On the Role of Nanofluids in Thermal-hydraulic Performance of Heat Exchangers—A Review
Abstract
1. Introduction
- Nanoparticles:
- Material type;
- Size and shape;
- Attraction/extraction characteristics with the hosting basefluid molecules;
- Volumetric concentration;
- Density;
- Specific heat capacity; and
- Thermal conductivity.
- Basefluids:
- Type;
- Temperature;
- pH value;
- Molecular attraction/extraction behaviour towards the dispersed particles;
- Density;
- Specific heat capacity;
- Viscosity; and
- Thermal conductivity.
- Preparation route:
- Single-step method; or
- Two-step approach.
- Chemical or physical dispersion/s (if added).
- Nanofluid long- and short–term dispersion, kinetic, and chemical stabilities.
2. Heat Exchangers
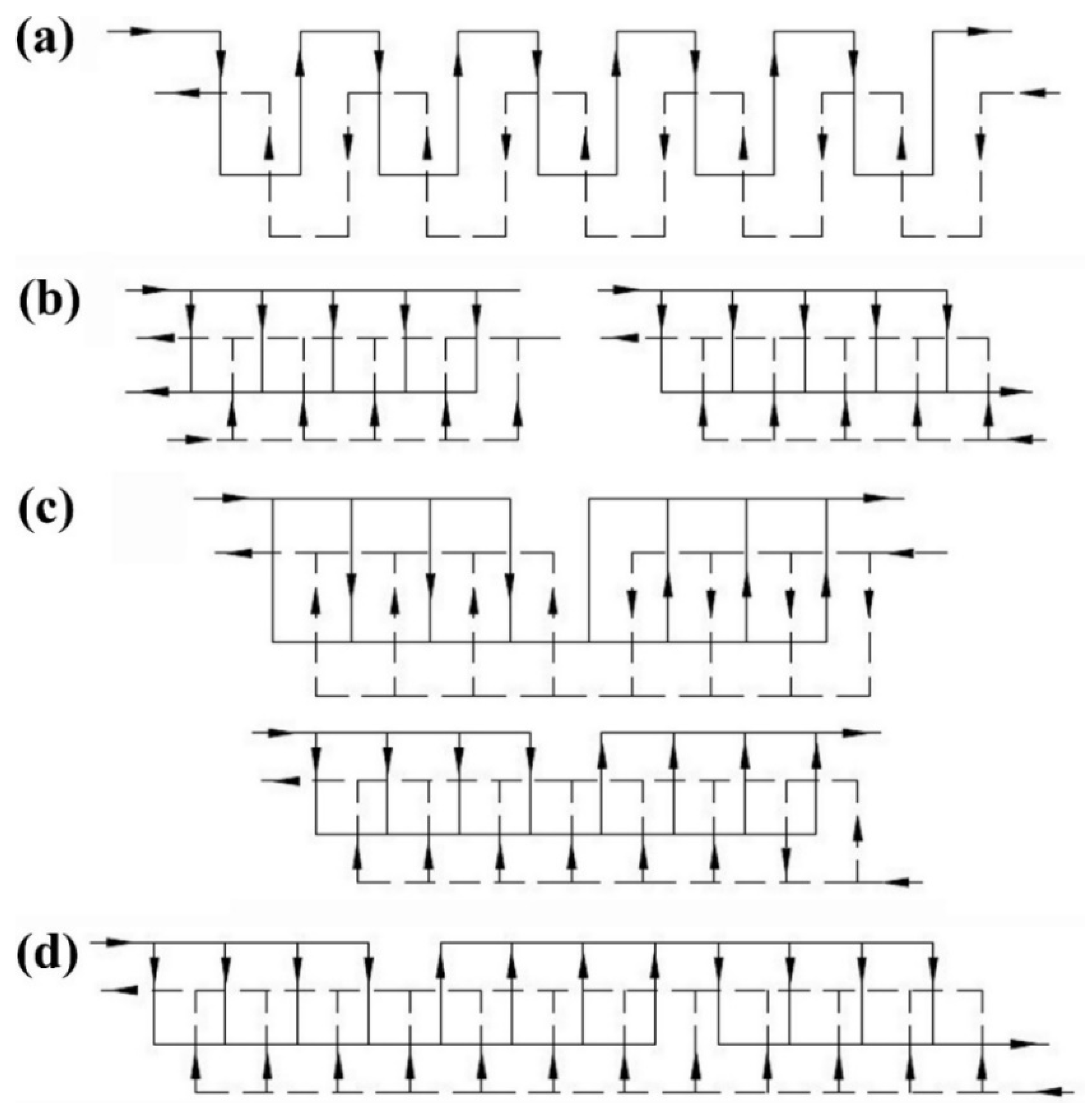
2.1. Plate Heat Exchangers
- -
- All-welded PHEs: The gaskets have been entirely eliminated, and as a result, the reliability of the HE has been enhanced by replacing a fully welded plate exchanger instead of the gaskets. This leads to eliminating the limitation associated with the operating pressure and temperature. The main constraint of this model is that no mechanical methods can be used for cleaning purposes, and therefore cleaning can only be achieved via chemical routes.
- -
- Wide-gap PHEs: Having the free-flow channel for highly viscous fluids and other products that contain coarse particles leads to eliminating the clogging problem that is usually encountered in HEs of the shell and tube type.
- -
- Free-flow PHEs: This unique design provides a wide flow path for fluids of high viscosity and fouling tendency and is also suitable for fluids that contain fibrous materials. This special design (free-flow design) can be considered as an improved design in comparison to the wide-gap PHEs. The main aspect of the design is that there is no contact point that can restrict the fluid flow in the flow path of the free-flow plates.
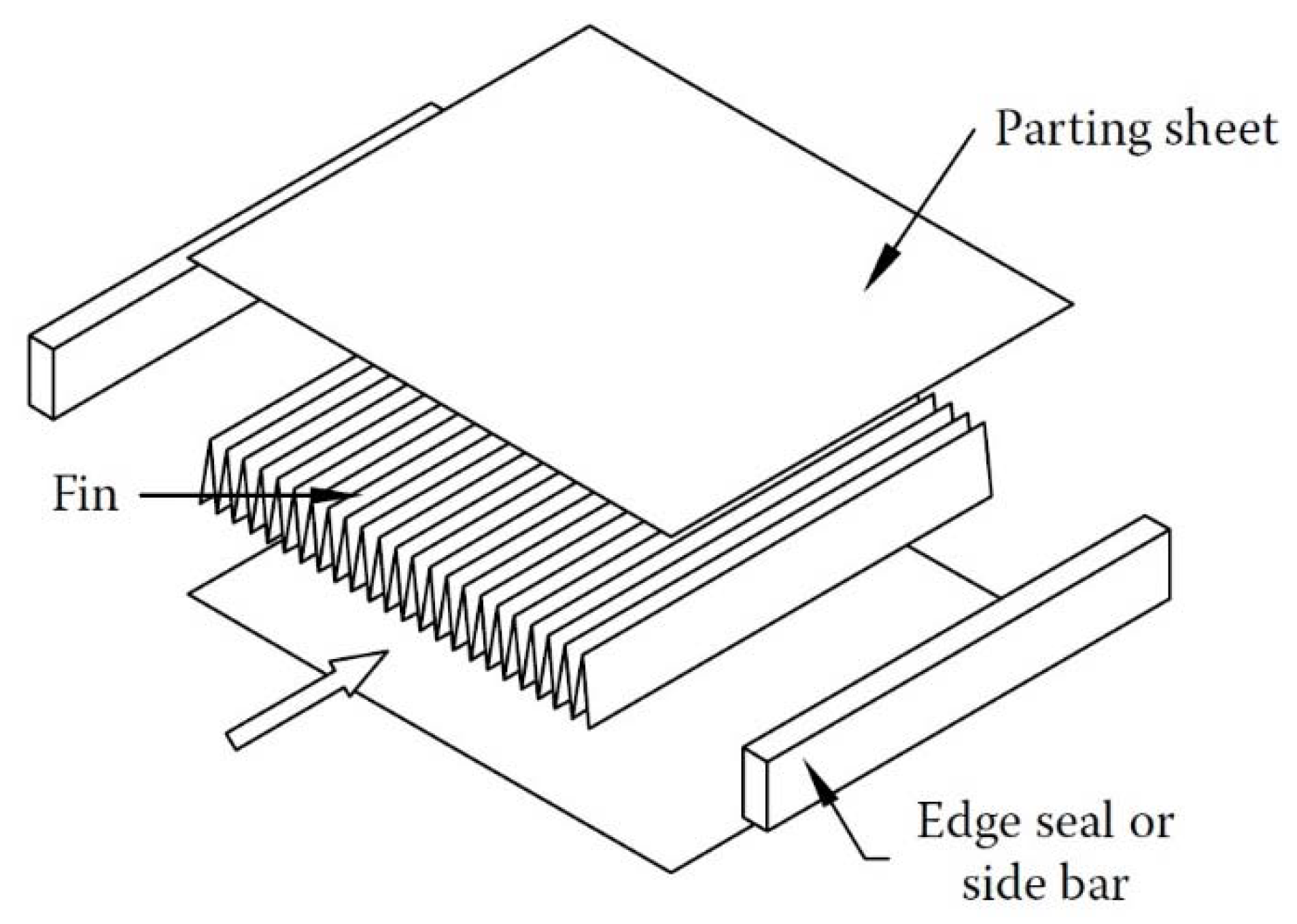
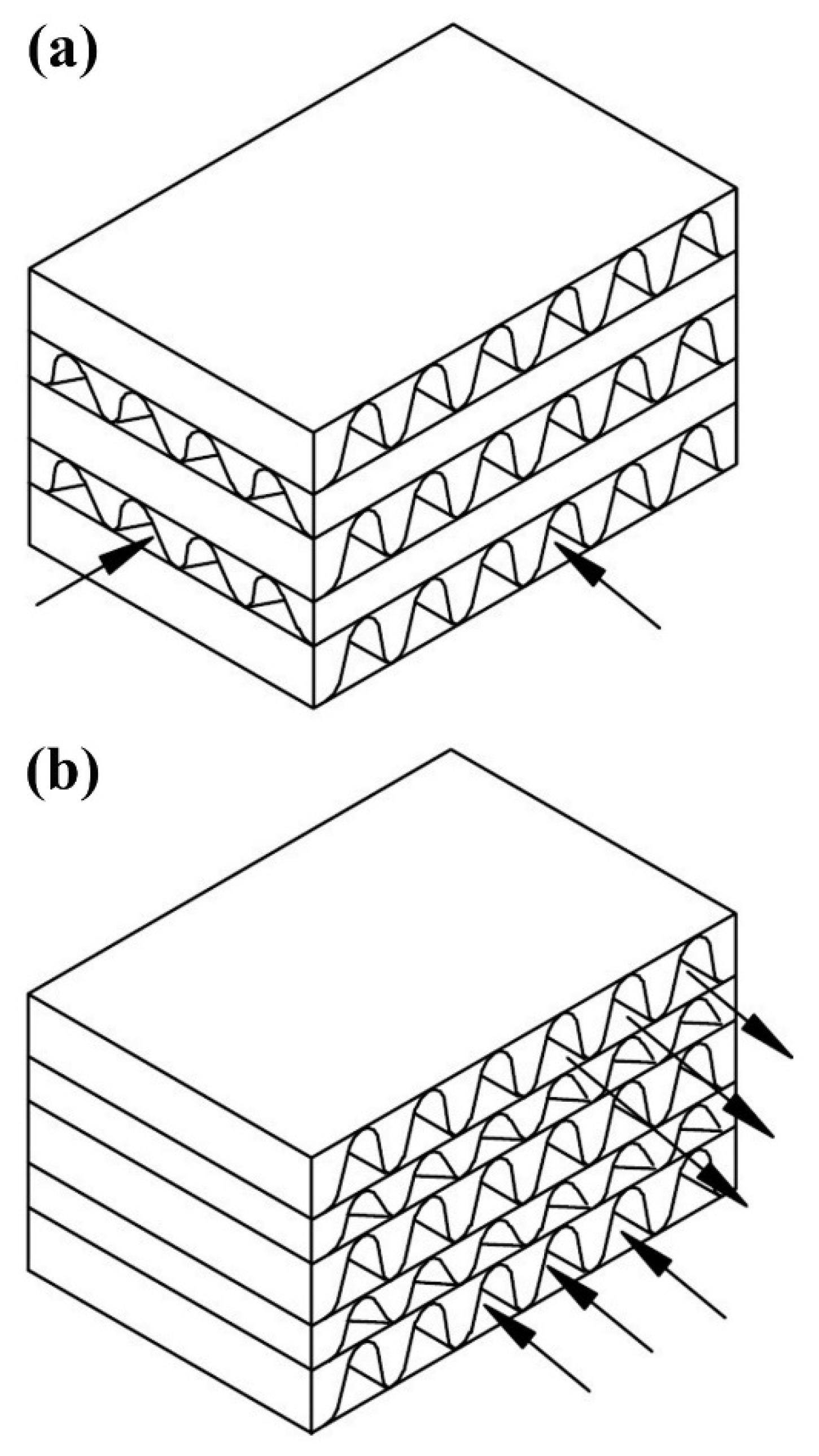
2.2. Plate-Fin Heat Exchangers
- Fluid leakage possibilities do not exist or rarely occur, and as such there is no risk associated with the fluid mixing or contamination.
- PFHEs are designed for low-pressure applications (i.e., less than 1000 kPa).
- This class of HEs are mainly used for gas-to-gas applications and, in particular cases, in gas-to-liquid systems (e.g., the WR-21 marine propulsion gas turbine cycle).
- PFHEs offer high area density (i.e., up to approximately 6000 m2/m3).
- Various fins geometries (rectangular, tubular, offset strip, and wavy fin) can be utilized between the plates for various applications.
- PFHEs are designed for operating temperatures up to approximately 800 °C. The type of fin-to-plate bonding and the materials define the maximum operating temperatures.
- PFHEs provide superior thermal performance compared to their counterparts by using extended surfaces.
- PFHEs can operate effectively with temperature differences as low as 1 °C for single phase streams; while between multiphase streams, the temperature difference can be as low as 3 °C.
- For cryogenic applications, brazed aluminum PFHEs are the optimum choice due to the high surface compactness, the capability of handling multiple streams, and the highly desirable low-temperature properties at which they are able to operate.
- In cryogenic applications, a thermal effectiveness of the order of 95% or higher can be attained.
- PFHEs have large heat transfer surface per unit volume, and low weight per unit heat transfer.
- Exchanging heat between many process streams is possible in PFHEs.
- PFHEs can be used in different temperatures (from 0 to 800 °C) and pressures (up to 140 bar) by selecting the proper materials. However, they rarely get exposed simultaneously to a high temperature and pressure operating environment [58].
