Studies on Sensing Properties and Mechanism of CuO Nanoparticles to H2S Gas
Abstract
1. Introduction
2. Experimental Scheme
2.1. Synthesis of CuO Nanoparticles
2.2. Characterization of Structures and Morphologies
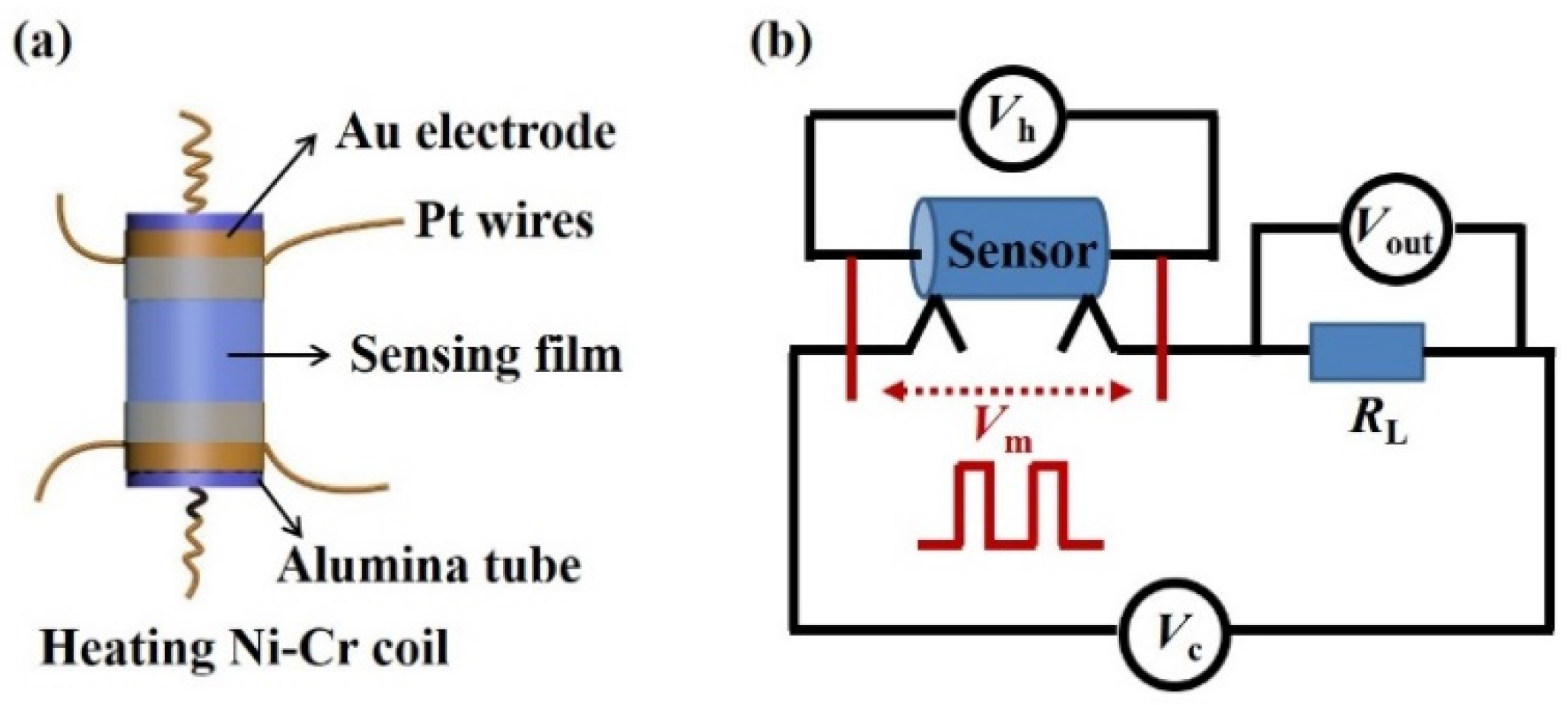
2.3. Preparation and Measurement of the Gas Sensor
3. Results and Discussions
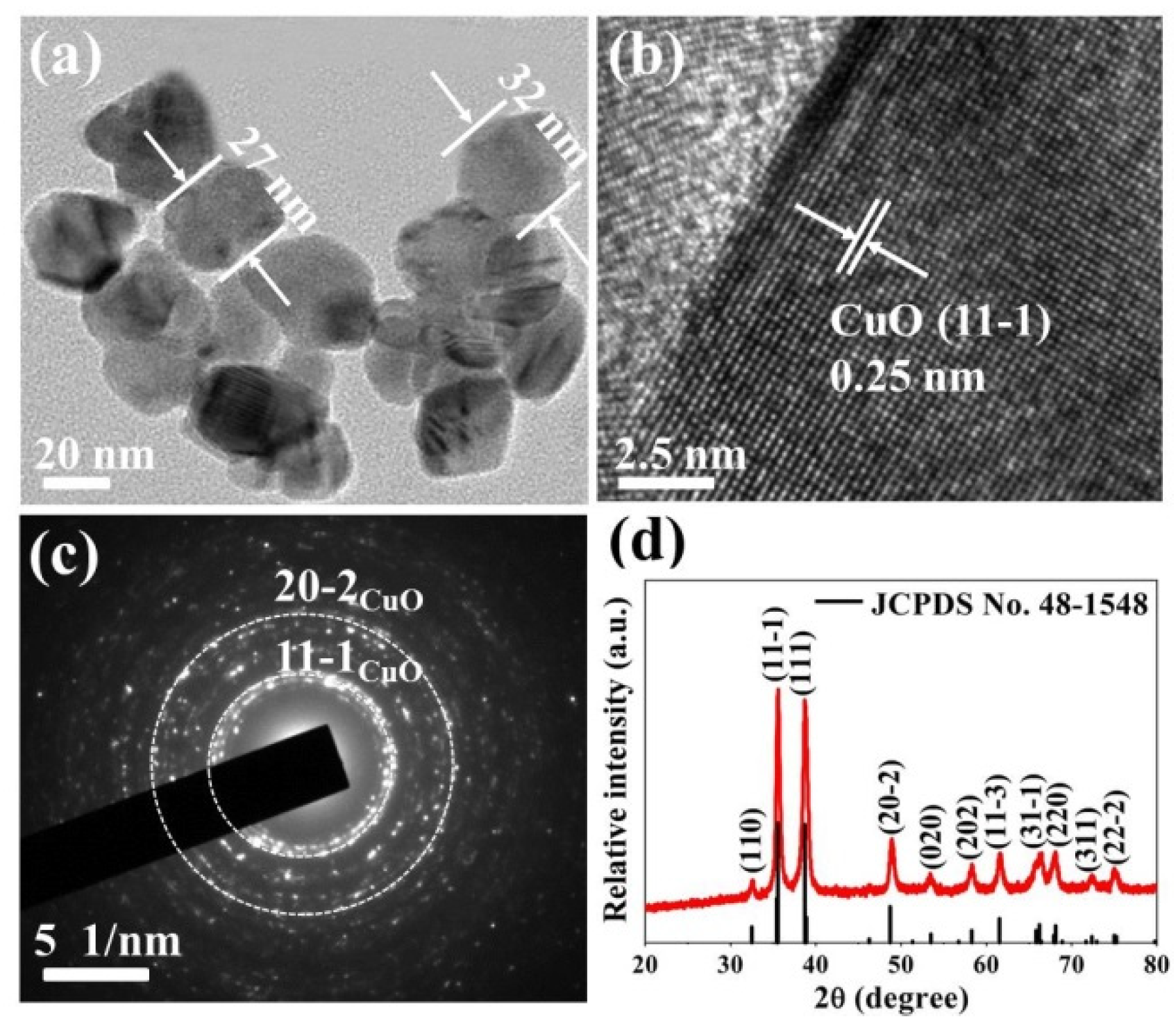
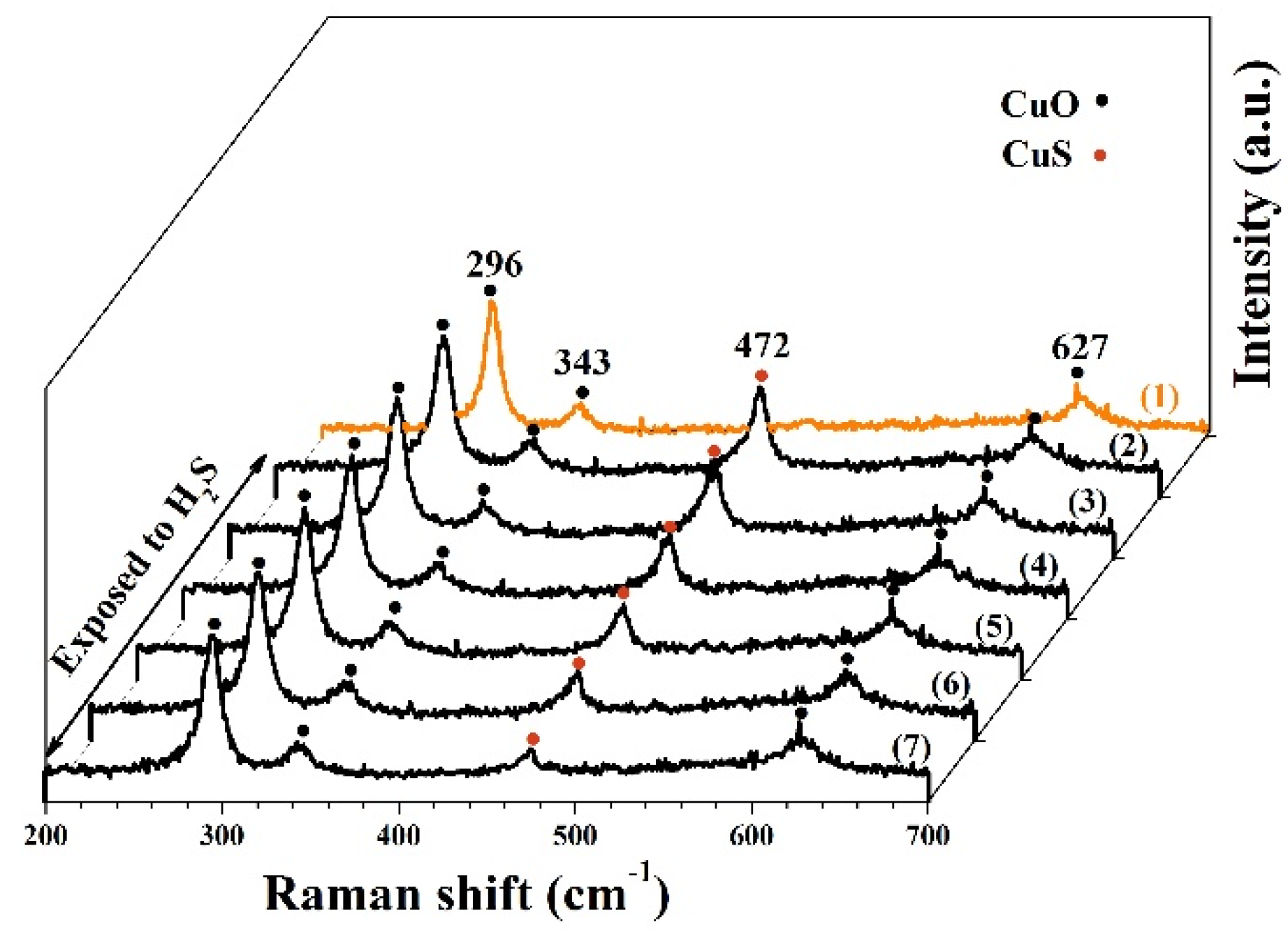
3.1. Morphological and Structural Characteristics
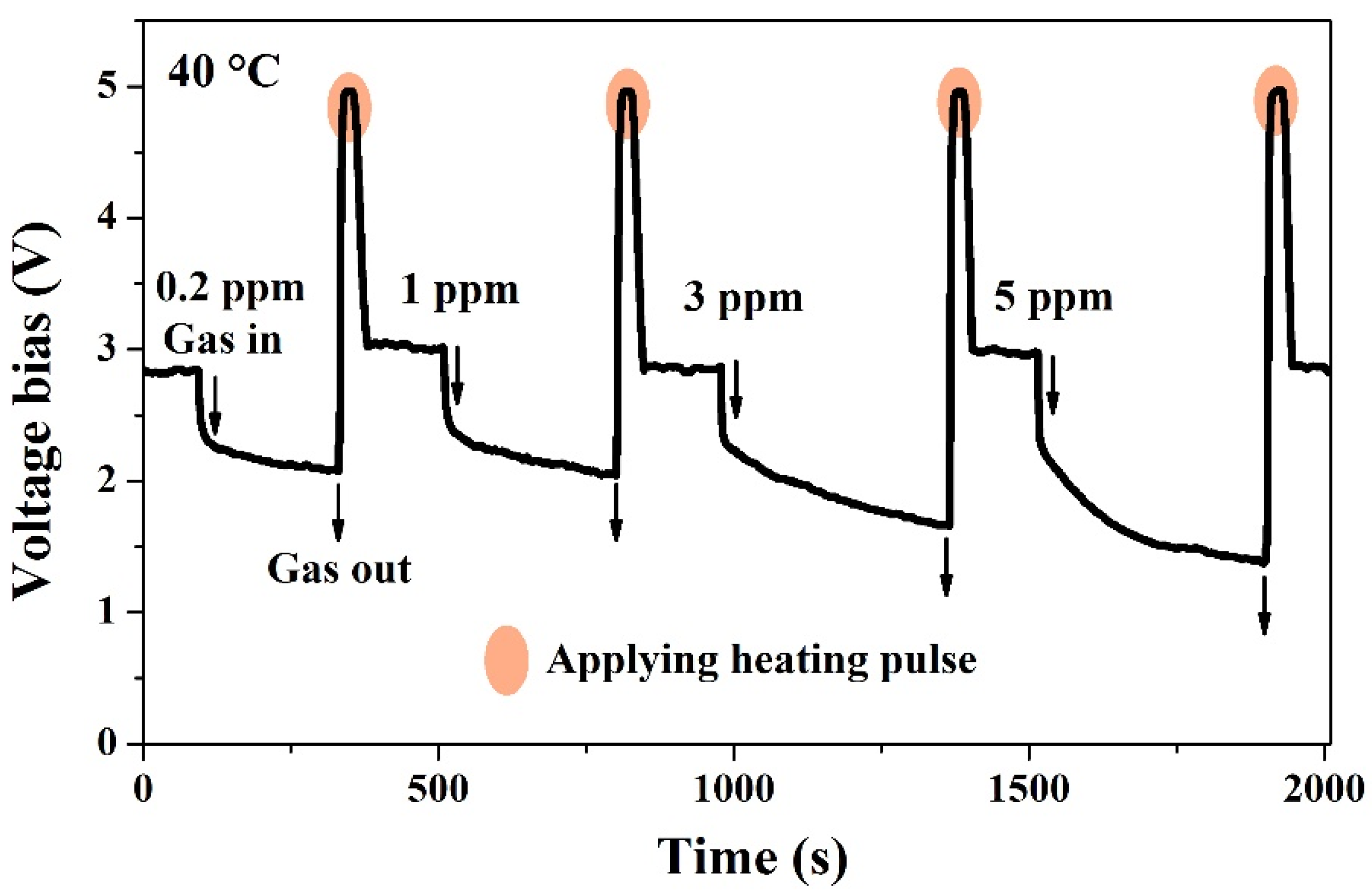
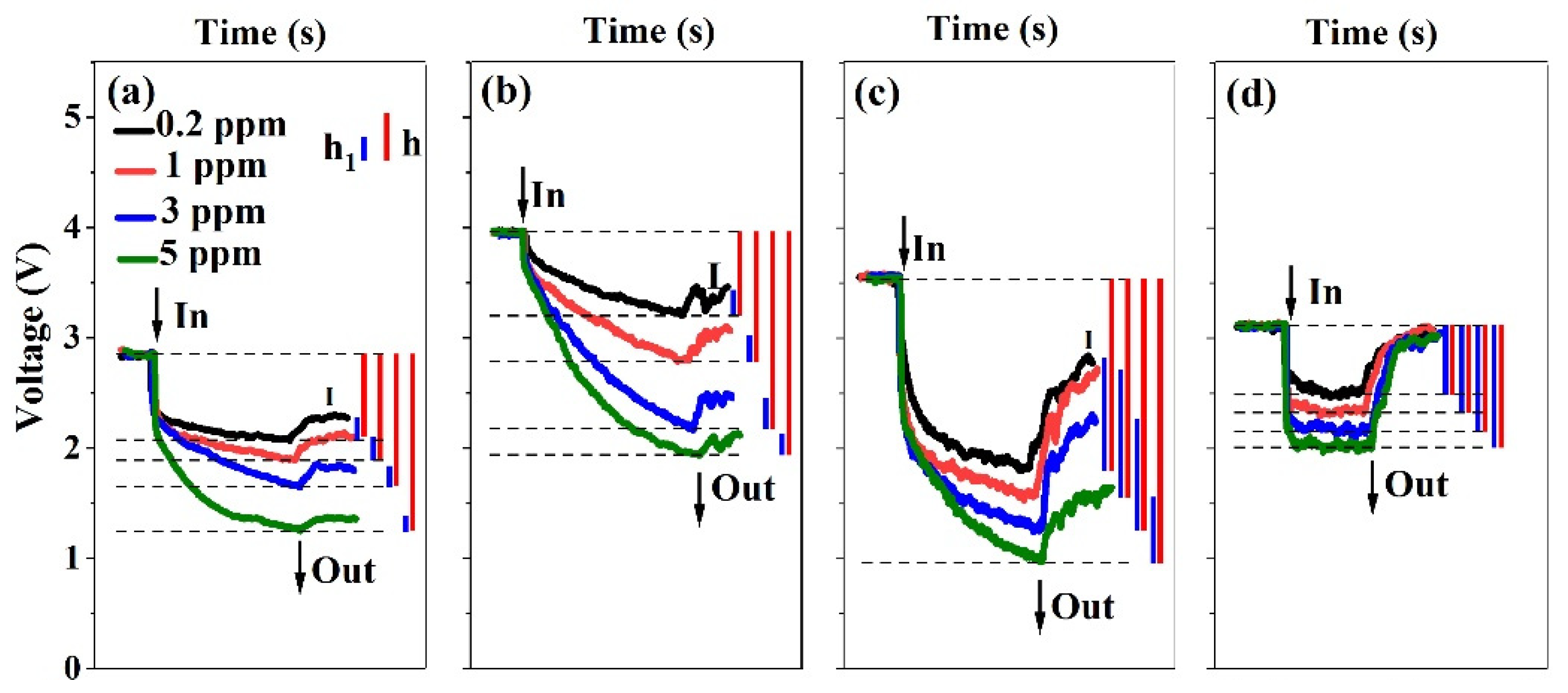
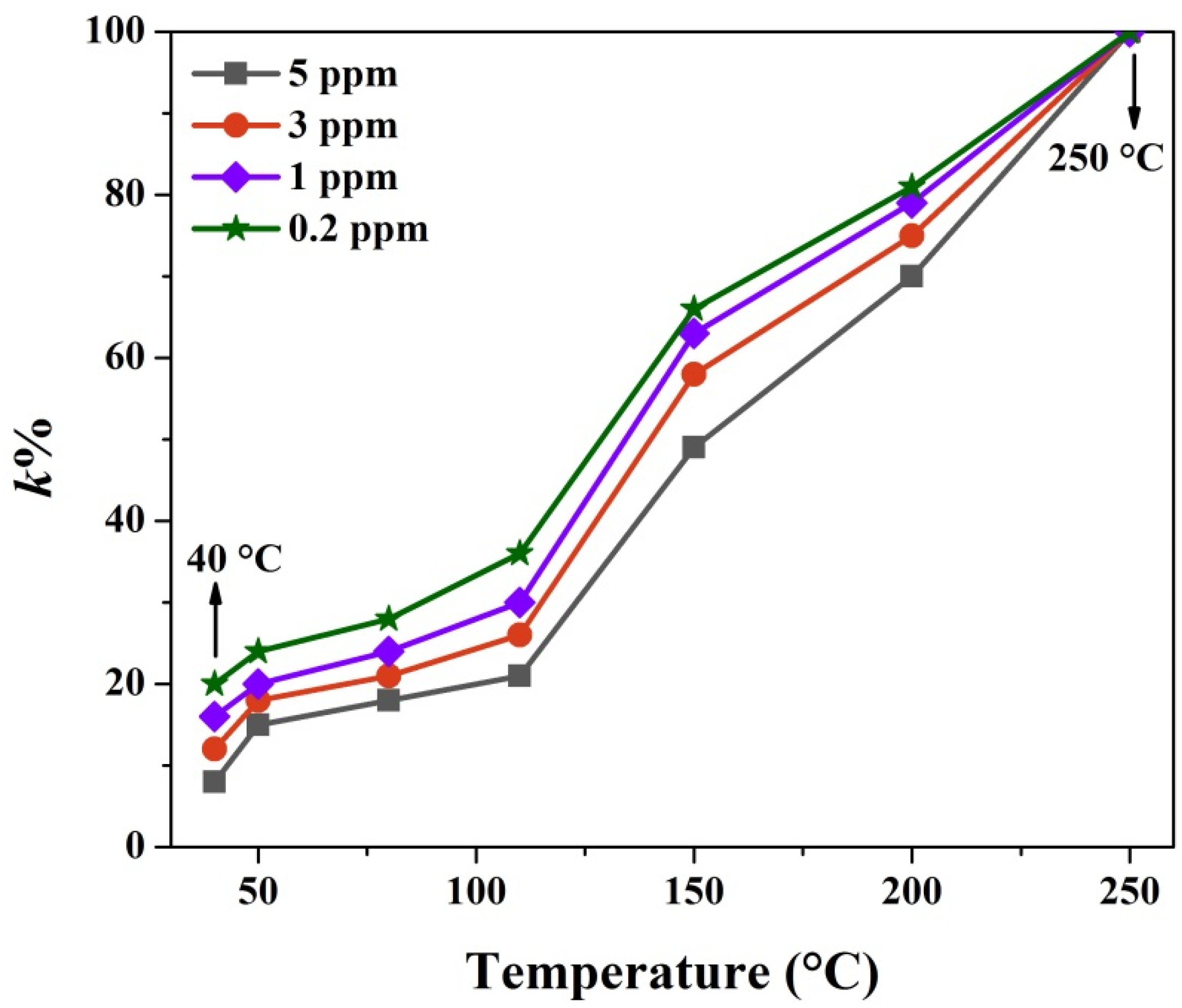
3.2. Gas Sensing Properties
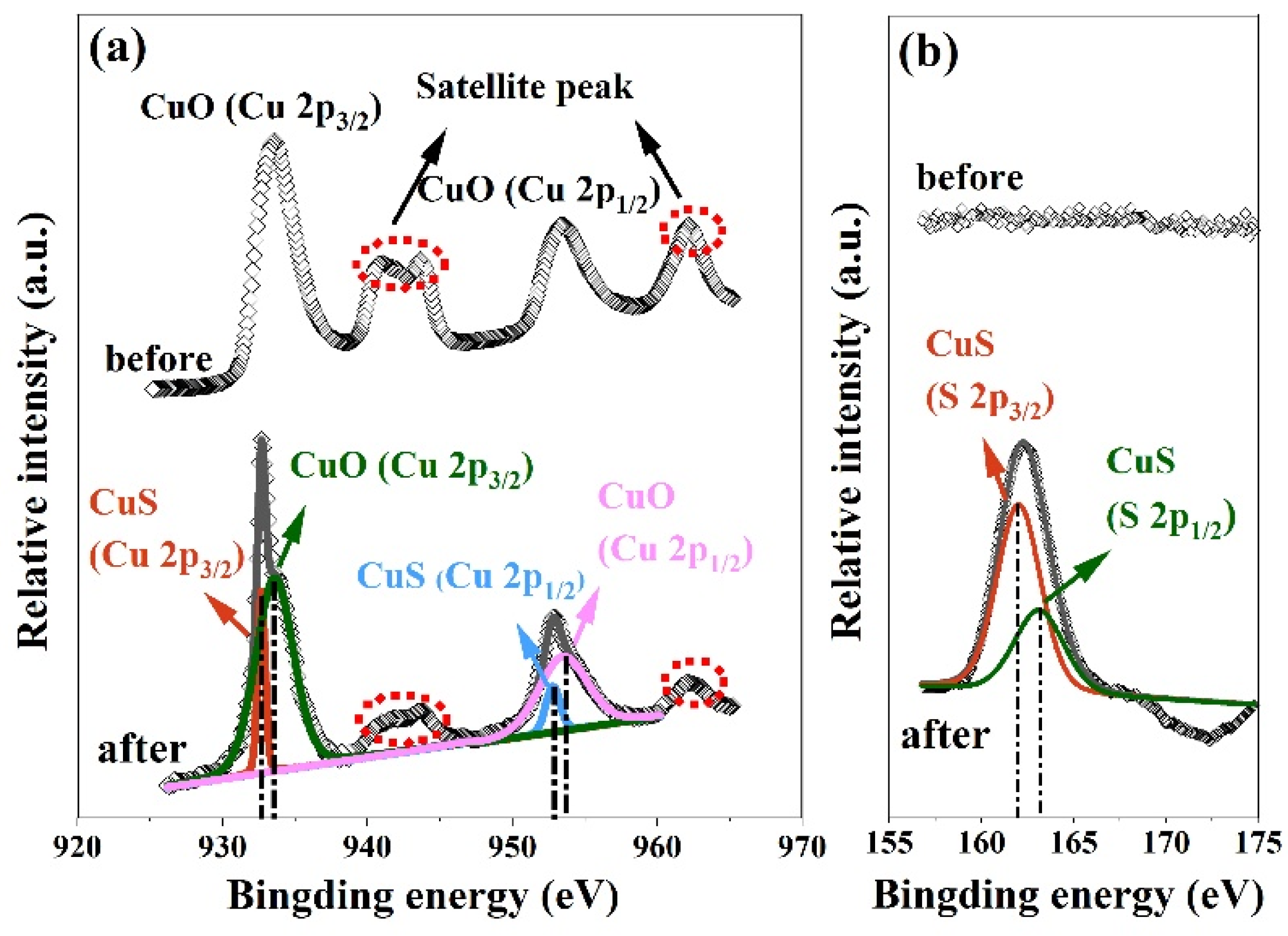
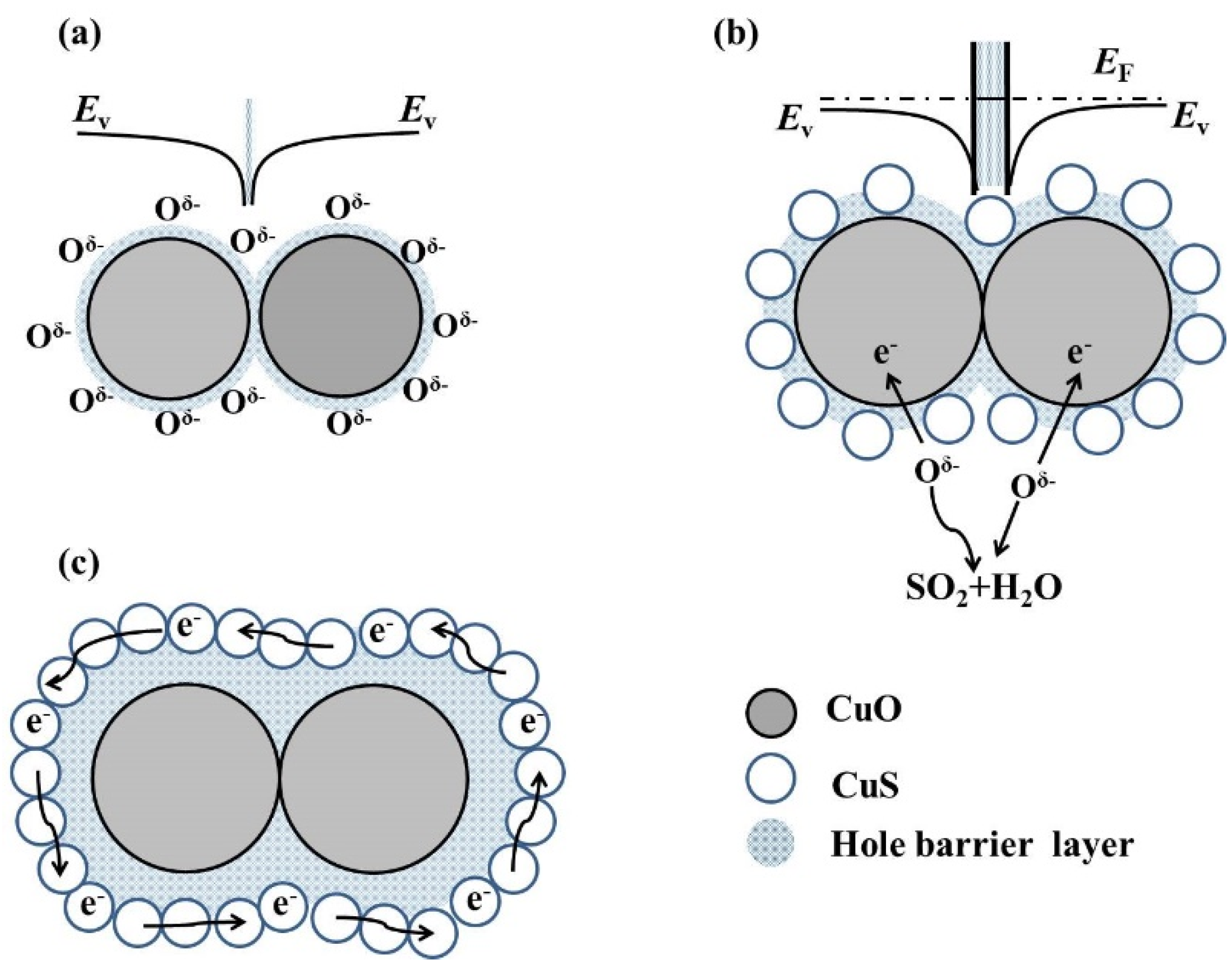
3.3. Sensing Mechanisms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Zhu, M.L.; Zhang, L.J. A Novel H2S Gas Sensor Applied to the Construction Workers During the Construction Process. Sci. Adv. Mater. 2019, 11, 143–146. [Google Scholar] [CrossRef]
- Meng, F.N.; Di, X.P.; Dong, H.W.; Zhang, Y.; Zhu, C.L.; Li, C.Y.; Chen, Y.J. Ppb H2S gas sensing characteristics of Cu2O/CuO sub-microspheres at low-temperature. Sensor Actuat. B Chem. 2013, 182, 197–204. [Google Scholar] [CrossRef]
- Patil, G.E.; Kajale, D.D.; Gaikwad, V.B.; Jain, G.H. Effect of Thickness on Nanostructured SnO2 Thin Films by Spray Pyrolysis as Highly Sensitive H2S Gas Sensor. J. Nanosci. Nanotechnol. 2012, 12, 6192–6201. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.L.; Chen, C.; Luo, R.X.; Chen, A.F.; Li, D.Q. Synthesis of MoO3/reduced graphene oxide hybrids and mechanism of enhancing H2S sensing performances. Sensor Actuat. B Chem. 2015, 216, 113–120. [Google Scholar] [CrossRef]
