Label-Free Imaging of Single Nanoparticles Using Total Internal Reflection-Based Leakage Radiation Microscopy
Abstract
1. Introduction
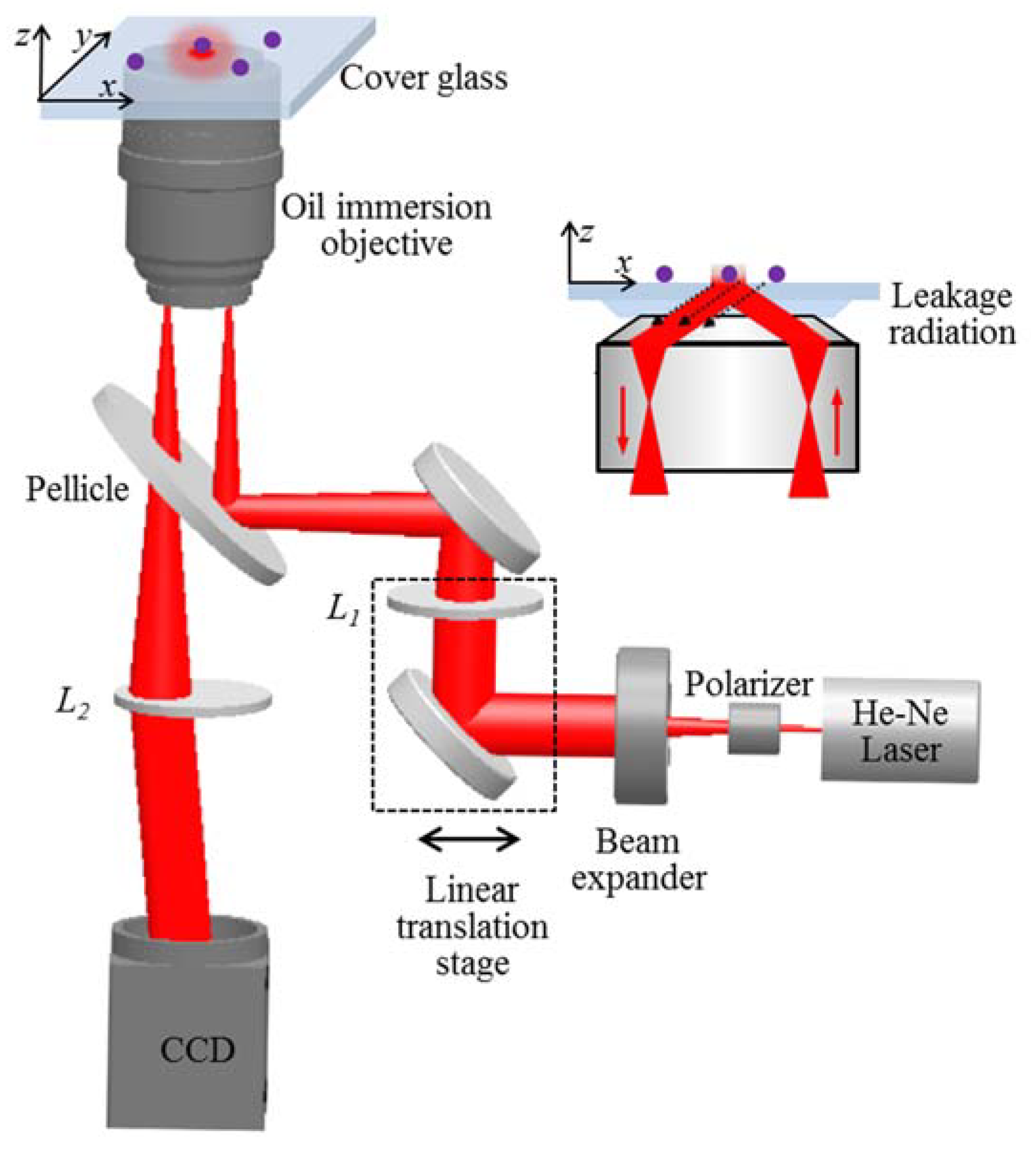
2. Materials and Methods
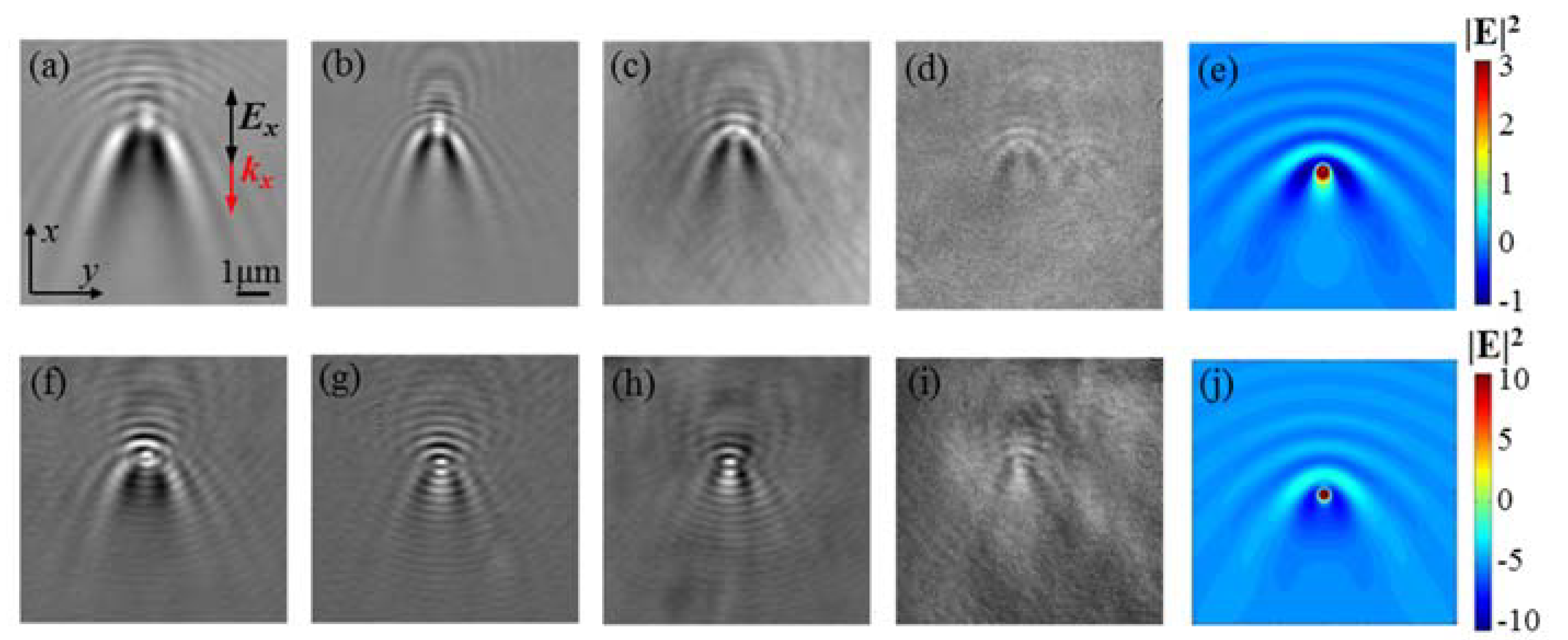
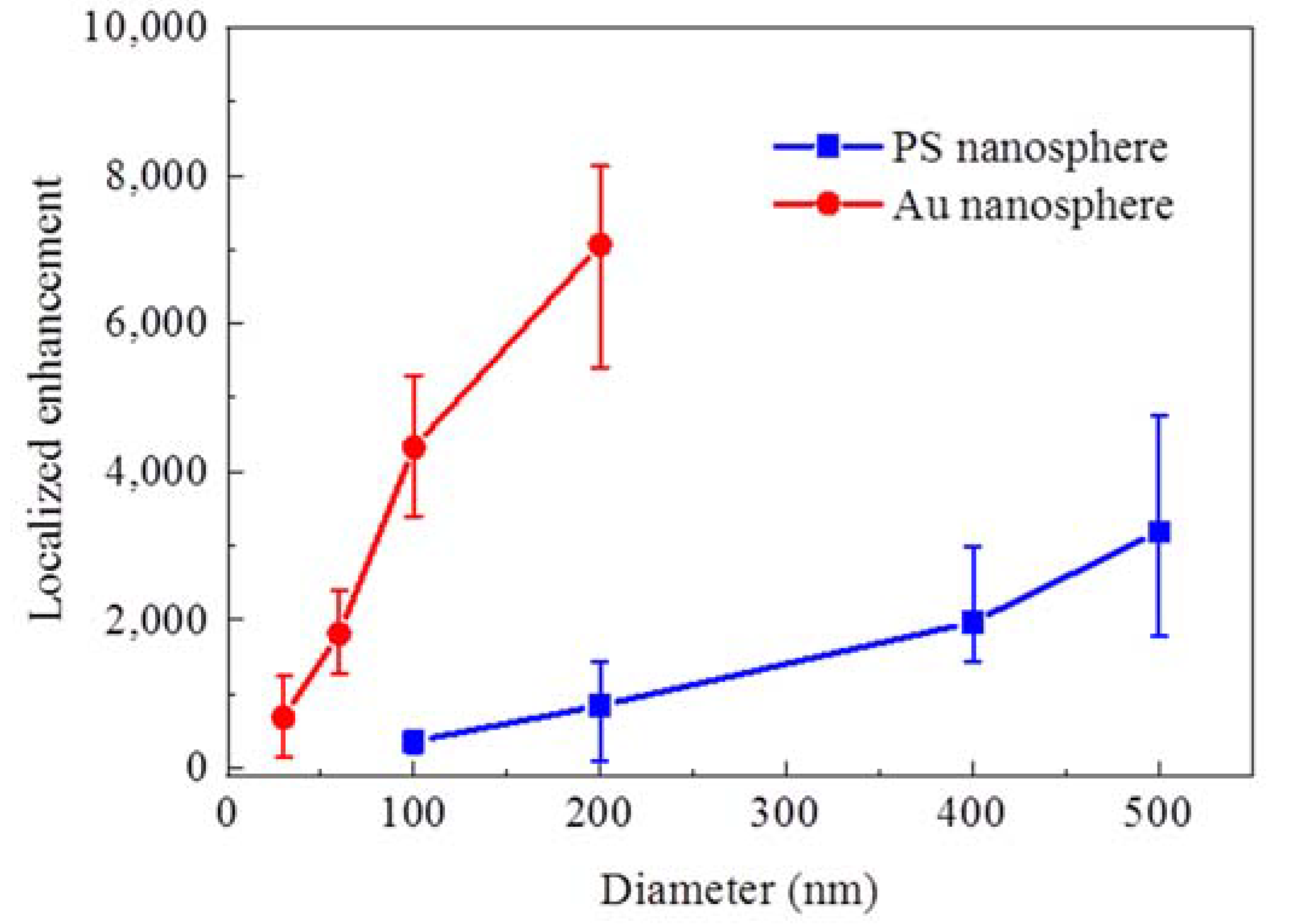
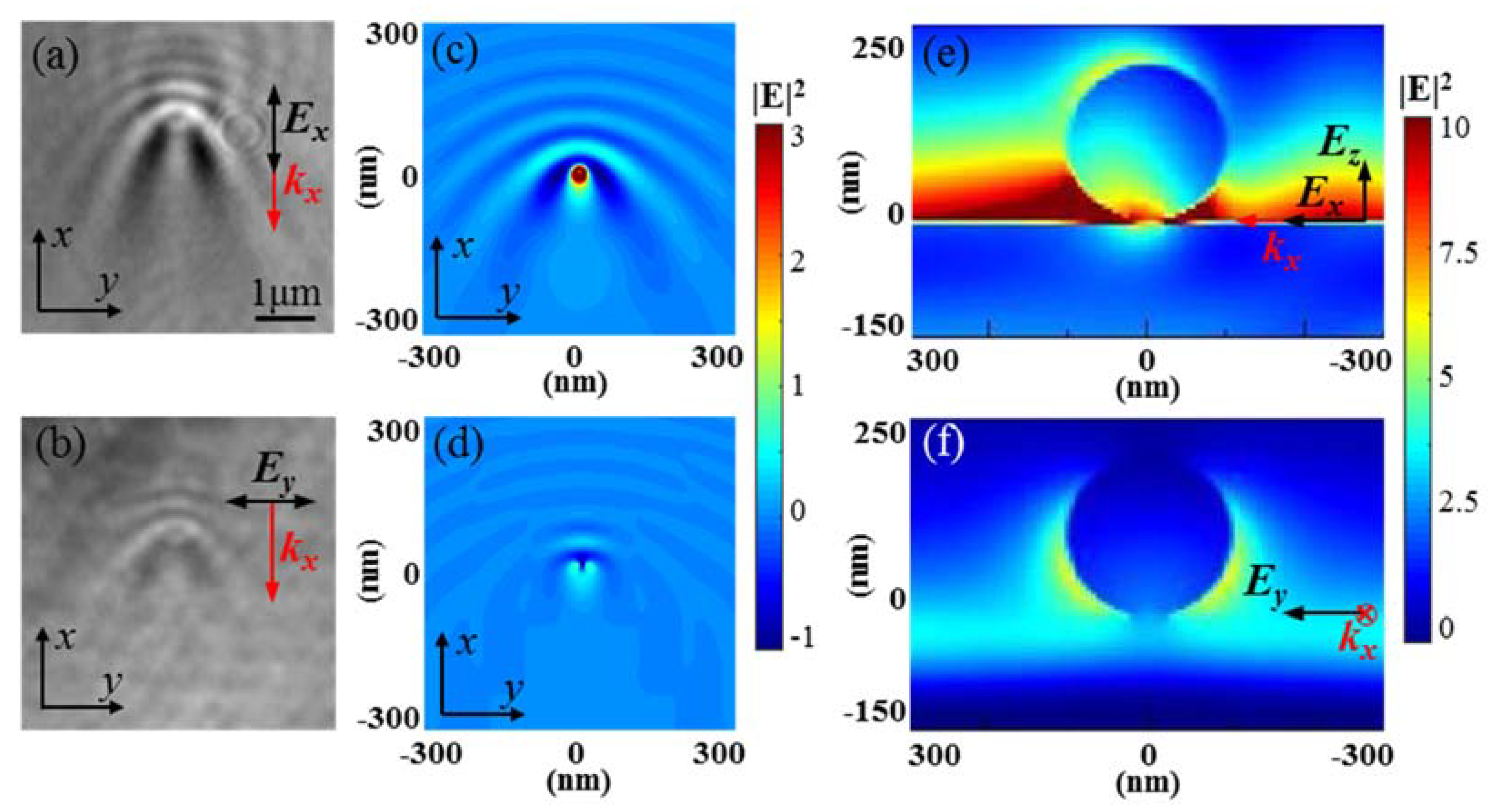
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhirde, A.; Xie, J.; Swierczewska, M.; Chen, X. Nanoparticles for cell labeling. Nanoscale 2011, 3, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Yezhelyev, M.V.; Gao, X.; Xing, Y.; Al-Hajj, A.; Nie, S.; O’Regan, R.M. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006, 7, 657–667. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.; Barton, J.; Halas, N.J.; West, J.; Drezek, R. Nanoshell-Enabled Photonics-Based Imaging and Therapy of Cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Stanton-Maxey, K.J.; Stanley, J.K.; Levin, C.S.; Bradhan, R.; Akin, D.; Badve, S.; Sturgis, J.; Robinson, J.P.; Bashir, R.; et al. A Cellular Trojan Horse for Delivery of Therapeutic Nanoparticles into Tumors. Nano Lett. 