1. Introduction
Friction-related reductions in energy consumption in friction joints and the prevention of component wear in these joints under adverse conditions (e.g., low temperatures, aggressive/oxidizing atmosphere) are important research problems in space engineering, cryogenics, micromechanics, and other fields [
1,
2,
3,
4]. These problems are solved by using organic/liquid lubricants or by applying solid lubricant coatings if liquid lubricants cannot be used. The most-studied tribological properties (under adverse low-temperature conditions) are molybdenum disulfide (MoS
2) solid lubricant coatings [
5,
6,
7]. A decrease in temperature during sliding friction in a vacuum did not have a noticeable effect on antifriction properties; moreover, it can reduce the volume of material removed due to wear [
8]. The tribological properties of MoS
2-based coatings can, however, significantly worsen under the influence of aggressive components (oxygen) fed into the vacuum chamber holding the sample [
9,
10].
The tribological properties of MoS
2-based solid lubricant coatings and other transition metal dichalcogenides have been extensively studied over the past 30 years. Research in this area has looked for ways to increase the wear resistance of solid lubricant coatings at different contact loads and over a relatively wide range of temperatures and air humidity. Moreover, researchers have sought to find fundamentally new forms/structural states that can lead to new properties [
11,
12,
13,
14]. The testing temperature range has been, as a rule, between room temperature and ~500 °C. In most cases, coatings were applied through ion (magnetron) sputtering [
10,
11,
12]. The structural and chemical state properties of these coatings have been achieved through pulsed laser deposition (PLD) [
15,
16,
17] and liquid-based electrodeposition [
18].
The most common structural state of deposited coatings is “amorphous”, which is generally assumed to form from nanoclusters with a lamellar structure typical of the 2H-MoS
2 phase [
19,
20,
21,
22]. Friction sliding is associated with the tribo-induced crystallization of such coatings. During this process, a tribo-layer with enhanced lamellar structure ordering forms on the coating surface in the friction zone [
22]. Most studies that have obtained Mo/W/S
x/Se
x coatings have done so with a chalcogen-to-metal ratio within ~1 <
x ≤ 2, and have tested these coatings for tribological properties. When the mentioned ratio declines, the coefficient of friction either decreases or grows as the composition of the coatings approaches stoichiometric values [
23,
24]. The nature of this dependence is affected to some degree by the structural state of the coatings (the texture).
In general, an MoS
x material with an amorphous structure that is obtained through physical vapor deposition and chemical synthesis has an S/Mo element ratio within a broader range (~1 ≤
x ≤ 10) [
25,
26]. The concentration of S atoms affects the formation of local atomic packing into the cluster units that form the MoS
x amorphous compounds [
26,
27,
28]. In the
Supplementary Materials (
Figure S1), we consider the possible variants for atomic packing and chemical bond organization in such nanoclusters. One of the main Mo
3–S-type nanostructures consists of clusters (Mo
3S
6, Mo
3S
9, Mo
3S
13, and Mo
3S
12), where three Mo atoms form a triangle and are held in this configuration by chemical bonds with S atoms located between them [
27,
28,
29,
30]. When the concentration of S atoms changes, the chemical bond organization may change both within and between Mo
3–S clusters.
To study atomic packaging in materials with an MoS
3 composition, the Mo–S
3 (-Mo-3S-Mo-3S-) linear chain model (Hibble et al. [
31,
32]) was employed alongside the model outlined above (Weber et al. [
30]). The structural element of this model is an MoS
6 cluster, where a Mo atom is surrounded by 6 S atoms. S
2− and S
22− ligands mold the Mo atoms into a curved chain. MoS
3-based nanomaterials may exhibit better antifriction behavior at normal temperatures and humidity when they are in the form of coatings or additives (to hydrocarbon lubricants) [
33,
34,
35]. The tribo-induced formation of a tribo-film with a 2H-MoS
2 lamellar structure was found on the contact surface of these nanomaterials in normal environmental conditions (in terms of sliding friction). The tribological properties of MoS
3 and other MoS
x>3 coatings have not been sufficiently studied in low-temperature adverse conditions.
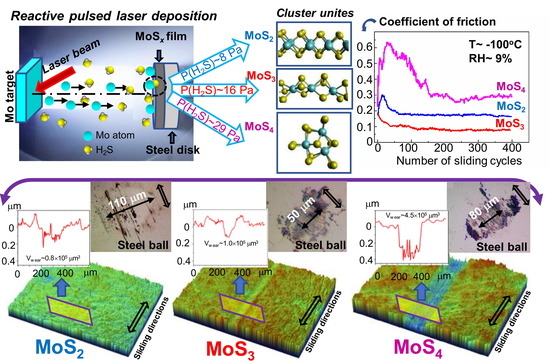
In the present work, we studied the possibility of applying laser-based processes to obtain sufficiently smooth and uniform low-friction nanocoatings with different sulfur contents (MoS2, MoS3, and MoS4). We determined the optimal structural and chemical states of clustered-type MoSx coatings, i.e., with the lowest coefficient of friction and most resistance to wear during sliding friction in an oxidizing environment at low temperatures. To prepare the thin-film coatings, a technique involving reactive pulsed laser deposition (RPLD) of laser-ablated plumes from the Mo target in reactive H2S gas was developed. Special deposition conditions (substrate temperature, laser fluence, hydrogen sulfide pressure, and distance from the substrate to the target) were selected to ensure that the growing coatings were bombarded by laser plasma ions. Ion bombardment of the growing coatings facilitated the formation of sufficiently dense amorphous structures with good adhesion to the steel substrate.
Analyses of the structure and chemical states of the elements in the obtained RPLD MoSx coatings (2 ≤ x ≤ 4) showed that sulfur content had an important effect on the local packing of atoms in the clustered-type structures of these coatings. An increase in sulfur content facilitated the transformation of the clustered-type structure in such a way that the combination of layered MoS2 clusters with weakly ordered Mo–S3/Mo3–S clusters was replaced by a polymer-like structure consisting of Mo3S12/Mo3S13 cluster units. It was apparent that the type of cluster and the characteristics of atomic packing had an important effect on the tribological properties of amorphous MoSx coatings at a low temperature in an oxidizing medium.
Comparative analyses of MoS2, MoS3, and MoS4 thin-film coatings showed that the best antifriction properties in adverse sliding friction conditions (a temperature of −100 °C and an environment containing water and oxygen molecules) were observed for MoS3 films composed of disordered Mo–S3/Mo3–S cluster units. In that case, the wear of the counterbody (a steel ball) when it slid over the surface of the MoS3 coating was at its lowest. During the sliding of the counterbody over MoS2 films containing the layered MoS2 cluster units, the removal of material from the wear track was minimal; however, the coefficient of friction and the wear of the counterbody were significantly higher than with MoS3 films. The tribological properties of MoS4 coatings that contained locally ordered Mo3S12/Mo3S13 cluster units were unsatisfactory. To explain these differences in the tribological properties of the coatings, we studied the tribo-induced modification of clustered-type coatings. Furthermore, we analyzed the mechanisms of structure modification due to tribo-impact and exposure to oxygen and water; these influences and their impacts on the tribological characteristics of the coatings were discussed.
It is important to note that homogeneous/single-layer MoSx coatings have a narrow scope, since they do not withstand a high load. Therefore, it is important to create nanolayered or nanocomposite coatings that contain an MoSx nanophase with enhanced tribo-characteristics. Our work thus focused on the study of single-layer MoSx coatings in order to then proceed to create MoSx-containing coatings with a more complex architecture. Previous studies have shown that nanolayered coatings containing alternating ultrathin MoSx layers (with an optimal concentration of sulfur) and diamond-like carbon (DLC) layers can exhibit a higher tribological performance during friction at a temperature of −100 °C in an oxidizing environment (compared to single-layer MoSx or DLC coatings). In our study, nanolayered coatings which contained an MoS3 solid lubricant demonstrated the best antifriction properties in complicated conditions.
