A General Protocol for Electrospun Non-Woven Fabrics of Dialdehyde Cellulose and Poly(Vinyl Alcohol)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
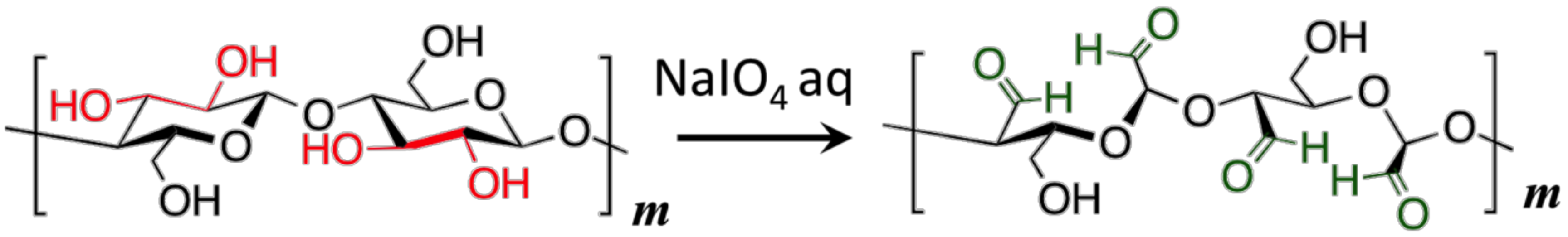
2.2. Preparation of Dialdehyde Cellulose (DAC)
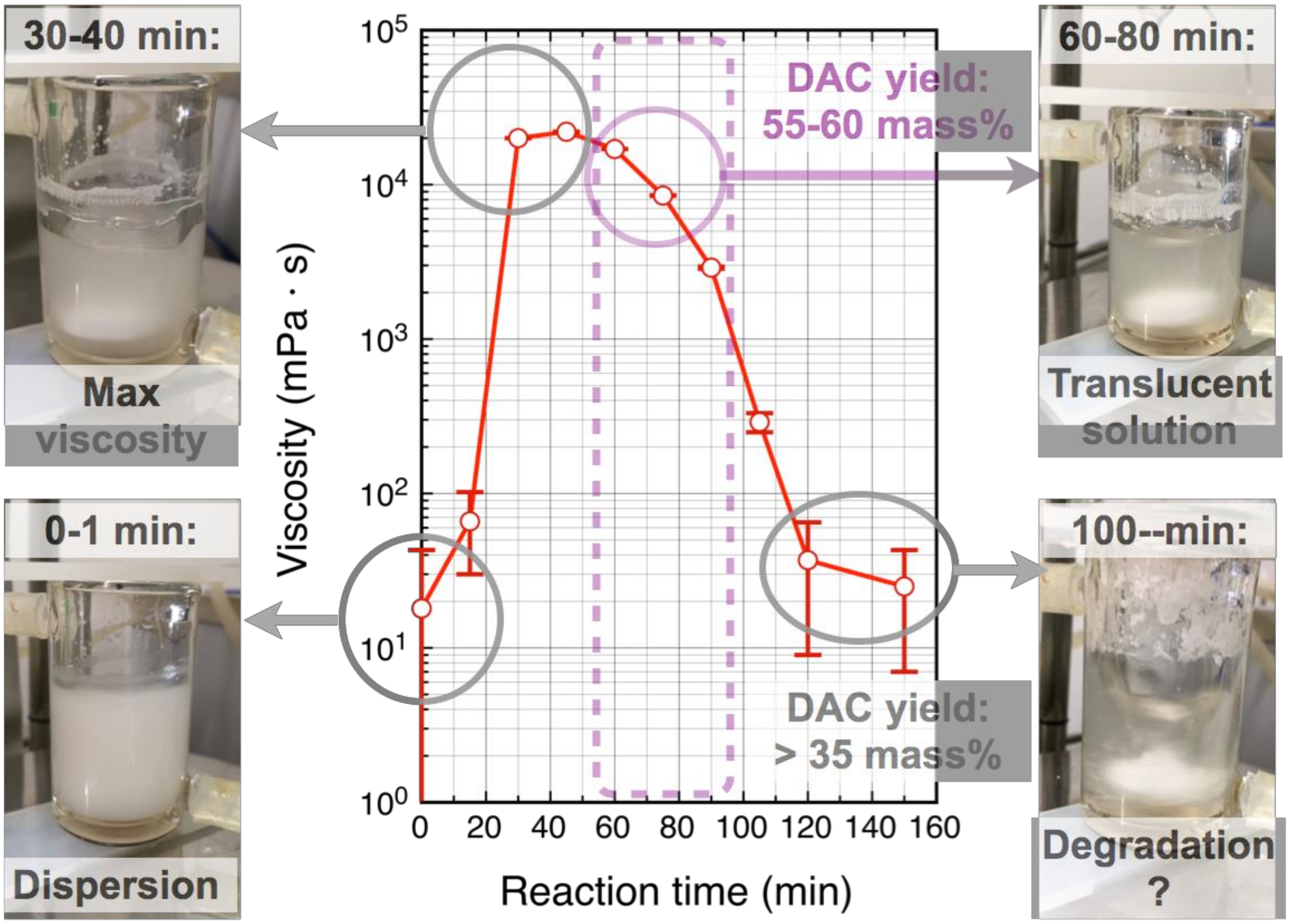
2.3. Viscosity Measurement
2.4. Quantification of Aldehyde Contents in DAC Preparations
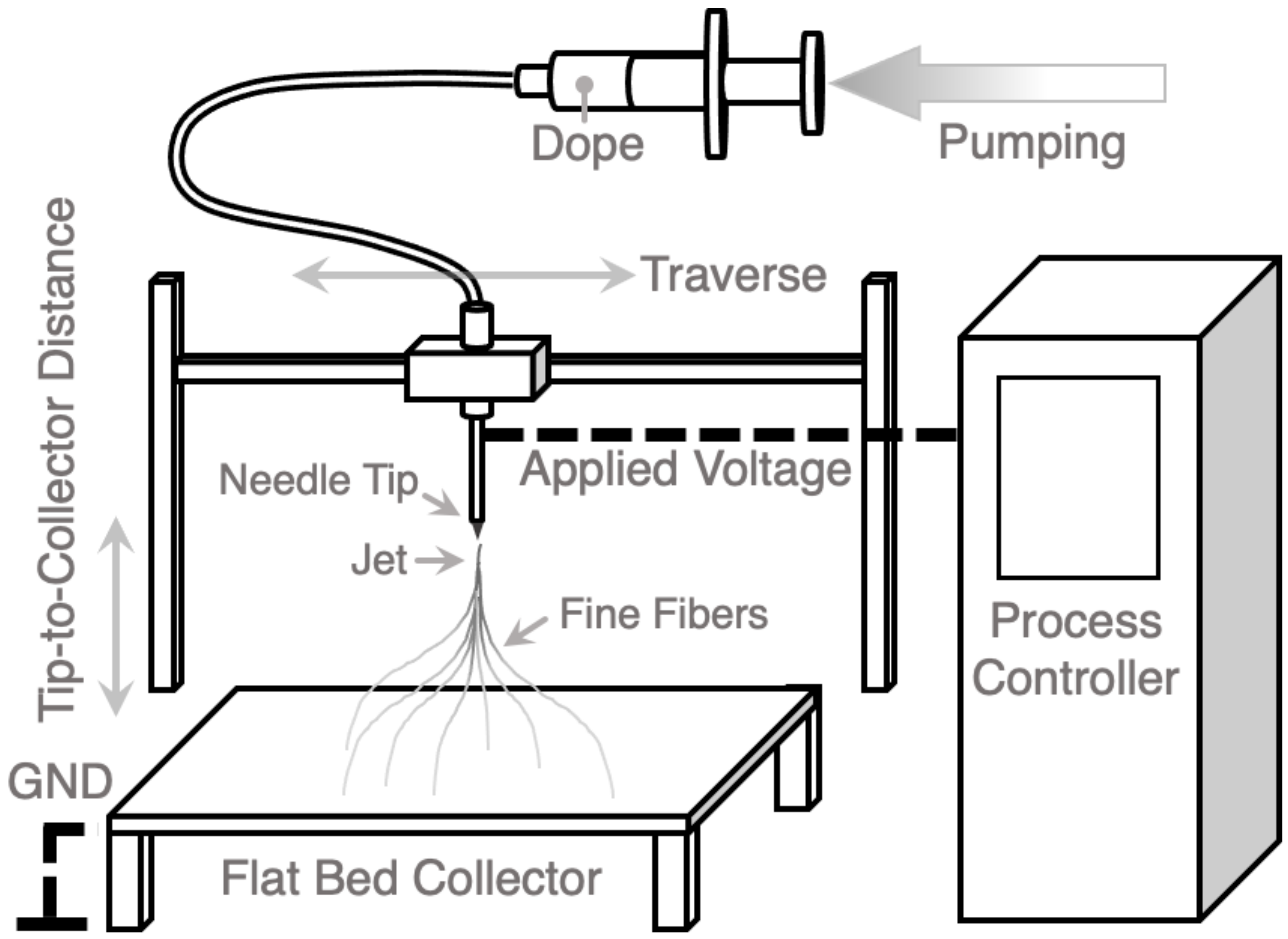
2.5. Electrospinning
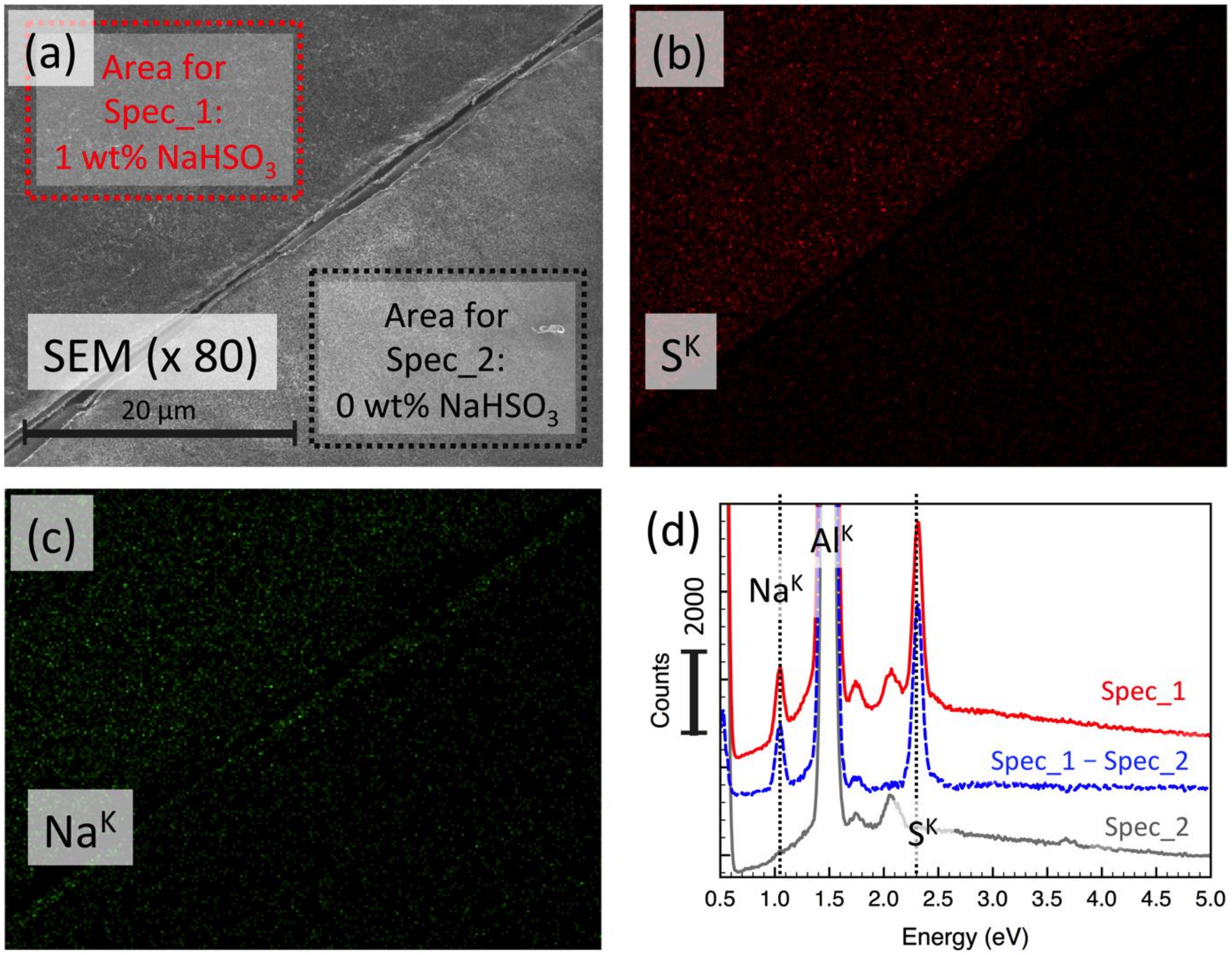
2.6. Scanning Electron Microscopy and Elemental Mapping
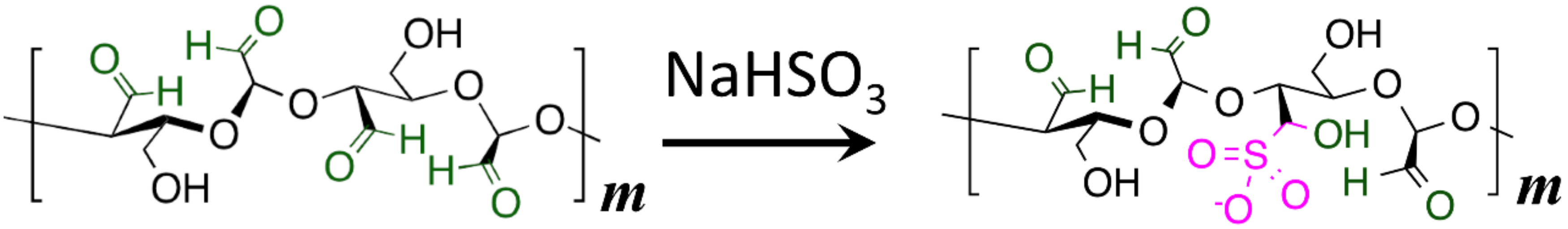
2.7. Post-Spun Treatment of Electrospun Non-Woven Fabrics (ESNWs)
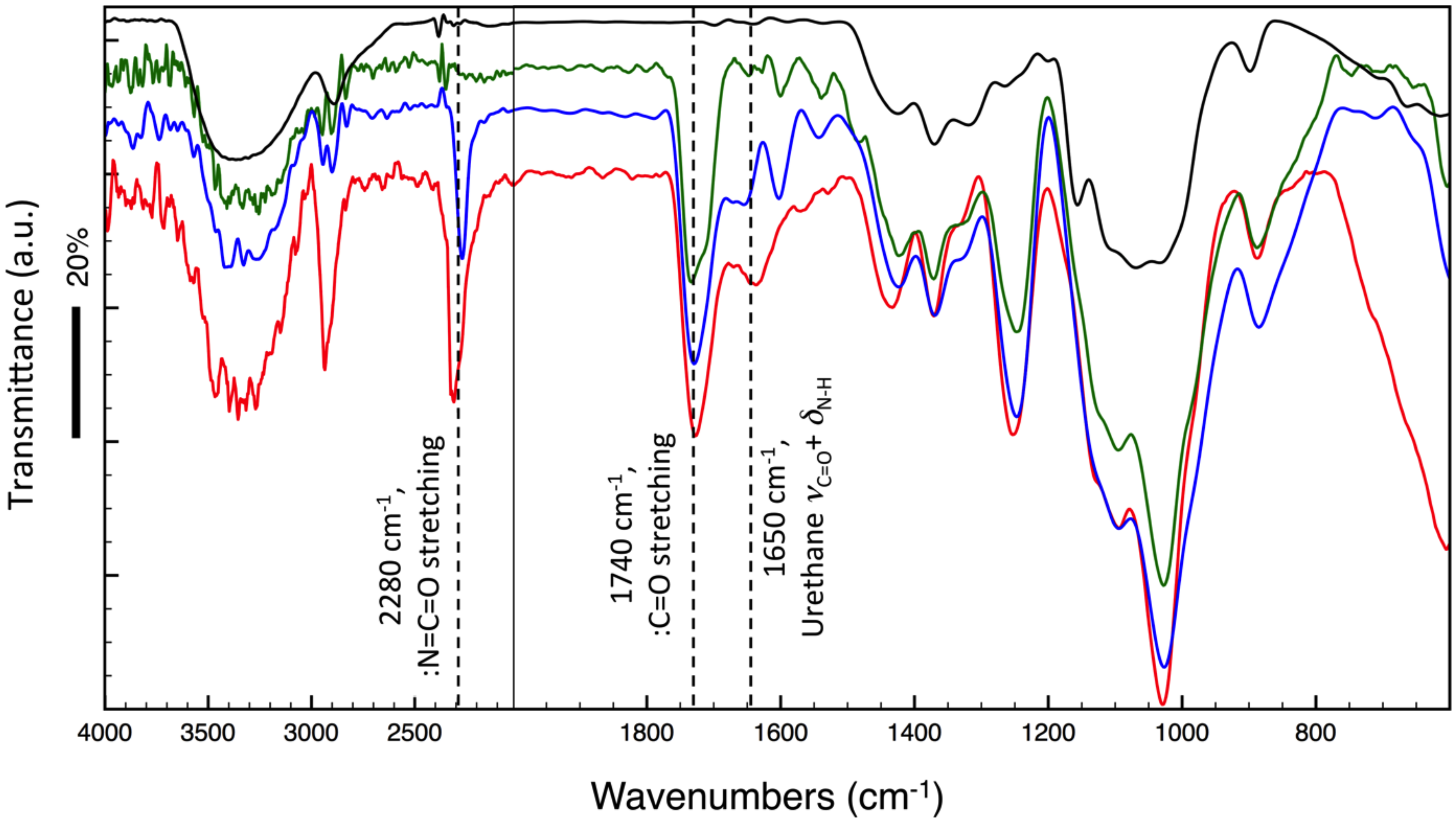
2.8. Vibrational Spectra
3. Results and Discussion
3.1. Water-Soluble DAC
3.2. Preparation of Dialdehyde Cellulose/Poly(Vinyl Alcohol) (DAC/PVA) Dope for Electrospinning
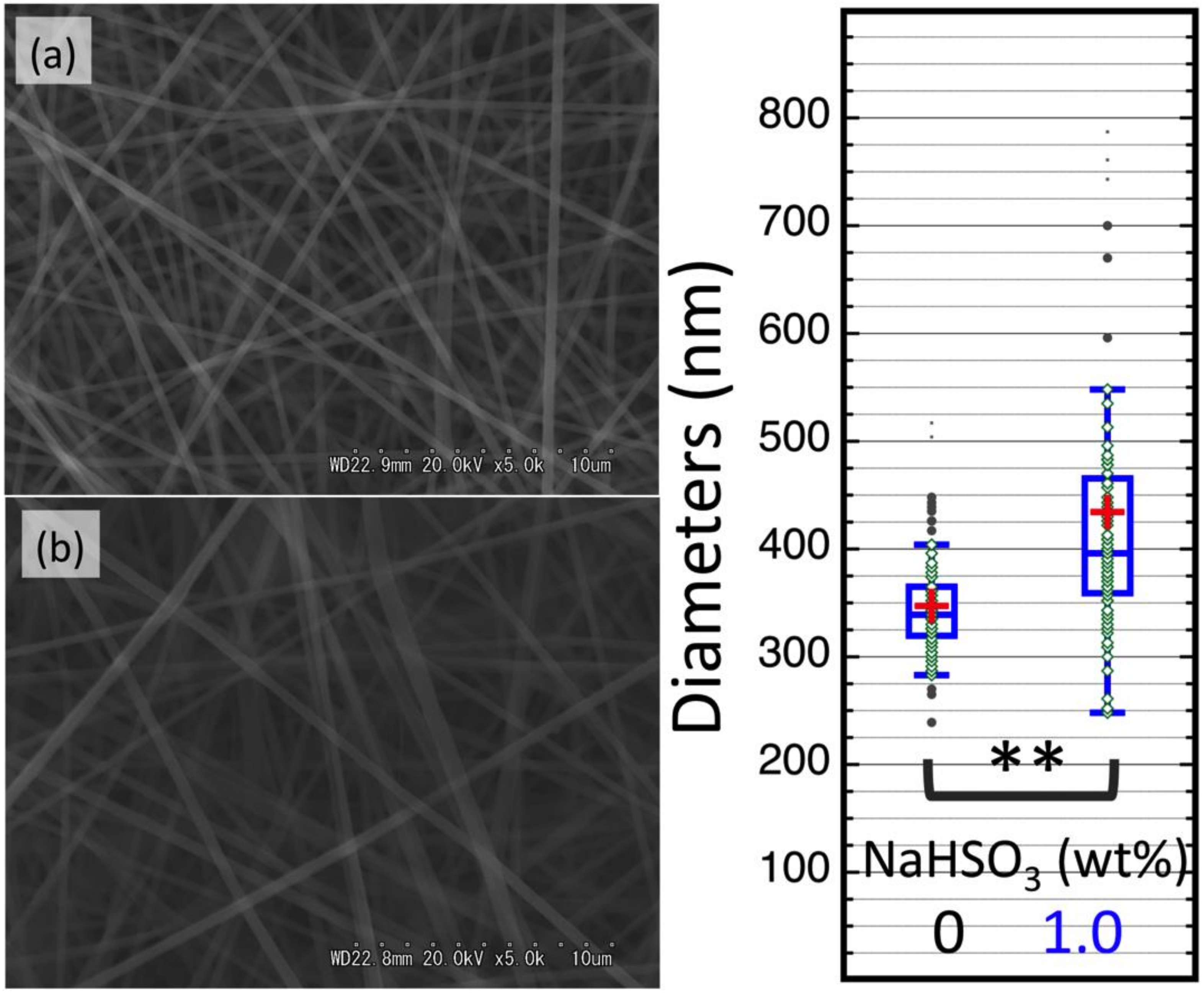
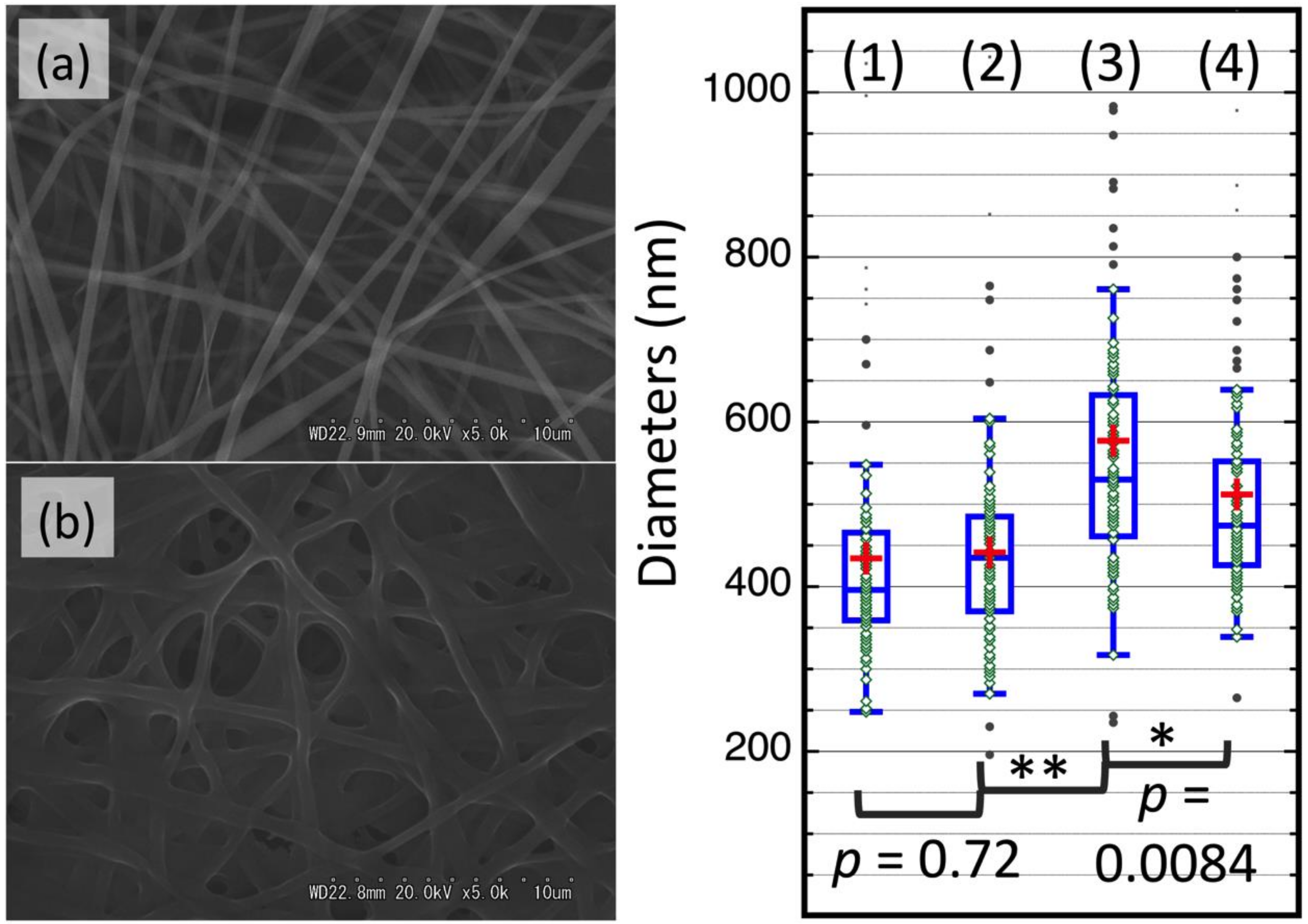
3.3. Characterization of DAC/PVA ESNWs
3.4. Post-Spun Treatment for Insolubilization of DAC/PVA ESNWs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Korotcenkov, G. Nanofibers. Miniat. Fluid. Devices Rapid Biol. Detect. 2013, 6, 33–45. [Google Scholar]
