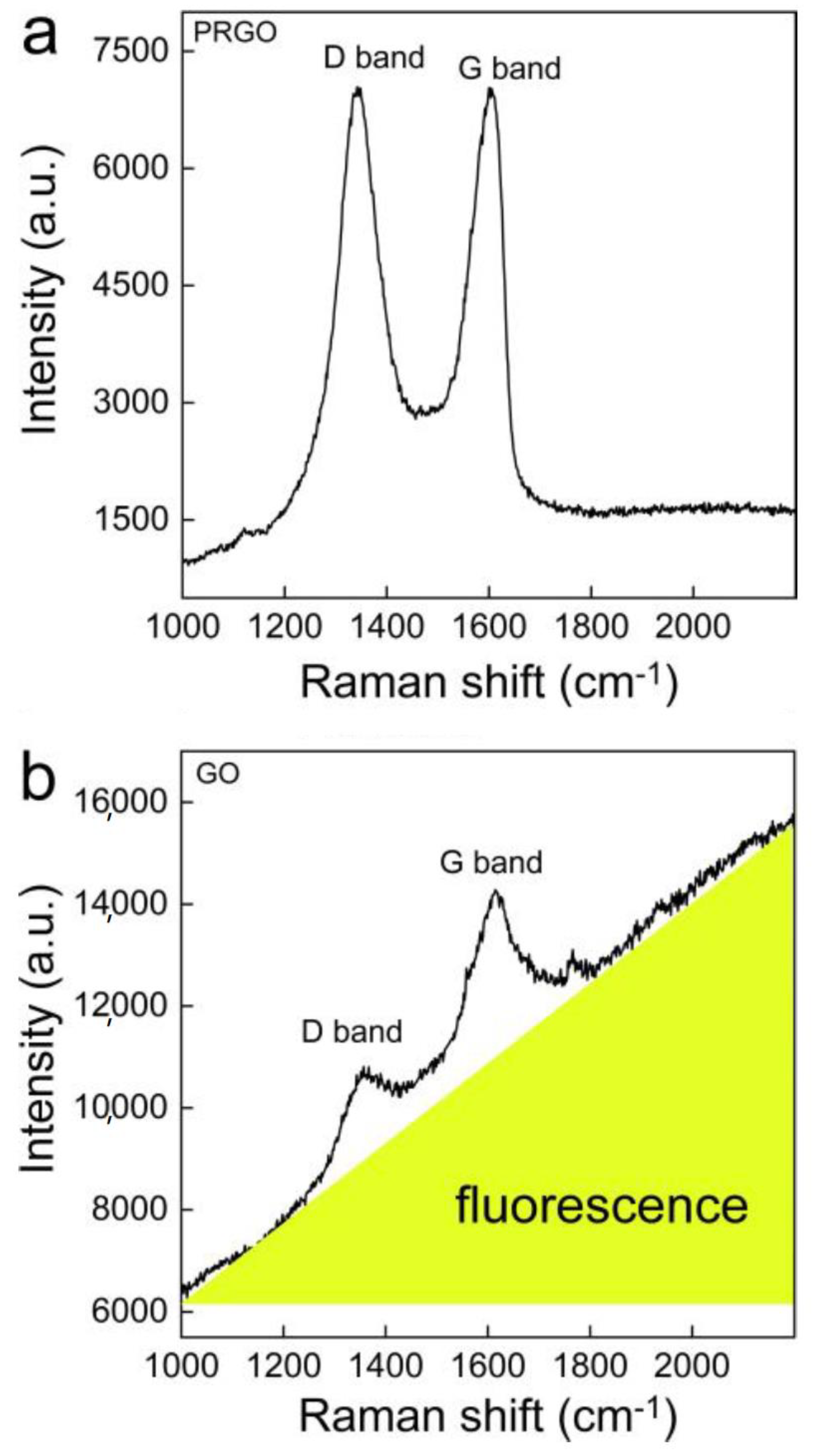
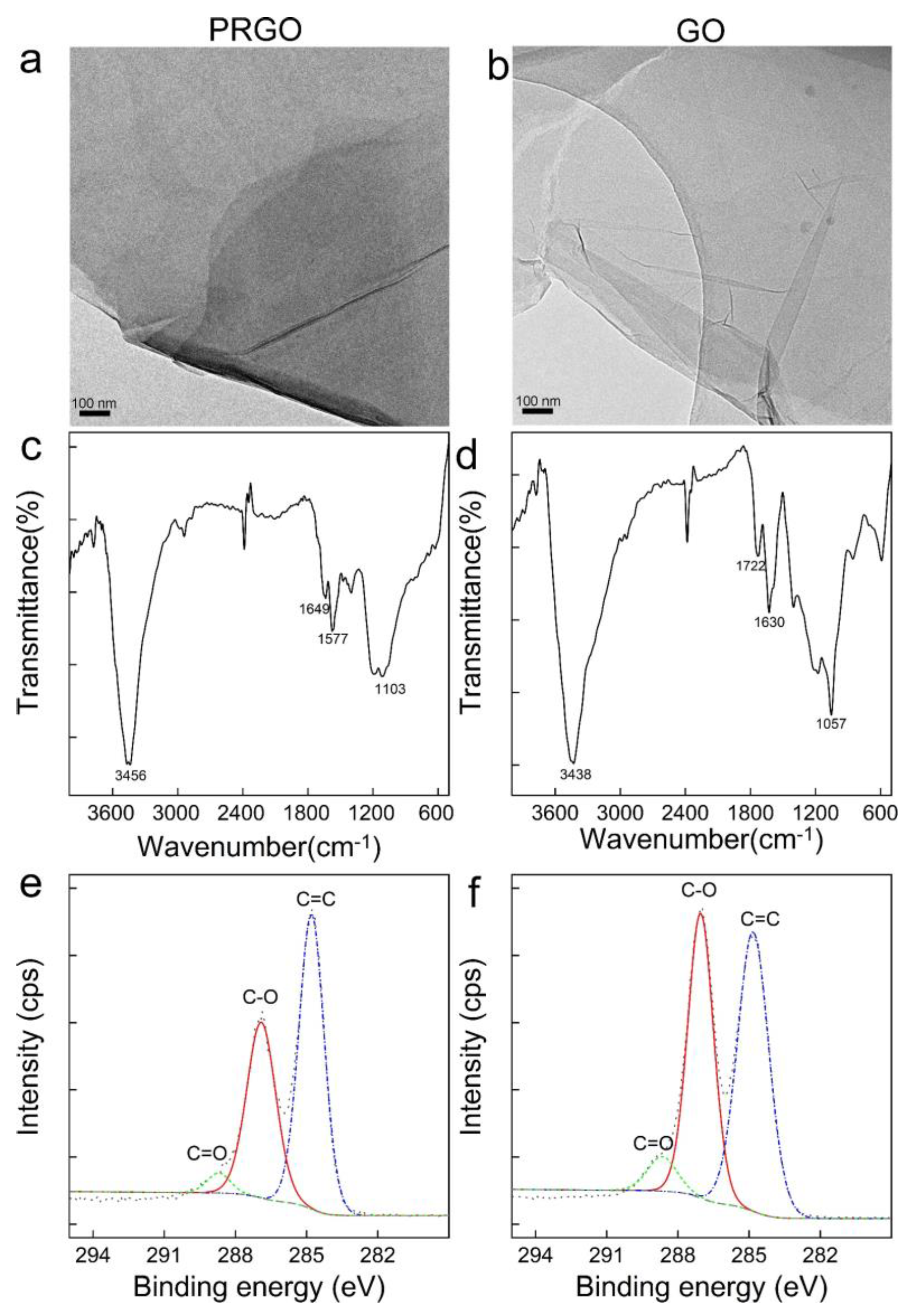
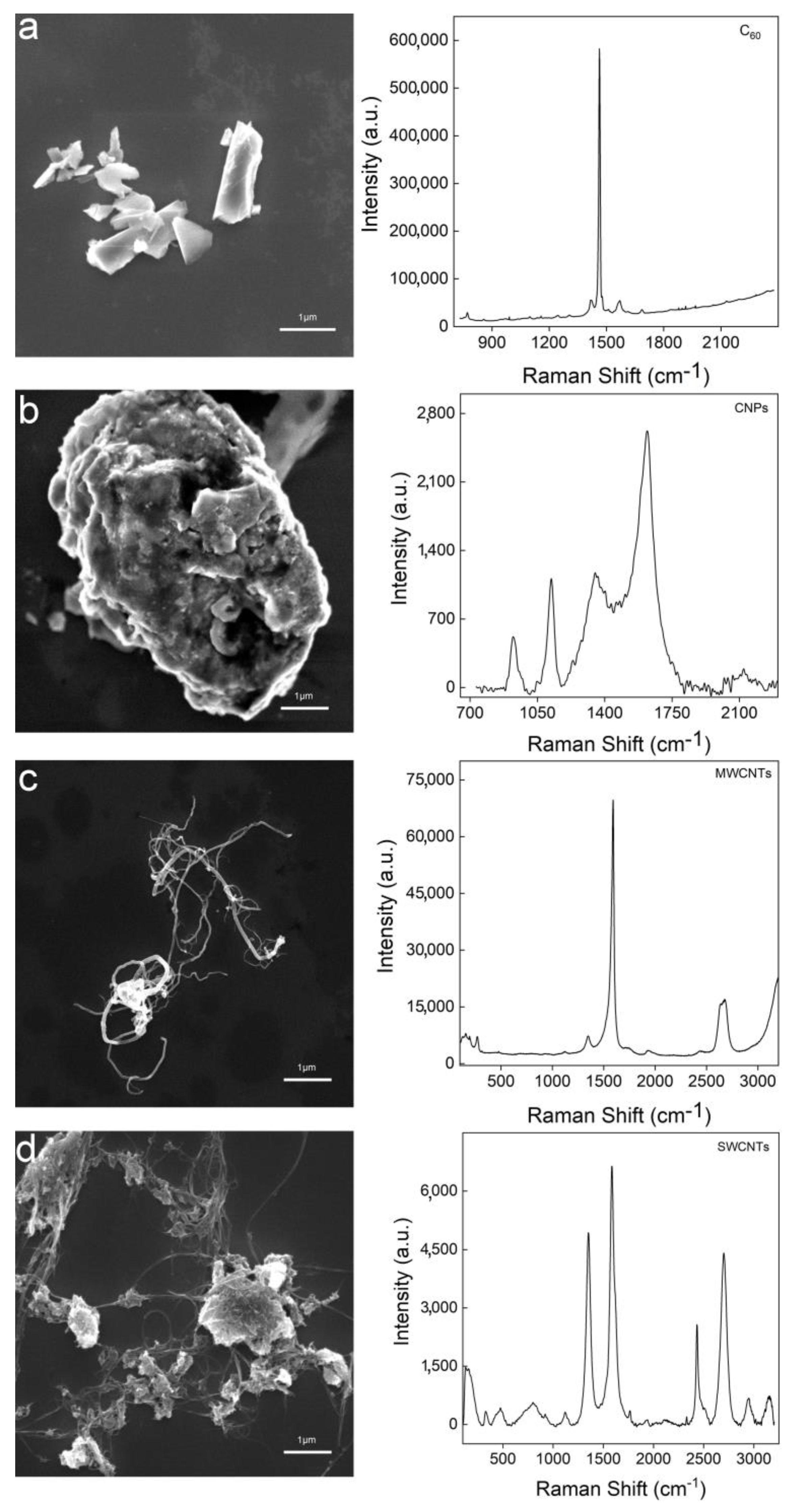
3.1. Characterization of GO and PRGO
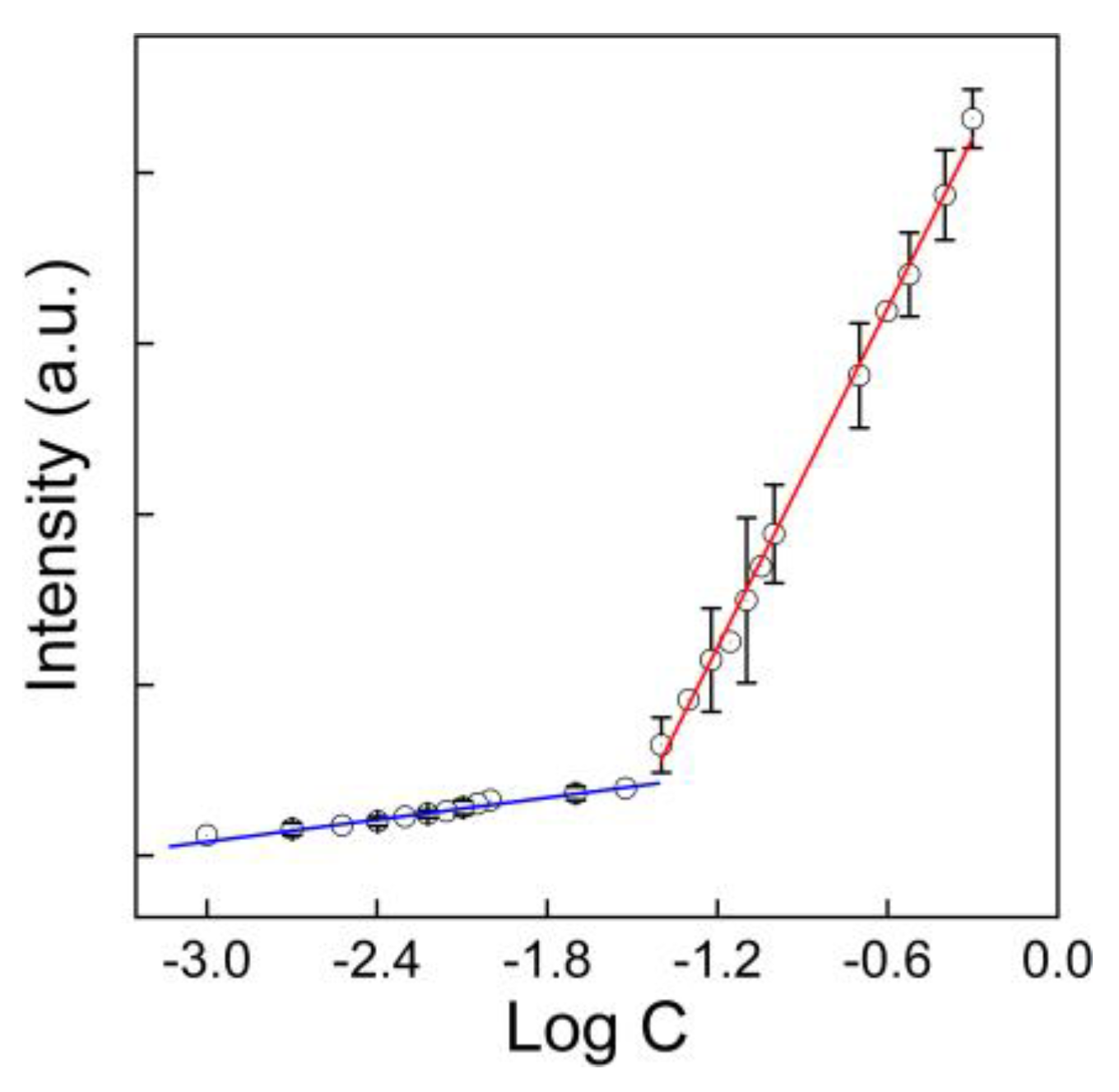
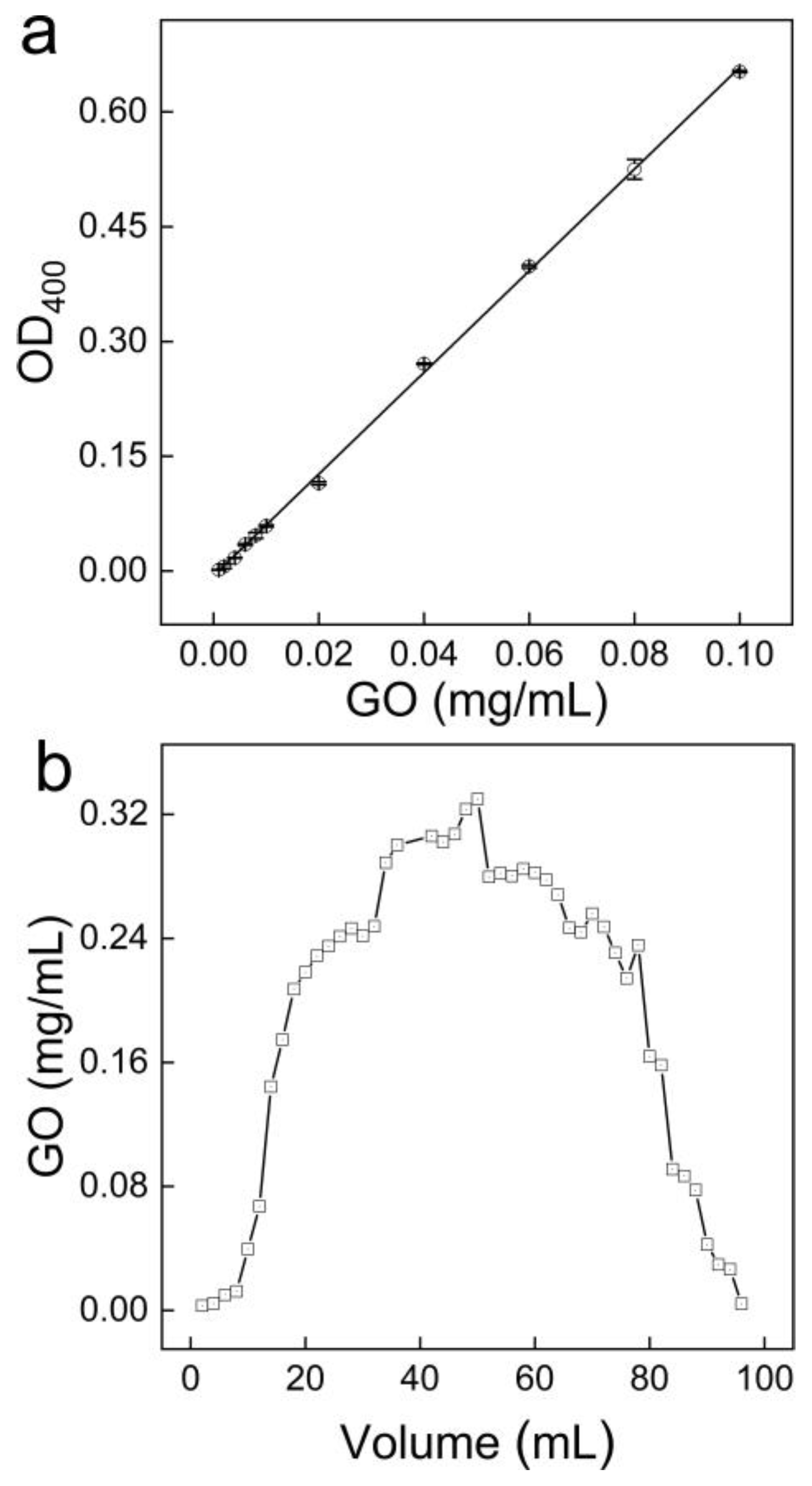
3.2. Quantification of GO in Water
3.3. Analyses of Local Water Samples
3.4. Transport of GO in Quartz Sands
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, D.; Tang, L.; Li, J. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef]
- Novoselov, K.S. Nobel lecture: Graphene: Materials in the flatland. Rev. Mod. Phys. 2011, 83, 837–849. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef]
- Kavitha, T.; Gopalan, A.I.; Lee, K.-P.; Park, S.-Y. Glucose sensing, photocatalytic and antibacterial properties of graphene–ZnO nanoparticle hybrids. Carbon 2012, 50, 2994–3000. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, S.-T.; Yilihamu, A.; Wu, D. Advances in the applications of graphene adsorbents: From water treatment to soil remediation. Rev. Inorg. Chem. 2019, 39, 47–76. [Google Scholar] [CrossRef]
- Zhao, L.; Guan, X.; Yu, B.; Ding, N.; Liu, X.; Ma, Q.; Yang, S.; Yilihamu, A.; Yang, S.-T. Carboxylated graphene oxide-chitosan spheres immobilize Cu2+ in soil and reduce its bioaccumulation in wheat plants. Environ. Int. 2019, 133, 105208. [Google Scholar] [CrossRef]
