Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance
Abstract
:1. Introduction
2. Importance of Flowers in Daily Life
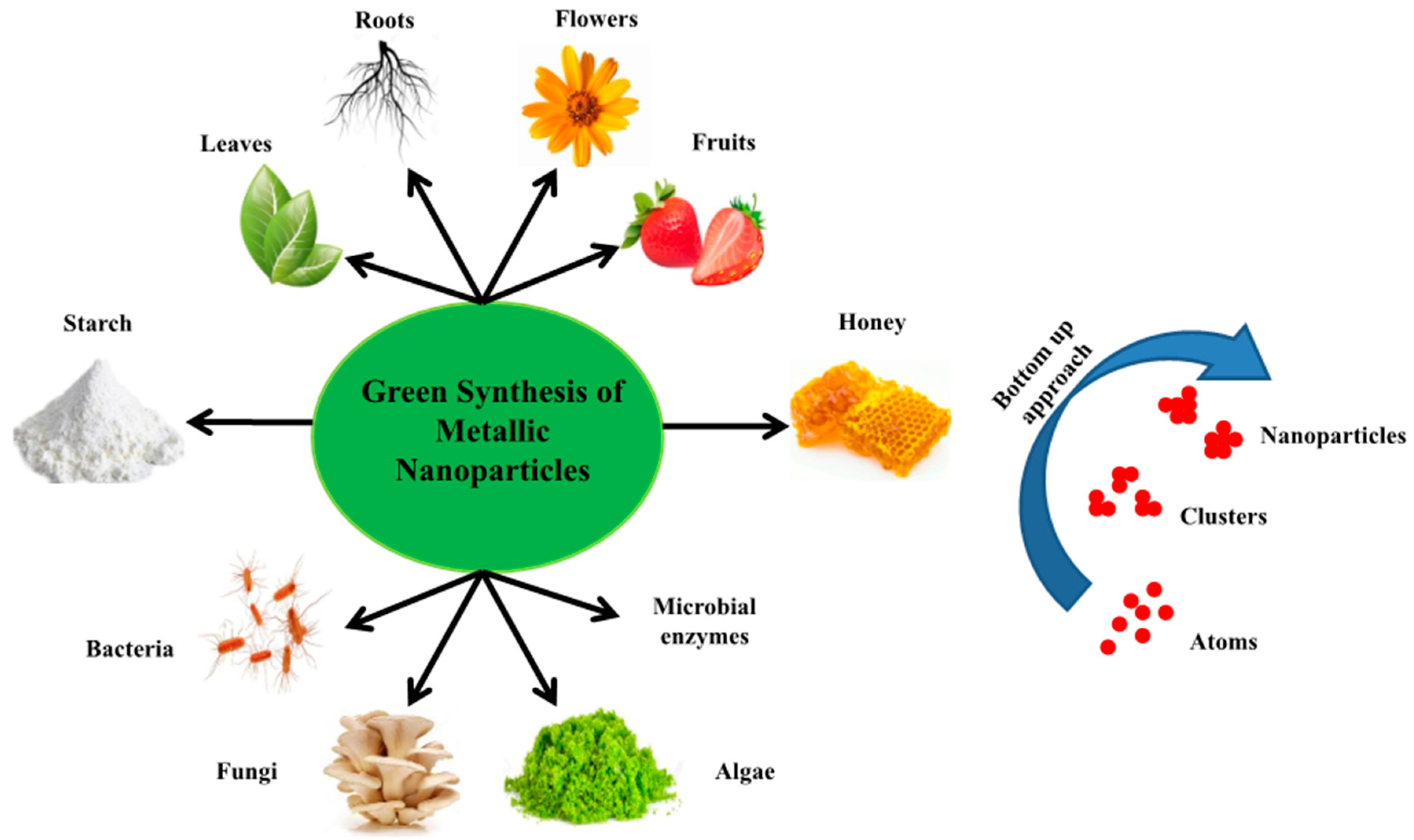
3. Green Synthesis of Nanoparticles (NPs)
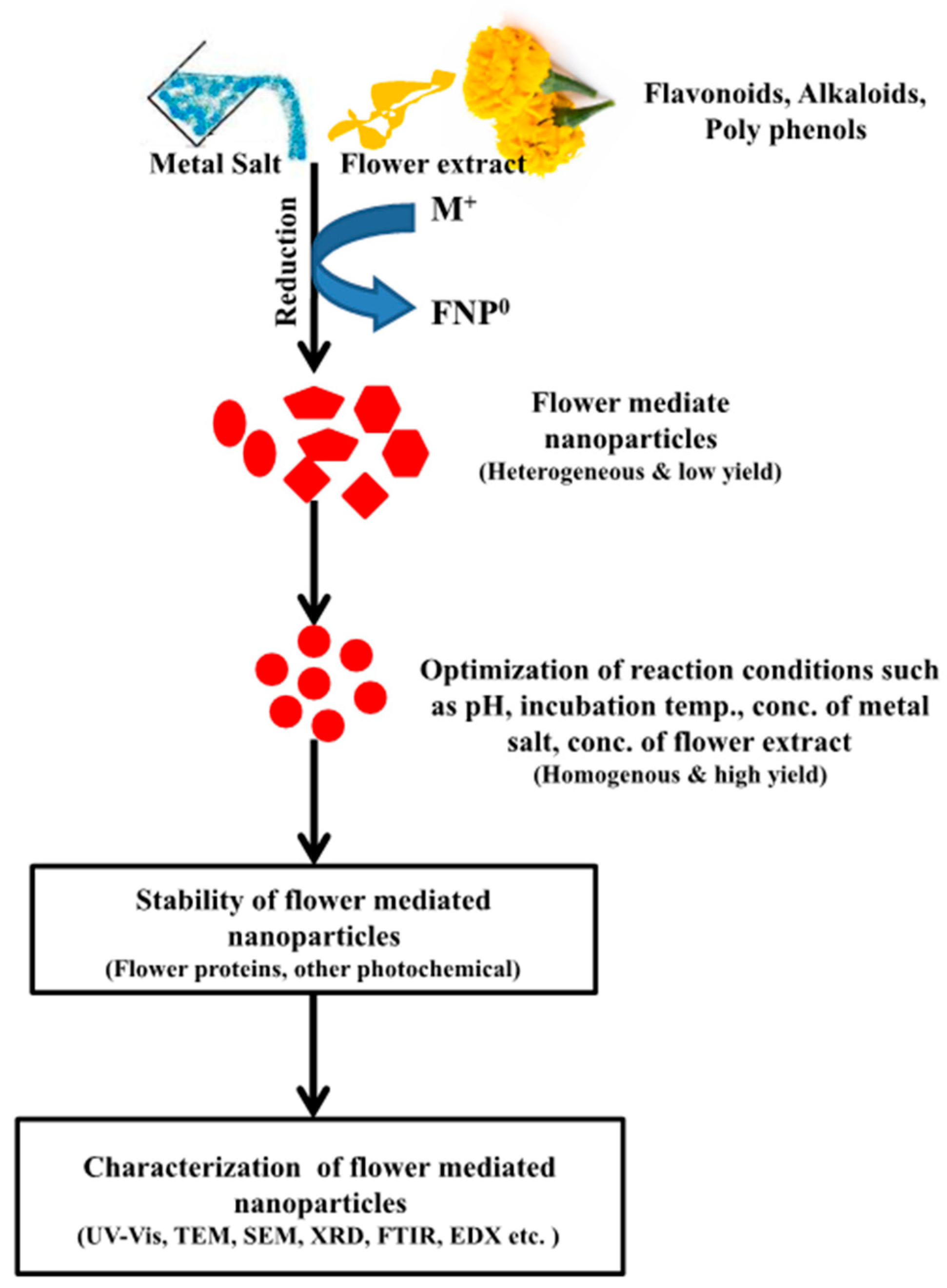
4. Green Synthesis of Nanoparticles Mediated by Flowers
4.1. Silver Nanoparticles (AgNPs)
4.2. Gold Nanoparticles (AuNPs)
4.3. Other Nanoparticles
5. Approaches Used in the Characterization of Nanoparticles
6. Antibacterial Activity of Flower-Derived NPs
7. Antioxidant Potentials of Flower-Derived NPs
8. Catalytic Properties of Flower-Derived NPs
9. Insecticidal Properties of Flower-Derived NPs against Parasites
10. Challenges in the Use of Flower-Mediated Nanoparticles
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhattacharyya, D.; Singh, S.; Satnalika, N. Nanotechnology, big things from a tiny world: A review. Int. J. u- e- Serv. Sci. Technol. 2009, 2, 29–38. [Google Scholar]
- Goddard, W.A., III; Brenner, D.; Lyshevski, S.E.; Iafrate, G.J. Handbook of Nanoscience, Engineering, and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Khandel, P.; Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. J. Nanostruct. Chem. 2018, 8, 369–391. [Google Scholar] [CrossRef] [Green Version]
- Bogunia-Kubik, K.; Sugisaka, M. From molecular biology to nanotechnology and nanomedicine. BioSystem 2002, 65, 123–138. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–306. [Google Scholar] [CrossRef]
- Zharov, V.P.; Kim, J.W.; Curiel, D.T.; Everts, M. Self-assembling nanoclusters in living systems: Application for integrated photothermal nanodiagnostics and nanotherapy. Nanomedicine 2005, 1, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxidenanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Cao, G. Nanastructures and Nanomaterials-Synthesis, Properties and Applications, 2nd ed.; World Scientific: Singapore, 2004. [Google Scholar]
- Varma, R.S. Greener approach to nanomaterials and their sustainable applications. Curr. Opin. Chem. Eng. 2012, 1, 123–128. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green and controlled synthesis of gold and platinum nanomaterials using vitamin B2: Density assisted self-assembly of nanospheres, wires and rods. Green Chem. 2006, 8, 516–518. [Google Scholar] [CrossRef]
- Comba, L.; Corbet, S.A.; Barron, A.; Bird, A.; Collinge, S.; Miyazaki, C.; Powell, M. Garden flowers: Insect visits and the floral reward of horticulturally-modified variants. Ann. Bot. 1999, 83, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Huss, E.; Yosef, K.B.; Zaccai, Z. Humans’ relationship to flowers as an example of the multiple components of embodied aesthetics. Behav. Sci. 2018, 8, E32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, F.J.; Thompson, E.; Rosch, E. The Embodied Mind: Cognitive Science and Human Experience; MIT Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Baron, R.A. The sweet smell of … helping: Effects of pleasant ambient fragrance on prosocial behavior in shopping malls. Pers. Soc. Psychol. Bull. 1997, 23, 498–503. [Google Scholar] [CrossRef]
- Sarid, O.; Zaccai, M. Changes in mood states are induced by smelling familiar and exotic fragrances. Front. Psychol. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shubhashree, M.N.; Shantha, T.R.; Ramarao, V.; Prathapa Reddy, M.P.; Venkateshawarulu, G. A review on therapeutic uses of flowers as depicted in classical texts of Ayurveda and Siddha. J. Res. Educ. Indian Med. 2015, 21, 1–14. [Google Scholar]
- Varadhan, K.P. Introduction to pushpaayurveda. Anc. Sci. Life. 1985, 4, 153–157. [Google Scholar]
- Nishteswar, K. Pushpayurveda (flowers of medicinal plants) delineated in Kaiyadevanighantu. Punarna V. 2015, 2, 1–10. [Google Scholar]
- Puckhaber, L.S.; Stipanovic, R.D.; Bost, G.A. Analyses for flavonoid aglycones in fresh and preserved Hibiscus flowers. In Trends in New Crops and New Uses; Jules, J., Anna, W., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 56–563. [Google Scholar]