- Huang, Z.-M.; Zhang, Y.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Ohkawa, K.; Minato, K.-I.; Kumagai, G.; Hayashi, S.; Yamamoto, H. Chitosan Nanofiber. Biomacromolecules 2006, 7, 3291–3294. [Google Scholar] [CrossRef]
- Ohkawa, K.; Cha, D.; Kim, H.; Nishida, A.; Yamamoto, H. Electrospinning of Chitosan. Macromol. Rapid Commun. 2004, 25, 1600–1605. [Google Scholar] [CrossRef]
- Du, J.; Hsieh, Y.-L. Cellulose/chitosan hybrid nanofibers from electrospinning of their ester derivatives. Cellulose 2008, 16, 247–260. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, Y.-L. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2119–2129. [Google Scholar] [CrossRef]
- Ohkawa, K.; Hayashi, S.; Nishida, A.; Yamamoto, H.; Ducreux, J. Preparation of Pure Cellulose Nanofiber via Electrospinning. Text. Res. J. 2009, 79, 1396–1401. [Google Scholar] [CrossRef]
- Devarayan, K.; Hanaoka, H.; Hachisu, M.; Araki, J.; Ohguchi, M.; Behera, B.K.; Ohkawa, K. Direct Electrospinning of Cellulose-Chitosan Composite Nanofiber. Macromol. Mater. Eng. 2013, 298, 1059–1064. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa, N.; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef]