- Kim, J.; Yong, K. Mechanism Study of ZnO Nanorod-Bundle Sensors for H2S Gas Sensing. J. Phys. Chem. C 2011, 115, 7218–7224. [Google Scholar] [CrossRef]
- Li, Z.J.; Huang, Y.W.; Zhang, S.C.; Chen, W.M.; Kuang, Z.; Ao, D.Y.; Liu, W.; Fu, Y.Q. A fast response & recovery H2S gas sensor based on α-Fe2O3 nanoparticles with ppb level detection limit. J. Hazard. Mater. 2015, 300, 167–174. [Google Scholar]
- Poongodi, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Meena, P.; Masuda, Y.; Lee, C. Electrodeposition of WO3 nanostructured thin films for electrochromic and H2S gas sensor applications. J. Alloy. Compd. 2017, 719, 71–81. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Tomescu-Stanoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sensor Actuat. B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Xu, Z.K.; Luo, Y.Y.; Duan, G.T. Self-Assembly of Cu2O Monolayer Colloidal Particle Film Allows the Fabrication of CuO Sensor with Super selectivity for Hydrogen Sulfide. ACS Appl. Mater. Interfaces 2019, 11, 8164–8174. [Google Scholar] [CrossRef]
- Kim, H.; Jin, C.; Park, S.; Kim, S.; Lee, C. H2S gas sensing properties of bare and Pd-functionalized CuO nanorods. Sensor Actuat. B Chem. 2012, 161, 594–599. [Google Scholar] [CrossRef]