- Khan, Z.S.; Shinde, V.N.; Bhosle, N.O.; Nasreen, S. Chemical composition and antimicrobial activity of angiospermic plants. Middle-East J. Sci. Res. 2010, 6, 56–61. [Google Scholar]
- Arullappan, S.; Zakaria, Z.; Basri, D.F. Preliminary screening of antibacterial activity using crude extracts of Hibiscus rosa-sinensis. Trol. Life Sci. Res. 2009, 20, 109–118. [Google Scholar]
- Ruban, P.; Gajalakshmi, K. In vitro antibacterial activity of Hibiscus rosa-sinensis flower extract against human pathogens. Asian Pac. J. Trop. Biomed. 2012, 2, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.A.; Naqvi, S.A.; Mukhtar, A.; Hussain, Z.; Shahzad, S.A.; Mansha, A.; Mahmood, N. Antioxidant and antibacterial activities of Hibiscus Rosa-sinensis Linn flower extracts. Pak. J. Pharm. Sci. 2014, 27, 469–474. [Google Scholar]
- Meena, A.K.; Patidar, D.; Singh, R.K. Ameliorative effect of Hibiscus rosa-sinensis on phenylhydrazine induced haematotoxicity. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 8678–8683. [Google Scholar]
- Wong, K.C.; Teng, Y.E. Volatile components of Mimusops elengi L. flowers. J. Essent. Oil. Res. 1994, 6, 453–458. [Google Scholar] [CrossRef]
- Rout, P.K.; Sahoo, D.; Misra, L.N. Comparison of extraction methods of Mimusops elengi L. flowers. Ind. Crops. Prod. 2010, 32, 678–680. [Google Scholar] [CrossRef]
- Sundari, U.T.; Rekha, S.; Parvathi, A. Phytochemical analysis of some therapeutic medicinal flowers. Int. J. Pharm. 2012, 2, 583–585. [Google Scholar]
- Koppula, S.B. Antimicrobial activity of floral extracts on selected human pathogens. Int. J. Bio-Pharm. Res. 2013, 2, 141–143. [Google Scholar]
- Reddy, L.J.; Jose, B. Evaluation of antibacterial activity of Mimusops elengi L. flowers and Trichosanthes cucumerina L. fruits from South India. Int. J. Pharm. Pharm. Sci. 2013, 5, 362–364. [Google Scholar]
- Tuntiwachwuttikul, P.; Rayanil, K.; Taylor, W.C. Chemical constituents from the flowers of Nyctanthes arbortristis. Sci. Asia. 2003, 29, 21–30. [Google Scholar] [CrossRef]
- Khatune, N.A.; Mossadik, M.A.; Rahman, M.M.; Khondkar, P.; Haque, M.E.; Gray, A.I. A benzofuranone from the flowers of Nyctanthes arbortristis and its antibacterial and cytotoxic activities. Dhaka Univ. J. Pharm. Sci. 2005, 4, 33–37. [Google Scholar] [CrossRef]
- Nanu, R.; Raghuveer, I.; Chitme, H.; Chandra, R. Antidiabetic activity of Nyctanthes arbortristis. Pharmacogn. Mag. 2008, 4, 335–340. [Google Scholar]
- Kim, M.R.; Lee, J.Y.; Lee, H.H.; Aryal, D.K.; Kim, Y.G.; Kim, S.K.; Woo, E.R.; Kang, K.W. Antioxidative effects of quercetin-glycosides isolated from the flower buds of Tussilago farfara L. Food Chem. Toxicol. 2006, 44, 1299–1307. [Google Scholar] [CrossRef]
- Maurya, S.; Bhardwaj, A.K.; Gupta, K.K.; Agarwal, S.; Kushwaha, A.; Chaturvedi, V.K.; Pathak, R.K.; Gopal, R.; Uttam, K.N.; Soingh, A.K.; et al. Green synthesis of silver nanoparticles using Pleurotus and bactericidal activity. Cell Mol. Biol. 2016, 62, 131. [Google Scholar]
- Baruwati, B.; Varma, R.S. High value products from waste: Grape pomace extract- a three-in-one package for the synthesis of metal nanoparticles. ChemSusChem 2009, 2, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Varma, R.S. A greener synthesis of core (Fe, Cu)-shell (Au, Pt, Pd, and Ag) nanocrystals using aqueous vitamin C. Cryst. Growth Des. 2007, 7, 2582–2587. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Microwave-assisted shape-controlled bulk synthesis of noble nanocrystals and their catalytic properties. Cryst. Growth Des. 2007, 7, 686–690. [Google Scholar]
- Baruwati, B.; Polshettiwara, V.; Varma, R.S. Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem. 2009, 11, 926–930. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Self-assembly of metal oxides into three-dimensional nanostructures: Synthesis and application in catalysis. ACS Nano 2009, 3, 728–736. [Google Scholar] [CrossRef]