3. Nanofluids: Fundamentals and Characteristics
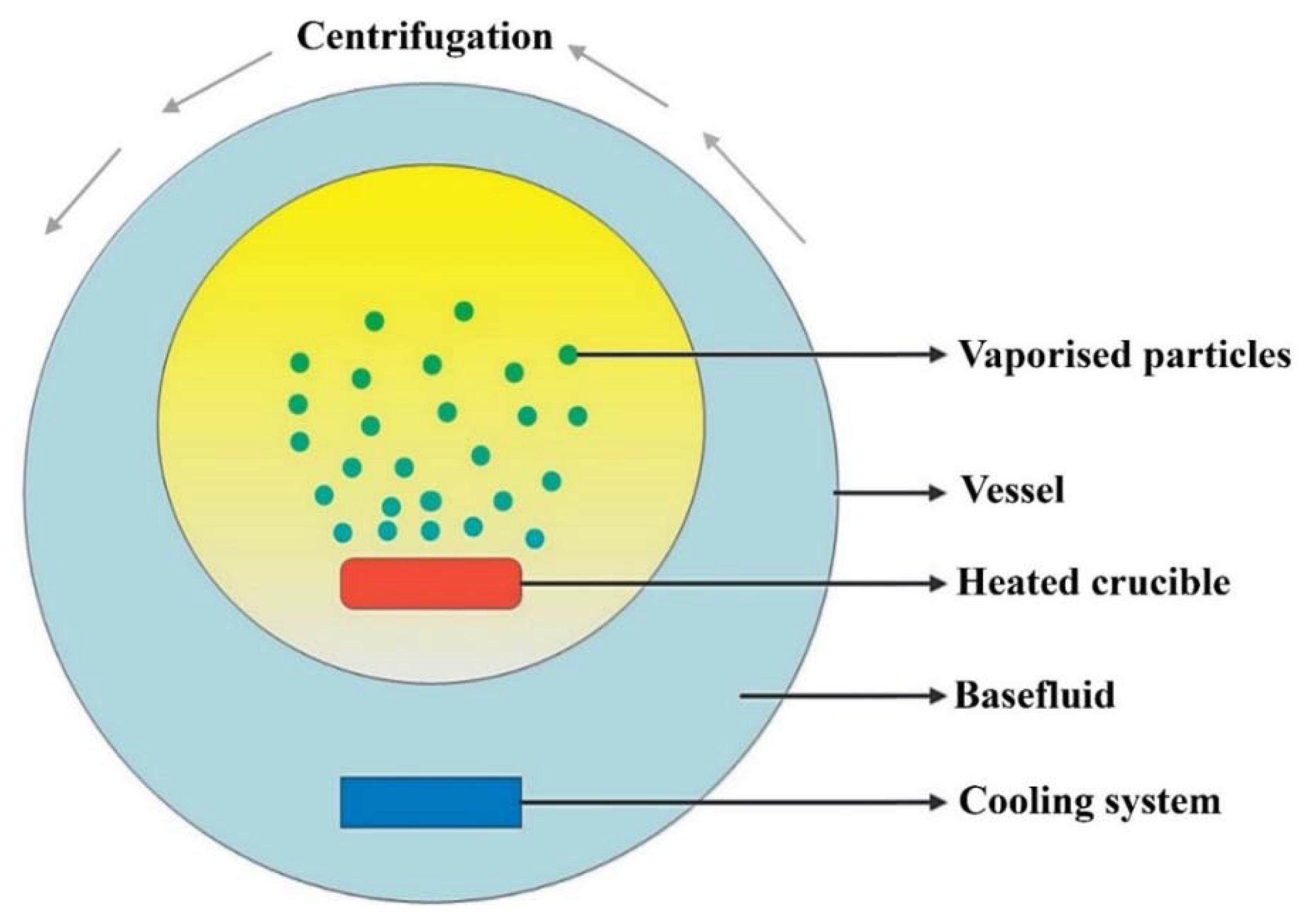
3.1. Fabrication Approaches
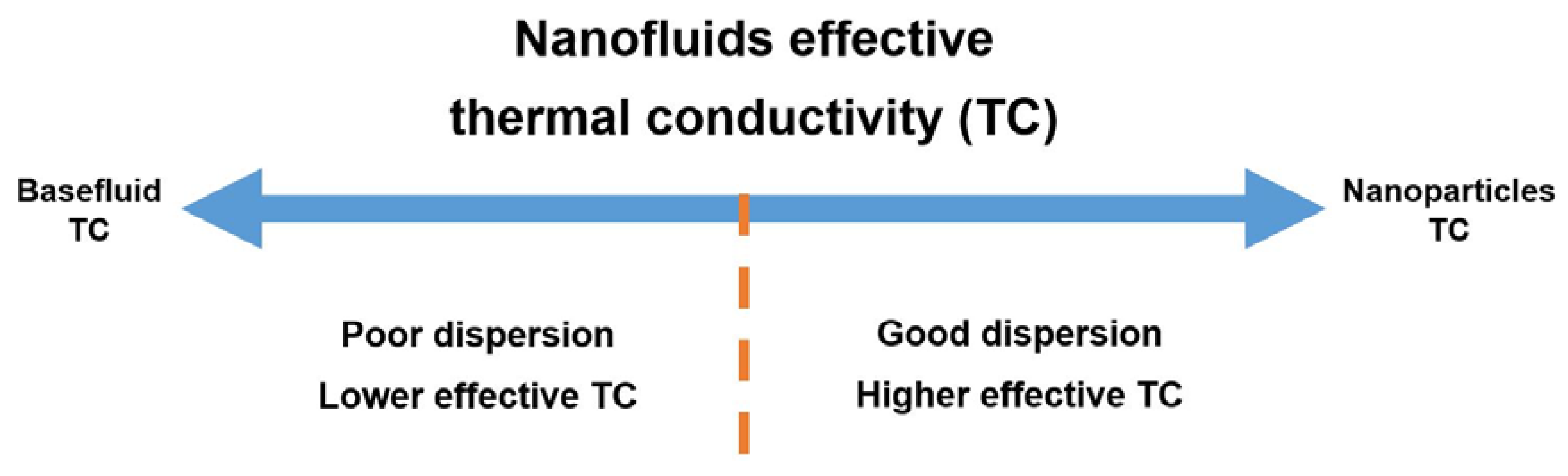
3.2. Dispersion Stability
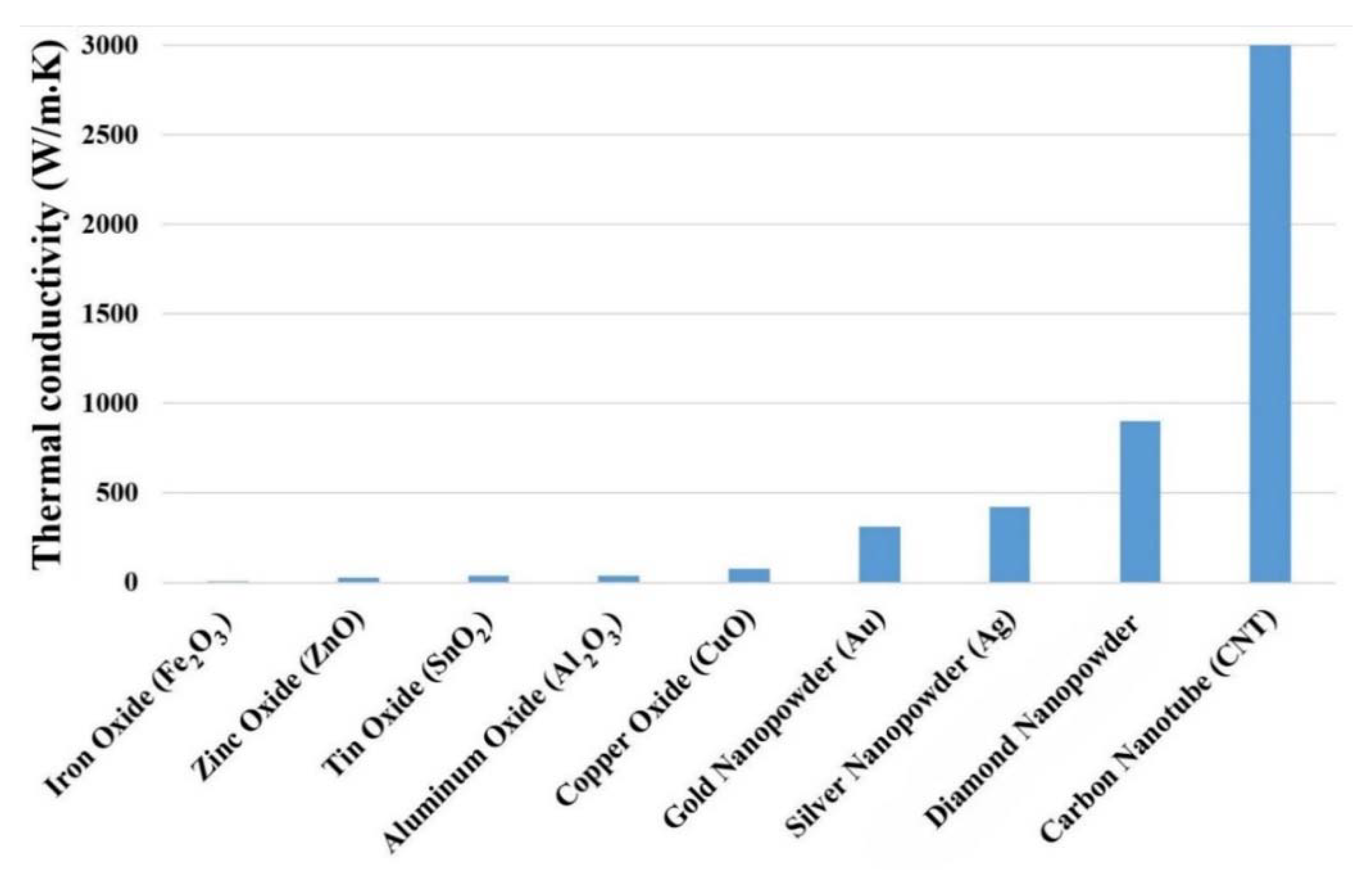
3.3. Nanofluids Thermophysical Properties
- Effective thermal conductivity:
- Cylindrical cell method;
- Steady-state parallel-plate method;
- Temperature oscillation approach;
- 3-ω method;
- Thermal comparator method;
- Thermal constants analyzer approach;
- Flash lamp method; and
- Transient hot-wire method.
- Effective viscosity:
- Capillary tube viscometer;
- Rotating viscometer;
- Capillary viscometer;
- Pressure differences over capillaries device; and
- Torsional oscillating cup.
4. Application of Nanofluids in Heat Exchangers
4.1. Plate Heat Exchangers
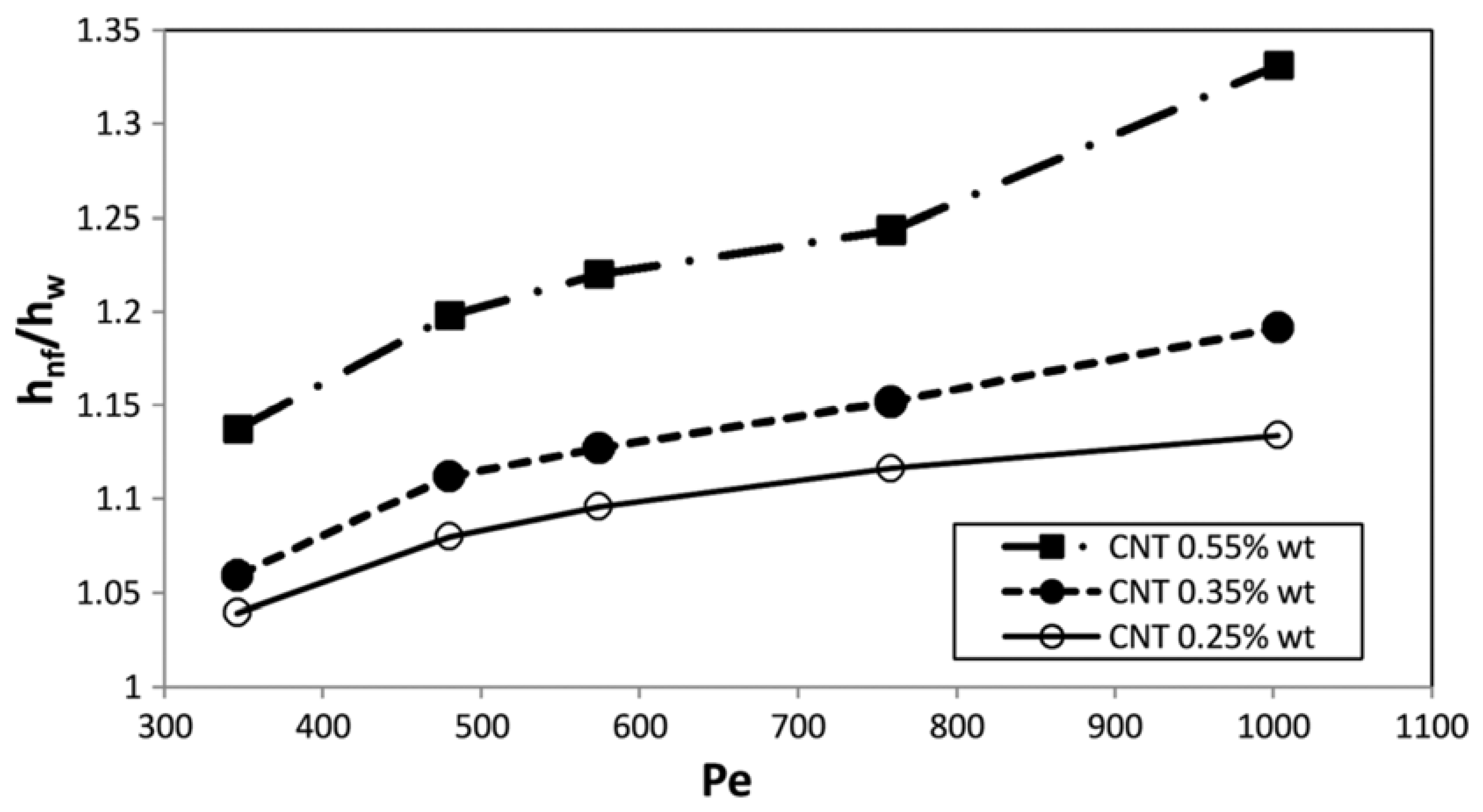
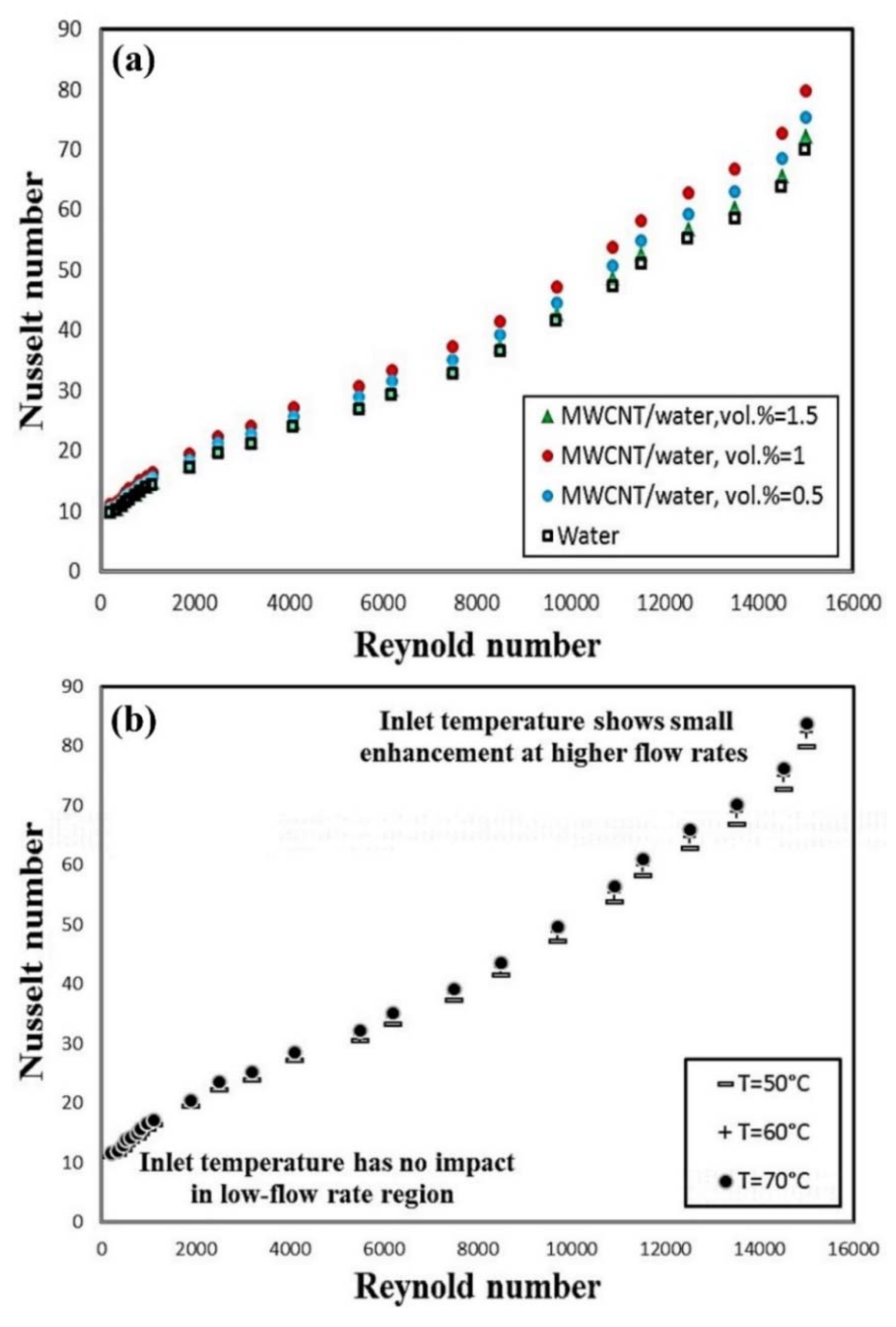
4.1.1. CNT-Based Nanofluids
4.1.2. Hybrid Nanofluids Containing CNT Nanoparticles
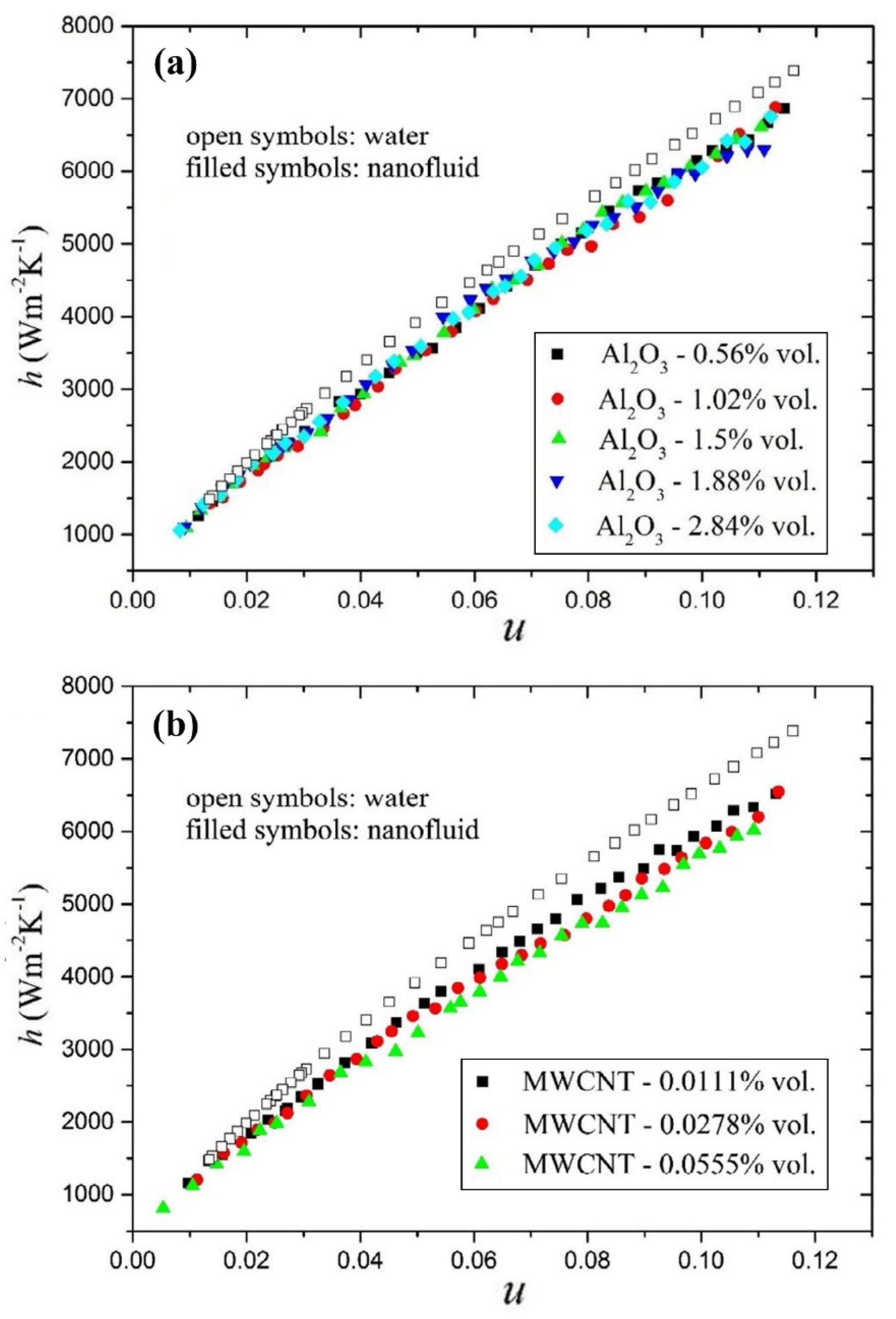
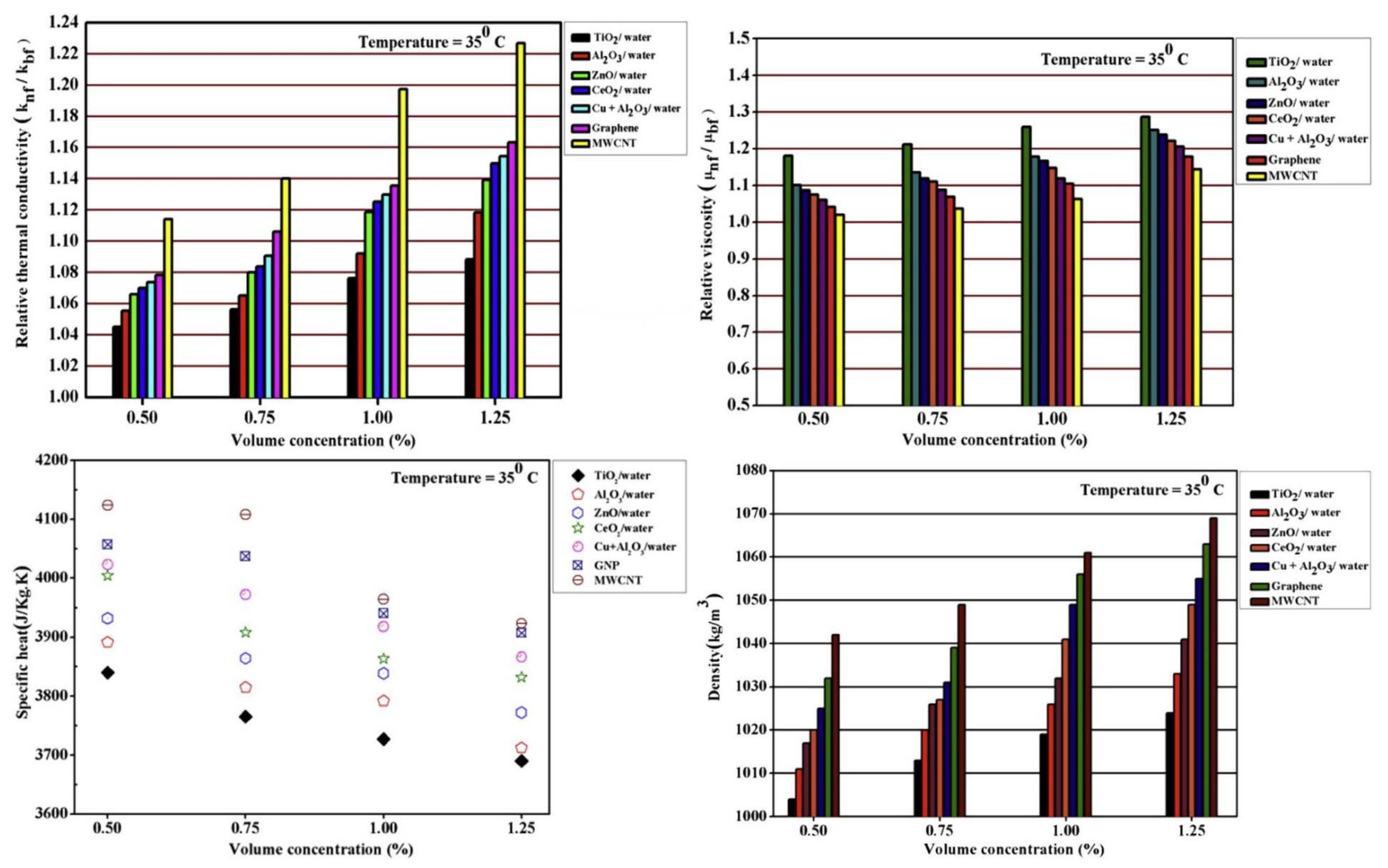
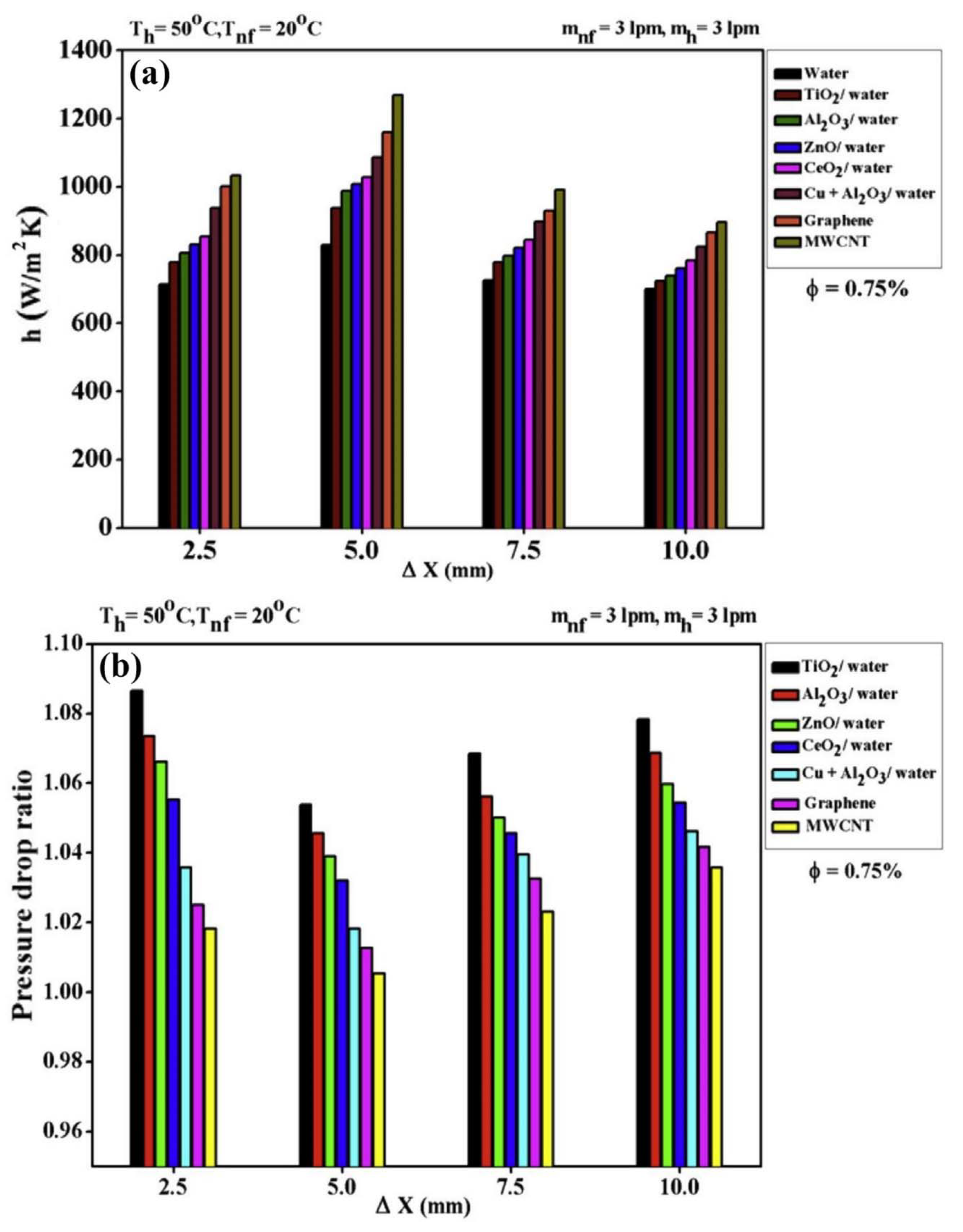
4.1.3. Other Types of Nanofluids
- It is reported by all the researchers that the Nu number and the convective heat transfer coefficient are enhanced by adding nanoparticles to the working fluids. Moreover, increasing the solid concentration of nanoparticles and the Re number leads to enhancing the Nu number and the convective heat transfer coefficient.
- Adding nanoparticles to the working fluids leads to increasing the dynamic viscosity of the resultant fluids (nanofluids) which, in turn, leads to increasing the pressure loss and pumping power. However, in some literature, it is reported that the increase in the pressure drop is negligible [190,195].
- Replacing the conventional working fluids with nanofluids will bring certain advantages from the heat transfer performance point of view. However, they impose some extra cost in terms of energy consumption; increasing the pressure drop leads to increasing the pumping power and energy consumption.