- Yu, W.W.; Sun, Y.; Zhang, T.N.; Zhang, K.N.; Wang, S.X.; Chen, X.; Dai, N. CuO/WO3 Hybrid Nanocubes for High-Responsivity and Fast-Recovery H2S Sensors Operated at Low Temperature. Part. Part. Syst. Char. 2016, 33, 15–20. [Google Scholar] [CrossRef]
- Xue, X.; Xing, L.; Chem, Y.; Shi, S.; Wang, Y.; Wang, T. Synthesis and H2S Sensing Properties of CuO−SnO2 Core/Shell PN-Junction Nanorods. J. Phys. Chem. C 2008, 112, 12157–12160. [Google Scholar] [CrossRef]
- Morrison, S.R. Selectivity in semiconductor gas sensors. Sensor Actuator. 1987, 12, 425–440. [Google Scholar] [CrossRef]
- Pizzini, S.; Butta, N.; Narducci, D.; Palladino, M. Thick film ZnO resistive gas sensors: Analysis of their kinetic behavior. J. Electrochem. Soc. 1989, 136, 1945–1948. [Google Scholar] [CrossRef]
- Moseley, P.T. Materials selection for semiconductor gas sensors. Sensor Actuat. B Chem. 1992, 6, 149–156. [Google Scholar] [CrossRef]
- Mu, C.; He, J.H. Confined conversion of CuS nanowires to CuO nanotubes by annealing-induced diffusion in nanochannels. Nanoscale Res. Lett. 2011, 6, 150. [Google Scholar] [CrossRef]
- Wang, S.H.; Huang, Q.J.; Wen, X.G.; Li, X.Y.; Yang, S.H. Thermal oxidation of Cu2S nanowires: A template method for the fabrication of mesoscopic CuxO (x = 1,2) wires. Phys. Chem. Chem. Phys. 2002, 4, 3425–3429. [Google Scholar] [CrossRef]
- Nie, Y.X.; Deng, P.; Zhao, Y.Y.; Wang, P.L.; Xing, L.L.; Zhang, Y.; Xue, X.Y. The conversion of PN-junction influencing the piezoelectric output of a CuO/ZnO nanoarray nanogenerator and its application as a room-temperature self-powered active H2S sensor. Nanotechnology 2014, 25, 265501. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, W.; Yong, K. CuO/ZnO Heterostructured Nanorods: Photochemical Synthesis and the Mechanism of H2S Gas Sensing. J. Phys. Chem. C 2012, 116, 15682–15691. [Google Scholar] [CrossRef]
- Wang, L.W.; Kang, Y.F.; Wang, Y.; Zhu, B.L.; Zhang, S.M.; Huang, W.P.; Wang, S.R. CuO nanoparticle decorated ZnO nanorod sensor for low-temperature H2S detection. Mat. Sci. Eng. C 2012, 32, 2079–2085. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Goyal, C.P.; Sharma, P.K.; Goutam, U.K.; Bhattacharya, S.; Datta, N.; Kaur, M.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Selective H2S sensing characteristics of CuO modified WO3 thin films. Sensor Actuat. B Chem. 2013, 188, 525–532. [Google Scholar] [CrossRef]
- Hu, X.B.; Zhu, Z.G.; Chen, C.; Wen, T.Y.; Zhao, X.L.; Xie, L.L. Highly sensitive H2S gas sensors based on Pd-doped CuO nanoflowers with low operating temperature. Sensor Actuat. B Chem. 2017, 253, 809–817. [Google Scholar] [CrossRef]
- Kim, J.H.; Katoch, A.; Choi, S.W.; Kim, S.S. Growth and sensing properties of networked p-CuO nanowires. Sensor Actuat. B Chem. 2015, 212, 190–195. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and Metal Oxide Nanoparticles in Chemiresistors: Does the Nanoscale Matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef]