- Baruwati, B.; Nadagouda, M.N.; Varma, R.S. Bulk synthesis of monodisperse ferrite nanoparticles at water−organic interfaces under conventional and microwave hydrothermal treatment and their surface functionalization. J. Phys. Chem. C 2008, 112, 18399–18404. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008, 10, 859–862. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003, 14, 824–828. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Kulkarni, A.R. Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surf. B Biointerfaces. 2009, 71, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Wong, C.W.; Yasumira, A.A.N. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E J. Chem. 2009, 6, 61–70. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K. Biosynthesis of CdS nanoparticles: An improved green and rapid procedure. J. Colloid. Interface Sci. 2010, 342, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Dhoondia, Z.H.; Chakraborty, H. Lactobacillus mediated synthesis of silver oxide nanoparticles. Nanomater. Nanotechno. 2012, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Karve, M.S.; Chopade, B.A. Novel polyhedral gold nanoparticles: Green synthesis, optimization and characterization by environmental isolate of Acinetobacter sp. SW30. World J. Microbiol. Biotechnol. 2014, 30, 2723–2731. [Google Scholar] [CrossRef]
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2003, 14, 95–100. [Google Scholar] [CrossRef]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol Sci. 2012, 13, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Korbekandi, H.; Ashari, Z.; Iravani, S.; Abbasi, S. Optimization of biological synthesis of silver nanoparticles using Fusarium oxysporum. Iran. J. Pharm. Res. 2013, 12, 289–298. [Google Scholar]
- Gholami-Shabani, M.; Akbarzadeh, A.; Norouzian, D.; Amini, A.; Gholami-Shabani, Z.; Imani, A.; Chiani, M.; Riazi, G.; Shams-Ghahfarokhi, M.; Razzaqhi-Abyaneh, M. Antimicrobial activity and physical characterization of silver nanoparticles green synthesized using nitrate reductase from Fusarium oxysporum. Appl. Biochem. Biotechnol. 2014, 172, 4084–4098. [Google Scholar] [CrossRef]
- Singaravelu, G.; Arockiamary, J.S.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces. 2007, 57, 97–101. [Google Scholar] [CrossRef]
- Venkatpurwar, V.; Pokharkar, V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Mater. Lett. 2011, 65, 999–1002. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Kannan, C.; Annadurai, G. Green synthesis of silver nanoparticles using marine brown algae Turbinaria conoides and its antibacterial activity. Int. J. Pharm. Bio Sci. 2012, 3, 502–510. [Google Scholar]
- El-Rafie, H.M.; El-Rafie, M.H.; Zahran, M.K. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Namvar, F.; Ahmad, M.B.; Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 2013, 18, 5954. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol. Program 2003, 19, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; Pyne, S.; Misra, A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 212–216. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 34–41. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces. 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jain, D.; Upadhyay, M.K.; Khandelwal, N.; Verma, H.N. Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Biost. 2010, 5, 483–489. [Google Scholar]
- Yang, X.; Li, Q.; Wang, H.; Huang, J.; Lin, L.; Wang, W.; Sun, D.; Su, Y.; Opiyo, J.B.; Hong, L.; et al. Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf. J. Nanopart Res. 2010, 12, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.G.; Gokavarapu, S.D.; Rajeswari, A.; Dhas, T.S.; Karthick, V.; Kapadia, Z.; Shrestha, T.; Barathy, I.A.; Roy, A.; Sinha, S. Facile green synthesis of gold nanoparticles using leaf extract of antidiabetic potent Cassia auriculata. Colloids Surf. B Biointerfaces 2011, 87, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Zargar, M.; Hamid, A.A.; Bakar, F.A.; Shamsudin, M.N.; Shameli, K.; Jahanshiri, F.; Farahani, F. Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules 2011, 16, 6667. [Google Scholar] [CrossRef]