4.2. Plate-Fin Heat Exchangers
5. Discussion and Future Direction
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| γ | Alpha phase |
| ANL | Argonne National Laboratory |
| CNTs | Carbon nanotubes |
| Specific heat capacity (kJ/kg·K) | |
| DIW | Deionized water |
| DW | Double-walled |
| EG | Ethylene glycol |
| thermal-hydraulic performance | |
| f | Friction factor |
| Volumetric concentration | |
| h | Convective heat transfer coefficient (W/m2·K) |
| HE | Heat exchanger |
| hnf/hw | Relative convective heat transfer coefficient |
| ṁ | Volume flow rate (lpm) |
| MW | Multi-walled |
| Nu | Nusselt number |
| P | Pressure (Pa) |
| Pe | Peclet number |
| PFHEs | Plate-fin heat exchangers |
| PHEs | Plate heat exchangers |
| Re | Reynolds number |
| SANSS | Submerged arc nanoparticles synthesis system |
| SEM | Scanning electron microscope |
| SW | Single-walled |
| T | Temperature |
| TC | Thermal conductivity (W/m·K) |
| TEM | Transmission electron microscope |
| u | Flow velocity |
| Volume (m3) | |
| VEROS | Vacuum evaporation onto a running oil substrate |
| vol. % | Volume percentage |
| wt. % | Wight percentage |
| X | spacing distance (mm) |
| Greek letters | |
| Density (kg/m3) | |
| Δ | Difference |
| Subscripts | |
| bf | Basefluid |
| h | Hot |
| i | Used nanofluid |
| nf | Nanofluid |
| np | Nanoparticles |
| ref | Reference liquid |
| w | Water |
References
- Dudley, B. BP statistical review of world energy. In BP Statistical Review of World Energy; BP Publishing: London, UK, 2019; p. 64. [Google Scholar]
- Exxonmobil. Outlook for Energy: Aperspective to 2040. p. 58. Available online: https://corporate.exxonmobil.com/Energy-and-environment/Looking-forward/Outlook-for-Energy/Outlook-for-Energy-A-perspective-to-2040#exxonMobilSupportsTheParisAgreement (accessed on 1 December 2019).