- Wang, Y.W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.H.; Liu, Y.; Wang, H. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef]
- Sun, C.; Yang, S.-T.; Gao, Z.; Yang, S.; Yilihamu, A.; Ma, Q.; Zhao, R.-S.; Xue, F. Fe3O4/TiO2/reduced graphene oxide composites as highly efficient Fenton-like catalyst for the decoloration of methylene blue. Mater. Chem. Phys. 2019, 223, 751–757. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food. Drug. Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.M.; Goulart, V.A.; Gomes, K.N.; Ladeira, M.S.; Santos, A.K.; Lorençon, E.; Ladeira, L.O.; Resende, R.R. Graphene based nanomaterials biological and medical applications and toxicity. Nanomedicine 2015, 2423–2450. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Li, H.; Qu, X.; Yang, S.-T.; Chang, X.-L. Bioaccumulation and toxicity of 13C-skeleton labeled graphene oxide in wheat. Environ. Sci. Technol. 2017, 51, 10146–10153. [Google Scholar] [CrossRef]
- Xie, J.; Ming, Z.; Li, H.; Yang, H.; Yu, B.; Wu, R.; Liu, X.; Bai, Y.; Yang, S.T. Toxicity of graphene oxide to white rot fungus. Phanerochaete chrysosporium. Chemosphere 2016, 151, 324–331. [Google Scholar] [CrossRef]