2. Materials and Methods
Nd/YAG (yttrium aluminum garnet) laser radiation at a wavelength of 1064 nm was used for the ablation of the Mo target. The duration of laser pulses was 15 ns, with an energy of ~85 mJ. The laser pulse frequency was 25 Hz. A laser fluence of ~20 J/cm
2 was chosen to minimize droplet formation during the pulsed laser ablation of the Mo target. A standard configuration of reactive pulsed laser deposition was used to obtain the MoS
x thin-film coatings. The angle between the laser beam and the target surface was ~45°. The substrate was placed parallel to the target surface, and thus, perpendicular to the laser plume flow. The distance between the Mo target and the substrate was ~3.5 cm. The substrate was kept at room temperature. The deposition chamber was evacuated to a pressure of 10
−2 Pa. Then, H
2S was injected into the deposition chamber. Film deposition was conducted at three different H
2S pressures. In accordance with preliminary studies by Fominski et al. [
36], H
2S gas pressures of 8, 16, and 29 Pa were selected. The deposition time of the films was 20 min.
The laser plasma generated upon the irradiation of the Mo target was studied through time-of-flight spectrometry. To collect the time-of-flight spectra of Mo ions, a Langmuir plasma probe was installed 3.5 cm away from the Mo target. A probe diameter of 0.8 cm was maintained at a bias of –40 V. This bias voltage was sufficient to reject all plasma electrons and obtain a pure ion signal. The collected ion signals were used to assess the Mo ion fluxes that could influence the film growth.
The deposition substrate was polished on 95Cr18 stainless steel discs (a C content of 0.95% and a Cr content of 18%). To determine the thicknesses of the MoSx films, the substrate was pressed tightly against the shield, which was removed after coating deposition. Then the thickness was measured using optical profilometry.
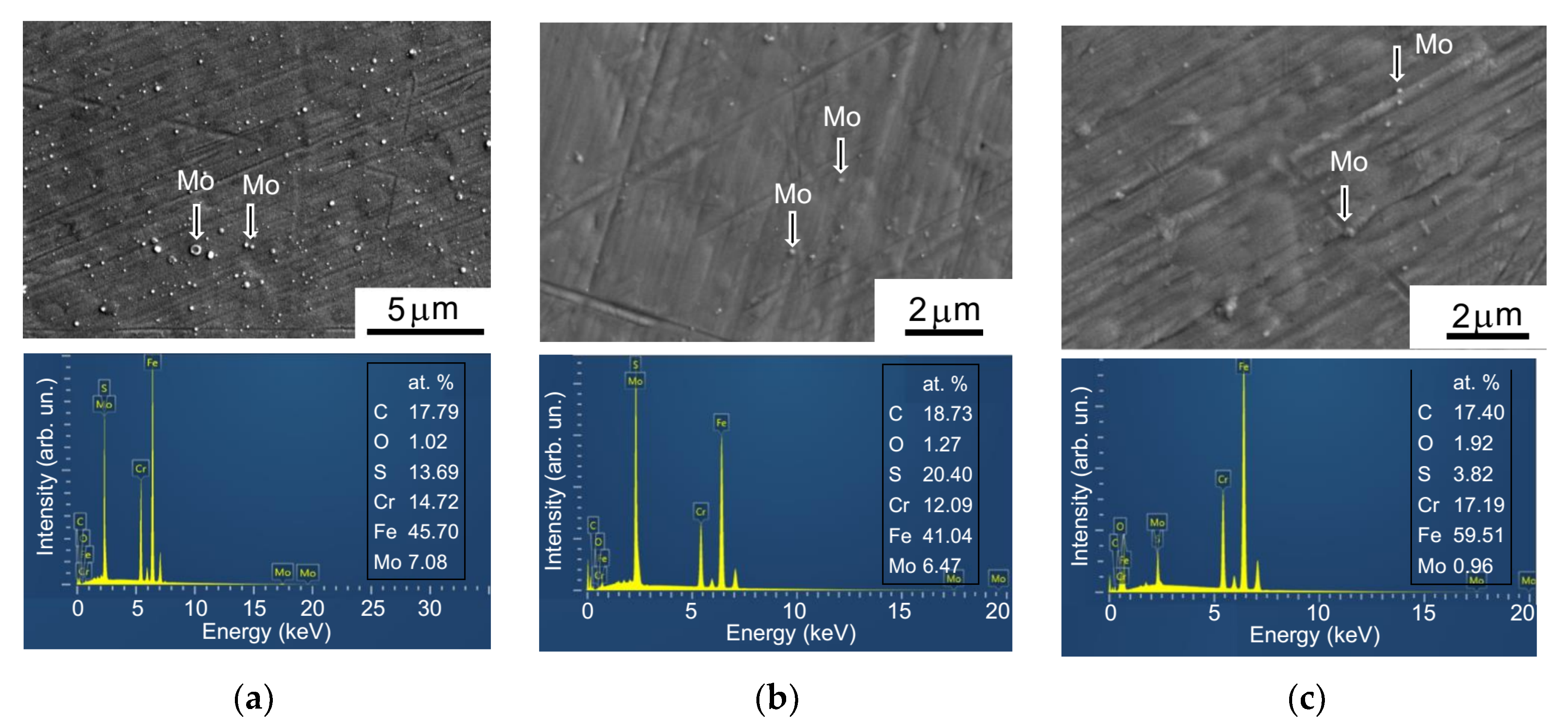
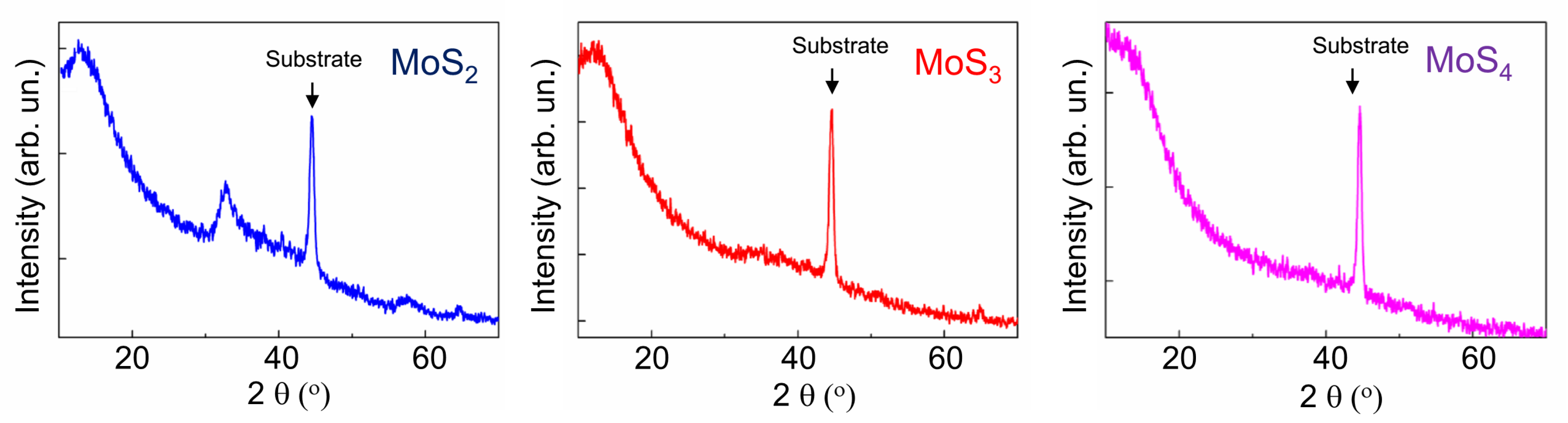
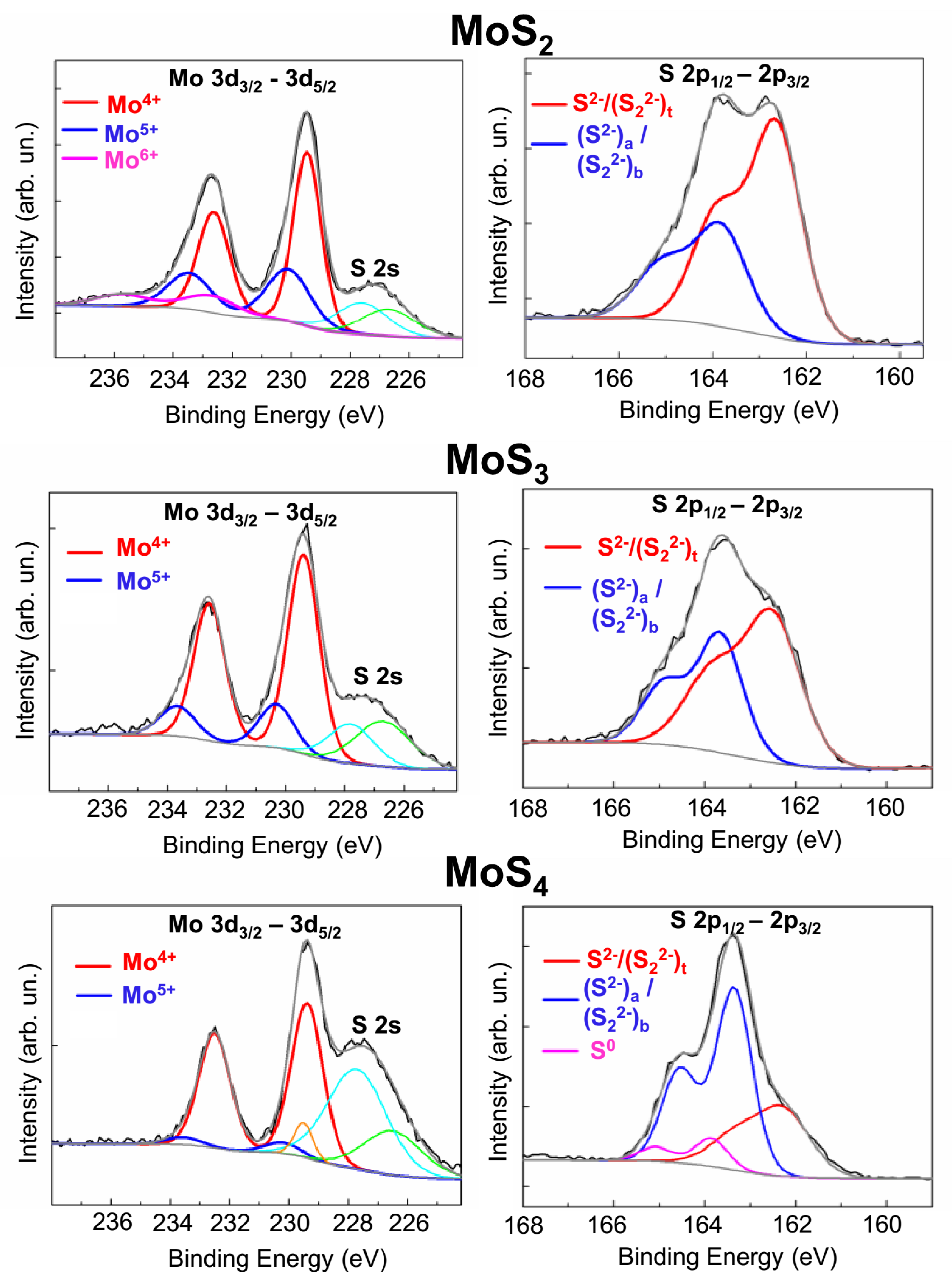