- Ibrahim, T.K.; Mohammed, M.K.; Al Doori, W.H.A.; Al-Sammarraie, A.T.; Basrawi, F. Study of the performance of the gas turbine power plants from the simple to complex cycle: A technical review. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 57, 228–250. [Google Scholar]
- Bhargava, R.K.; Bianchi, M.; De Pascale, A.; Negri Di Montenegro, G.; Peretto, A. Gas turbine based power cycles—A state-of-the-art review. In Challenges on Power Engineering and Environment, Proceedings of the International Conference on Power Engineering Hangzhou, China, 23–27 October 2007; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Olumayegun, O.; Wang, M.; Kelsall, G. Closed-cycle gas turbine for power generation: A state-of-the-art review. Fuel 2016, 180, 694–717. [Google Scholar] [CrossRef]
- Nascimento Silva, F.C.; Flórez-Orrego, D.; de Oliveira Junior, S. Exergy assessment and energy integration of advanced gas turbine cycles on an offshore petroleum production platform. Energy Convers. Manag. 2019, 197, 111846. [Google Scholar] [CrossRef]
- Blakey, S.; Rye, L.; Wilson, C.W. Aviation gas turbine alternative fuels: A review. Proc. Combust. Inst. 2011, 33, 2863–2885. [Google Scholar] [CrossRef]
- Sayyaadi, H.; Mehrabipour, R. Efficiency enhancement of a gas turbine cycle using an optimized tubular recuperative heat exchanger. Energy 2012, 38, 362–375. [Google Scholar] [CrossRef]
- Siddique, M.; Khaled, A.-R.A.; Abdulhafiz, N.I.; Boukhary, A.Y. Recent Advances in Heat Transfer Enhancements: A Review Report. Int. J. Chem. Eng. 2010, 2010, 28. [Google Scholar] [CrossRef]
- Huminic, G.; Huminic, A. Application of nanofluids in heat exchangers: A review. Renew. Sustain. Energy Rev. 2012, 16, 5625–5638. [Google Scholar] [CrossRef]
- Masuda, H.; Ebata, A.; Teramae, K. Alteration of Thermal Conductivity and Viscosity of Liquid by Dispersing Ultra-Fine Particles (Dispersion of Al2o3, Sio2 and Tio2 Ultra-Fine Particles); ScienceOpen Inc.: Boston, MA, USA; Berlin, Germany; Budapest, Hungary, 1993; pp. 227–233. [Google Scholar]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the Conference 1995 International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995. Other Information: PBD: October 1995; Argonne National Lab.: Lemont, IL, USA, 1995. [Google Scholar]
- Ali, N.; Teixeira, J.A.; Addali, A. New pH Correlations for Stainless Steel 316L, Alumina. and Copper(I) Oxide Nanofluids Fabricated at Controlled Sonication Temperatures. J. Nano. Res. 2019, 58, 125–138. [Google Scholar]
- Asadi, A. A guideline towards easing the decision-making process in selecting an effective nanofluid as a heat transfer fluid. Energy Convers. Manag. 2018, 175, 1–10. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A. Aluminium Nanofluids Stability: A Comparison between the Conventional Two-Step Fabrication Approach and the Controlled Sonication Bath Temperature Method. J. Nanomater. 2019, 2019, 9. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A. A Review on Nanofluids: Fabrication, Stability. and Thermophysical Properties. J. Nanomater. 2018, 2018, 33. [Google Scholar]
- Saffarian, M.R.; Moravej, M.; Doranehgard, M.H. Heat transfer enhancement in a flat plate solar collector with different flow path shapes using nanofluid. Renew. Energy 2020, 146, 2316–2329. [Google Scholar] [CrossRef]
- Bozorg, M.V.; Hossein Doranehgard, M.; Hong, K.; Xiong, Q. CFD study of heat transfer and fluid flow in a parabolic trough solar receiver with internal annular porous structure and synthetic oil–Al2O3 nanofluid. Renew. Energy 2020, 145, 2598–2614. [Google Scholar] [CrossRef]
- Gholamalipour, P.; Siavashi, M.; Doranehgard, M.H. Eccentricity effects of heat source inside a porous annulus on the natural convection heat transfer and entropy generation of Cu-water nanofluid. Int. Commun. Heat Mass Transf. 2019, 109, 104367. [Google Scholar] [CrossRef]
- Xiong, Q.; Bozorg, M.V.; Doranehgard, M.H.; Hong, K.; Lorenzini, G. A CFD investigation of the effect of non-Newtonian behavior of Cu–water nanofluids on their heat transfer and flow friction characteristics. J. Therm. Anal. Calorim. 2020, 139, 2601–2621. [Google Scholar] [CrossRef]
- Xiong, Q.; Khosravi, A.; Nabipour, N.; Doranehgard Mohammad, H.; Sabaghmoghadam, A.; Ross, D. Nanofluid flow and heat transfer due to natural convection in a semi-circle/ellipse annulus using modified lattice Boltzmann method. Int. J. Numer. Methods Heat Fluid Flow 2019, 29, 4746–4763. [Google Scholar] [CrossRef]
- Siavashi, M.; Karimi, K.; Xiong, Q.; Doranehgard, M.H. Numerical analysis of mixed convection of two-phase non-Newtonian nanofluid flow inside a partially porous square enclosure with a rotating cylinder. J. Therm. Anal. Calorim. 2019, 137, 267–287. [Google Scholar] [CrossRef]
- Rahimi, A.; Kasaeipoor, A.; Amiri, A.; Doranehgard, M.H.; Malekshah, E.H.; Kolsi, L. Lattice Boltzmann method based on Dual-MRT model for three-dimensional natural convection and entropy generation in CuO–water nanofluid filled cuboid enclosure included with discrete active walls. Comput. Math. Appl. 2018, 75, 1795–1813. [Google Scholar] [CrossRef]
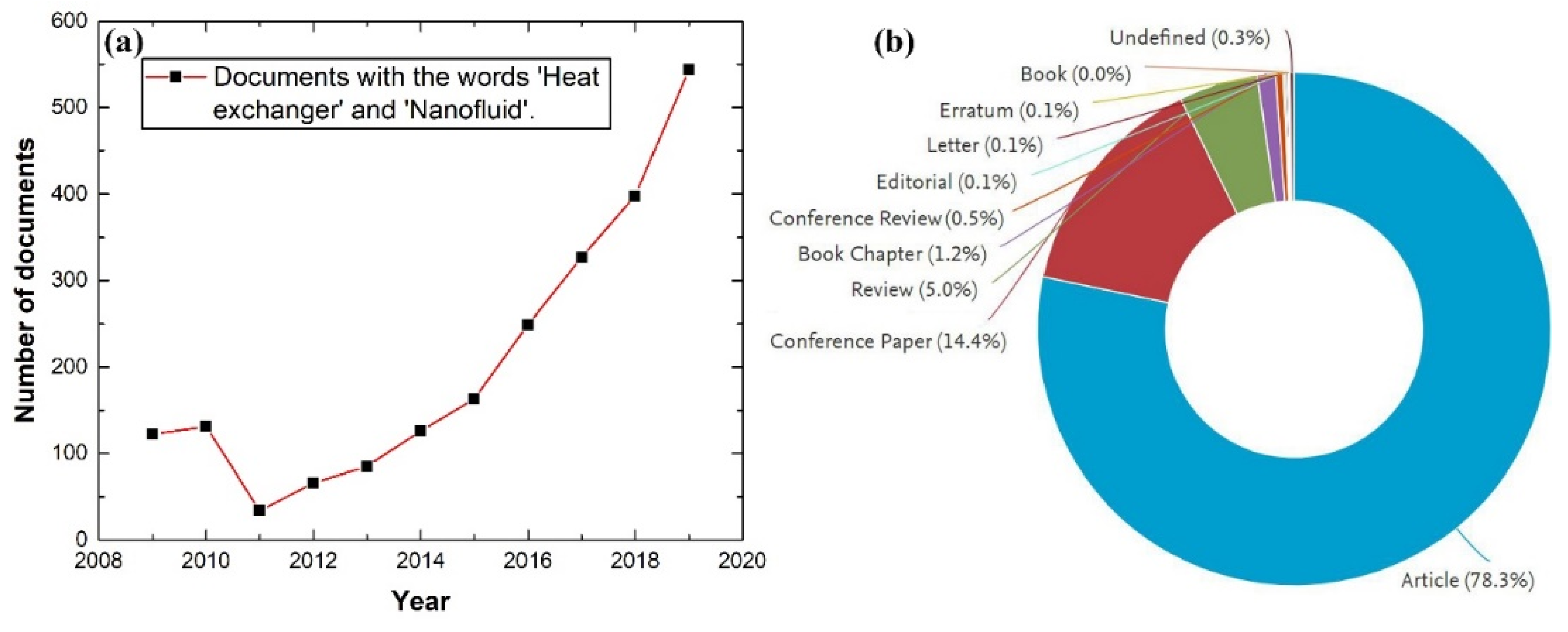
- Scopus-Database. Analysis of Scopus Search Result for the Words ‘Heat Exchanger’ and ‘Nanofluid’ from 1996 to 2020; Elsevier: Amsterdam, The Netherlands, 2020; Available online: www.scopus.com (accessed on 1 January 2020).
- Yang, L.; Huang, J.-N.; Ji, W.; Mao, M. Investigations of a new combined application of nanofluids in heat recovery and air purification. Powder Technol. 2020, 360, 956–966. [Google Scholar] [CrossRef]
- Patra, N.; Gupta, V.; Singh, R.; Singh, R.S.; Ghosh, P.; Nayak, A. An experimental analysis of quenching of continuously heated vertical rod with aqueous Al2O3 nanofluid. Resour.-Effic. Technol. 2017, 3, 378–384. [Google Scholar]
- Ebrahim, S.A.; Chang, S.; Cheung, F.-B.; Bajorek, S.M. Parametric investigation of film boiling heat transfer on the quenching of vertical rods in water pool. Appl. Therm. Eng. 2018, 140, 139–146. [Google Scholar] [CrossRef]
- Ramadhani, C.A.; Putra, W.N.; Rakhman, D.; Oktavio, L.; Harjanto, S. A Comparative Study on Commercial Grade and Laboratory Grade of TiO2 particle in Nanofluid for Quench Medium in Rapid Quenching Process. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kazimierz Dolny, Poland, 21–23 November 2019. [Google Scholar]
- Wang, X.; Zhang, J.; Ma, Y.; Wang, G.; Han, J.; Dai, M.; Sun, Z.Y. A comprehensive review on the properties of nanofluid fuel and its additive effects to compression ignition engines. Appl. Surf. Sci. 2020, 504, 144581. [Google Scholar] [CrossRef]
- Saleh, H.; Alali, E.; Ebaid, A. Medical applications for the flow of carbon-nanotubes suspended nanofluids in the presence of convective condition using Laplace transform. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 24, 206–212. [Google Scholar] [CrossRef]
- Li, Z.G.; Zhang, J.R.; Li, B.; Liu, X.B.; Yang, F.Y. Investigation of friction power consumption and the performance of a water turbine seal based on the imbalanced rotation of magnetic nanofluids. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 26–27 September 2018. [Google Scholar]
- Asadi, A.; Asadi, M.; Rezaei, M.; Siahmargoi, M.; Asadi, F. The effect of temperature and solid concentration on dynamic viscosity of MWCNT/MgO (20–80)–SAE50 hybrid nano-lubricant and proposing a new correlation: An experimental study. Int. Commun. Heat Mass Transf. 2016, 78, 48–53. [Google Scholar] [CrossRef]
- Asadi, M.; Asadi, A. Dynamic viscosity of MWCNT/ZnO–engine oil hybrid nanofluid: An experimental investigation and new correlation in different temperatures and solid concentrations. Int. Commun. Heat Mass Transf. 2016, 76, 41–45. [Google Scholar] [CrossRef]
- Ebrahimnia-Bajestan, E.; Niazmand, H.; Duangthongsuk, W.; Wongwises, S. Numerical investigation of effective parameters in convective heat transfer of nanofluids flowing under a laminar flow regime. Int. J. Heat Mass Transf. 2011, 54, 4376–4388. [Google Scholar] [CrossRef]
- Martínez-Cuenca, R.; Mondragón, R.; Hernández, L.; Segarra, C.; Jarque, J.C.; Hibiki, T.; Juliá, J.E. Forced-convective heat-transfer coefficient and pressure drop of water-based nanofluids in a horizontal pipe. Appl. Therm. Eng. 2016, 98, 841–849. [Google Scholar] [CrossRef]
- Yazid, M.N.A.W.M.; Sidik, N.A.C.; Mamat, R.; Najafi, G. A review of the impact of preparation on stability of carbon nanotube nanofluids. Int. Commun. Heat Mass Transf. 2016, 78, 253–263. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Eres, G. Order in vertically aligned carbon nanotube arrays. Appl. Phys. Lett. 2006, 88, 213111. [Google Scholar] [CrossRef]
- Ngoc Hong, P.; Minh, D.N.; Van Hung, N.; Minh, P.N.; Khoi, P.H. Carbon nanotube and graphene aerogels – the world’s 3D lightest materials for environment applications: A review. Int. J. Mater. Sci. Appl. 2017, 6, 277. [Google Scholar] [CrossRef]
- Askari, S.; Lotfi, R.; Seifkordi, A.; Rashidi, A.M.; Koolivand, H. A novel approach for energy and water conservation in wet cooling towers by using MWNTs and nanoporous graphene nanofluids. Energy Convers. Manag. 2016, 109, 10–18. [Google Scholar] [CrossRef]
- Neuberger, N.; Adidharma, H.; Fan, M. Graphene: A review of applications in the petroleum industry. J. Pet. Sci. Eng. 2018, 167, 152–159. [Google Scholar] [CrossRef]
- Shanbedi, M.; Heris, S.Z.; Amiri, A.; Hosseinipour, E.; Eshghi, H.; Kazi, S.N. Synthesis of aspartic acid-treated multi-walled carbon nanotubes based water coolant and experimental investigation of thermal and hydrodynamic properties in circular tube. Energy Convers. Manag. 2015, 105, 1366–1376. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Mehrali, M.; Saidur, R.; Mehrali, M.; Tahan Latibari, S.; Akhiani, A.R.; Metselaar, H.S.C. A comprehensive review on graphene nanofluids: Recent research, development and applications. Energy Convers. Manag. 2016, 111, 466–487. [Google Scholar] [CrossRef]
- Cooper, J.K. Heat exchangers: Characteristics. In Types and Emerging Applications; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2016. [Google Scholar]
- Rao, C.S.; Hartel, R.W. Scraped Surface Heat Exchangers. Crit. Rev. Food Sci. Nutr. 2006, 46, 207–219. [Google Scholar] [CrossRef]
- Büyükalaca, O.; Yılmaz, T. Influence of rotational speed on effectiveness of rotary-type heat exchanger. Heat Mass Transf. 2002, 38, 441–447. [Google Scholar] [CrossRef]
- Purandare, P.S.; Lele, M.M.; Gupta, R. Parametric Analysis of Helical Coil Heat Exchanger. Int. J. Eng. Res. Technol. (IJERT) 2012, 1, 1–5. [Google Scholar]
- Master, B.I.; Chunangad, K.S.; Boxma, A.J.; Kral, D.; Stehlík, P. Most Frequently Used Heat Exchangers from Pioneering Research to Worldwide Applications. Heat Transf. Eng. 2006, 27, 4–11. [Google Scholar] [CrossRef]
- Thulukkanam, K. Heat Exchanger Design Handbook; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Kumar, V.; Tiwari, A.K.; Ghosh, S.K. Application of nanofluids in plate heat exchanger: A review. Energy Convers. Manag. 2015, 105, 1017–1036. [Google Scholar] [CrossRef]
- Al-asadi, M.T.; Mohammed, H.A.; Kherbeet, A.S.; Al-aswadi, A.A. Numerical study of assisting and opposing mixed convective nanofluid flows in an inclined circular pipe. Int. Commun. Heat Mass Transf. 2017, 85, 81–91. [Google Scholar] [CrossRef]
- Gholami, A.; Wahid, M.A.; Mohammed, H.A. Thermal–hydraulic performance of fin-and-oval tube compact heat exchangers with innovative design of corrugated fin patterns. Int. J. Heat Mass Transf. 2017, 106, 573–592. [Google Scholar] [CrossRef]
- Abed, A.M.; Alghoul, M.A.; Sopian, K.; Mohammed, H.A.; Majdi, H.S.; Al-Shamani, A.N. Design characteristics of corrugated trapezoidal plate heat exchangers using nanofluids. Chem. Eng. Process. Process. Intensif. 2015, 87, 88–103. [Google Scholar] [CrossRef]
- Gholami, A.A.; Wahid, M.A.; Mohammed, H.A. Heat transfer enhancement and pressure drop for fin-and-tube compact heat exchangers with wavy rectangular winglet-type vortex generators. Int. Commun. Heat Mass Transf. 2014, 54, 132–140. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Hasan, H.A.; Wahid, M.A. Heat transfer enhancement of nanofluids in a double pipe heat exchanger with louvered strip inserts. Int. Commun. Heat Mass Transf. 2013, 40, 36–46. [Google Scholar] [CrossRef]
- Hajatzadeh Pordanjani, A.; Aghakhani, S.; Afrand, M.; Mahmoudi, B.; Mahian, O.; Wongwises, S. An updated review on application of nanofluids in heat exchangers for saving energy. Energy Convers. Manag. 2019, 198, 111886. [Google Scholar] [CrossRef]
- Shah, R. Compact heat exchanger technology and applications. Heat Exch. Eng. 1991, 2, 1–23. [Google Scholar]
- Shah, R.K.; Robertson, J.M. Compact Heat Exchangers for the Process Industry. In Energy Efficiency in Process Technology Pilavachi; Pilavachi, P.A., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 565–580. [Google Scholar]
- Taylor, M.A. Plate-fin heat exchangers: Guide to their specification and use. In Heat Transfer and Fluid Flow Services; Oxfordshire: England, UK, 1987. [Google Scholar]
- Ali, N.; Teixeira, J.A.; Addali, A.; Al-Zubi, F.; Shaban, E.; Behbehani, I. The effect of aluminium nanocoating and water pH value on the wettability behavior of an aluminium surface. Appl. Surf. Sci. 2018, 443, 24–30. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A.; Saeed, M.; Al-Zubi, F.; Sedaghat, A.; Bahzad, H. Deposition of Stainless Steel Thin Films: An Electron Beam Physical Vapour Deposition Approach. Materials 2019, 12, 571. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A. Effect of Water Temperature. pH Value. and Film Thickness on the Wettability Behaviour of Copper Surfaces Coated with Copper Using EB-PVD Technique. J. Nano. Res. 2019, 60, 124–141. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism, 2nd ed.; Clarendon Press: Hauppauge, NY, USA, 1881. [Google Scholar]
- Choi, S.U.S.; Cho, Y.I.; Kasza, K.E. Degradation Effects of Dilute Polymer-Solutions on Turbulent Friction and Heat-Transfer Behavior. J. Non-Newton Fluid Mech. 1992, 41, 289–307. [Google Scholar] [CrossRef]
- Choi, U.; France, D.M.; Knodel, B.D. Impact of Advanced Fluids on costs of District Cooling Systems; Argonne National Lab.: Lemont, IL, USA, 1992.
- Choi, U.; Tran, T. Experimental studies of the effects of non-Newtonian surfactant solutions on the performance of a shell-and-tube heat exchanger. In Recent Developments in non-Newtonian Flows and Industrial Applications; The American Society of Mechanical Engineers: New York, NY, USA, 1991; pp. 47–52. [Google Scholar]
- Liu, K.; Choi, U.; Kasza, K.E. Measurements of Pressure Drop and Heat Transfer in Turbulent Pipe Flows of Particulate Slurries; Argonne National Lab.: Lemont, IL, USA, 1988.