- Yilihamu, A.; Ouyang, B.; Ouyang, P.; Bai, Y.; Zhang, Q.; Shi, M.; Guan, X.; Yang, S.-T. Interaction between graphene oxide and nitrogen-fixing bacterium Azotobacter chroococcum: Transformation, toxicity and nitrogen fixation. Carbon 2020, 160, 5–13. [Google Scholar] [CrossRef]
- Liu, J.-H.; Yang, S.-T.; Wang, H.; Chang, Y.; Cao, A.; Liu, Y. Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine 2012, 7, 1801–1812. [Google Scholar] [CrossRef]
- Li, B.; Yang, J.; Huang, Q.; Zhang, Y.; Peng, C.; Zhang, Y.; He, Y.; Shi, J.; Li, W.; Hu, J.; et al. Biodistribution and pulmonary toxicity of intratracheally instilled graphene oxide in mice. NPG Asia Mater. 2013, 5, e44. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Yang, S.; Guan, X.; Zhang, Q.; Shi, M.; Yang, S.-T.; Chen, C.; Chang, X.-L. Chemical reduction of graphene enhances in vivo translocation and photosynthetic inhibition in pea plants. Environ. Sci. Nano 2019, 6, 1077–1088. [Google Scholar] [CrossRef]
- Navarro, D.A.; Kah, M.; Losic, D.; Kookana, R.S.; McLaughlin, M.J. Mineralisation and release of 14C-graphene oxide (GO) in soils. Chemosphere 2020, 238, 124558. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Hu, M.; Pan, B.; Xie, Y.; Petersen, E.J. Biodistribution and toxicity of radio-labeled few layer graphene in mice after intratracheal instillation. Part. Fibre Toxicol. 