The surface morphologies of MoSx coatings and their chemical compositions were studied by scanning electron microscopy and by energy-dispersive X-ray spectroscopy (SEM/EDS, Tescan LYRA 3) both before and after tribo-testing. To determine the S/Mo atom content ratio, thin MoSx films were deposited on the polished Si substrates for 2 min. These samples were studied using Rutherford backscatter spectroscopy (RBS) on He ions (an ion energy of 1.5 MeV, a scattering angle of 160°, and an ion beam diameter of 100 μm). Mathematical modeling of the RBS spectra was performed using the SIMNRA program. The crystal structure of the coatings deposited on the steel substrates was examined using grazing incidence (10°) X-ray diffraction (XRD) with Cu Kα radiation (λ = 0.15406 nm) in an Ultima IV (Rigaku) diffractometer. The chemical states of the MoSx coatings were studied by X-ray photoelectron spectroscopy (XPS). XPS spectra were obtained by a Theta Probe Thermo Fisher Scientific spectrometer with a monochromatic Al Kα X-ray source (1486.7 eV) and an X-ray spot size of 400 μm. The photoelectrons had a take-off angle of 50° with respect to the surface plane. The spectrometer energy scale was calibrated using Au4f7/2 core level lines with a binding energy of 84.0 eV. The structure of the coatings before and after tribo-testing was studied by means of micro-Raman spectroscopy (MRS) using a 632.8-nm (He–Ne) laser, where the laser beam cross-section was less than 1 μm. The modes of the MRS spectrum measurements (laser beam intensity, measurement time) were selected in such a way that structural and chemical changes would not occur in the MoSx coatings.
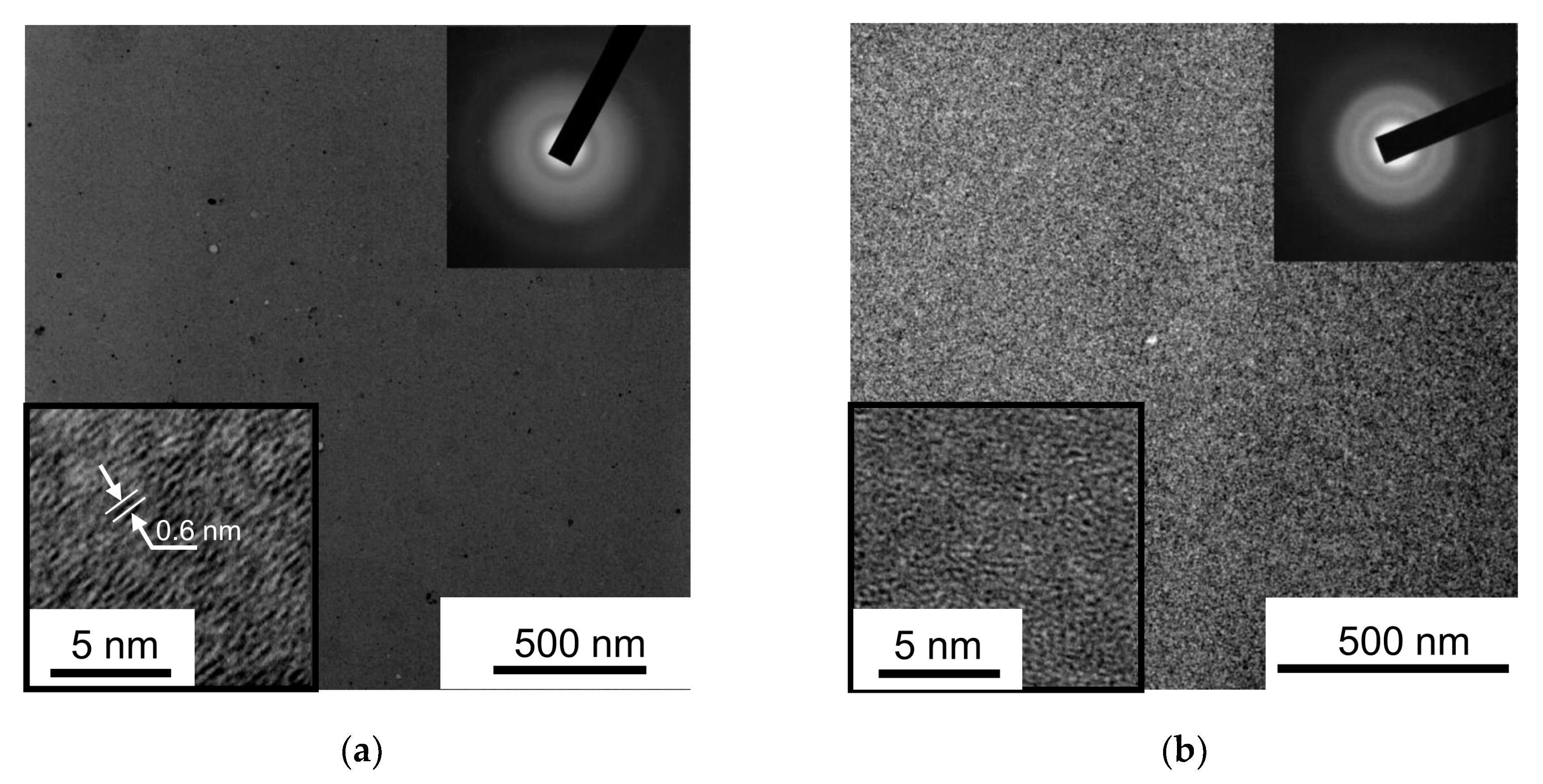
To study the structure of MoSx coatings at the nanoscale level, thin MoSx films were deposited on NaCl substrates. The conditions for obtaining the thin MoSx films were the same as those used for producing the coatings on steel discs. The thin films were studied using transmission electron microscopy (TEM, including high-resolution TEM) and selected area diffraction (SAED) in a JEM-2100 (200 keV, JEOL, Japan) microscope. The samples were submerged in water and later the films were transferred to the microscope to produce a planar image.