- Xuan, Y.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.U.S. Application of Metallic Nanoparticle Suspensions in Advanced Cooling Systems; American Society of Mechanical Engineers: New York, NY, USA, 1996; Volume 72, pp. 227–234. [Google Scholar]
- Alarifi, I.M.; Nguyen, H.M.; Naderi Bakhtiyari, A.; Asadi, A. Feasibility of ANFIS-PSO and ANFIS-GA Models in Predicting Thermophysical Properties of Al2O3-MWCNT/Oil Hybrid Nanofluid. Materials 2019, 12, 3628. [Google Scholar] [CrossRef] [PubMed]
- Alarifi, I.M.; Alkouh, A.B.; Ali, V.; Nguyen, H.M.; Asadi, A. On the rheological properties of MWCNT-TiO2/oil hybrid nanofluid: An experimental investigation on the effects of shear rate, temperature. and solid concentration of nanoparticles. Powder Technol. 2019, 355, 157–162. [Google Scholar] [CrossRef]
- Asadi, M.; Asadi, A.; Aberoumand, S. An experimental and theoretical investigation on the effects of adding hybrid nanoparticles on heat transfer efficiency and pumping power of an oil-based nanofluid as a coolant fluid. Int. J. Refrig. 2018, 89, 83–92. [Google Scholar] [CrossRef]
- Asadi, A.; Asadi, M.; Rezaniakolaei, A.; Rosendahl, L.A.; Afrand, M.; Wongwises, S. Heat transfer efficiency of Al2O3-MWCNT/thermal oil hybrid nanofluid as a cooling fluid in thermal and energy management applications: An experimental and theoretical investigation. Int. J. Heat Mass Transf. 2018, 117, 474–486. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Investigation on convective heat transfer and flow features of nanofluids. J. Heat Transf. 2003, 125, 151–155. [Google Scholar] [CrossRef]
- Zhou, D. Heat transfer enhancement of copper nanofluid with acoustic cavitation. Int. J. Heat Mass Transf. 2004, 47, 3109–3117. [Google Scholar] [CrossRef]
- Jwo, C.-S.; Teng, T.-P. Experimental study on thermal properties of brines containing nanoparticles. Rev. Adv. Mater. Sci. 2005, 10, 79–83. [Google Scholar]
- Jana, S.; Salehi-Khojin, A.; Zhong, W.H. Enhancement of fluid thermal conductivity by the addition of single and hybrid nano-additives. Thermochim. Acta 2007, 462, 45–55. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, D.; Wang, J.; Chen, L.; Sundén, B. Experimental investigation on heat transfer characteristics of various nanofluids in an indoor electric heater. Renew. Energy 2020, 147, 1011–1018. [Google Scholar] [CrossRef]
- Kang, H.U.; Kim, S.H.; Oh, J.M. Estimation of Thermal Conductivity of Nanofluid Using Experimental Effective Particle Volume. Exp. Heat Transf. 2006, 19, 181–191. [Google Scholar] [CrossRef]
- Patel, H.E.; Das, S.; Sundararajan, T. Thermal conductivities of naked and monolayer protected metal nanoparticle based nanofluids: Manifestation of anomalous enhancement and chemical effects. Appl. Phys. Lett. 2003, 83, 2931. [Google Scholar] [CrossRef]
- Buongiorno, J.; Venerus, D.C.; Prabhat, N.; McKrell, T.; Townsend, J.; Christianson, R.; Tolmachev, Y.V.; Keblinski, P.; Hu, L.-W.; Alvarado, J.L. A benchmark study on the thermal conductivity of nanofluids. J. Appl. Phys. 2009, 106, 094312. [Google Scholar] [CrossRef]
- Putnam, S.A.; Cahill, D.G.; Braun, P.V.; Ge, Z.; Shimmin, R.G. Thermal conductivity of nanoparticle suspensions. J. Appl. Phys. 2006, 99, 084308. [Google Scholar] [CrossRef]
- Keykhosravi, A.; Simjoo, M. Enhancement of capillary imbibition by Gamma-Alumina nanoparticles in carbonate rocks: Underlying mechanisms and scaling analysis. J. Pet. Sci. Eng. 2020, 187, 106802. [Google Scholar] [CrossRef]
- Vasheghani, M. Enhancement of the thermal conductivity and viscosity of aluminum component−engine oil nanofluids. Nanosci. Technol. Int. J. 2012, 3, 333–340. [Google Scholar] [CrossRef]
- Behzad, K.; Raoufi, R.; Bahrami, A. The effect of concentration on thermal and optical behaviours of aluminium nanofluids. Dig. J. Nanomater. Biostruct. 2016, 11, 357–363. [Google Scholar]
- Kumar, A.; Chamoli, A.; Khan, S.; Anwar, A.A.; Jeyan, J.M.L. Density of Kerosene Aluminium Nanofluid used for Regenerative Cooling Applications of Thrust Chambers. Int. J. Eng. Adv. Technol. (IJEAT) 2019, 9, 176–180. [Google Scholar]
- Huang, D.; Wu, Z.; Sunden, B. Pressure drop and convective heat transfer of Al2O3/water and MWCNT/water nanofluids in a chevron plate heat exchanger. Int. J. Heat Mass Transf. 2015, 89, 620–626. [Google Scholar] [CrossRef]
- Haghighi, E.B.; Nikkam, N.; Saleemi, M.; Behi, M.; Mirmohammadi, S.A.; Poth, H.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M.; Palm, B. Shelf stability of nanofluids and its effect on thermal conductivity and viscosity. Meas. Sci. Technol. 2013, 24, 105301. [Google Scholar] [CrossRef]
- Soltani, S.; Etemad, S.G.; Thibault, J. Pool boiling heat transfer of non-Newtonian nanofluids. Int. Commun. Heat Mass Transf. 2010, 37, 29–33. [Google Scholar] [CrossRef]
- Beck, M.P.; Sun, T.; Teja, A.S. The thermal conductivity of alumina nanoparticles dispersed in ethylene glycol. Fluid Phase Equilib. 2007, 260, 275–278. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K.; Kulkarni, D.P. Development of new correlations for convective heat transfer and friction factor in turbulent regime for nanofluids. Int. J. Heat Mass Transf. 2010, 53, 4607–4618. [Google Scholar] [CrossRef]
- Khairul, M.A.; Saidur, R.; Hossain, A.; Alim, M.A.; Mahbubul, I.M. Heat transfer performance of different nanofluids flows in a helically coiled heat exchanger. Adv. Mater. Res. 2014, 832, 160–165. [Google Scholar] [CrossRef]
- Mondragón, R.; Segarra, C.; Jarque, J.C.; Julia, J.E.; Hernández, L.; Martínez-Cuenca, R. Characterization of physical properties of nanofluids for heat transfer application. J. Phys. Conf. Ser. 2012, 395, 012017. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Khattab, R.M.; Girgis, L.G.; El Daidamony, H.; Abdel Aziz, R.E. Stability and electrical conductivity of water-base Al2O3 nanofluids for different applications. HBRC J. 2016, 12, 227–234. [Google Scholar] [CrossRef]
- Ahammed, N.; Asirvatham, L.G.; Wongwises, S. Thermoelectric cooling of electronic devices with nanofluid in a multiport minichannel heat exchanger. Exp. Therm. Fluid Sci. 2016, 74, 81–90. [Google Scholar] [CrossRef]
- Manimaran, R.; Palaniradja, K.; Alagumurthi, N.; Sendhilnathan, S.; Hussain, J. Preparation and characterization of copper oxide nanofluid for heat transfer applications. Appl. Nanosci. 2014, 4, 163–167. [Google Scholar] [CrossRef]
- Drzazga, M.; Dzido, G.; Lemanowicz, M.; Gierczycki, A. Influence of nonionic surfactant on nanofluid properties. In Proceedings of the 14th European Conference on Mixing, Warszawa, Poland, 10–13 September 2012; pp. 89–94. [Google Scholar]
- Kedzierski, M.A. Effect of CuO Nanoparticle Concentration on R134a/Lubricant Pool-Boiling Heat Transfer. J. Heat Transf. 2009, 131, 043205. [Google Scholar] [CrossRef]
- Liu, M.-S.; Lin, M.-C.; Huang, I.-T.; Wang, C.-C. Enhancement of Thermal Conductivity with CuO for Nanofluids. Chem. Eng. Technol. 2006, 29, 72–77. [Google Scholar] [CrossRef]
- Pourfattah, F.; Abbasian Arani, A.A.; Babaie, M.R.; Nguyen, H.M.; Asadi, A. On the thermal characteristics of a manifold microchannel heat sink subjected to nanofluid using two-phase flow simulation. Int. J. Heat Mass Transf. 2019, 143, 118518. [Google Scholar] [CrossRef]
- Asadi, A.; Pourfattah, F. Heat transfer performance of two oil-based nanofluids containing ZnO and MgO nanoparticles; a comparative experimental investigation. Powder Technol. 2019, 343, 296–308. [Google Scholar] [CrossRef]
- Haghshenas, F.M.; Talaie, M.R.; Nasr, S. Numerical and experimental investigation of heat transfer of ZnO/water nanofluid in the concentric tube and plate heat exchangers. Therm. Sci. 2011, 15, 183–194. [Google Scholar] [CrossRef]
- Bhagat, U.; More, P.; Khanna, P. Study of Zinc Oxide Nanofluids for Heat Transfer Application. SAJ Nanosci. Nanotechnol. 2015, 1, 1–7. [Google Scholar]
- Raykar, V.S.; Singh, A.K. Thermal and rheological behavior of acetylacetone stabilized ZnO nanofluids. Thermochim. Acta 2010, 502, 60–65. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Majdan-Cegincara, R. Effect of temperature on volumetric and transport properties of nanofluids containing ZnO nanoparticles poly(ethylene glycol) and water. J. Chem. Thermodyn. 2012, 54, 55–67. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Majdan-Cegincara, R. Investigation on stability and rheological properties of nanofluid of ZnO nanoparticles dispersed in poly(ethylene glycol). Fluid Phase Equilib. 2013, 354, 102–108. [Google Scholar] [CrossRef]
- Adiwibowo, M.T.; Ibadurrohman, M.; Slamet. Synthesis of Zno Nanoparticles and their nanofluid stability in the presence of a Palm Oil-Based Primary Alkyl Sulphate surfactant for detergent application. Int. J. Technol. 2018, 9, 307–316. [Google Scholar] [CrossRef]
- Abdollahi, A.; Mohammed, H.; Vanaki, S.M.; Sharma, R. Numerical investigation of fluid flow and heat transfer of nanofluids in microchannel with longitudinal fins. Ain Shams Eng. J. 2018, 9, 3411–3418. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced thermal conductivity of TiO2—Water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Prakash, D.S.B.; Kotin, K.N.; Kumar, P. Preparation and characterization of Nanofluid (CuO—Water, TiO2—Water). EPH—Int. J. Sci. Eng. 2015, 1, 14–20. [Google Scholar]
- Hosseinzadeh, K.; Afsharpanah, F.; Zamani, S.; Gholinia, M.; Ganji, D.D. A numerical investigation on ethylene glycol-titanium dioxide nanofluid convective flow over a stretching sheet in presence of heat generation/absorption. Case Stud. Therm. Eng. 2018, 12, 228–236. [Google Scholar] [CrossRef]
- Saleh, R.; Putra, N.; Wibowo, R.E.; Septiadi, W.N.; Prakoso, S.P. Titanium dioxide nanofluids for heat transfer applications. Exp. Therm. Fluid Sci. 2014, 52, 19–29. [Google Scholar] [CrossRef]
- Wei, B.; Zou, C.; Li, X. Experimental investigation on stability and thermal conductivity of diathermic oil based TiO2 nanofluids. Int. J. Heat Mass Transf. 2017, 104, 537–543. [Google Scholar] [CrossRef]
- Abdolbaqi, M.K.; Sidik, N.A.C.; Aziz, A.; Mamat, R.; Azmi, W.H.; Yazid, M.N.A.W.M.; Najafi, G. An experimental determination of thermal conductivity and viscosity of BioGlycol/water based TiO2 nanofluids. Int. Commun. Heat Mass Transf. 2016, 77, 22–32. [Google Scholar] [CrossRef]
- Leong, K.Y.; Azmi, W.H. Nanofluids Containing Titanium Dioxide: Thermo-Physical Properties and Energy Saving Applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–20. [Google Scholar]