- Pierre, G.; Punta, C.; Delattre, C.; Melone, L.; Dubessay, P.; Fiorati, A.; Pastori, N.; Galante, Y.; Michaud, P. TEMPO-mediated oxidation of polysaccharides: An ongoing story. Carbohydr. Polym. 2017, 165, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, K. Nanofibers of cellulose and its derivatives fabricated using direct electrospinning. Molecules 2015, 20, 9139–9154. [Google Scholar] [CrossRef] [PubMed]
- Nypelo, T.; Amer, H.; Konnerth, J.; Potthast, A.; Rosenau, T. Self-standing nanocellulose janus-type films with aldehyde and carboxyl functionalities. Biomacromolecules 2018, 19, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Koprivica, S.; Siller, M.; Hosoya, T.; Roggenstein, W.; Rosenau, T.; Potthast, A. Regeneration of aqueous periodate solutions by ozone treatment: A sustainable approach for dialdehyde cellulose production. ChemSusChem 2016, 9, 825–833. [Google Scholar] [CrossRef]
- Potthast, A.; Schiehser, S.; Rosenau, T.; Kostic, M. Oxidative modifications of cellulose in the periodate system-Reduction and beta-elimination reactions. Holzforschung 2009, 63, 12–17. [Google Scholar] [CrossRef]