2015, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Coleman, J.N. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chang, P.R.; Zheng, P.; Ma, X. The composites based on plasticized starch and graphene oxide/reduced graphene oxide. Carbohyd. Polym. 2013, 94, 63–70. [Google Scholar] [CrossRef]
- Zhi, M.; Huang, W.; Shi, Q.; Ran, K. Improving water dispersibility of non-covalent functionalized reduced graphene oxide with l-tryptophan via cleaning oxidative debris. J. Mater. Sci.-Mater. Electron. 2016, 27, 7361–7368. [Google Scholar] [CrossRef]
- Xia, T.; Ma, P.; Qi, Y.; Zhu, L.; Qi, Z.; Chen, W. Transport and retention of reduced graphene oxide materials in saturated porous media: Synergistic effects of enhanced attachment and particle aggregation. Environ. Pollut. 2019, 247, 383–391. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Chu, B.T.T.; Tobias, G.; Llewellyn, S.A.; Green, M.L.H. Quantitative assessment of carbon nanotube dispersions by Raman spectroscopy. Carbon 2007, 10, 907–912. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, W.; He, L.; Nakayama, N.; Chen, K.; Sun, X.; Chen, X.; Dai, H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2007, 2, 47–52. [Google Scholar] [CrossRef]
- Cao, L.; Meziani, M.J.; Sahu, S.; Sun, Y.P. Photoluminescence properties of graphene versus other carbon nanomaterials. Acc. Chem. Res. 2013, 46, 171–180. [Google Scholar] [CrossRef]
- Zhu, P.Z.; Shen, M.; Xiao, S.H.; Zhang, D. Experimental study on the reducibility of graphene oxide by hydrazine hydrate. Physical B 2011, 406, 498–502. [Google Scholar] [CrossRef]
- Wu, R.H.; Yu, B.W.; Liu, X.Y.; Li, H.L.; Wang, W.X.; Chen, L.Y.; Bai, Y.T.; Ming, Z. One-pot hydrothermal preparation of graphene sponge for the removal of oils and organic solvents. Appl. Surf. Sci. 2016, 362, 56–62. [Google Scholar] [CrossRef]
- Shang, Z.J.; Ma, L.; Li, J.W.; Ai, W.; Yu, T.; Gurzadyan, G.G. The origin of fluorescence from graphene oxide. Sci. Rep. 2012, 2, 792. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Wu, S.; Wang, Y.; Wu, L.; Yuan, P.; Wang, X. Self-assembly of mildly reduced graphene oxide monolayer for enhanced Raman scattering. J. Solid. Chem. 2016, 237, 57–63. [Google Scholar] [CrossRef]
- He, D.N.; Peng, Z.; Gong, W.; Luo, Y.Y.; Zhao, P.F.; Kong, L.X. Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv. 2015, 5, 11966. [Google Scholar] [CrossRef]