- Vassallo, P.; Kumar, R.; D’Amico, S. Pool boiling heat transfer experiments in silica–water nano-fluids. Int. J. Heat Mass Transf. 2004, 47, 407–411. [Google Scholar] [CrossRef]
- Akilu, S.; Baheta, A.T.; Sharma, K.V.; Said, M.A. Experimental determination of nanofluid specific heat with SiO2 nanoparticles in different base fluids. AIP Conf. Proc. 2017, 1877, 090001. [Google Scholar]
- Anoop, K.; Sadr, R.; Al-Jubouri, M.; Amani, M. Rheology of mineral oil-SiO2 nanofluids at high pressure and high temperatures. Int. J. Therm. Sci. 2014, 77, 108–115. [Google Scholar] [CrossRef]
- Mohammed, H.; Alawi, O.; Che Sidik, N.A.; Akademia Baru, P. Mixed Convective Nanofluids Flow in a Channel having Forward-Facing Step with Baffle. J. Adv. Res. Appl. Mech. 2016, 24, 2289–7895. [Google Scholar]
- Mohammed, H.; Fathinia, F.; Vuthaluru, H.; Liu, S. CFD based investigations on the effects of blockage shapes on transient mixed convective nanofluid flow over a backward facing step. Powder Technol. 2019, 346, 441–451. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Saedodin, S.; Mahian, O.; Wongwises, S. Thermophysical properties. heat transfer and pressure drop of COOH-functionalized multi walled carbon nanotubes/water nanofluids. Int. Commun. Heat Mass Transf. 2014, 58, 176–183. [Google Scholar] [CrossRef]
- Delfani, S.; Karami, M.; Behabadi, M.A.A. Performance characteristics of a residential-type direct absorption solar collector using MWCNT nanofluid. Renew. Energy 2016, 87, 754–764. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.K.; Lee, C.H.; Jung, Y.M.; Cheong, S.I.; Lee, C.G.; Ku, B.C.; Jang, S.P. Stability and thermal conductivity characteristics of nanofluids. Thermochim. Acta 2007, 455, 70–74. [Google Scholar] [CrossRef]
- Asadi, A.; Alarifi, I.M.; Ali, V.; Nguyen, H.M. An experimental investigation on the effects of ultrasonication time on stability and thermal conductivity of MWCNT-water nanofluid: Finding the optimum ultrasonication time. Ultrason. Sonochem. 2019, 58, 104639. [Google Scholar] [CrossRef]
- Kolsi, L.; Oztop, H.F.; Ghachem, K.; Almeshaal, M.A.; Mohammed, H.A.; Babazadeh, H.; Abu-Hamdeh, N. Numerical Study of Periodic Magnetic Field Effect on 3D Natural Convection of MWCNT-Water/Nanofluid with Consideration of Aggregation. Processes 2019, 7, 957. [Google Scholar] [CrossRef]
- Pourfattah, F.; Sabzpooshani, M.; Toghraie, D.; Asadi, A. On the optimization of a vertical twisted tape arrangement in a channel subjected to MWCNT–water nanofluid by coupling numerical simulation and genetic algorithm. J. Therm. Anal. Calorim. 2020, 1–13. [Google Scholar] [CrossRef]
- Lyu, Z.; Asadi, A.; Alarifi, I.M.; Ali, V.; Foong, L.K. Thermal and Fluid Dynasmics Performance of MWCNT-Water Nanofluid Based on Thermophysical Properties: An Experimental and Theoretical Study. Sci. Rep. 2020, 10, 5185. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Saedodin, S.; Mahian, O.; Wongwises, S. Heat transfer characteristics and pressure drop of COOH-functionalized DWCNTs/water nanofluid in turbulent flow at low concentrations. Int. J. Heat Mass Transf. 2014, 73, 186–194. [Google Scholar] [CrossRef]
- Shamaeil, M.; Firouzi, M.; Fakhar, A. The effects of temperature and volume fraction on the thermal conductivity of functionalized DWCNTs/ethylene glycol nanofluid. J. Therm. Anal. Calorim. 2016, 126, 1455–1462. [Google Scholar] [CrossRef]
- Said, Z.; Saidur, R.; Sabiha, M.A.; Rahim, N.A.; Anisur, M.R. Thermophysical properties of Single Wall Carbon Nanotubes and its effect on exergy efficiency of a flat plate solar collector. Solar Energy 2015, 115, 757–769. [Google Scholar] [CrossRef]
- Mercatelli, L.; Sani, E.; Zaccanti, G.; Martelli, F.; Di Ninni, P.; Barison, S.; Pagura, C.; Agresti, F.; Jafrancesco, D. Absorption and scattering properties of carbon nanohorn-based nanofluids for direct sunlight absorbers. Nanosc. Res. Lett. 2011, 6, 282. [Google Scholar] [CrossRef] [PubMed]
- Mashali, F.; Languri, E.M.; Davidson, J.; Kerns, D.; Johnson, W.; Nawaz, K.; Cunningham, G. Thermo-physical properties of diamond nanofluids: A review. Int. J. Heat Mass Transf. 2019, 129, 1123–1135. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Togun, H.; Mehrali, M.; Sadeghi Nejad, P.; Tahan Latibari, S.; Abdulrazzaq, T.; Kazi, S.N.; Metselaar, H.S.C. An experimental and numerical investigation of heat transfer enhancement for graphene nanoplatelets nanofluids in turbulent flow conditions. Int. J. Heat Mass Transf. 2015, 81, 41–51. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ganji, D.D.; Jafarkazemi, F. Analysis of utilizing Graphene nanoplatelets to enhance thermal performance of flat plate solar collectors. Energy Convers. Manag. 2016, 126, 1–11. [Google Scholar] [CrossRef]
- Bioucas, F.E.B.; Vieira, S.I.C.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A. Performance of heat transfer fluids with nanographene in a pilot solar collector. Sol. Energy 2018, 172, 171–176. [Google Scholar] [CrossRef]
- Otanicar, T.P.; Phelan, P.E.; Prasher, R.S.; Rosengarten, G.; Taylor, R.A. Nanofluid-based direct absorption solar collector. J. Renew. Sustain. Energy 2010, 2, 033102. [Google Scholar] [CrossRef]
- Gorji, T.B.; Ranjbar, A.A. A numerical and experimental investigation on the performance of a low-flux direct absorption solar collector (DASC) using graphite. magnetite and silver nanofluids. Sol. Energy 2016, 135, 493–505. [Google Scholar]
- Luo, Z.; Wang, C.; Wei, W.; Xiao, G.; Ni, M. Performance improvement of a nanofluid solar collector based on direct absorption collection (DAC) concepts. Int. J. Heat Mass Transf. 2014, 75, 262–271. [Google Scholar] [CrossRef]
- Asadi, A.; Aberoumand, S.; Moradikazerouni, A.; Pourfattah, F.; Żyła, G.; Estellé, P.; Mahian, O.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A. Recent advances in preparation methods and thermophysical properties of oil-based nanofluids: A state-of-the-art review. Powder Technol. 2019, 352, 209–226. [Google Scholar] [CrossRef]
- Asadi, A.; Asadi, M.; Siahmargoi, M.; Asadi, T.; Gholami Andarati, M. The effect of surfactant and sonication time on the stability and thermal conductivity of water-based nanofluid containing Mg(OH)2 nanoparticles: An experimental investigation. Int. J. Heat Mass Transf. 2017, 108, 191–198. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A.; Moravej, M.; Aberoumand, H.; Javaherdeh, K. Experimental study on the rheological behavior of silver-heat transfer oil nanofluid and suggesting two empirical based correlations for thermal conductivity and viscosity of oil based nanofluids. Appl. Therm. Eng. 2016, 101, 362–372. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A. Experimental study on synthesis, stability. thermal conductivity and viscosity of Cu–engine oil nanofluid. J. Taiwan Inst. Chem. Eng. 2017, 71, 315–322. [Google Scholar] [CrossRef]
- Akoh, H.; Tsukasaki, Y.; Yatsuya, S.; Tasaki, A. Magnetic-Properties of Ferromagnetic Ultrafine Particles Prepared by Vacuum Evaporation on Running Oil Substrate. J. Cryst. Growth 1978, 45, 495–500. [Google Scholar] [CrossRef]
- Wagener, M.; Murty, B.S.; Guenther, B. Preparation of metal nanosuspensions by high-pressure dc-sputtering on running liquids. In Proceedings of the 1996 MRS Fall Symposium, Boston, MA, USA, 2–5 December 1996; George, E.P., Gotthardt, R., Otsuka, K., Trolier-McKinstry, S., Wun-Fogle, M., Eds.; Materials Research Society: Pittsburgh, PA. USA, 1997; pp. 149–154. [Google Scholar]
- Eastman, J.A.; Choi, U.S.; Li, S.; Thompson, L.J.; Lee, S. Enhanced thermal conductivity through the development of nanofluids. In Proceedings of the 1996 MRS Fall Symposium, Boston, MA, USA, 2–5 December 1996; George, E.P., Gotthardt, R., Otsuka, K., Trolier-McKinstry, S., Wun-Fogle, M., Eds.; Materials Research Society: Pittsburgh, PA, USA, 1997; pp. 3–11. [Google Scholar]
- Mukherjee, S.; Mishra, P.C.; Chaudhuri, P. Stability of Heat Transfer Nanofluids—A Review. ChemBioEng Rev. 2018, 5, 312–333. [Google Scholar] [CrossRef]
- Sabiha, M.A.; Mostafizur, R.M.; Saidur, R.; Mekhilef, S. Experimental investigation on thermo physical properties of single walled carbon nanotube nanofluids. Int. J. Heat Mass Transf. 2016, 93, 862–871. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, S.U.S.; Jang, S.P.; Lee, S.Y. Production of aqueous spherical gold nanoparticles using conventional ultrasonic bath. Nanosc. Res. Lett. 2012, 7, 420. [Google Scholar] [CrossRef]
- Asadi, A.; Pourfattah, F.; Miklós Szilágyi, I.; Afrand, M.; Żyła, G.; Seon Ahn, H.; Wongwises, S.; Minh Nguyen, H.; Arabkoohsar, A.; Mahian, O. Effect of sonication characteristics on stability thermophysical properties and heat transfer of nanofluids: A comprehensive review. Ultrason. Sonochem. 2019, 58, 104701. [Google Scholar] [CrossRef]
- Duangthongsuk, W.; Wongwises, S. Measurement of temperature-dependent thermal conductivity and viscosity of TiO2-H2O nanofluids. Exp. Therm. Fluid Sci. 2009, 33, 706–714. [Google Scholar] [CrossRef]
- Sandhu, H.; Dasaroju, G. An Experimental Study on Stability and Some Thermophysical Properties of Multiwalled Carbon Nanotubes with Water-Ethylene Glycol Mixtures. Part. Sci. Technol. 2017, 35, 547–554. [Google Scholar] [CrossRef]
- Ilyas, S.; Pendyala, R.; Marneni, N.; Lim, S. Stability, rheology and thermal analysis of functionalized alumina- thermal oil-based nanofluids for advanced cooling systems. Energy Convers. Manag. 2017, 142, 215–229. [Google Scholar] [CrossRef]
- Wen, D.; Ding, Y. Experimental investigation into the pool boiling heat transfer of aqueous based γ-alumina nanofluids. J. Nanopart. Res. 2005, 7, 265–274. [Google Scholar] [CrossRef]
- Fontes, D.H.; Ribatski, G.; Bandarra Filho, E.P. Experimental evaluation of thermal conductivity. viscosity and breakdown voltage AC of nanofluids of carbon nanotubes and diamond in transformer oil. Diam. Relat. Mater. 2015, 58, 115–121. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Moradikazerouni, A.; Bakhtiari, R.; Asadi, A.; Afrand, M. An experimental study on stability and thermal conductivity of water/silica nanofluid: Eco-friendly production of nanoparticles. J. Clean. Prod. 2019, 206, 1089–1100. [Google Scholar] [CrossRef]
- Song, Y.Y.; Bhadeshia, H.K.D.H.; Suh, D.-W. Stability of stainless-steel nanoparticle and water mixtures. Powder Technol. 2015, 272, 34–44. [Google Scholar] [CrossRef]
- Saidina, D.S.; Abdullah, M.Z.; Hussin, M. Metal oxide nanofluids in electronic cooling: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 4381–4398. [Google Scholar] [CrossRef]