- Potthast, A.; Kostic, M.; Schiehser, S.; Kosma, P.; Rosenau, T. Studies on oxidative modifications of cellulose in the periodate system: Molecular weight distribution and carbonyl group profiles. Holzforschung 2007, 61, 662–667. [Google Scholar] [CrossRef]
- Calvini, P.; Conio, G.; Lorenzoni, M.; Pedemonte, E. Viscometric determination of dialdehyde content in periodate oxycellulose. Part I. Methodology. Cellulose 2004, 11, 99–107. [Google Scholar] [CrossRef]
- Rahn, K.; Heinze, T. New cellulosic polymers by subsequent modification of 2,3-dialdehyde cellulose. Cell Chem. Technol. 1998, 32, 173–183. [Google Scholar]
- Syamala Devi, K.; Sinha, T.J.; Vasudevan, P. Biosoluble surgical material from 2,3-dialdehyde cellulose. Biomaterials 1986, 7, 193–196. [Google Scholar] [CrossRef]
- Amer, H.; Nypelo, T.; Sulaeva, I.; Bacher, M.; Henniges, U.; Potthast, A.; Rosenau, T. Synthesis and characterization of periodate-oxidized polysaccharides: Dialdehyde xylan (DAX). Biomacromolecules 2016, 17, 2972–2980. [Google Scholar] [CrossRef]
- Hell, S.; Ohkawa, K.; Amer, H.; Potthast, A.; Rosenau, T. “Dialdehyde cellulose” nanofibers by electrospinning as polyvinyl alcohol blends: Manufacture and product characterization. J. Wood Chem. Technol. 2018, 38, 96–110. [Google Scholar] [CrossRef]