2007, 7, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Hund-Rinke, K.; Simon, M. Ecotoxic Effect of Photocatalytic Active Nanoparticles (TiO2) on Algae and Daphnids (8 pp). Environ. Sci. Pollut. Res. 2006, 13, 225–232. [Google Scholar] [CrossRef]
- He, H.; Ren, J. A novel evanescent wave scattering imaging method for single gold particle tracking in solution and on cell membrane. Talanta 2008, 77, 166–171. [Google Scholar] [CrossRef]
- Enoki, S.; Lino, R.; Morone, N.; Kaihatsu, K.; Sakakihara, S.; Kato, N.; Noji, H. Label-free single-particle imaging of the influenza virus by objective-type Total Internal Reflection Dark-Field Microscopy. PLoS ONE 2012, 7, e49208. [Google Scholar] [CrossRef]
- Drezet, A.; Hohenau, A.; Koller, D.; Stepanov, A.; Ditlbacher, H.; Steinberger, B.; Aussenegg, F.R.; Leitner, A.; Krenn, J.R. Leakage radiation microscopy of surface plasmon polaritons. Mater. Sci. Eng. B 2010, 49, 220–229. [Google Scholar] [CrossRef]
- Zybin, A.; Kuritsyn, Y.; Gurevich, E.; Temchura, V.; Überla, K.; Niemax, K. Real-time Detection of Single Immobilized Nanoparticles by Surface Plasmon Resonance Imaging. Plasmonics 2010, 5, 31–35. [Google Scholar] [CrossRef]