- Asadi, A.; Alarifi, I.M.; Nguyen, H.M.; Moayedi, H. Feasibility of least-square support vector machine in predicting the effects of shear rate on the rheological properties and pumping power of MWCNT–MgO/oil hybrid nanofluid based on experimental data. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Halelfadl, S.; Maré, T.; Estellé, P. Efficiency of carbon nanotubes water based nanofluids as coolants. Exp. Therm. Fluid Sci. 2014, 53, 104–110. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Casanova, C.; Páramo, R.; Barbés, B.; Legido, J.L.; Piñeiro, M.M. A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. J. Appl. Phys. 2009, 106, 064301. [Google Scholar] [CrossRef]
- Saini, A.; Sandhu, H.; Sharma, S.; Dasaroju, G. Nanofluids: A Review Preparation, Stability. Properties and Applications. Int. J. Eng. Res. Technol. (IJERT) 2016, 5, 11–16. [Google Scholar]
- Zhou, S.-Q.; Ni, R. Measurement of the specific heat capacity of water-based Al2O3 nanofluid. Appl. Phys. Lett. 2008, 92, 093123. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Gracia-Fernández, C.; Legido, J.L.; Lugo, L. Specific heat of metal oxide nanofluids at high concentrations for heat transfer. Int. J. Heat Mass Transf. 2015, 88, 872–879. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Wang, X. Enhanced Thermal Conductivity of Liquid Paraffin Based Nanofluids Containing Copper Nanoparticles. J. Dispers. Sci. Technol. 2011, 32, 948–951. [Google Scholar] [CrossRef]
- Li, X.F.; Zhu, D.S.; Wang, X.J.; Wang, N.; Gao, J.W.; Li, H. Thermal conductivity enhancement dependent pH and chemical surfactant for Cu-H2O nanofluids. Thermochim. Acta 2008, 469, 98–103. [Google Scholar] [CrossRef]
- Srivastava, S. Effect of aggregation on thermal conductivity and viscosity of nanofluids. Appl. Nanosci. 2012, 2, 325–331. [Google Scholar]
- Sadri, R.; Ahmadi, G.; Togun, H.; Dahari, M.; Kazi, S.N.; Sadeghinezhad, E.; Zubir, N. An experimental study on thermal conductivity and viscosity of nanofluids containing carbon nanotubes. Nanosc. Res. Lett. 2014, 9, 151. [Google Scholar] [CrossRef]
- Raja, M.; Vijayan, R.; Dineshkumar, P.; Venkatesan, M. Review on nanofluids characterization. heat transfer characteristics and applications. Renew. Sustain. Energy Rev. 2016, 64, 163–173. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Saha, B.B.; Irshad, K. Comprehensive study on nanofluid and ionanofluid for heat transfer enhancement: A review on current and future perspective. J. Mol. Liq. 2020, 305, 112787. [Google Scholar] [CrossRef]
- Paul, G.; Chopkar, M.; Manna, I.; Das, P.K. Techniques for measuring the thermal conductivity of nanofluids: A review. Renew. Sustain. Energy Rev. 2010, 14, 1913–1924. [Google Scholar] [CrossRef]
- Doganay, S.; Alsangur, R.; Turgut, A. Effect of external magnetic field on thermal conductivity and viscosity of magnetic nanofluids: A review. Mater. Res. Express 2019, 6, 112003. [Google Scholar] [CrossRef]
- Pozhar, L.A. Structure and dynamics of nanofluids: Theory and simulations to calculate viscosity. Phys. Rev. E 2000, 61, 1432–1446. [Google Scholar] [CrossRef] [PubMed]
- Khanafer, K.; Vafai, K.; Lightstone, M. Buoyancy-driven heat transfer enhancement in a two-dimensional enclosure utilizing nanofluids. Int J. Heat Mass Transf. 2003, 46, 3639–3653. [Google Scholar] [CrossRef]
- Wang, B.G.; Wang, X.B.; Lou, W.J.; Hao, J.C. Thermal conductivity and rheological properties of graphite/oil nanofluids. Colloids Surf. Physicochem. Eng. Asp. 2012, 414, 125–131. [Google Scholar] [CrossRef]
- Bahiraei, M.; Rahmani, R.; Yaghoobi, A.; Khodabandeh, E.; Mashayekhi, R.; Amani, M. Recent research contributions concerning use of nanofluids in heat exchangers: A critical review. Appl. Therm. Eng. 2018, 133, 137–159. [Google Scholar] [CrossRef]
- Maré, T.; Halelfadl, S.; Sow, O.; Estellé, P.; Duret, S.; Bazantay, F. Comparison of the thermal performances of two nanofluids at low temperature in a plate heat exchanger. Exp. Therm. Fluid Sci. 2011, 35, 1535–1543. [Google Scholar] [CrossRef]
- Safi, M.A.; Ghozatloo, A.; Hamidi, A.A.; Shariaty-Niassar, M. Calculation of Heat Transfer Coefficient of MWCNT-TiO2 Nanofluid in Plate Heat Exchanger. Int. J. Nanosci. Nanotechnol. 2014, 10, 153–162. [Google Scholar]
- Rashidi, S.; Karimi, N.; Mahian, O.; Abolfazli Esfahani, J. A concise review on the role of nanoparticles upon the productivity of solar desalination systems. J. Therm. Anal. Calorim. 2019, 135, 1145–1159. [Google Scholar] [CrossRef]
- Tabari, Z.T.; Heris, S.Z. Heat Transfer Performance of Milk Pasteurization Plate Heat Exchangers Using MWCNT/Water Nanofluid. J. Dispers. Sci. Technol. 2015, 36, 196–204. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Hormozi, F. Heat transfer pressure drop and fouling studies of multi-walled carbon nanotube nano-fluids inside a plate heat exchanger. Exp. Therm. Fluid Sci. 2016, 72, 1–11. [Google Scholar] [CrossRef]
- Goodarzi, M.; Amiri, A.; Goodarzi, M.S.; Safaei, M.R.; Karimipour, A.; Languri, E.M.; Dahari, M. Investigation of heat transfer and pressure drop of a counter flow corrugated plate heat exchanger using MWCNT based nanofluids. Int. Commun. Heat Mass Transf. 2015, 66, 172–179. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwari, A.K.; Ghosh, S.K. Effect of variable spacing on performance of plate heat exchanger using nanofluids. Energy 2016, 114, 1107–1119. [Google Scholar] [CrossRef]
- Asadi, A.; Alarifi, I.M.; Foong, L.K. An experimental study on characterization. stability and dynamic viscosity of CuO-TiO2/water hybrid nanofluid. J. Mol. Liq. 2020, 307, 112987. [Google Scholar] [CrossRef]
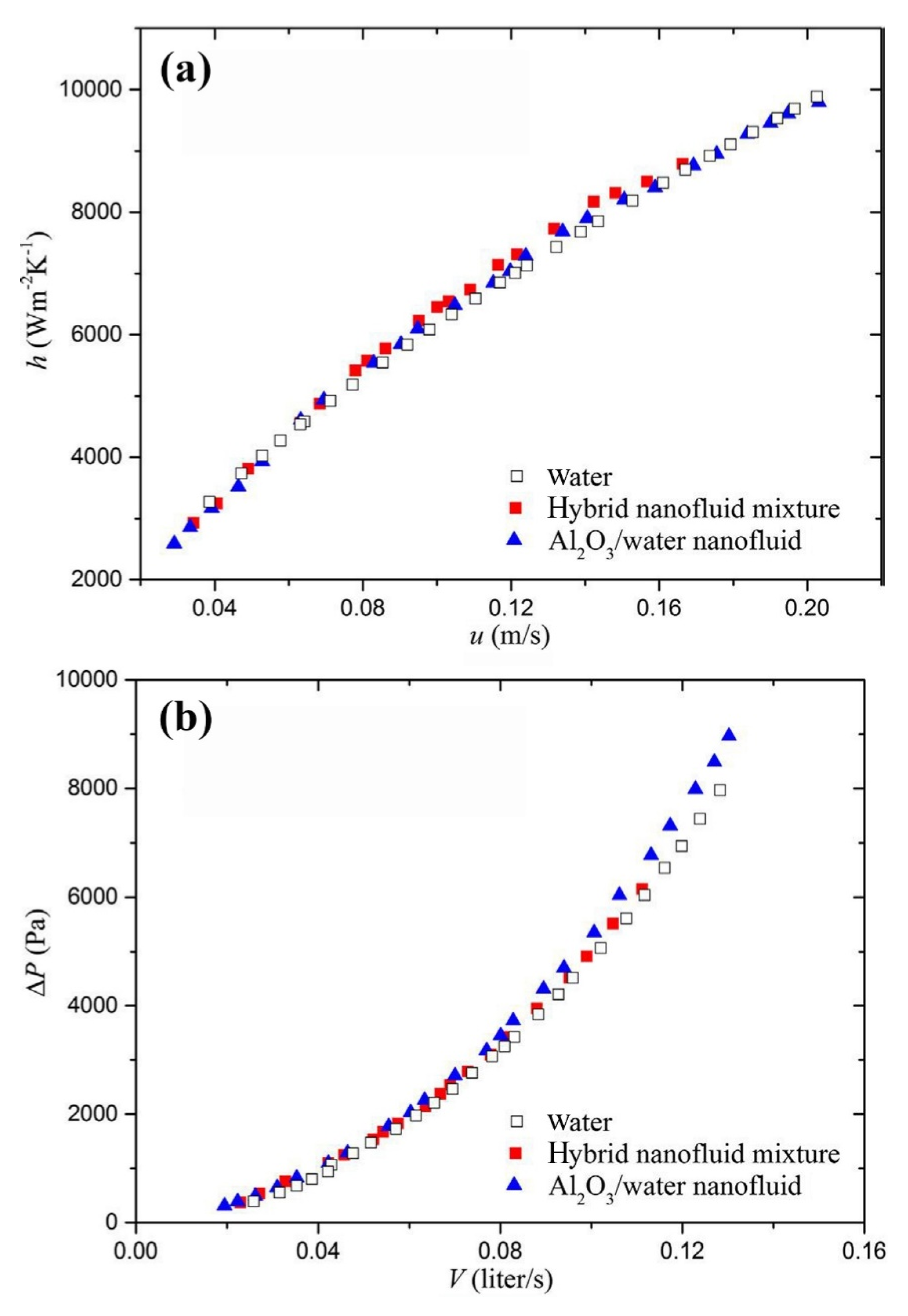
- Huang, D.; Wu, Z.; Sunden, B. Effects of hybrid nanofluid mixture in plate heat exchangers. Exp. Therm. Fluid Sci. 2016, 72, 190–196. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwari, A.K.; Ghosh, S.K. Exergy analysis of hybrid nanofluids with optimum concentration in a plate heat exchanger. Mater. Res. Express 2018, 5, 065022. [Google Scholar] [CrossRef]
- Mouromtseff, I.E. Water and Forced-Air Cooling of Vacuum Tubes Nonelectronic Problems in Electronic Tubes. Proc. IRE 1942, 30, 190–205. [Google Scholar] [CrossRef]
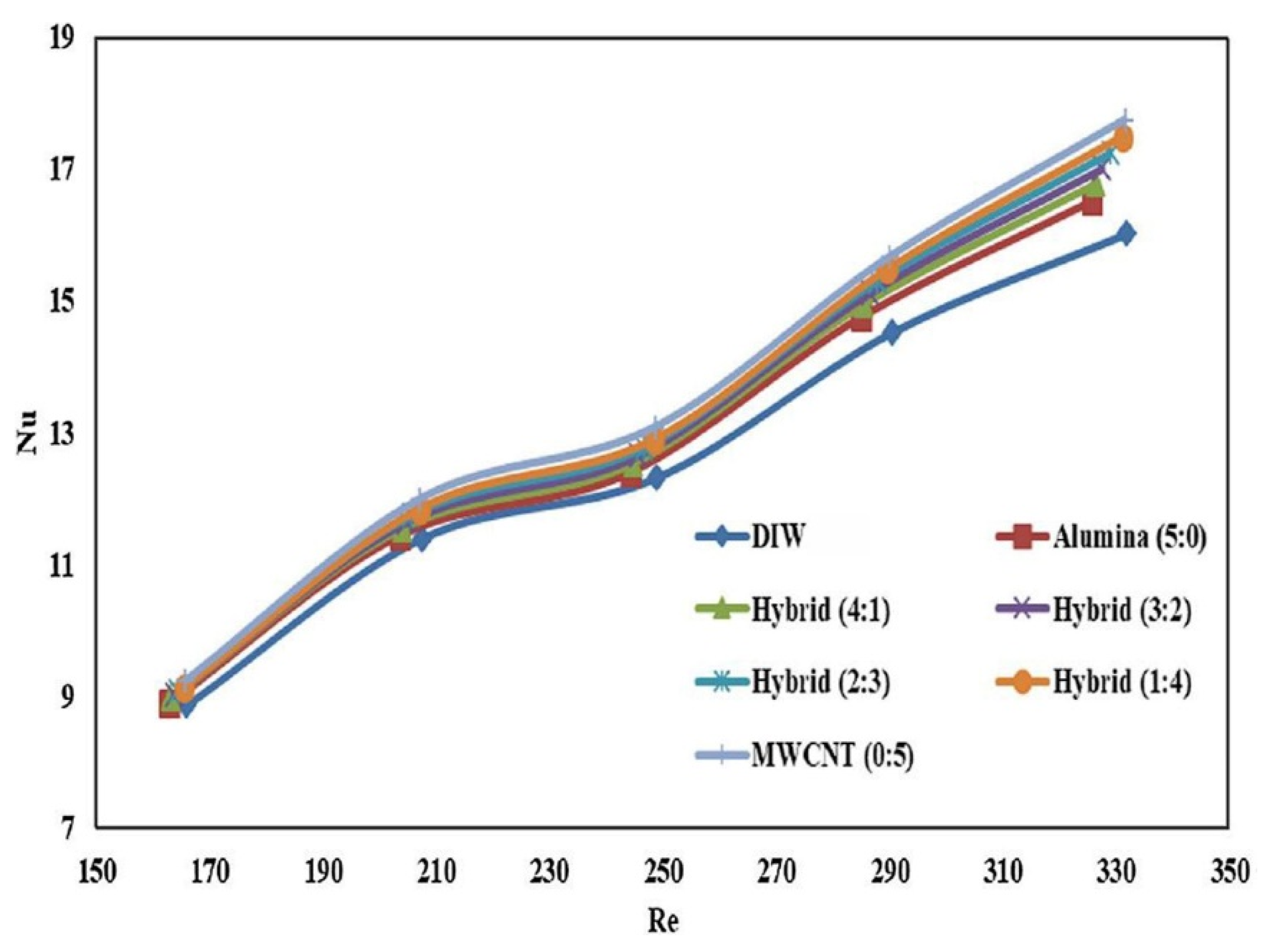
- Bhattad, A.; Sarkar, J.; Ghosh, P. Experimentation on effect of particle ratio on hydrothermal performance of plate heat exchanger using hybrid nanofluid. Appl. Therm. Eng. 2019, 162, 114309. [Google Scholar] [CrossRef]
- Bhattad, A.; Sarkar, J.; Ghosh, P. Hydrothermal performance of different alumina hybrid nanofluid types in plate heat exchanger. J. Therm. Anal. Calorim. 2019, 139, 3777–3787. [Google Scholar] [CrossRef]
- Barzegarian, R.; Moraveji, M.K.; Aloueyan, A. Experimental investigation on heat transfer characteristics and pressure drop of BPHE (brazed plate heat exchanger) using TiO2–water nanofluid. Exp. Therm. Fluid Sci. 2016, 74, 11–18. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwari, A.K.; Ghosh, S.K. Effect of chevron angle on heat transfer performance in plate heat exchanger using ZnO/water nanofluid. Energy Convers. Manag. 2016, 118, 142–154. [Google Scholar] [CrossRef]
- Pourhoseini, S.H.; Naghizadeh, N.; Hoseinzadeh, H. Effect of silver-water nanofluid on heat transfer performance of a plate heat exchanger: An experimental and theoretical study. Powder Technol. 2018, 332, 279–286. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Han, F.; Wadsö, L.; Sundén, B. Experimental comparative evaluation of a graphene nanofluid coolant in miniature plate heat exchanger. Int. J. Therm. Sci. 2018, 130, 148–156. [Google Scholar] [CrossRef]
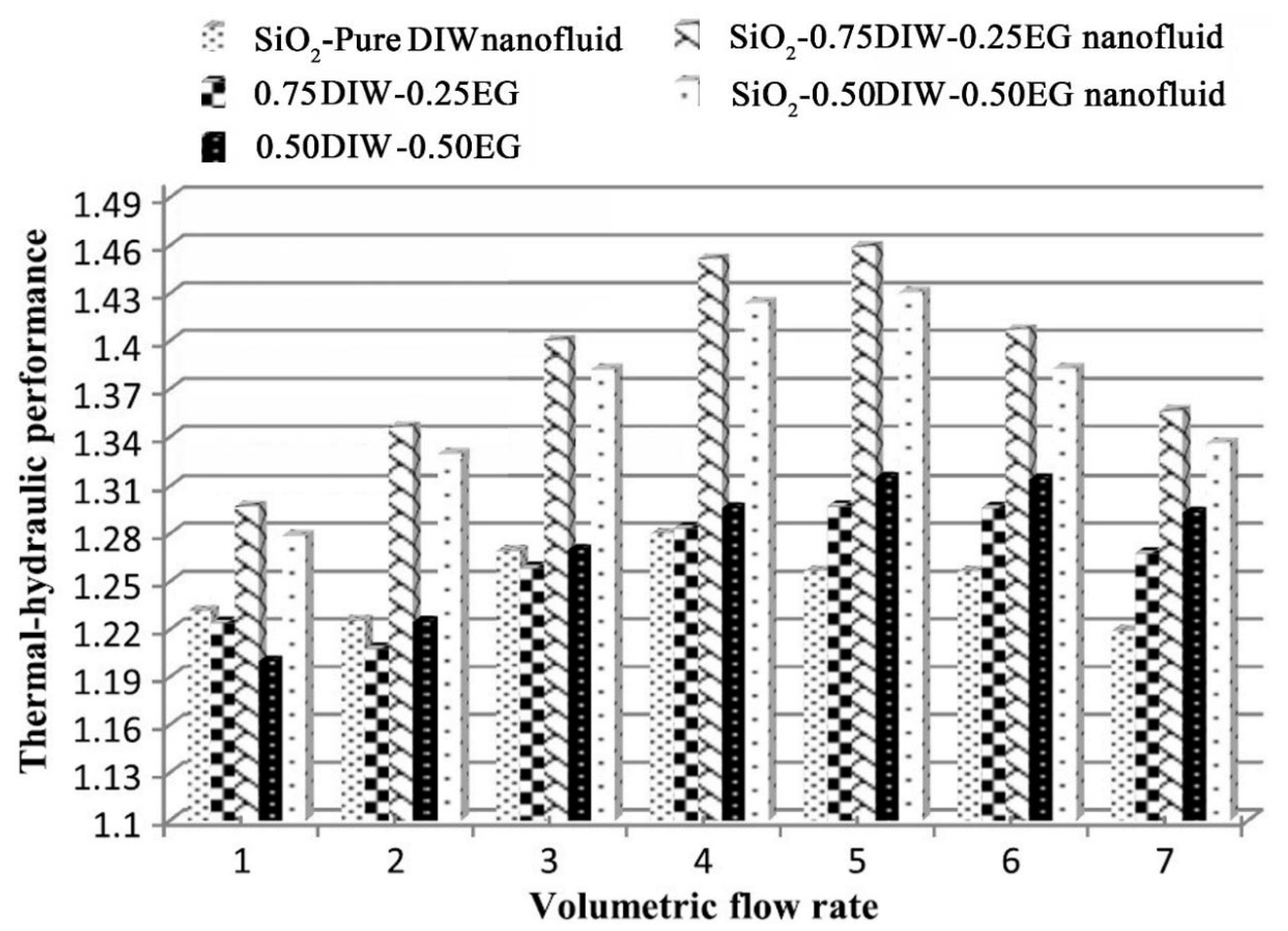
- Mansoury, D.; Doshmanziari, F.I.; Kiani, A.; Chamkha, A.J.; Sharifpur, M. Heat Transfer and Flow Characteristics of Al2O3/Water Nanofluid in Various Heat Exchangers: Experiments on Counter Flow. Heat Transf. Eng. 2020, 41, 220–234. [Google Scholar] [CrossRef]
- Talari Vijaya, K.; Thamida Sunil, K.; Sastry, R.C. Determination of Optimum Concentration of Nanofluid for Process Intensification of Heat Transfer Using Corrugated Plate Type Heat Exchanger. Chem. Prod. Proc. Model. 2019, 14. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Ghosh, P.; Sarkar, J. Heat transfer and pressure drop characteristics of CeO2/water nanofluid in plate heat exchanger. Appl. Therm. Eng. 2013, 57, 24–32. [Google Scholar] [CrossRef]
- Unverdi, M.; Islamoglu, Y. Characteristics of heat transfer and pressure drop in a chevron-type plate heat exchanger with Al2O3/water nanofluids. Therm. Sci. 2017, 21, 2379–2391. [Google Scholar] [CrossRef]
- Elias, M.M.; Saidur, R.; Ben-Mansour, R.; Hepbasli, A.; Rahim, N.A.; Jesbains, K. Heat transfer and pressure drop characteristics of a plate heat exchanger using water based Al2O3 nanofluid for 30° and 60° chevron angles. Heat Mass Transf. 2018, 54, 2907–2916. [Google Scholar] [CrossRef]
- Tariq, R.; Sheikh, K.A.; Shayyan, M. Study of Thermal Performance of Common Heat Exchangers by Using Nanofluids. In Proceedings of the 2018 International Conference on Applied and Engineering Mathematics (ICAEM), London, UK, 4–6 July 2018; pp. 130–135. [Google Scholar]
- Attalla, M.; Maghrabie, H.M. An experimental study on heat transfer and fluid flow of rough plate heat exchanger using Al2O3/water nanofluid. Exp. Heat Transf. 2020, 33, 261–281. [Google Scholar] [CrossRef]
- Sözen, A.; Khanları, A.; Çiftçi, E. Heat transfer enhancement of plate heat exchanger utilizing kaolin-including working fluid. Proceedings of the Institution of Mechanical Engineers. Part A J. Power Energy 2019, 233, 626–634. [Google Scholar] [CrossRef]
- Meisam, A.; Ahmad, A.; Hamed, M. Experimental Investigation of Metal Oxide Nanofluids in a Plate Heat Exchanger. J. Therm. Heat Transf. 2019, 33, 994–1005. [Google Scholar] [CrossRef]
- Soman, D.P.; Karthika, S.; Kalaichelvi, P.; Radhakrishnan, T.K. Experimental study of turbulent forced convection heat transfer and friction factor in dimpled plate heat exchanger. Appl. Therm. Eng. 2019, 162, 114254. [Google Scholar] [CrossRef]
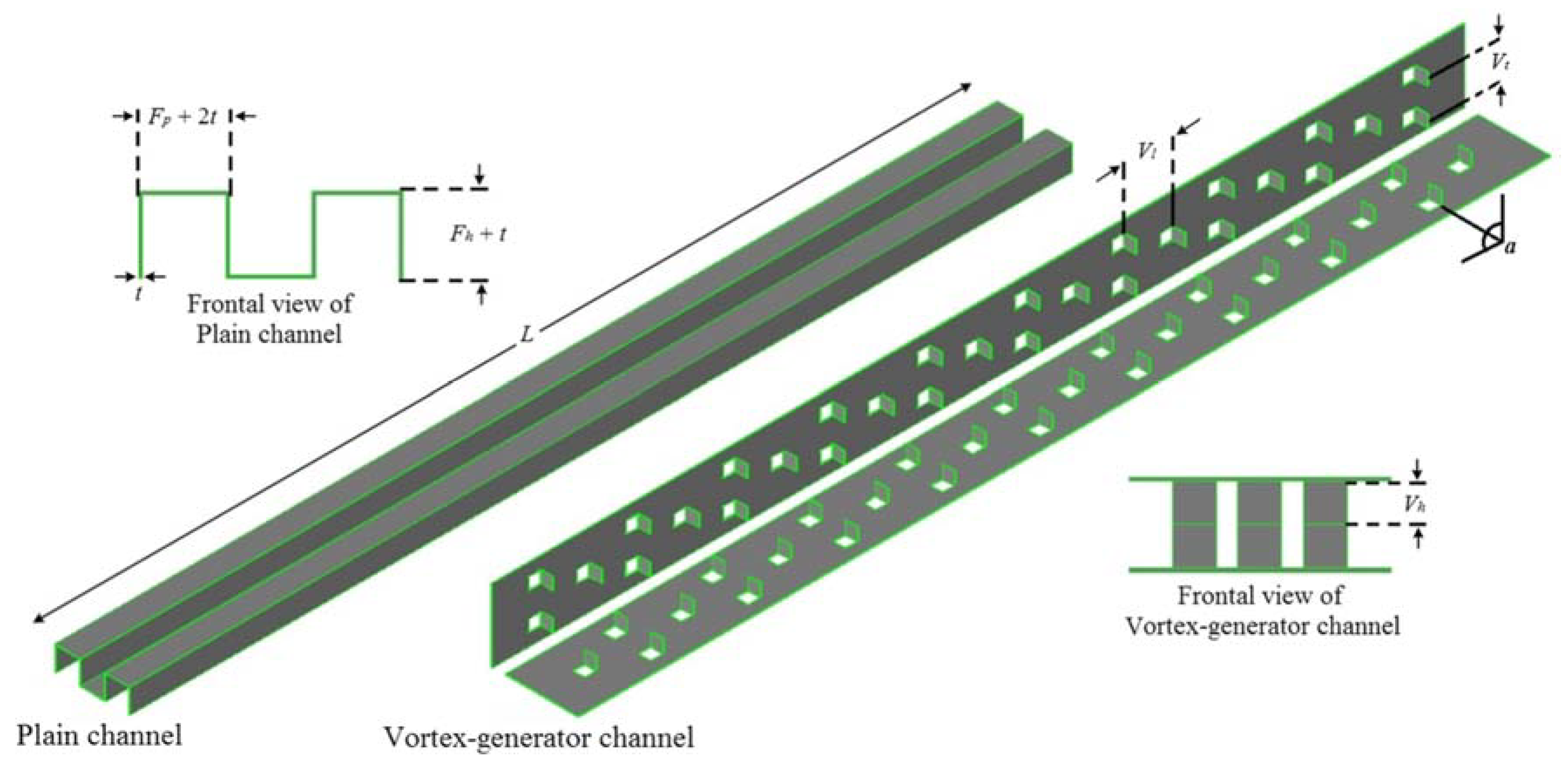
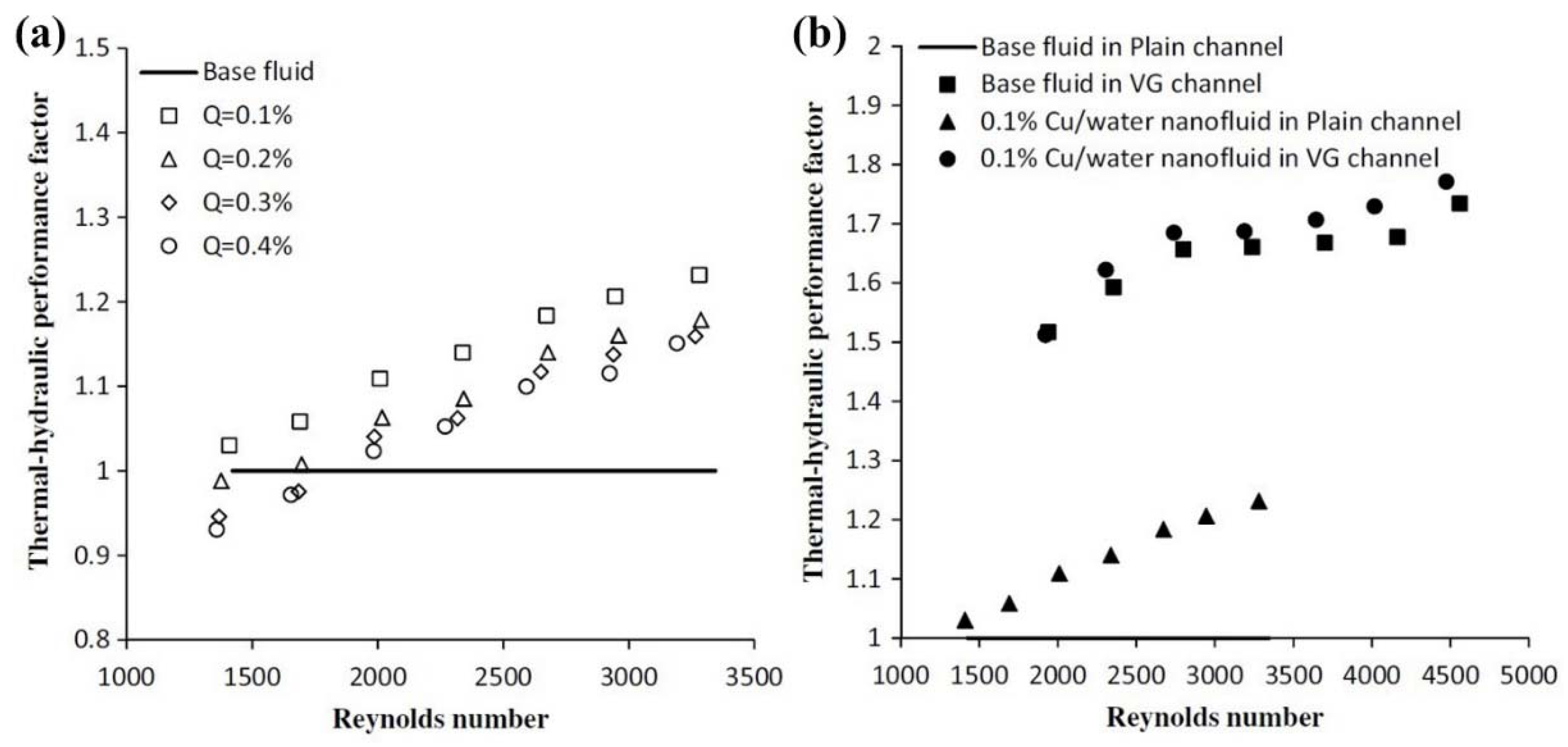
- Khoshvaght-Aliabadi, M.; Hormozi, F.; Zamzamian, A. Effects of geometrical parameters on performance of plate-fin heat exchanger: Vortex-generator as core surface and nanofluid as working media. Appl. Therm. Eng. 2014, 70, 565–579. [Google Scholar] [CrossRef]
- Khoshvaght-Aliabadi, M.; Hormozi, F.; Zamzamian, A. Experimental analysis of thermal–hydraulic performance of copper–water nanofluid flow in different plate-fin channels. Exp. Therm. Fluid Sci. 2014, 52, 248–258. [Google Scholar] [CrossRef]