- Kim, U.J.; Wada, M.; Kuga, S. Solubilization of dialdehyde cellulose by hot water. Carbohyd Polym. 2004, 56, 7–10. [Google Scholar] [CrossRef]
- Devarayan, K.; Miyamoto, M.; Hachisu, M.; Araki, J.; Periasamy, V.; Ohkawa, K. Cationic derivative of electrospun non-woven cellulose-chitosan composite fabrics for immobilization of aminoacylase-I. Text. Res. J. 2013, 83, 1918–1925. [Google Scholar] [CrossRef]
- Lukáš, D.; Sarkar, A.; Martinová, L.; Vodsed’álková, K.; Lubasová, D.; Chaloupek, J.; Pokorný, P.; Mikeš, P.; Chvojka, J.; Komárek, M. Physical principles of electrospinning (Electrospinning as a nano-scale technology of the twenty-first century). Text. Prog. 2009, 41, 59–140. [Google Scholar] [CrossRef]
- Ohkawa, K.; Hayashi, S.; Kameyama, N.; Yamamoto, H.; Yamaguchi, M.; Kimoto, S.; Kurata, S.; Shinji, H. Synthesis of Collagen-like sequential polypeptides containing O-phospho-L-hydroxyproline and preparation of electrospun composite fibers for possible dental application. Macromol. Biosci. 2009, 9, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Saitoh, A.; Ohkawa, K. Synthesis of sequential polypeptides containing O-phospho-L-serine. Macromol. Biosci. 2003, 3, 354–363. [Google Scholar] [CrossRef]