- Hafiz, S.M.; Ritikos, R.; Whitcher, T.J.; Razib, N.M.; Bien, D.C.S.; Chanlek, N.; Nakajima, H.; Saisopa, T.; Songsiriritthigul, P.; Huang, N.M.; et al. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuators B 2014, 193, 692–700. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, Z.B.; Pu, X.M.; Shang, L.; Yin, G.F.; Chen, X.C.; Cheng, S. Fabrication of carboxylic graphene oxide-composited polypyrrole film for neurite growth under electrical stimulation. Front. Mater. Sci. 2019, 13, 258–267. [Google Scholar] [CrossRef]


| Sample | Location | Latitude | Longitude |
|---|---|---|---|
| No. 1 | Wanli bridge | 30.64723 | 104.06004 |
| No. 2 | Lotus pond of Sichuan University | 30.63913 | 104.06933 |
| No. 3 | Wenshu monastery | 30.67574 | 104.07183 |
| No. 4 | Culture park | 30.6605 | 104.0431 |
| No. 5 | Drunk plum garden | 30.65801 | 104.04321 |
| No. 6 | Baihuatan park | 30.65612 | 104.04365 |
| No. 7 | Library of Southwest Minzu University | 30.63849 | 104.04742 |
| No. 8 | Rain in Southwest Minzu University | 30.63849 | 104.04742 |
| Spiked GO (mg/mL) | Determined GO (mg/mL) | Recovery (%) |
|---|---|---|
| 0.25 | 0.2505 ± 0.0051 | 100.2% |
| 0.07 | 0.0634 ± 0.0065 | 90.6% |
| 0.05 | 0.0499 ± 0.0064 | 99.7% |
| 0.03 | 0.0345 ± 0.0029 | 115.1% |
| 0.007 | 0.0072 ± 0.0071 | 103.2% |
| 0.005 | 0.0047 ± 0.0003 | 94.6% |