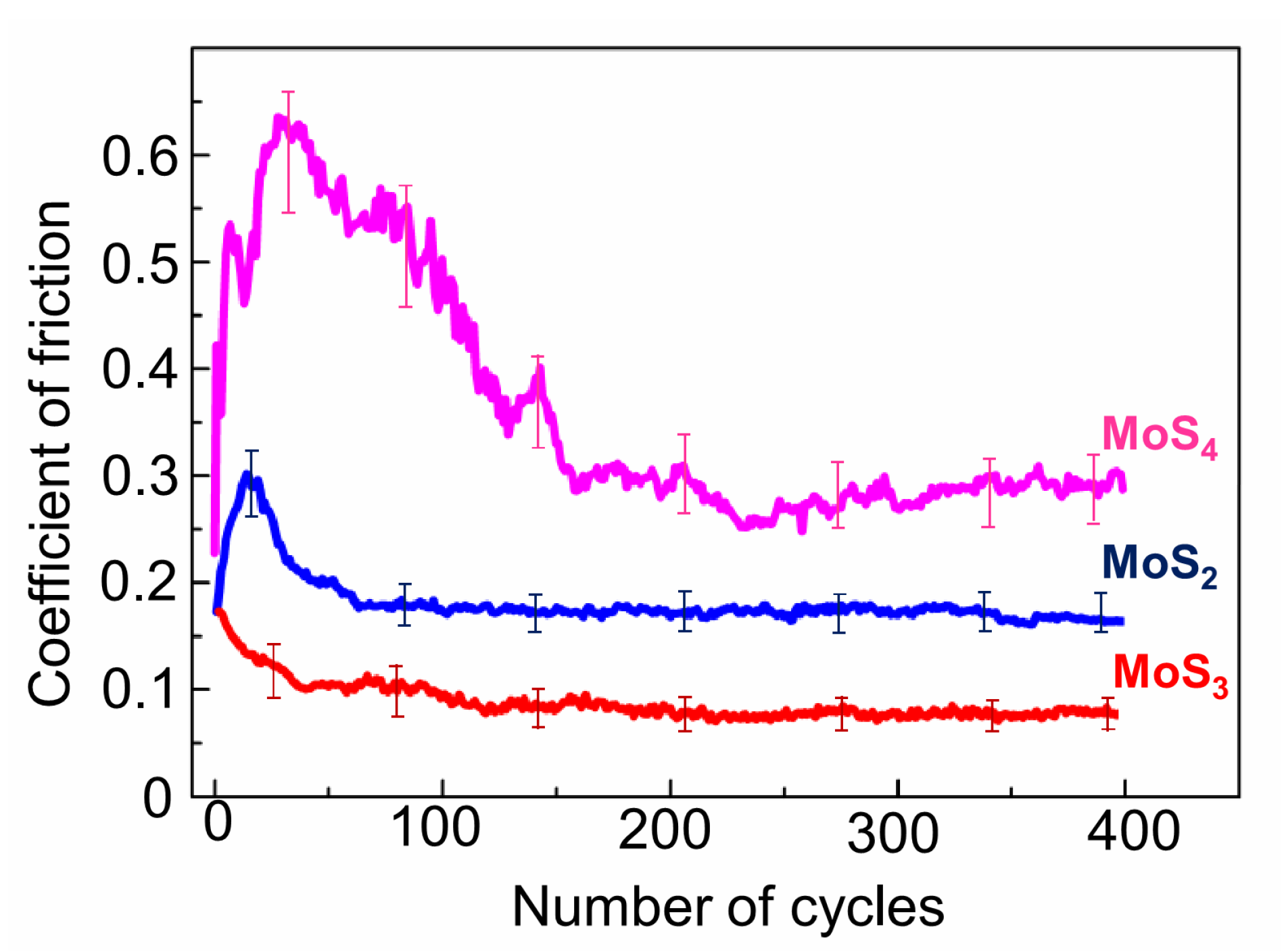
The tribo-testing of the thin-film coatings was carried out on an Anton Paar TRB3 tribometer in reciprocating motion mode, using a steel ball (100Cr6) with a diameter of 6 mm as a counterbody. The load on the ball was 1 N, and the Hertzian contact stress was 660 MPa. The average speed of the ball over a substrate with an MoS
x coating was 1 cm/s. The length of the wear track was 5 mm. The tested steel discs were mounted in a holder cooled with liquid nitrogen. To prevent water vapor condensation and ice layer formation, friction tests were conducted in a mixture of air and argon. The tribometer, which was modified by the authors, is shown in
Figure S2. An optimal argon flow rate allowed us to keep the disc temperature at −100 °C and to control the partial pressure of the air in the gas mixture surrounding the tested sample. The relative humidity (RH) of the air–argon mixture around the tested sample did not exceed RH ≤ 9%. For comparison, tribo-tests were performed at 22 °C in an air–argon mixture (RH ≤ 9%) and in air at RH ~ 50%. Four-hundred cycles of tribo-tests were conducted. The duration of testing revealed the key friction and wear properties of the obtained thin-film MoS
x coatings.