- Ohkawa, K.; Saitoh, A.; Yamamoto, H. Synthesis of poly(O-phospho-L-serine) and its structure in aqueous solution. Macromol. Rapid. Comm. 1999, 20, 619–621. [Google Scholar] [CrossRef]
- Ohkawa, K.; Nishibayashi, M.; Devarayan, K.; Hachisu, M.; Araki, J. Synthesis of peptide-cellulose conjugate mediated by a soluble cellulose derivative having beta-Ala esters. Int. J. Biol. Macromol. 2013, 53, 150–159. [Google Scholar] [CrossRef]
- Ohkawa, K.; Hachisu, M.; Devarayan, K.; Araki, J. Design and synthesis of peptide-cellulose conjugate molecules -Aspects from energy/steric profiles-. Fiber. Polym. 2013, 14, 1970–1974. [Google Scholar] [CrossRef]
- Devarayan, K.; Hayashi, T.; Hachisu, M.; Araki, J.; Ohkawa, K. Correlations between steric/thermochemical parameters and O-/N-acylation reactions of cellulose. Carbohyd Polym. 2013, 94, 468–478. [Google Scholar] [CrossRef]
- Devarayan, K.; Hachisu, M.; Araki, J.; Ohkawa, K. Synthesis of peptide-cellulose conjugate mediated by a soluble cellulose derivative having beta-Ala esters (II): Conjugates with O-phospho-L-serine-containing peptides. Cellulose 2013, 20, 365–378. [Google Scholar] [CrossRef]