- Philip, D. Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 650–653. [Google Scholar] [CrossRef]
- Venu, R.; Ramulu, T.S.; Anandakumar, S.; Rani, V.S.; Kim, C.G. Bio-directed synthesis of platinum nanoparticles using aqueous honey solutions and their catalytic applications. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 733–738. [Google Scholar] [CrossRef]
- Reddy, S.M.; Datta, K.K.R.; Sreelakshmi, C.; Eswaramoorthy, M.; Reddy, B.V.S. Honey mediated green synthesis of Pd nanoparticles for suzuki coupling and hydrogenation of conjugated olefins. Nanosci. Nanotechnol. Lett. 2012, 4, 420–425. [Google Scholar] [CrossRef]
- Haiza, H.; Azizan, A.; Mohidin, A.H.; Halin, D.S.C. Green synthesis of silver nanoparticles using local honey. Nano Hybrids. 2013, 4, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Cai, X.; Nelson, K.; Xing, W.; Xia, J.; Zhang, R.; Stacy, A.J.; Luderer, M.; Lanza, G.M.; Wang, L.V.; et al. A green synthesis of carbon nanoparticles from honey and their use in real-time photoacoustic imaging. Nano Res. 2013, 6, 312–325. [Google Scholar] [CrossRef] [Green Version]
- Chidambaram, J.; Saritha, K.; Maheshwari, R.; Muzammil, M.S. Efficacy of green synthesis of silver nanoparticles using flowers of Calendula officinalis. Chem. Sci. Trans. 2014, 3, 773–777. [Google Scholar]
- Esfanddarani, H.M.; Kajani, A.A.; Bordbar, A.K. Green synthesis of silver nanoparticles using flower extract of Malva sylvestris and investigation of their antibacterial activity. IET Nanobiotechnol. 2018, 12, 412–416. [Google Scholar] [CrossRef]
- Surya, S.; Kumar, G.D.; Rajakumar, R. Green synthesis of silver nanoparticles from flower extract of Hibiscus rosa-sinensis and its antibacterial activity. Int. J. Innov Res. Sci. Eng. Technol. 2016, 5, 5242–5247. [Google Scholar]
- Patil, M.P.; Singh, R.D.; Koli, P.B.; Patil, K.T.; Jagdale, B.S.; Tipare, A.R.; Kim, G.D. Antibacterial potential of silver nanoparticles synthesized using Madhuca longifolia flower extract as a green resource. Micro Pathog. 2018, 121, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Manisha, D.R.; Alwala, J.; Kudle, K.R.; Rudra, M.P.P. Biosynthesis of silver nanoparticles using flower extracts of Catharanthus roseus and evaluation of its antibacterial efficacy. World J. Pharm. Pharm. Sci. 2014, 3, 877–885. [Google Scholar]
- Lee, Y.J.; Song, K.; Cha, S.H.; Cho, S.; Kim, Y.S.; Park, Y. Sesquiterpenoids from Tussilago farfara flower bud extract for the eco-friendly synthesis of silver and gold nanoparticles possessing antibacterial and anticancer activities. Nanomaterials 2019, 9, E819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Gurav, D.D.; Jabgunde, A.M.; Kale, S.; Pardesi, K.; Shinde, V.; Bellare, J.; et al. Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J. Nanobiotechnol. 2012, 10, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayan, V.; Onteru, S.K.; Singh, D. Mangifera indica flower extract mediated biogenic green gold nanoparticles: Efficient nanocatalyst for reduction of 4-nitrophenol. Environ. Prog. Sustain. Energy 2018, 37, 283–294. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, M.I.; Kanchi, S.; Mdluli, P.S.; Singh, G.; Stenström, T.A.; Bisetty, K. Biosynthesis of ZnO nanoparticles using Jacaranda mimosifolia flowers extract: Synergistic antibacterial activity and molecular simulated facet specific adsorption studies. J. Photoc. Photobiol. B Biol. 2016, 162, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Hajra, A.; Dutta, S.; Mondal, N.K. Mosquito larvicidal activity of cadmium nanoparticles synthesized from petal extracts of marigold (Tagetes sp.) and rose (Rosa sp.) flower. J. Parasit Dis. 2016, 40, 1519–1527. [Google Scholar] [CrossRef] [Green Version]