- Sun, X.; Liu, H.; Jiang, L.; Wei, R.; Wang, X.; Wang, C.; Lu, X.; Huang, C. Detecting a single nanoparticle by imaging the localized enhancement and interference of surface plasmon polaritons. Opt. Lett. 2019, 44, 5707–5710. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Wang, S.; Tao, N. Study of single particle charge and Brownian motions with surface plasmon resonance. Appl. Phys. Lett. 2010, 97, 223703. [Google Scholar] [CrossRef]
- Wang, S.; Shan, X.; Patel, U.; Huang, X.; Lu, J.; Li, J.; Tao, N. Label-free imaging, detection, and mass measurement of single viruses by surface plasmon resonance. Proc. Natl. Acad. Sci. USA 2010, 107, 16028–16032. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, G.; Wang, H.; Li, H.; Zhang, T.; Tao, N.; Ding, X.; Yu, H. Interferometric plasmonic imaging and detection of single exosomes. Proc. Natl. Acad. Sci. USA 2018, 115, 10275–10280. [Google Scholar] [CrossRef]
- Yu, H.; Shan, X.; Wang, S.; Chen, H.; Tao, N. Plasmonic Imaging and Detection of Single DNA Molecules. ACS Nano 2014, 8, 3427–3433. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Wang, S.; Nagaraj, V.J.; Liu, Q.; Wu, J.; Tao, N. Label-free measuring and mapping of binding kinetics of membrane proteins in single living cells. Nat. Chem. 2012, 4, 846–853. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, H.; Shan, X.; Wang, W.; Liu, X.; Wang, S.; Tao, N. Label-free tracking of single organelle transportation in cells with nanometer precision using a plasmonic imaging technique. Small 2015, 11, 2878–2884. [Google Scholar] [CrossRef]
- Lumerical Solutions Inc. Available online: http://lumerical.com/ (accessed on 1 March 2018).
- Axelrod, D. Total Internal Reflection Fluorescence Microscopy in Cell Biology. Traffic 2001, 2, 764–774. [Google Scholar] [CrossRef]
- Goos, F.; Hanchen, H. A new and fundamental experiment on total reflection. Ann. Phys. 1947, 1, 333–346. [Google Scholar] [CrossRef]
- Puri, A.; Birman, J.L. Goos-Hanchen beam shift at total internal reflection with application to spatially dispersive media. J. Opt. Soc. Am. 1986, 3, 543–549. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Sun, X.; Liu, H.; Wei, R.; Wang, X.; Wang, C.; Lu, X.; Huang, C. Label-Free Imaging of Single Nanoparticles Using Total Internal Reflection-Based Leakage Radiation Microscopy. Nanomaterials 2020, 10, 615. https://doi.org/10.3390/nano10040615
Jiang L, Sun X, Liu H, Wei R, Wang X, Wang C, Lu X, Huang C. Label-Free Imaging of Single Nanoparticles Using Total Internal Reflection-Based Leakage Radiation Microscopy. Nanomaterials. 2020; 10(4):615. https://doi.org/10.3390/nano10040615
Chicago/Turabian StyleJiang, Liwen, Xuqing Sun, Hongyao Liu, Ruxue Wei, Xue Wang, Chang Wang, Xinchao Lu, and Chengjun Huang. 2020. "Label-Free Imaging of Single Nanoparticles Using Total Internal Reflection-Based Leakage Radiation Microscopy" Nanomaterials 10, no. 4: 615. https://doi.org/10.3390/nano10040615
APA StyleJiang, L., Sun, X., Liu, H., Wei, R., Wang, X., Wang, C., Lu, X., & Huang, C. (2020). Label-Free Imaging of Single Nanoparticles Using Total Internal Reflection-Based Leakage Radiation Microscopy. Nanomaterials, 10(4), 615. https://doi.org/10.3390/nano10040615