- Khatri, Z.; Ahmed, F.; Jhatial, A.K.; Abro, M.I.; Mayakrishnan, G.; Kim, I.-S. Cold pad-batch dyeing of cellulose nanofibers with reactive dyes. Cellulose 2014, 21, 3089–3095. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, K.V. Biopolymers for biomedical and pharmaceutical applications: Recent advances and overview of alginate electrospinning. Nanomaterials 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Papaparaskeva, G.; Dinev, M.M.; Krasia-Christoforou, T.; Turcu, R.; Porav, A.S.; Balanean, F.; Socoliuc, V. White magnetic paper with zero remanence based on electrospun cellulose microfibers doped with iron oxide nanoparticles. Nanomaterials 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Massaglia, G.; Frascella, F.; Chiadò, A.; Sacco, A.; Marasso, L.S.; Cocuzza, M.; Pirri, F.C.; Quaglio, M. Electrospun nanofibers: From food to energy by engineered electrodes in microbial fuel cells. Nanomaterials 2020, 10. [Google Scholar] [CrossRef] [PubMed]
| NaHSO3 (wt%) | 0 | 1.0 | 2.5 | 5.0 | 7.5 | 10.0 |
| pH of medium | 6.4 | 3.2 | 3.0 | 3.0 | 2.9 | 2.7 |
| (a) NaHSO3 (µmol) 1 | 0 | 69.1 | 240 | 481 | 721 | 961 |
| (b) Aldehyde (µmol) 1 | 561 | 562 | 553 | 551 | 562 | 558 |