- Marimuthu, S.; Rahuman, A.A.; Jayaseelan, C.; Kirthi, A.V.; Santhoshkumar, T.; Velayutham, K.; Bagavan, A.; Kamaraj, C.; Elango, G.; Iyappan, M.; et al. Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropisgigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian J. Trop. Med. 2013, 6, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, Y.; Ogunyemi, S.O.; Abdelazez, A.; Zhang, M.; Hong, X.; Ibrahim, E.; Hossain, A.; Fouad, H.; Li, B.; Chen, J. The green synthesis of MgO nano-flowers using Rosmarinus officinalis L. (Rosemary) and the antibacterial activities against Xanthomonas oryzae pv. Oryzae. BioMed. Res. Int. 2019, 2019, 5620989. [Google Scholar] [CrossRef] [Green Version]
- Igwe, O.U.; Nwamezie, F. Green synthesis of iron nanoparticles using flower extract of Piliostigma thonningii and their antibacterial activity evaluation. Chem. Int. 2018, 4, 60–66. [Google Scholar]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Mashwani, Z.R.; Khan, M.A.; Khan, T.; Nadhman, A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141. [Google Scholar] [CrossRef]
- Muruganantham, N.; Govindharaju, R.; Anitha, P.; Anusuya, V. Synthesis and Characterization of silver nanoparticles using Lablab purpureus flowers (Purple colour) and its anti-microbial activities. Int. J. Sci. Res. Biol. Sci. 2018, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mandal, P. Biosynthesis of silver nanoparticles by Plumeria rubra flower extract: Characterization and their antimicrobial activities. Int. J. Eng. Sci. Inv. 2018, 7, 1–6. [Google Scholar]
- Bharathi, D.; Bhuvaneshwari, V. Evaluation of the cytotoxic and antioxidant activity of phyto-synthesized silver nanoparticles using Cassia angustifolia flowers. BioNanoScience 2018, 9, 155–163. [Google Scholar] [CrossRef]
- Karnuakaran, G.; Jagathambal, M.; Gusev, A.; Kloesnikov, E.; Mandal, A.R.; Kuznestov, D. Allamanda cathartica flower’s aqueous extract-mediated green synthesis of silver nanoparticles with excellent antioxidant and antibacterial potential for biomedical application. MRS Commun. 2016, 6, 41–46. [Google Scholar] [CrossRef]
- Moteriya, P.; Chanda, S. Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1556–1567. [Google Scholar] [CrossRef] [Green Version]
- Padalia, H.; Moteriya, P.; Chanda, S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem. 2014, 8, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Varadavenkatesan, T.; Selvaraj, R.; Vinayagam, R. Dye degradation and antibacterial activity of green synthesized silver nanoparticles using Ipomoea digitata Linn. flower extract. Int. J. Environ. Sci. Te. 2019, 16, 2395–2404. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Kumar, V.D.; Elakya, V.; Kamala, T.; Park, S.K.; Saravanan, M.; Bououdina, M.; Arasu, M.V.; Kovendan, K.; Vincent, S. Biosynthesized silver nanoparticles using floral extract of Chrysanthemum indicum L.-potential for malaria vector control. Environ. Sci. Pollut. Res. 2015, 22, 9759–9765. [Google Scholar] [CrossRef] [PubMed]
- Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z.; Zeiri, Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014, 9, 4007–4021. [Google Scholar]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-functionalized gold nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef]
- Qian, X.; Peng, X.H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008, 26, 83–90. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045–3059. [Google Scholar] [CrossRef]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Nagaraj, B.; Malakar, B.; Divya, T.K.; Krishnamurthy, N.B.; Liny, P.; Dinesh, R. Environmental benign synthesis of gold nanoparticles from the flower extracts of Plumeria alba Linn. (Frangipani) and evaluation of their biological activities. Int. J. Drug Dev. Res. 2012, 4, 144–150. [Google Scholar]