- Christy, A.J.; Nehru, L.C.; Umadevi, M. A novel combustion method to prepare CuO nanorods and its antimicrobial and photocatalytic activities. Powder Technol. 2013, 235, 783–786. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Ganapathi, S.K.; Kaur, M.; Datta, N.; Muthe, K.P.; Aswal, D.K.; Gupta, S.K.; Yakhmi, J.V. Sub-ppm H2S sensing at room temperature using CuO thin films. Sensor Actuat. B Chem. 2010, 151, 90–96. [Google Scholar] [CrossRef]
- Guo, D.M.; Cai, P.J.; Sun, J.; He, W.N.; Wu, X.H.; Zhang, T.; Wang, X.; Zhang, X.T. Reduced-graphene-oxide/metal-oxide p-n heterojunction aerogels as efficient 3D sensing frameworks for phenol detection. Carbon 2016, 99, 571–578. [Google Scholar] [CrossRef]
- Yang, D.; Kang, K.; Kim, D.; Li, Z.Y.; Park, I. Fabrication of heterogeneous nanomaterial array by programmable heating and chemical supply within microfluidic platform towards multiplexed gas sensing application. Sci. Rep. 2015, 5, 8149. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Z.; Yan, B.; Zheng, Z.; Bao, Q.L.; Wu, T.; Li, C.M.; Shen, Z.X.; Zhang, J.X.; Gong, H. Multifunctional CuO nanowire devices: P-type field effect transistors and CO gas sensors. Nanotechnology 2009, 20, 085203. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, M. Effect of particle size and dopant on properties of SnO2-based gas sensors. Sensor Actuat. B Chem. 2000, 69, 144–152. [Google Scholar] [CrossRef]
- Kumar, A.; Sanger, A.; Kumar, A.; Chandra, R. Highly sensitive and selective CO gas sensor based on a hydrophobic SnO2/CuO bilayer. RSC Adv. 2016, 6, 47178–47184. [Google Scholar] [CrossRef]