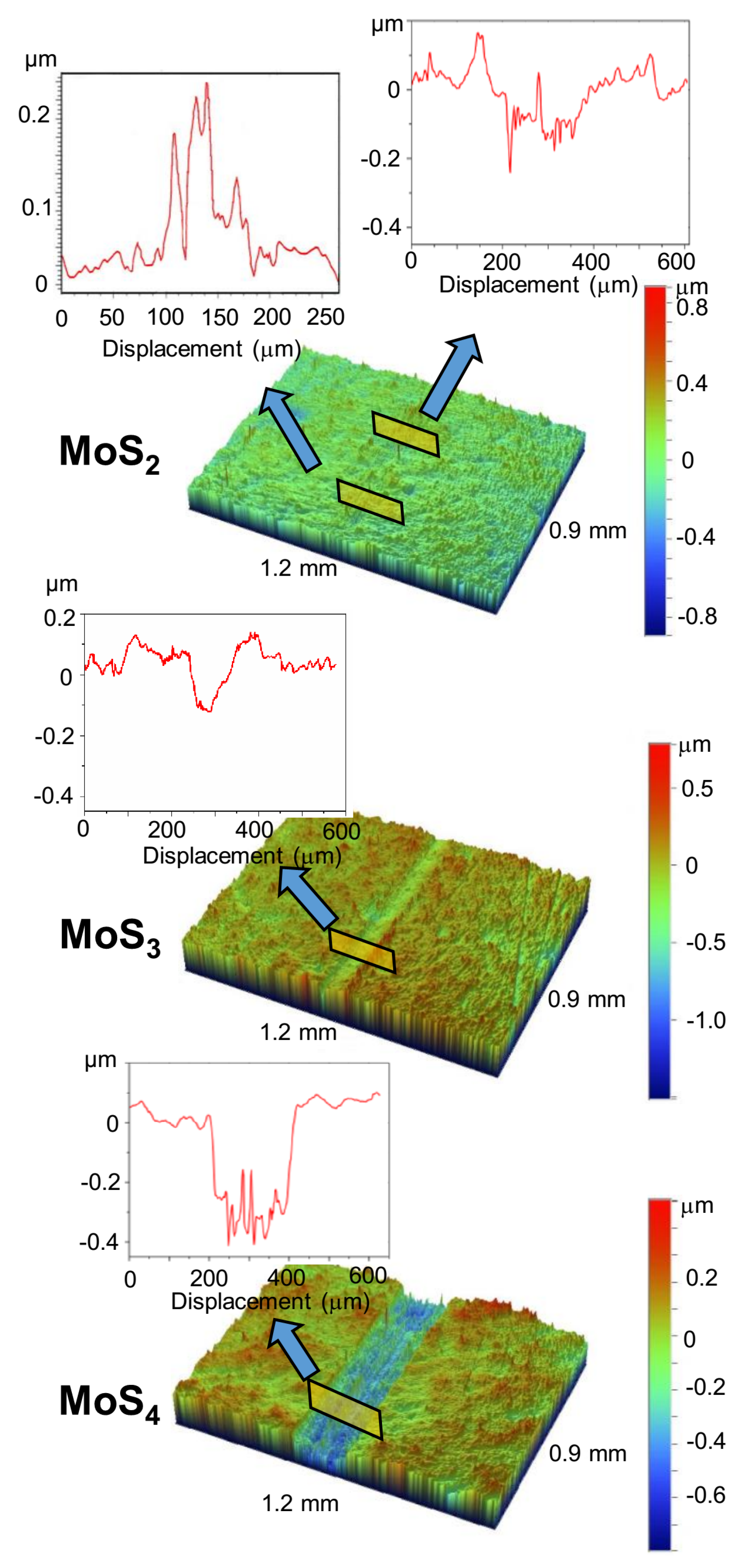
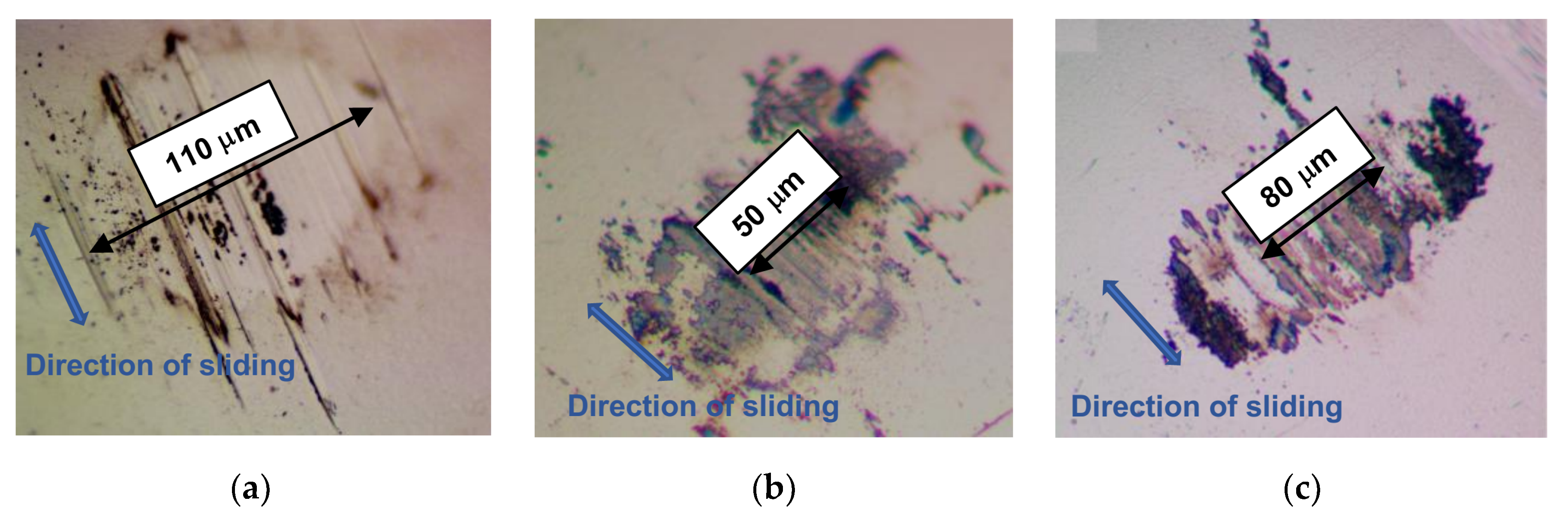
Characterization of the wear tracks on the coatings, the wear scars on the balls, and the wear debris was carried out using a Wyko optical profilometer and optical microscopy. The tests were performed both before and after ultrasonic cleaning of the disk surface. The cleaning made it possible to remove low-adhesion wear particles. After tribo-testing, additional studies of the samples were carried out using SEM, EDS, and MRS techniques.
4. Discussion
Further studies of the wear tracks using EDS confirmed the differences in the wear mechanisms, which were characteristic of MoS
2 and sulfur-rich MoS
x coatings (see
Supplementary Materials, Figures S8–S10). For the MoS
2 coating, when the analyzing electron beam moved across the wear track, the measurements of the intensity of the Mo Lα and S Kα peaks did not reveal any noticeable change in the intensity either in the region where the counterbody slid or at the borders of the track. Thus, as it barely moved from the track, the coating material was not transferred to the boundaries during tribo-testing. For the MoS
3 and MoS
4 coatings, the intensity of the Mo Lα and S Kα peaks markedly decreased in the ball-sliding region and increased at the boundaries of the track. These changes can be explained by both the transfer of material from the track to its boundaries in the course of wear and by the deformation of coating material under pressure from the counterbody.