- Khoshvaght-Aliabadi, M.; Zamzamian, A.; Hormozi, F. Wavy Channel and Different Nanofluids Effects on Performance of Plate-Fin Heat Exchangers. J. Therm. Heat Transf. 2014, 28, 474–484. [Google Scholar] [CrossRef]
- Khoshvaght-Aliabadi, M. Thermal performance of plate-fin heat exchanger using passive techniques: Vortex-generator and nanofluid. Heat Mass Transf. 2016, 52, 819–828. [Google Scholar] [CrossRef]
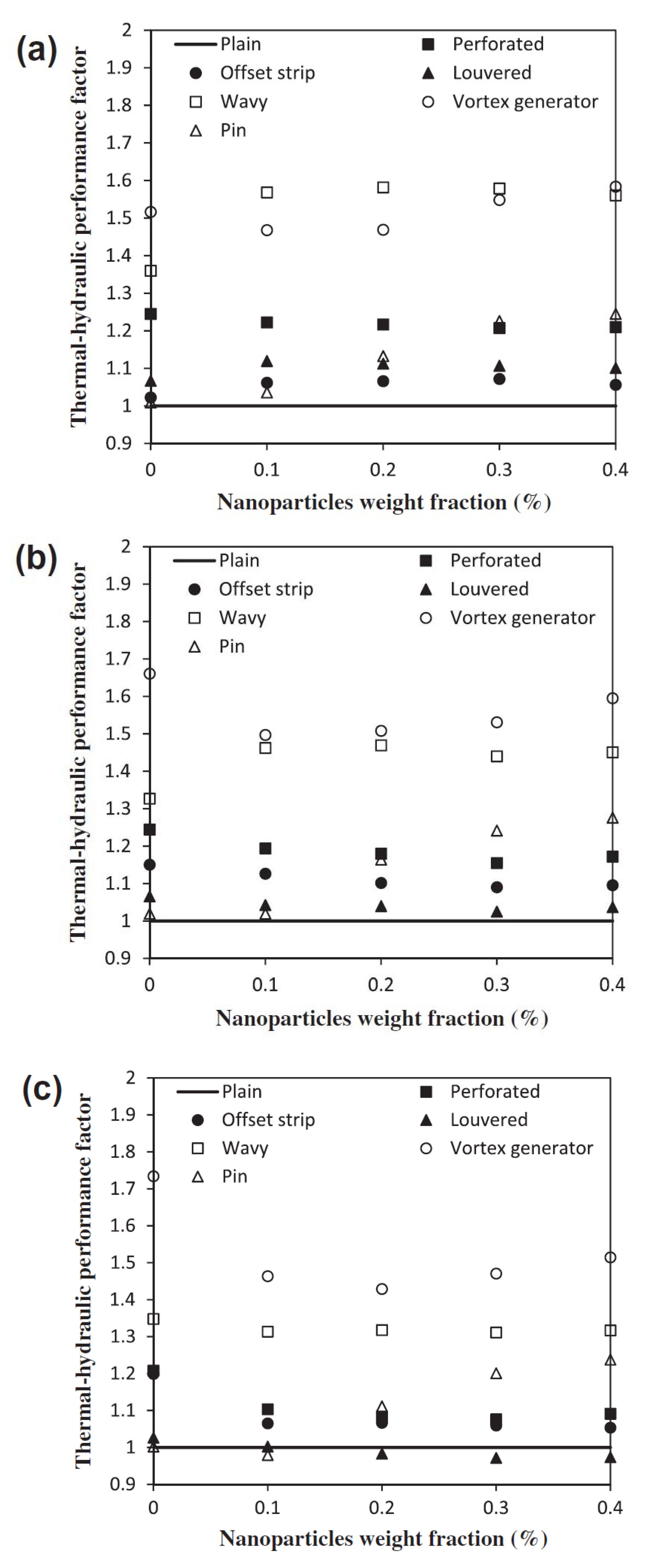
- Khoshvaght-Aliabadi, M.; Jafari, A.; Sartipzadeh, O.; Salami, M. Thermal–hydraulic performance of wavy plate-fin heat exchanger using passive techniques: Perforations, winglets. and nanofluids. Int. Commun. Heat Mass Transf. 2016, 78, 231–240. [Google Scholar] [CrossRef]
- Khoshvaght-Aliabadi, M.; Ariana, H.; Khaligh, S.F.; Salami, M. Effects of delta winglets on performance of wavy plate-fin in PFHEs. J. Therm. Anal. Calorim. 2018, 131, 1625–1640. [Google Scholar] [CrossRef]
- Khoshvaght-Aliabadi, M.; Salami, M. Turbulent flow of Al2O3-water nanofluid through plate-fin heat exchanger (PFHE) with offset-strip channels. Therm. Sci. Eng. Prog. 2018, 6, 164–176. [Google Scholar] [CrossRef]
- Morteza, K.-A.; Mortazavi, S. Combined effects of holes and winglets on chevron plate-fins to enhance the performance of a plate-fin heat exchanger working with nanofluid. Exp. Heat Transf. 2019, 32, 584–599. [Google Scholar] [CrossRef]
- Khoshvaght-Aliabadi, M.; Hassani, S.M.; Mazloumi, S.H. Comparison of hydrothermal performance between plate fins and plate-pin fins subject to nanofluid-cooled corrugated miniature heat sinks. Microelectron. Reliab. 2017, 70, 84–96. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Yan, Y.; Reay, D. A review on graphene based nanofluids: Preparation. characterization and applications. J. Mol. Liq. 2019, 279, 444–484. [Google Scholar] [CrossRef]

| Origin | Nanoparticles | Basefluids | Source |
|---|---|---|---|
| Metals | Cu * | Water, EG, oil, acetone, and water & EG mixture. | [68,74,75,76,77,78,79] |
| Ag * | Water, and toluene. | [80,81] | |
| Au * | Water, and toluene. | [81,82,83] | |
| Al * | Water, oil, EG, kerosene. | [15,84,85,86,87] | |
| Oxides | Al2O3 * | Water, EG, oil, and water & glycerine mixture. | [82,88,89,90,91,92,93,94,95,96] |
| CuO * | Water, oil, and R-134a *. | [92,93,97,98,99,100,101] | |
| ZnO * | Water, EG, and oil. | [102,103,104,105,106,107,108,109] | |
| TiO2 * | Water, EG, oil, water & EG mixture, and bioglycol & water mixture. | [110,111,112,113,114,115,116] | |
| SiO2 * | Water, EG, glycerol, oil, and glycerol & EG mixture. | [80,117,118,119,120,121] | |
| Carbon-based | MWCNTs * | Water, EG, water & EG mixture, and fullerenes oil. | [88,122,123,124,125,126,127,128] |
| DWCNTs * | Water, and EG. | [129,130] | |
| SWCNTs * | Water, water & EG mixture. | [131,132] | |
| Nanodiamond | Water, EG, propylene glycol, midel oil, silicone oil, mineral oil, transformer oil, and engine oil. | [133] | |
| Graphene | Water, water & EG mixture. | [134,135,136] | |
| Graphite | Water, texatherm oil. | [137,138,139] |
| Reference | Nanofluid | Considered Conditions and Objectives | Type of HE | Findings |
|---|---|---|---|---|
| Tiwari et al. [196] | CeO2-water* |
| Chevron corrugated PHE | They found that the optimum solid concentration (0.75 vol. %) in which the heat transfer reached its maximum enhancement by 39%. They reported that increasing the flow rate of the nanofluid and the hot water leads to enhancing the heat transfer coefficient. Moreover, the increase in the pressure drop at the optimum solid concentration is negligible while the heat transfer has been significantly improved. |
| Barzegarian et al. [190] | TiO2-water |
| Brazed PHE | Their results revealed that increasing the Re number and solid concentration results in enhancing the convective heat transfer coefficient, and the maximum enhancement took place at the highest solid concentration by 23.7%. They also reported that the increase in pressure drop by increasing the solid concentration is negligible. |
| Kumar et al. [191] | ZnO-water |
| Chevron-type PHE | They reported that the solid concentration of 1.0 vol. % is the optimum solid concentration where the maximum heat transfer rate is achieved. |
| Unverdi and Islamoglu [197] | Al2O3-water |
| Chevron-type PHE | They reported that increasing the solid concentration and flow rate results in enhancing the Nu number by the maximum of 42.4%. They also reported that the maximum increase in the heat transfer and pressure drop took place at the highest solid concentration and Re number by 6.4% and 8.4%, respectively. |
| Pourhoseini et al. [192] | Ag-water |
| CR14-45 COMER PHE | They found that the effect of flow rate on heat transfer performance is more significant than the effect of solid concentration. |
| Wang et al. [193] | Graphene nanoplatelets-EG/water (50:50) |
| Miniature PHE | They reported the maximum enhancement of 4% in heat transfer as the solid concentration increased. Moreover, they reported that the increase in Re number leads to enhancing the heat transfer performance in all the studied solid concentrations. The same trend as was observed for the pressure drop; increasing the solid concentration and Re number leads to increasing the pressure drop. |
| Mansoury et al. [194] | Al2O3-water |
| Different HEs; a Double-pipe, a Shell and tube, and a PHE | They reported that the maximum heat transfer of 60% is achieved in the double-pipe HE, while the minimum enhancement took place in the PHE by 11%. Moreover, the minimum increase in pressure drop has been experienced in the PHE. |
| Elias et al. [198] | Al2O3-water |
| Chevron-type PHE | The results revealed the maximum enhancement of 7.8% in the heat transfer coefficient at the solid concentration of 0.5 vol. %. Moreover, increasing the solid concentration leads to increasing the pressure drop. |
| Tayyab at al. [199] | CuO-water |
| Different HEs: Shell and tube, concentric, spiral, and PHE | The results revealed that the heat transfer performance of the nanofluid in the PHE is better than the other studied HEs. The maximum enhancement in heat transfer for the PHE is 26% while for the other HEs, 21% is reported. |
| Attalla and Maghrabie [200] | Al2O3-water |
| PHE | The results revealed that the heat transfer performance and the pressure drop has been increased as the solid concentration and surface roughness increased. Moreover, it is found that the influence of the surface roughness is more noticeable than the solid concentration. |
| Talari et al. [195] | Al2O3-water |
| Corrugated PHE | They declared that since the heat transfer enhancement of the nanofluid showed a monotonic increase, it is not possible to find an optimum solid concentration. |
| Sözen et al. [201] | Kaolin-water |
| Spiral PHE | It is revealed that using nanofluid instead of the based fluid leads to having 17.6% enhancement in heat transfer rate. Moreover, increasing the Re number leads to decreasing the effectiveness of the PHE. |
| Meisam et al. [202] | Al2O3-water TiO2-water SiO2-water |
| PHE | The results revealed that adding nanoparticles to the basefluid leads to considerable enhancement in heat transfer performance. The maximum enhancement in the heat transfer achieved by using SiO2-water nanofluid at the highest solid concentration and Re number of 37 by 2.82%, while the minimum enhancement has been experienced by using Al2O3-water nanofluid at the solid concentration of 0.1 wt. % and Re number 158 by 1.64%. |
| Soman et al. [203] | γ-Al2O3-water* |
| Dimpled PHE | It is revealed that increasing the mass flow rate leads to increasing the heat transfer rate in the PHE. Moreover, increasing the mass flow rate has a direct effect on the heat transfer performance. A new correlation for predicting the Nu number has also been proposed. |
| Reference | Nanofluid | Considered Conditions and Objectives | Type of HE | Findings |
|---|---|---|---|---|
| Aliabadi et al. [208] | Al2O3-water |
| Wavy plate-fin HE | It is revealed that the Nu number for the wavy channel is higher than that of the plain channel. Moreover, the performance factor values show that applying these three techniques leads to improving the thermal-hydraulic performance of the HE. |
| Aliabadi et al. [209] | Al2O3-water |
| Wavy plate-fin HE | It is reported that using the nanofluid instead of the basefluid leads to increasing the thermal performance and pressure drop by 11.3% and 6.2%, respectively. Moreover, increasing the waviness aspect ratio and winglets height results in increasing the heat transfer and pressure drop. |
| Aliabadi and Salami [210] | Al2O3-water |
| Offset-strip | It is reported that the most effective factor on the thermal-hydraulic performance is the channel height. Moreover, using nanofluid results in having better thermal performance compared to the basefluid. |
| Aliabadi and Mortazavi [211] | Al2O3-water |
| Chevron plate-fin HE combined with holes and winglets | It is found that the HE equipped with holes and winglets showed enhanced Nu number by a maximum of 1.6%. Moreover, it is reported that employing the nanofluid as the working fluid also leads to enhancing the Nu number. The optimum solid concentration has been reported as 0.3%. |
| Aliabadi et al. [212] | Al2O3-water |
| Plate and plate pin fin HE | It is reported that the plate-pin fin showed better heat transfer performance and lower pressure drop. Moreover, using nanofluid leads to enhancing the heat transfer coefficient and the best performance achieved at the solid concentration of 0.3 wt. %. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almurtaji, S.; Ali, N.; Teixeira, J.A.; Addali, A. On the Role of Nanofluids in Thermal-hydraulic Performance of Heat Exchangers—A Review. Nanomaterials 2020, 10, 734. https://doi.org/10.3390/nano10040734
Almurtaji S, Ali N, Teixeira JA, Addali A. On the Role of Nanofluids in Thermal-hydraulic Performance of Heat Exchangers—A Review. Nanomaterials. 2020; 10(4):734. https://doi.org/10.3390/nano10040734
Chicago/Turabian StyleAlmurtaji, Salah, Naser Ali, Joao A. Teixeira, and Abdulmajid Addali. 2020. "On the Role of Nanofluids in Thermal-hydraulic Performance of Heat Exchangers—A Review" Nanomaterials 10, no. 4: 734. https://doi.org/10.3390/nano10040734
APA StyleAlmurtaji, S., Ali, N., Teixeira, J. A., & Addali, A. (2020). On the Role of Nanofluids in Thermal-hydraulic Performance of Heat Exchangers—A Review. Nanomaterials, 10(4), 734. https://doi.org/10.3390/nano10040734