- Xie, Y.; Xing, R.; Li, Q.; Xu, L.; Song, H. Three-dimensional ordered ZnO–CuO inverse opals toward low concentration acetone detection for exhaled breath sensing. Sensor Actuat. B Chem. 2015, 211, 255–262. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, X.G.; Lin, T. Highly sensitive SnO2 nanofiber chemiresistors with a low optimal operating temperature: Synergistic effect of Cu2+/Au co-doping. J. Mater. Chem. A 2014, 2, 13655–13660. [Google Scholar] [CrossRef]
- Yu, J.G.; Zhang, J.; Liu, S.W. Ion-Exchange Synthesis and Enhanced Visible-Light Photoactivity of CuS/ZnS Nanocomposite Hollow Spheres. J. Phys. Chem. C 2010, 114, 13642–13649. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.H.; van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11330. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Qiao, T.; Hu, X.Y. A simple hydrothermal route to nanocrystalline CuS. J. Cryst. Growth. 2004, 268, 64–70. [Google Scholar] [CrossRef]
- Dubale, A.A.; Tamirat, A.G.; Chen, H.M.; Berhe, T.A.; Pan, C.J.; Su, W.N.; Hwang, B.J. A highly stable CuS and CuS–Pt modified Cu2O/CuO heterostructure as an efficient photocathode for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 2205–2216. [Google Scholar] [CrossRef]
- Yua, T.; Zhao, X.; Shen, Z.X.; Wu, Y.H.; Su, W.H. Investigation of individual CuO nanorods by polarized micro-Raman scattering. J. Cryst. Growth 2004, 268, 590–595. [Google Scholar] [CrossRef]
- Chen, X.K.; Irwin, J.C.; Franck, J.P. Evidence for a strong spin-phonon interaction in cupric oxide. Phys. Rev. B 1995, 52, R13130. [Google Scholar] [CrossRef]