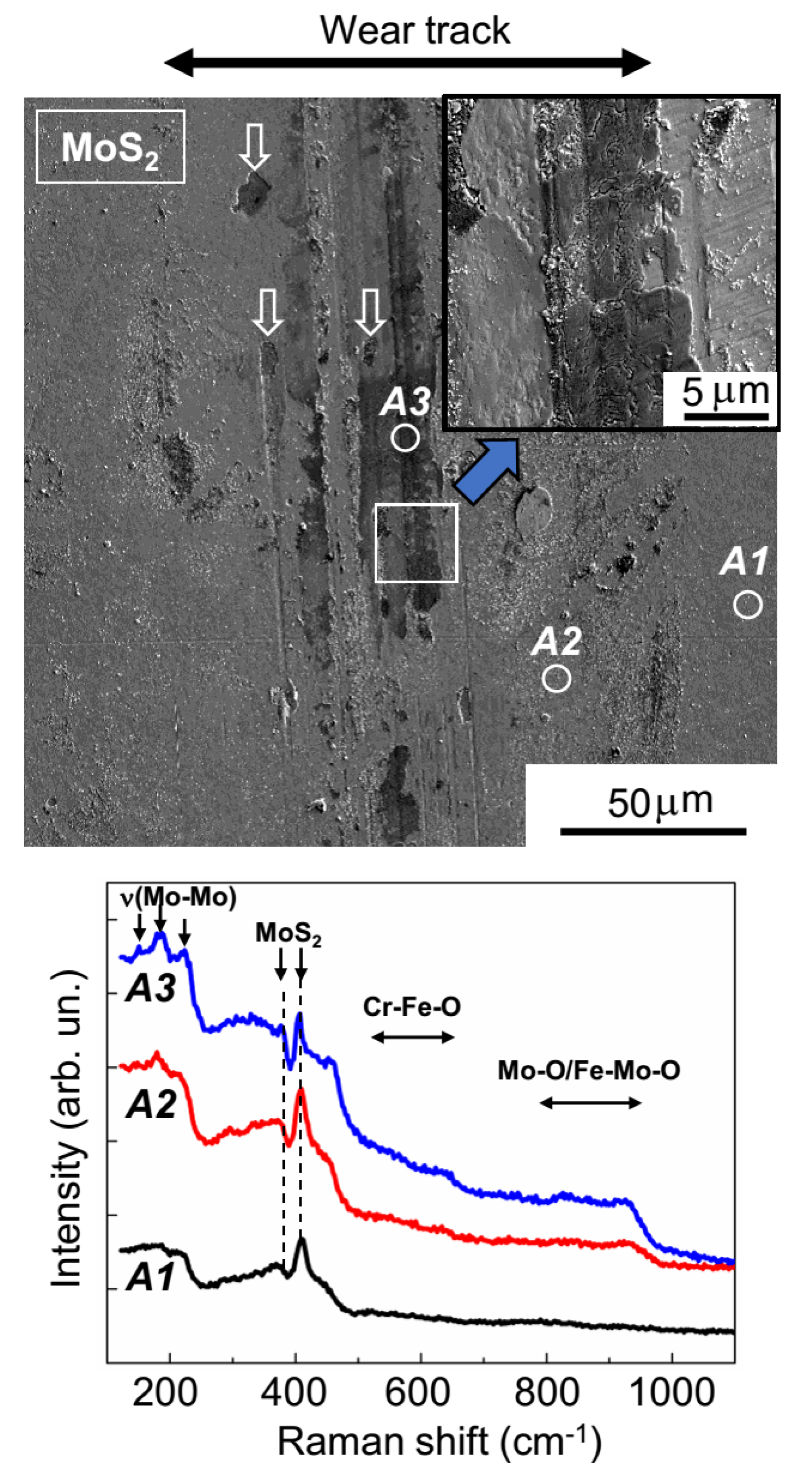
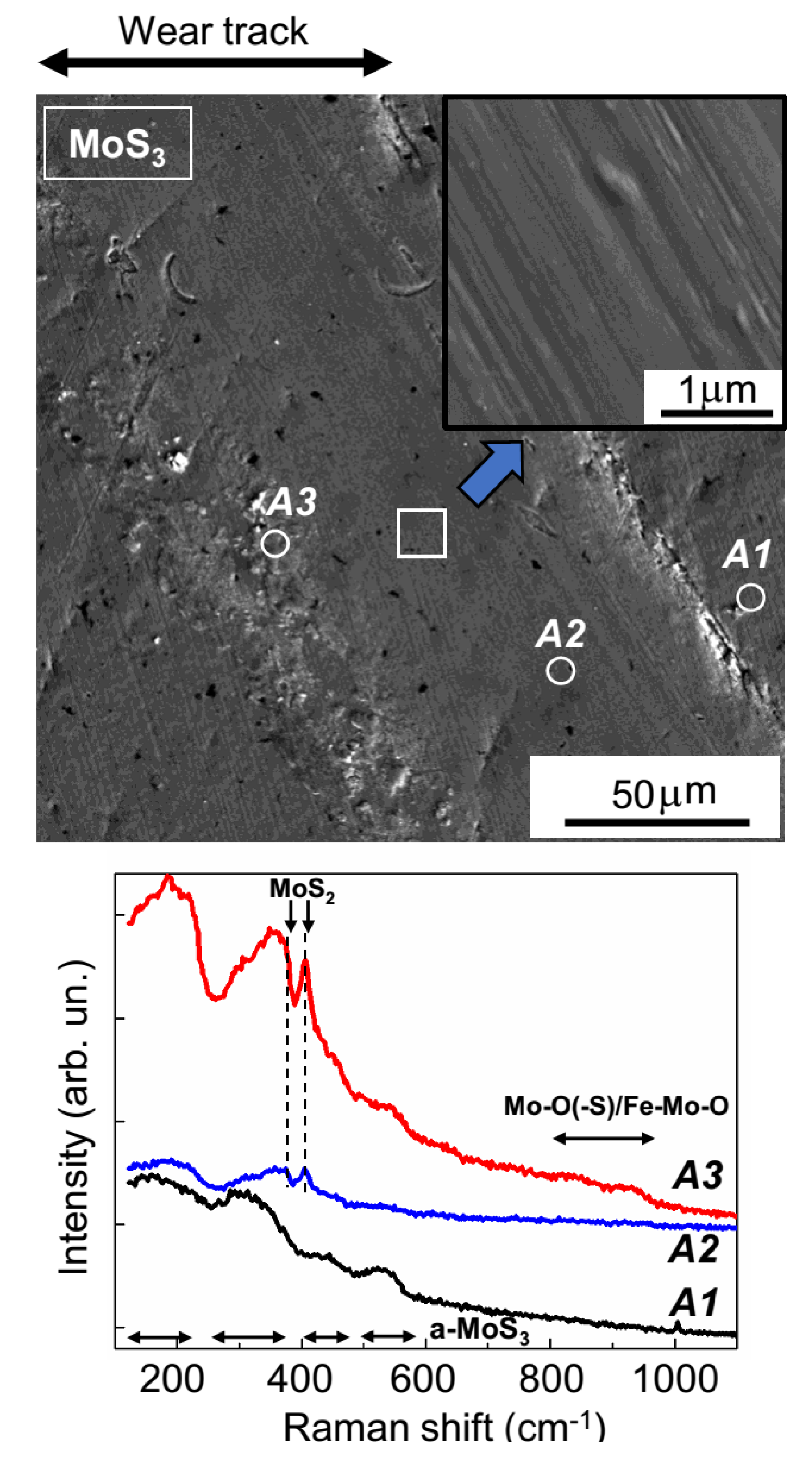
The SEM results (shown in
Figure 8) confirmed the uneven wear of the MoS
2 coating. On the track surface, there were regions that had barely any wear, and there were also areas formed by the deformation of wear particles. In all track areas, the sliding of the counterbody activated the structural rearrangement of the MoS
2 coating material. The MRS spectrum of the unworn coating (area
A1,
Figure 8) was attributable to its amorphous structure, in which there were regions of local atomic ordering into lamellar clusters (layer-type MoS
2); this was evidenced by narrow peaks at 410 and 370 cm
−1, which stood out clearly against the wide bands of the amorphous MoS
2. These peaks were caused by the vibrations of A
1g and E
2g1, respectively, in the hexagonal 2H-MoS
2 structure [
34,
35,
41]. For the MoS
2 coating, in the regions of the sliding track that were not subject to substantial wear (area
A2,
Figure 8), the peak intensity grew significantly at 410 cm
−1; this points to an increased content of lamellar material and/or increased crystallinity in these regions. During the activation of the development of the layered MoS
2 structure, the sliding friction caused the formation of Mo–O and Fe–Mo–O compounds, which was evidenced by the emergence of a broadened band in the Raman spectrum, at 800–980 cm
−1 [
42,
43]. An Fe–Mo–O compound (e.g., with Mo = O stretching in FeMoO
4, the Raman shift was 925 cm
−1) was probably formed during a reaction between molybdenum-based compounds and iron oxides on the surface of the steel ball.
The formation of an Mo–O compound could have been due to the environment containing O
2 and H
2O on the MoS
2 surface layer. In an orthorhombic a-MoO
3 compound, the most intense peak in Raman spectra is located near 820 cm
−1 (an O‒Mo
2 stretching vibration) [
44]. Changing the packing of the MoO
6 octahedra (the main structural element of all polymorphic modifications of MoO
3) may shift this line. For hexagonal h-MoO
3, the strongest peaks are located between 830 and 930 cm
−1 [
45]. Although the h-MoO
3 phase is metastable, when the ball comes into contact with the coating, local, nonequilibrium rapid heating and/or cooling of this material may occur due to friction. Therefore, the possibility that h-MoO
3 nano-inclusions formed cannot be completely excluded. Furthermore, the condensation of H
2O molecules can be accompanied by tribo-induced electrochemical corrosion. In this case, various Mo–O(–S) bonds can form whose vibration band range is between 800 to 1000 cm
−1 [
46].