| 0.003 | 0.0024 ± 0.0004 | 80.8% |
| 0.001 | 0.0011 ± 0.0001 | 114.3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Chen, Q.; Shi, M.; Zhang, Q.; Lan, S.; Maimaiti, T.; Li, Q.; Ouyang, P.; Tang, K.; Yang, S.-T. Fast Identification and Quantification of Graphene Oxide in Aqueous Environment by Raman Spectroscopy. Nanomaterials 2020, 10, 770. https://doi.org/10.3390/nano10040770
Yang S, Chen Q, Shi M, Zhang Q, Lan S, Maimaiti T, Li Q, Ouyang P, Tang K, Yang S-T. Fast Identification and Quantification of Graphene Oxide in Aqueous Environment by Raman Spectroscopy. Nanomaterials. 2020; 10(4):770. https://doi.org/10.3390/nano10040770
Chicago/Turabian StyleYang, Shengnan, Qian Chen, Mengyao Shi, Qiangqiang Zhang, Suke Lan, Tusunniyaze Maimaiti, Qun Li, Peng Ouyang, Kexin Tang, and Sheng-Tao Yang. 2020. "Fast Identification and Quantification of Graphene Oxide in Aqueous Environment by Raman Spectroscopy" Nanomaterials 10, no. 4: 770. https://doi.org/10.3390/nano10040770
APA StyleYang, S., Chen, Q., Shi, M., Zhang, Q., Lan, S., Maimaiti, T., Li, Q., Ouyang, P., Tang, K., & Yang, S.-T. (2020). Fast Identification and Quantification of Graphene Oxide in Aqueous Environment by Raman Spectroscopy. Nanomaterials, 10(4), 770. https://doi.org/10.3390/nano10040770