- Minceva-Sukarovaa, B.; Najdoskia, M.; Grozdanova, I.; Chunnilallb, C.J. Raman spectra of thin solid films of some metal sulfides. J. Mol. Struct. 1997, 410, 267–270. [Google Scholar] [CrossRef]
- Ishii, M.; Shibata, K.; Nozaki, H. Anion Distributions and Phase Transitions in CuS1-xSex(x = 0-1) Studied by Raman Spectroscopy. J. Solid. State. Chem. 1993, 105, 504–511. [Google Scholar] [CrossRef]
- McAleer, J.F.; Moseley, P.T.; Norris, J.O.W.; Williams, D.E. Tin dioxide gas sensors. Part 1.—Aspects of the surface chemistry revealed by electrical conductance variations. J. Chem. Soc. Faraday Trans. 1 1987, 83, 1323–1346. [Google Scholar] [CrossRef]
- McAleer, J.F.; Moseley, P.T.; Norris, J.O.W.; Williams, D.E.; Tofield, B.C. Tin dioxide gas sensors. Part 2.—The role of surface additives. J. Chem. Soc. Faraday Trans. 1 1988, 84, 441–457. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, D.W.; Zhou, X.; Sun, P.; Wang, H.Y.; Ma, J.; Lu, G.Y. Hydrothermal preparation and gas sensing properties of Zn-doped SnO2 hierarchical architectures. Sensor Actuat. B Chem. 2014, 191, 45–52. [Google Scholar] [CrossRef]
- Datta, N.; Ramgir, N.S.; Kumar, S.; Veerender, P.; Kaur, M.; Kailasaganapathi, S.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Role of various interfaces of CuO/ZnO random nanowire networks in H2S sensing: An impedance and Kelvin probe analysis. Sensor Actuat. B Chem. 2014, 202, 1270–1280. [Google Scholar] [CrossRef]