| Molar ratio %(a/b) | 0 | 17.1 | 43.5 | 87.1 | 128 | 172 |
| Status (after 24 h) | Gel | T.L. 2 | Aggr. 3 | Aggr. | Aggr. | Aggr. |
| Appearances |  | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hell, S.; Ohkawa, K.; Amer, H.; Potthast, A.; Rosenau, T. A General Protocol for Electrospun Non-Woven Fabrics of Dialdehyde Cellulose and Poly(Vinyl Alcohol). Nanomaterials 2020, 10, 671. https://doi.org/10.3390/nano10040671
Hell S, Ohkawa K, Amer H, Potthast A, Rosenau T. A General Protocol for Electrospun Non-Woven Fabrics of Dialdehyde Cellulose and Poly(Vinyl Alcohol). Nanomaterials. 2020; 10(4):671. https://doi.org/10.3390/nano10040671
Chicago/Turabian StyleHell, Slavica, Kousaku Ohkawa, Hassan Amer, Antje Potthast, and Thomas Rosenau. 2020. "A General Protocol for Electrospun Non-Woven Fabrics of Dialdehyde Cellulose and Poly(Vinyl Alcohol)" Nanomaterials 10, no. 4: 671. https://doi.org/10.3390/nano10040671
APA StyleHell, S., Ohkawa, K., Amer, H., Potthast, A., & Rosenau, T. (2020). A General Protocol for Electrospun Non-Woven Fabrics of Dialdehyde Cellulose and Poly(Vinyl Alcohol). Nanomaterials, 10(4), 671. https://doi.org/10.3390/nano10040671