- Lakshmeesha, T.R.; Kalagatur, N.K.; Mudili, V.; Mohan, C.D.; Rangappa, S.; Prasad, B.D.; Ashwini, B.S.; Hashem, A.; Alqarawi, A.A.; Malik, J.A.; et al. Biofabrication of zinc oxide nanoparticles with Syzygium aromaticum flower buds extract and finding its novel application in controlling the growth and mycotoxins of Fusarium graminearum. Front. Microbiol. 2019, 10, 1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarah, S.L.R.; Iyer, P.R. Green synthesis of copper nanoparticles from the flowers of Mimusops elengi. Int. J. Recent. Sci. Res. 2019, 10, 32956–32963. [Google Scholar]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud. Univ. Sci. 2016, 30, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Tripathy, S.K.; Yun, S.I. Fungus mediated synthesis of gold nanoparticles and their conjugation with genomic DNA isolated from Escherichia coli and Staphylococcus aureus. Process. Biochem. 2012, 47, 701–711. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticles dispersions for toxicological studies. J. Nanopart Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle adhesion to the cell membrane and its effect on nano particle uptake efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. [Google Scholar] [CrossRef] [Green Version]
- Panigrahi, S.; Basu, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, A.; Ghosh, S.K.; Pal, T. Synthesis and size-selective catalysis by supported gold nanoparticles: Study on heterogeneous and homogeneous catalytic process. J. Phys. Chem. C 2007, 111, 4596–4605. [Google Scholar] [CrossRef]
- Woo, Y.; Lai, D.Y. Aromatic amino and nitro-amino compounds and their halogenated derivatives. In Patty’s Toxicology; Bingham, E., Cohrssen, B., Powell, C.H., Eds.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Sharma, J.K.; Akhtar, M.S.; Ameen, S.; Srivastva, P.; Singh, G. Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloys Compd. 2015, 632, 321–325. [Google Scholar] [CrossRef]
- Lim, S.H.; Ahn, E.Y.; Park, Y. Green synthesis and catalytic activity of gold nanoparticles synthesized by Artemisia capillaries water extract. Nanoscale Res. Lett. 2016, 11, 474. [Google Scholar] [CrossRef] [Green Version]
- Rostami-Vartooni, A.; Nasrollahzadeh, M.; Alizadeh, M. Green synthesis of perlite supported silver nanoparticles using Hamamelis virginiana leaf extract and investigation of its catalytic activity for the reduction of 4-nitrophenol and Congo red. J. Alloys Compd. 2016, 680, 309–314. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Loganathan, B.; Dinesh, S.; Raghu, K. Strategic green synthesis, characterization and catalytic application to 4-nitrophenol reduction of palladium nanoparticles. J. Clust. Sci. 2017, 28, 2123–2131. [Google Scholar] [CrossRef]
- Senobari, S.; Nezamzadeh-Ejhieh, A. A comprehensive study on the enhanced photocatalytic activity of CuO-NiO nanoparticles: Designing the experiments. J. Mol. Liq. 2018, 261, 208–217. [Google Scholar] [CrossRef]
- Chen, A.; Chen, W.; Latham, P. 10 Fatal Cholangiocarcinoma in the setting of treatment-resistant hepatitis C virus infection. Am. J. ClinPathol. 2018, 149, S4. [Google Scholar] [CrossRef]
- Sultan, M.; Waheed, A.; Bibi, I.; Islam, A. Ecofriendly reduction of methylene blue with polyurethane catalyst. Int. J. Polym Sci. 2019, 2019, 3168618. [Google Scholar] [CrossRef]
- Begum, R.; Najeeb, J.; Sattar, A.; Naseem, K.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Chemical reduction of methylene blue in the presence of nanocatalysts: A critical review. Rev. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Matus, K.J.M.; Hutchison, J.E.; Peoples, R.; Rung, S.; Tanguay, R.L. Green Nanotechnology Challenges and Opportunities. Available online: https://greennano.org/sites/greennano2.uoregon.edu/files/GCI_WP_GN10.pdf (accessed on 2 November 2019).