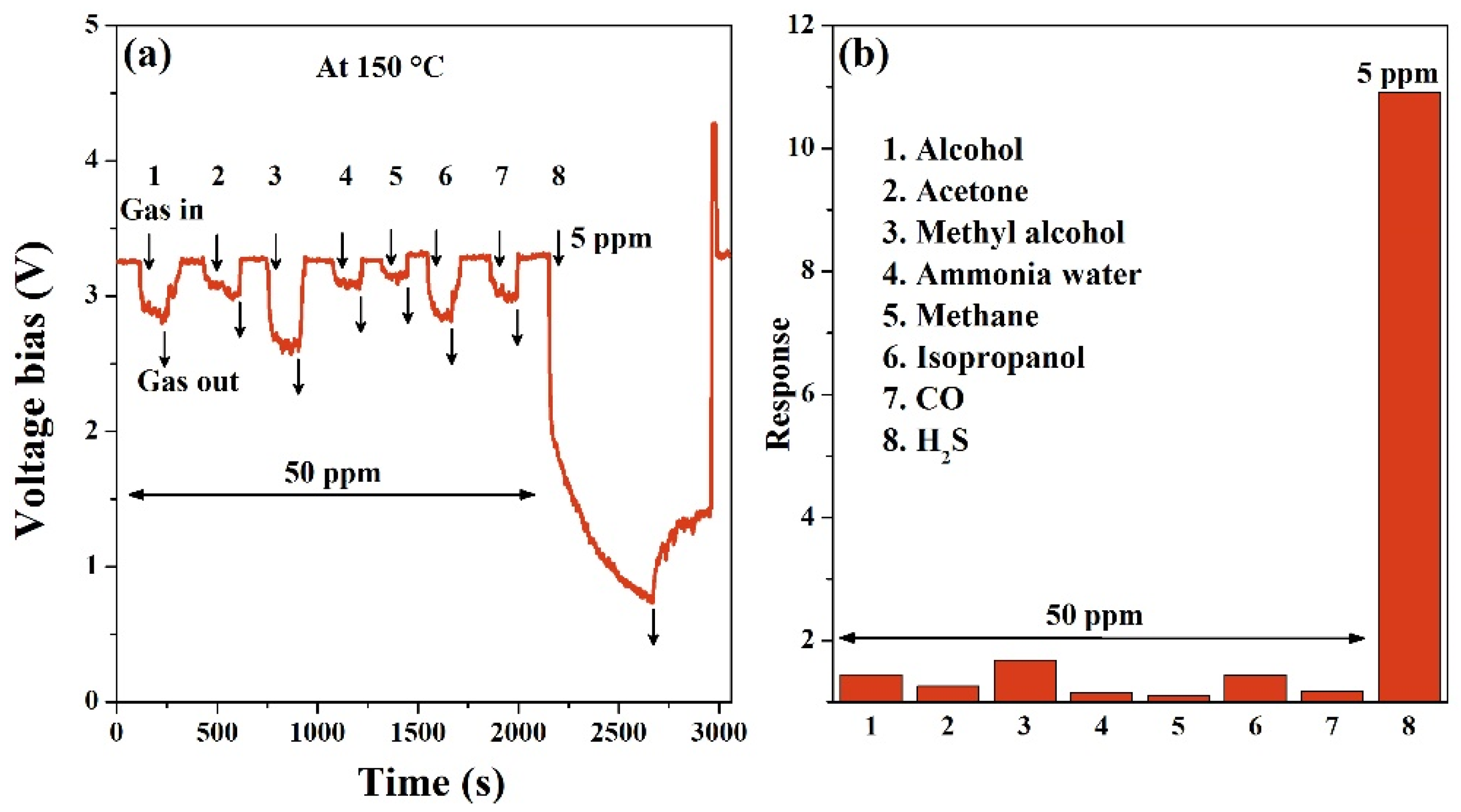
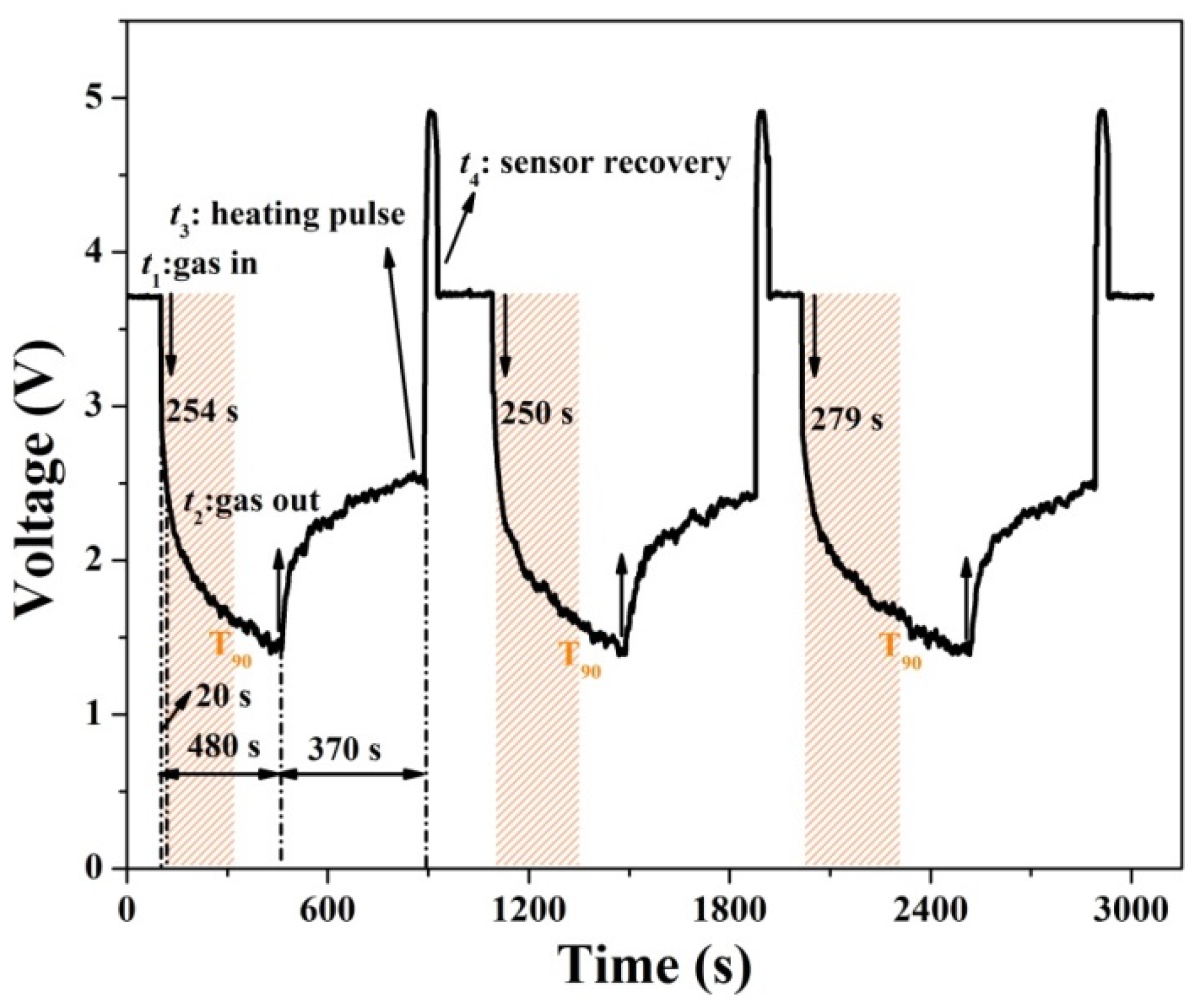
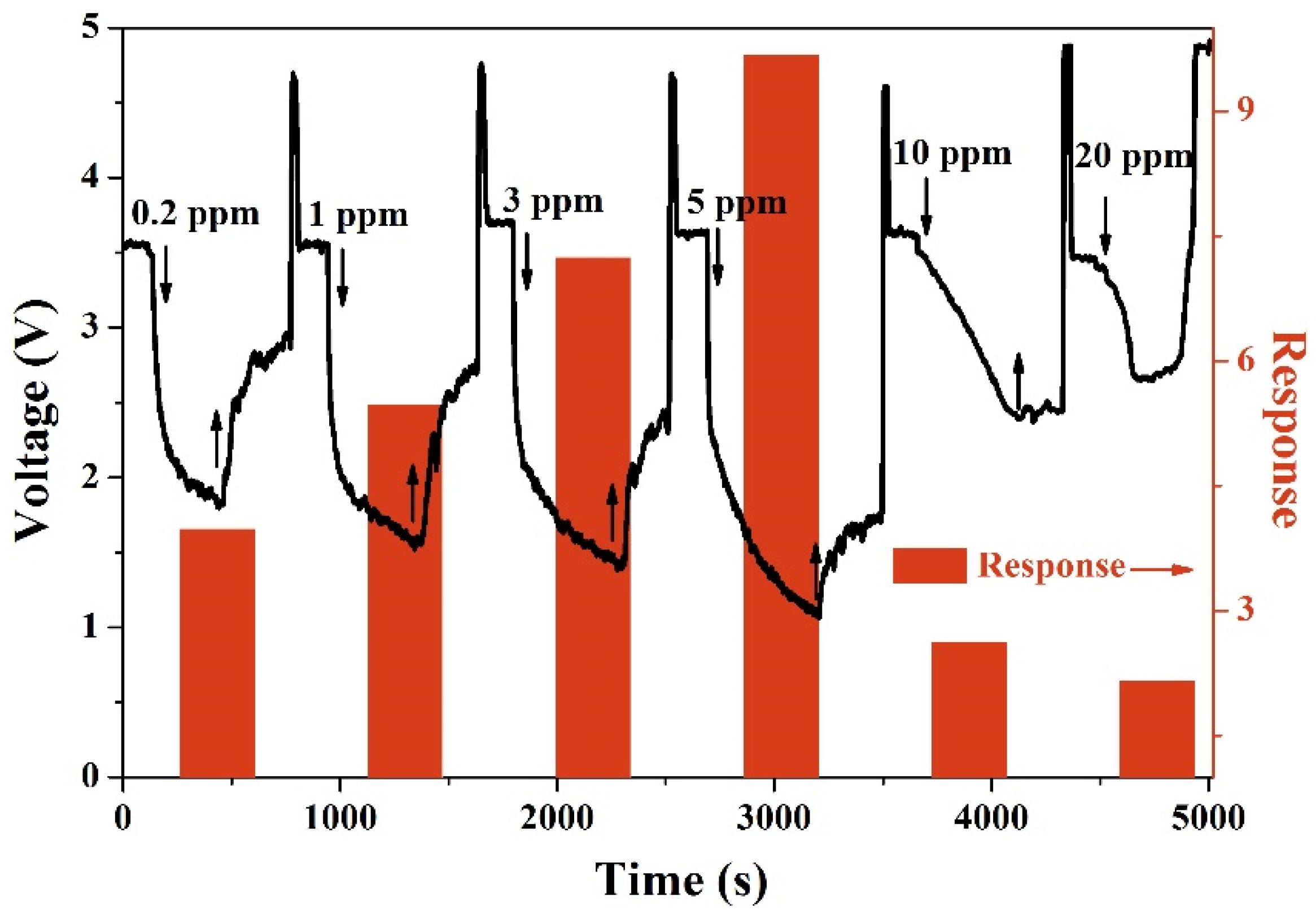
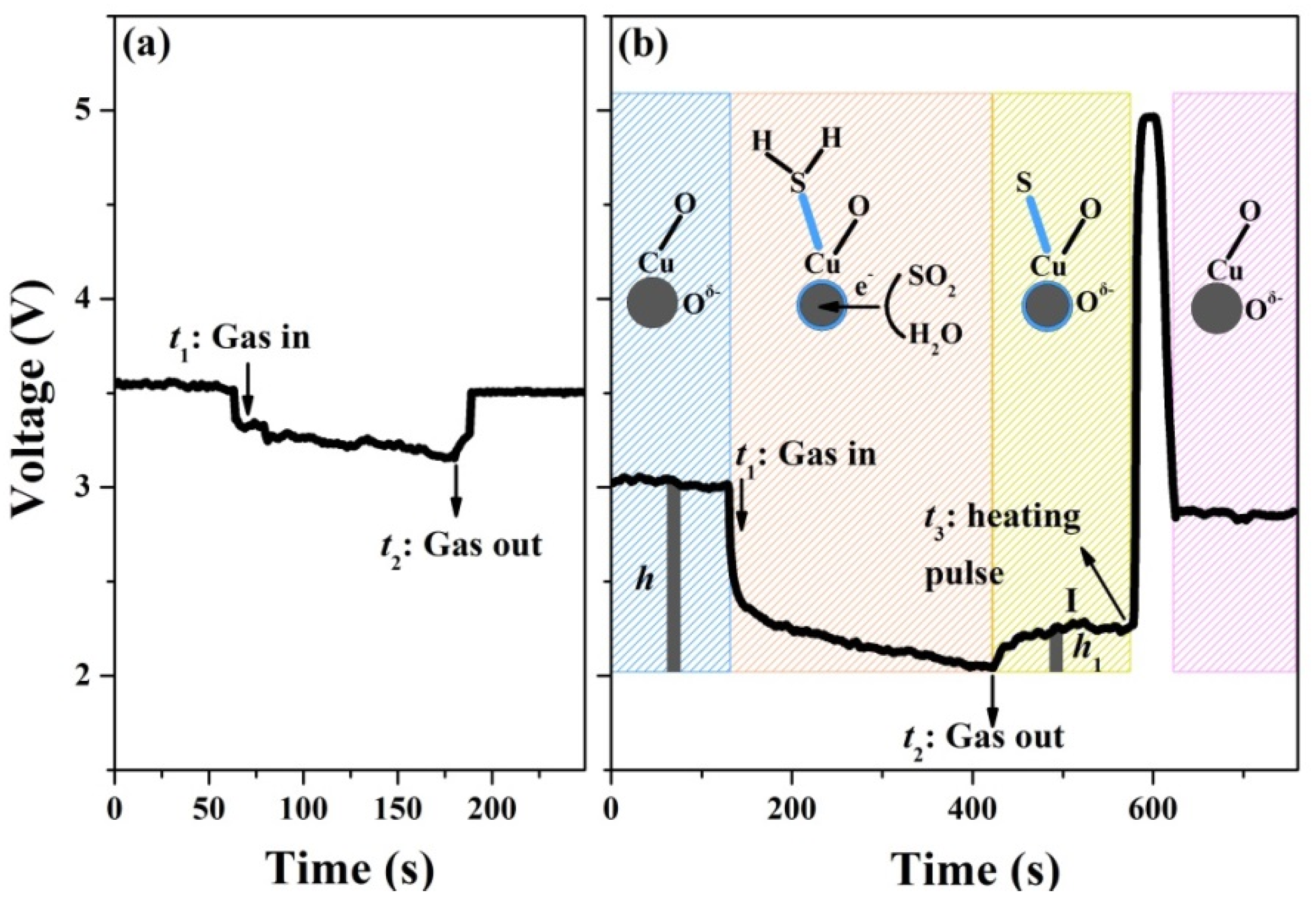
| Materials | Concentration (ppm) | Temperature | Response | Response Time (s) | Recovery Time (s) | Reference |
|---|---|---|---|---|---|---|
| Cu2O/CuO Cu2O | 0.1 0.1 | 95 °C RT | 2.5 1.7 | 76 100 | 75 / | [2] [9] |
| CuO thin film | 5 | RT | 3.5 | >100 | >4000 | [26] |
| CuO nanoparticles | 5 | 40 °C | 4.9 ± 0.43 | 297.5 ± 9.2 | 54 ± 7.1 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, F.; Sun, Y.; Lu, Y.; Yu, W.; Ge, M.; Shi, J.; Cong, R.; Hao, J.; Dai, N. Studies on Sensing Properties and Mechanism of CuO Nanoparticles to H2S Gas. Nanomaterials 2020, 10, 774. https://doi.org/10.3390/nano10040774
Peng F, Sun Y, Lu Y, Yu W, Ge M, Shi J, Cong R, Hao J, Dai N. Studies on Sensing Properties and Mechanism of CuO Nanoparticles to H2S Gas. Nanomaterials. 2020; 10(4):774. https://doi.org/10.3390/nano10040774
Chicago/Turabian StylePeng, Fang, Yan Sun, Yue Lu, Weiwei Yu, Meiying Ge, Jichao Shi, Rui Cong, Jiaming Hao, and Ning Dai. 2020. "Studies on Sensing Properties and Mechanism of CuO Nanoparticles to H2S Gas" Nanomaterials 10, no. 4: 774. https://doi.org/10.3390/nano10040774
APA StylePeng, F., Sun, Y., Lu, Y., Yu, W., Ge, M., Shi, J., Cong, R., Hao, J., & Dai, N. (2020). Studies on Sensing Properties and Mechanism of CuO Nanoparticles to H2S Gas. Nanomaterials, 10(4), 774. https://doi.org/10.3390/nano10040774