The relative intensity of the peaks characteristic of Mo–O and Fe–Mo–O peaks significantly increased in the MRS spectrum. This was measured in the region where there was wear debris adhesion (area
A3,
Figure 8). In this spectrum, there were broadened peaks at 530–660 cm
−1. One of the most probable causes of these peaks was the formation of an Fe–Cr–O compound [
47]. The production of this compound in the track is easy to explain, given the relatively intense wear of the steel ball and the effective adhesion (and deformation) of wear debris on the MoS
2 surface in the sliding track region. EDS studies confirmed the pronounced oxygen saturation of some local sections of the tracks that formed on the MoS
2 coating after tribo-testing (
Figure S8). The MRS spectra indicated an improvement in the quality of the local packing of MoS
2 in these regions. This improvement manifested itself both in the narrowing of the main MoS
2 peaks (at 370 and 410 cm
−1) and in the appearance of additional peaks in the range of 150–225 cm
−1 (the ν(Mo–Mo) vibration band) [
46]. Wear debris with a high MoS
2 content could have formed through local delamination of the MoS
2 coating (indicated by arrows on the SEM images in
Figure 8).
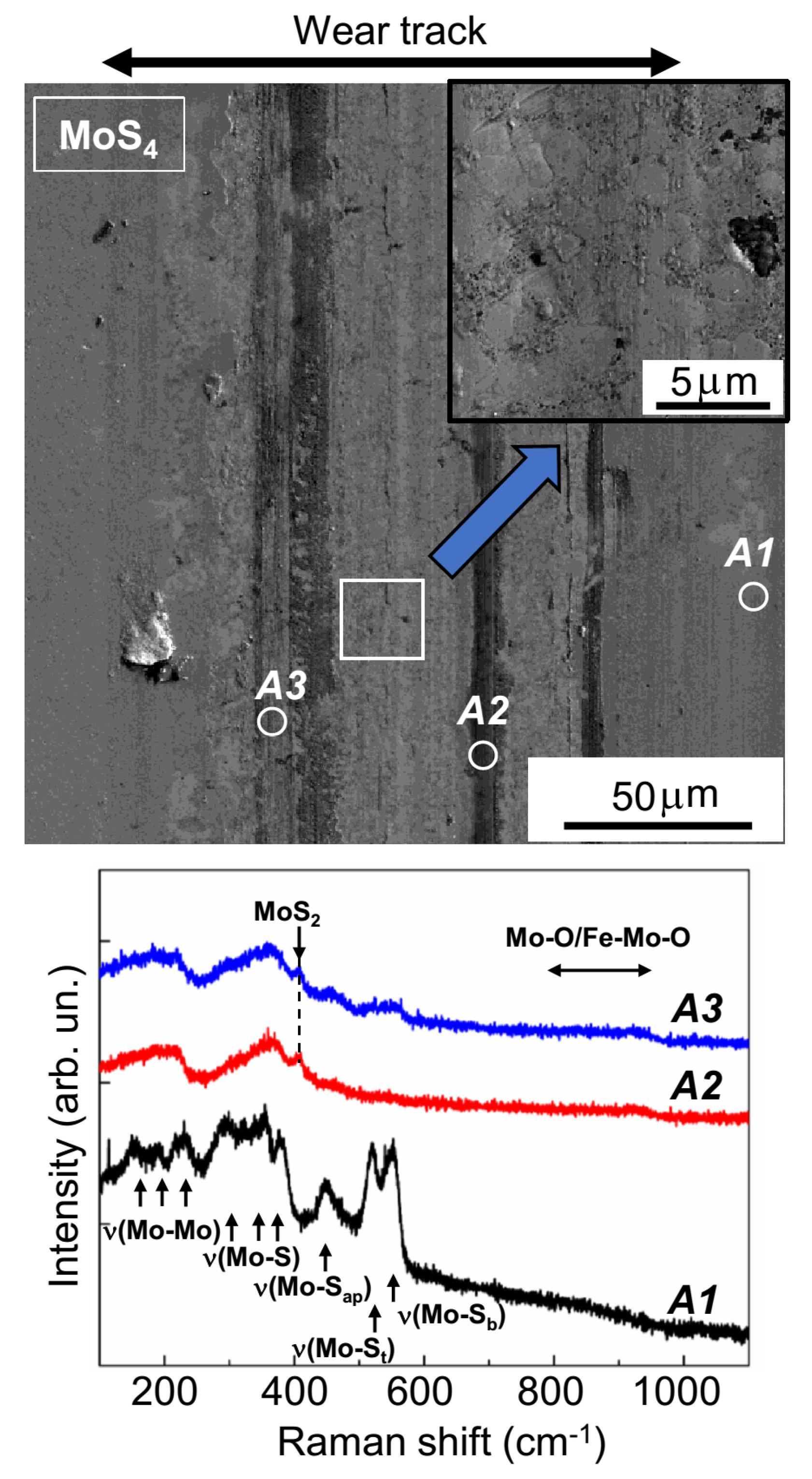
Figure 9 and
Figure 10 show the results from the SEM and MRS studies of wear tracks on the surfaces of MoS
3 and MoS
4 coatings, respectively. The Raman spectrum of pristine MoS
3 coatings (area
A1,
Figure 9) had four broad peaks at 200, 320, 450, and 540 cm
−1. This spectrum is typical of amorphous MoS
x~3 materials that lack qualitative ordering into clusters (Mo
3S
12/Mo
3S
13-type) [
25,
36,
39]. These clusters have narrower peaks that occur at the following vibration modes: ν(Mo-Mo) at ~200 cm
−1, ν(Mo-S)
coupled at ~320 cm
−1, ν(Mo-S
apical) at ~450 cm
−1, ν(S-S)
terminal at ~520 cm
−1, and ν(S-S)
bridging at 540 cm
−1 [
46]. The
Supplementary Materials (
Figure S1) provide further details on the various sulfur ligands in the clusters of the Mo
3S
12/Mo
3S
13-type. In unworn MoS
4 coatings, a spectrum with these peaks as well as other additional peaks was observed (area
A1,
Figure 10). Thus, the MRS studies confirmed a significant difference in terms of local atomic packing in the MoS
2, MoS
3, and MoS
4 coatings.
The central area of the wear track that formed on the MoS
3 coating had a rather smooth surface, indicating the perfect ductility of the coating material. After tribo-testing, sharp peaks appeared at 370 and 410 cm
−1 in the MRS spectrum (area
A2,
Figure 9) over a background of broad bands from the amorphous structure; this indicated the modification of the amorphous structure and the formation of layered-type MoS
2 inclusions in the amorphous MoS
2 phase. No noticeable peaks attributable to Mo–O and/or Fe–Mo–O compounds were visible in the MRS spectrum in the center of the track. Noticeable bands in a frequency range from 800 to 950 cm
−1 were detected in the spectra measured at the track boundaries (e.g., area
A3,
Figure 9). In these areas, the intensity of peaks of the MoS
2 phase increased significantly. Given the EDS results, which indicated an increase in the Mo, S, and O contents at the track boundaries (
Figure S9), it should be assumed that during testing of the MoS
3 coatings, wear particles containing both Mo–O/Fe–Mo–O compounds and sufficiently crystallized MoS
2 nanoparticles accumulated at the track boundaries.