- Balasooriya, E.R.; Jayasinghe, C.D.; Jayawardena, U.A.; Ruwanthika, R.W.D.; de Silva, R.M.; Udagama, P.V. Honey mediated green synthesis of nanoparticles: New era of safe nanotechnology. J. Nanomater. 2017, 2017, 5919876. [Google Scholar] [CrossRef]
| Nanoparticle Types | Reducing Agent | Stabilizing Agent | Specific Temperature | Ref |
|---|---|---|---|---|
| Silver | chlorine ions | proteins present in the flower | RT | [75,76] |
| water-soluble heterocyclic components, polyols, and certain proteins | flower | RT | [77] | |
| flower | flower | 40 °C | [78] | |
| flower | flower | 60 °C | [79] | |
| sesquiterpenoids | DMEM + FBS | 80 °C | [80] | |
| Gold | sesquiterpenoids | DMEM + FBS | 80 °C | [80] |
| flower | flower | 40 °C | [81] | |
| polyphenols and flavonols | flower | 25–60 °C | [82] | |
| Zinc | flower | flower | microwave irradiation | [83] |
| Cadmium | tannins, flavonoids, alkaloids, and carotenoids | flower | RT | [84] |
| Titanium | flower | flower | 60 °C | [85] |
| Magnesium | flower | flower | 70 °C | [86] |
| Iron | flower | flower | RT | [87] |
| Family | Flower Variety | Applications | Ref |
|---|---|---|---|
| Fabaceae | Lablab purpureus | Antibacterial activityagainst Escherichia coli and Staphylococcus aureus | [90] |
| Apocynaceae | Plumeria rubra | Antibacterial activity against Escherichia coli and Bacillus sp. | [91] |
| Apocynaceae | Catharanthus roseus | Antibacterial activity against Escherichia coli, Pseudomonas putida, Staphylococcus aureus, Klebsiella pneumoniae, and Bacillus subtilus | [79] |
| Fabaceae | Cassia angustifolia | Antioxidant and cytotoxicity activity | [92] |
| Apocynaceae | Allamanda cathartica | Antioxidant activity and antibacterial activity against Salmonella typhimurium, Staphylococcus aureus, Escherichia coli,and Klebsiella pneumoniae | [93] |
| Malvaceae | Malva sylvestris | Antibacterial activity against Escherichia coli, Staphylococcus aureus, and Streptococcus pyogenes | [76] |
| Fabaceae | Caesalpinia pulcherrima | Antibacterial activity against Staphylococcus aureus; antifungal activity against Candida glabrata; antioxidant activity; cytotoxicity activity | [94] |
| Asteraceae | Tussilago farfara | Antibacterial activity against Enterococcus faecium; cyrotoxicity activity | [80] |
| Asteraceae | Tagetes erecta | Antibacterial activity against Escherichia coli and Pseudomonas aeruginosa; antifungal activity against Candida albicans | [95] |
| Sapotaceae | Madhuca longifolia | Antibacterial activity against Bacillus cereus and Staphylococcus saprophyticus | [78] |
| Malvaceae | Hibiscus rosa-sinensis | antibacterial activity against Aeromonas hydrophila | [77] |
| Convolvulaceae | Ipomoea digitata Linn | Antibacterial activity against Staphylococcus epidermidis; catalytic activity against methylene blue | [96] |
| Asteraceae | Chrysanthemum indicum L. | Larvicidal and pupicidalactivity against Anopheles stephenis | [97] |
| Family | Flower Variety | Applications | Ref |
|---|---|---|---|
| Apocynaceae | Plumeria alba Linn | Antibacterial activity against Escherichia coli | [104] |
| Thymelaeaceae | Gnidia glauca | Chemocatalytic activity against 4-nitrophenol | [81] |
| Anacardiaceae | Mangifera indica | Catalytic activity against 4-nitrophenol | [82] |
| Asteraceae | Tussilago farfara | Antibacterial activity against Enterococcus faecium; cyrotoxicity activity | [80] |
| Family | Flower Variety | Types of Nanoparticles Synthesized | Applications | Ref |
|---|---|---|---|---|
| Sapotaceae | Mimusops elengi | Copper | Antibactrial activity against Escherichia coli, Streptococcus, Staphylococcus, Pseudomonas, and Bacillus subtilis; antifungal activity Aspergillus flavus, Candida albicans, Penicillium and Aspergillus fumigates; antioxidant activity; thrombolytic activity; anti-larval activity; cytotoxicity activity; heavy metals removal | [106] |
| Fabaceae | Piliostigma thonningii | Iron | Antibacterial activity against Escherichia coli and Staphylococcus aureus | [87] |
| Oleaceae | Nyctanthes arbor-tristis | Zinc | Antifungal activity against Alternaria alternate, Aspergillus niger, Botrytis cinerea, Fusarium oxysporum, and Penicillium expansum | [107] |
| Myrtaceae | Syzygium aromaticum | Zinc | Antifungal activity against Fusarium graminearum | [105] |
| Bignoniaceae | Jacaranda mimosifolia | Zinc | Antibacterial activity against Enterococcus faecium | [83] |
| Asteraceae | Tagetes sp. | Cadmium | Larvicidal activity against Aedes albopictus | [84] |
| Apocynaceae | Calotropis gigantean | Titanium | Acaricidal activity against Rhipicephalus microplus and Haemaphysalis bispinosa | [85] |
| Lamiaceae | Rosmarinus officinalis L. | Magnesium | Antibacterial activity against Xanthomonas oryzae pv. oryzae | [86] |
| Family | Flower Variety | Types of Nanoparticles Synthesized | Methods Used for NPs Characterization | Size | Morphology | Ref | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UV–vis | TEM | SEM | FT-IR | XRD | EDX | DLS | Zeta Potential | HRTEM | AFM | GC-MS | ||||||
| Fabaceae | Lablab purpureus | Silver | √ | - | √ | √ | √ | - | - | - | - | - | - | 5–50 nm | Spherical | [90] |
| Sapotaceae | Mimusopselengi | Copper | √ | - | √ | √ | √ | - | - | - | - | - | - | 42–90 nm | Rod and spherical | [106] |
| Fabaceae | Piliostigma thonningii | Iron | √ | - | √ | √ | √ | - | - | - | - | - | - | 20–100µm | Rod and spherical | [87] |
| Oleaceae | Nyctanthes arbor-tristis | Zinc | √ | √ | - | √ | √ | - | √ | - | - | - | - | 12–32 nm | Aggregate | [107] |
| Apocynaceae | Plumeria rubra | Silver | √ | √ | - | - | - | - | - | - | - | - | - | 20–80 nm | Spherical and irregular | [91] |
| Apocynaceae | Catharanthus roseus | Silver | √ | √ | - | √ | - | - | - | - | - | - | - | 6–25 nm | spherical | [79] |
| Fabaceae | Cassia angustifolia | Silver | √ | - | √ | √ | √ | √ | - | - | - | - | - | 10–80 nm | Spherical | [92] |
| Apocynaceae | Plumeria alba Linn | Gold | √ | - | - | - | - | - | - | - | √ | - | - | 20–30 and 80–150 nm | Spherical | [104] |
| Myrtaceae | Syzygium aromaticum | Zinc | √ | √ | √ | √ | √ | - | - | - | - | - | - | 30–40 nm | Triangular and hexagonal | [105] |
| Thymelaeaceae | Gnidia glauca | Gold | √ | √ | - | √ | √ | - | √ | - | √ | - | - | 50–150 nm | Spherical | [81] |
| Apocynaceae | Allamanda cathartica | Sliver | √ | √ | - | √ | √ | - | - | - | - | - | - | 39 nm | Spherical | [93] |
| Malvaceae | Malva sylvestris | Silver | √ | √ | - | √ | - | √ | - | - | - | √ | - | 20–40 nm | Spherical | [76] |
| Fabaceae | Caesalpinia pulcherrima | Silver | √ | √ | - | √ | √ | - | - | - | - | - | - | 12 nm | Spherical | [94] |
| Asteraceae | Tussilago farfara | Silver and Gold | √ | √ | - | - | √ | - | - | √ | - | √ | - | 13.57 and 18.20 nm | Spherical | [80] |
| Anacardiaceae | Mangifera indica | Gold | √ | √ | - | - | √ | √ | - | - | √ | - | - | 10–60 nm | Spherical | [82] |
| Asteraceae | Tagetes erecta | Silver | √ | √ | - | √ | √ | - | - | - | - | - | - | 10–90 nm | Spherical, hexagonal, and irregular | [95] |
| Sapotaceae | Madhuca longifolia | Silver | √ | √ | √ | √ | √ | √ | - | √ | - | - | - | 30–50 nm | Spherical and oval | [78] |
| Bignoniaceae | Jacaranda mimosifolia | Zinc | √ | - | - | √ | √ | - | - | - | √ | - | √ | 2–4 nm | Spherical | [83] |
| Malvaceae | Hibiscus rosa-sinensis | Silver | √ | - | √ | √ | - | - | - | - | - | - | - | 5–40 nm | Spherical | [77] |
| Convolvulaceae | Ipomoea digitata Linn | Silver | √ | - | √ | √ | √ | √ | - | - | - | - | - | 111 nm | Spherical | [96] |
| Asteraceae | Tagetes sp. | Cadmium | √ | - | √ | √ | - | - | - | - | - | - | - | 50 µm | Spherical | [84] |
| Apocynaceae | Calotropis gigantean | Titanium | - | - | √ | √ | √ | √ | - | - | - | - | - | 160–220 nm | Spherical | [85] |
| Lamiaceae | Rosmarinus officinalis L. | Magnesium | √ | √ | - | √ | √ | - | - | - | - | - | - | 20 nm | Spherical | [86] |
| Asteraceae | Chrysanthemum indicum L. | Silver | √ | √ | - | - | √ | √ | - | - | - | - | 25–59 nm | Spherical | [97] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials 2020, 10, 766. https://doi.org/10.3390/nano10040766
Kumar H, Bhardwaj K, Kuča K, Kalia A, Nepovimova E, Verma R, Kumar D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials. 2020; 10(4):766. https://doi.org/10.3390/nano10040766
Chicago/Turabian StyleKumar, Harsh, Kanchan Bhardwaj, Kamil Kuča, Anu Kalia, Eugenie Nepovimova, Rachna Verma, and Dinesh Kumar. 2020. "Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance" Nanomaterials 10, no. 4: 766. https://doi.org/10.3390/nano10040766
APA StyleKumar, H., Bhardwaj, K., Kuča, K., Kalia, A., Nepovimova, E., Verma, R., & Kumar, D. (2020). Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials, 10(4), 766. https://doi.org/10.3390/nano10040766