The SEM studies showed that the surfaces of the wear track on the MoS
4 coatings were less smooth than they were on the MoS
3 coatings (
Figure 10). According to the Raman spectra shown in
Figure 10, when sliding, the counterbody caused the formation of layered-type MoS
2 clusters in all track sections, accompanied by the formation of Mo–O and/or Fe–Mo–O compounds (the band at 800–930 cm
−1). The local packing typical of Mo
3S
12 and Mo
3S
13 moieties was completely distorted; this was evidenced by the disappearance of narrow peaks and the appearance of wide bands in the Raman spectra, which were measured in the region where the counterbody slid. EDS studies confirmed the relatively increased and fairly uniform distribution of oxygen atoms over the track surface on the MoS
4 coatings (
Figure S10).
Thus, the sliding friction of the steel counterbody at −100 °C on coatings with different sulfur contents caused the transformation of the amorphous structure and probably the formation of a tribo-film with a layered-type MoS
2 structure. It is well-known that the formation of such tribo-films at the sliding interface ensures sufficient low-friction properties [
34,
41,
48,
49]. The coefficient of friction and the wear resistance of the MoS
2, MoS
3, and MoS
4 coatings, however, differed significantly. All coatings had a rather dense structure. Thus, here it is difficult to accept the common explanation of the influence of an oxidizing environment on the tribological properties of MoS
x-based coatings. One of the main reasons an oxidizing environment has an influence is the penetration of O-containing molecules into the coating through microcracks and structure defects [
50]. It is unlikely the same influence exists in terms of sliding friction on MoS
2, MoS
3, and MoS
4 coatings.
Another important factor affecting the tribological properties of MoS
x coatings is the orientation of basal planes in the MoS
2 lamellar structure with respect to the surface of the coating [
49]. The orientation of reference planes can be seen in A
1g and E
2g1 peak intensities in Raman spectra. In Raman spectra of terrace-terminated MoS
2 films, which have the most qualitative tribological properties, the contribution of the E
2g1 Raman mode increases. In Raman spectra of edge-terminated MoS
2 films, the A
1g Raman mode may be more pronounced. In Raman spectra of most tribo-films, the peak at 370 cm
−1, which is attributable to the E
2g1 Raman mode, is expressed weakly; this could be due to signal overlap from resonance scattering on wear particles [
41]. Here, the Raman spectra of the tribo-films formed on the MoS
4 coatings had virtually no peak at 370 cm
−1, which can be explained by the tribo-film’s specific texture, as the basal planes were oriented mainly perpendicularly to the surface of the coating.
MoS
x structure modification and tribo-film formation can be influenced by the atmosphere during sliding friction, primarily by the concentration of O
2 and H
2O molecules. The role of these reagents can differ [
51,
52]. Additional tribo-testing of the MoS
2, MoS
3, and MoS
4 thin-film coatings was carried out at room temperature and at varying atmospheric humidity. In the
Supplementary Materials, Figures S11–S14 present the results of this testing. The MoS
4 coatings showed unsatisfactory tribological properties under all tribo-testing conditions, which was evident in the high coefficient of friction and fast wear. These results indicate that atomic packing in an amorphous MoS
4 structure could not ensure the effective tribological adaptation of the structure in all friction conditions. Probably, the layered-type MoS
2 clusters detected did not form a continuous tribo-film, but rather, mixed with metal oxide clusters.
The coefficient of friction for the MoS
2 coatings clearly depended on the humidity of the environment (
Figures S11 and S13). The coefficient of friction was increased to 0.15 at RH ~ 50% but did not exceed 0.08 at RH ~ 9%. The wear of the MoS
2 coating, however, was extremely weak in both humidity conditions (
Figures S12 and S14). The coefficient of friction of the MoS
3 coating was less dependent on moisture than was that of the MoS
2 coating. After 400 cycles, the coefficient of friction was 0.1 and 0.14 at RH ~ 9% and RH ~ 50%, respectively. Apparently, an increase in the temperature to 22 °C caused some growth in the coefficient of friction in the MoS
3 coating. A study of the optical images of the wear tracks after tribo-testing at RH ~ 9% and RH ~ 50% did not reveal a significant influence of humidity on the wear of this coating (
Figures S12 and S14).
Thus, the differences in the local atomic packing of the MoS2 and MoS3 coatings caused a noticeable variation in their tribological properties both at low temperatures and at room temperature. The MoS3 coating was associated with the lowest coefficient of friction under adverse conditions, i.e., in the presence of an oxidizing environment at low temperatures and at room temperature. The wear of the MoS2 coating, however, was less pronounced; this was due to the good adhesion of the wear debris to the surface of the MoS2 coating. The adhesion of wear debris to the MoS2 coating on the track could have been due to the fact that the original amorphous MoS2 coating contained MoS2 clusters, which could easily reorient on the surface layer to form terrace-terminated MoS2 tribo-films.
The surfaces of these films interacted weakly with the surfaces of the steel counterbodies, so the exfoliated particles of the tribo-film did not adhere to the ball surface, but rather moved to another region of the track. The layered wear particles interacted with the oxidizing environment at the edges of layers, which caused the formation of metal oxide compounds. These compounds worsened the ductile properties of the MoS
2-containing wear particles and had a negative impact on the coefficient of friction. These impacts increased the wear of the counterbody. As humidity decreased, the efficiency of the oxidation processes declined and the coefficient of friction of the MoS
2 coating significantly diminished. The dependence of the tribological properties on air humidity is quite typical of MoS
2-based coatings [
51,
52,
53,
54]. Even at low humidity (RH ~ 9%), a decrease in temperature to −100 °C promoted the adsorption of H
2O molecules into the coating surface. Thus, the tribological properties of the coating were similar to the properties observed at 22 °C and RH ~ 50%.
Forming an MoS
2 tribo-film on the surface of an amorphous MoS
3 coating requires the removal of excess sulfur and atom transfer, which are the processes that contribute to the formation of a layered-type MoS
2 structure. Lince et al. [
34] assumed that, in the absence of oxygen and water, this is the result of thermally activated pyrolysis of MoS
3. The necessary energy input is supplied by high local temperatures in the contact region due to mechanical energy that causes deformation in the MoS
3 clusters. Reactions leading to the formation of Mo–O–S compounds are more likely in the presence of oxygen. According to the MRS study, these compounds were present in the wear debris of the MoS
3 coating. There were, however, no obvious signs of an oxidation reaction in the coating material on the track. It can be assumed that, in the presence of water, a reaction occurred that is typical of the initial stage of the electrochemical process of hydrogen evolution on amorphous MoS
x≥2 films. In this reaction, excess sulfur is removed because of the formation of H
2S molecules [
27,
28]. The introduction of hydrogen into the MoS
3 structure can lead to the reorganization of chemical bonds and the formation of MoS
2-like clusters [
40]. During the formation of MoS
2 compounds in the area of contact between the MoS
3 coating and the steel counterbody, this compound probably has a very defective structure, and thus it can interact effectively with the surface of the counterbody [
51] and adhere to it. Therefore, in the area of contact between MoS
3 and the steel counterbody, competing processes can take place, i.e., structural rearrangement (MoS
3 to MoS
2) and the adherence of Mo and S atoms to the counterbody. Adhesive interactions between the MoS
3 surface layer and the steel ball can cause wear on this coating.
To activate the H
2S molecule evolution reaction, it is necessary to electrically polarize local regions of the track surface, and thus produce negative potential. MoS
x films have semiconductor properties [
53]. In the contact area between the metal counterbody and the semiconductor, a rapid flow of electrons from the metal to the semiconductor is possible. This flow causes the formation of a built-in negative charge in the local regions of the MoS
3 coatings. This charge repels (OH)
− molecules formed by the dissociative adsorption of H
2O molecules. As a result, the flow of O-containing polarized molecules toward the MoS
3 coating surface in the counterbody contact area may decrease.
A comparison between the tribo-testing results of the MoS3 coatings and the MoS4 coatings showed that the tribo-chemical processes and the structural modification of MoSx materials in the steel counterbody contact area significantly depended on the concentration of sulfur. This concentration largely determined local atomic packing into nanoclusters. Understanding the mechanisms of wear of these materials also requires knowledge of their mechanical properties, which are currently being studied and will be discussed in future papers.