Imprinted Polymeric Beads-Based Screen-Printed Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) for Selective Recognition of Fluoxetine
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Apparatus
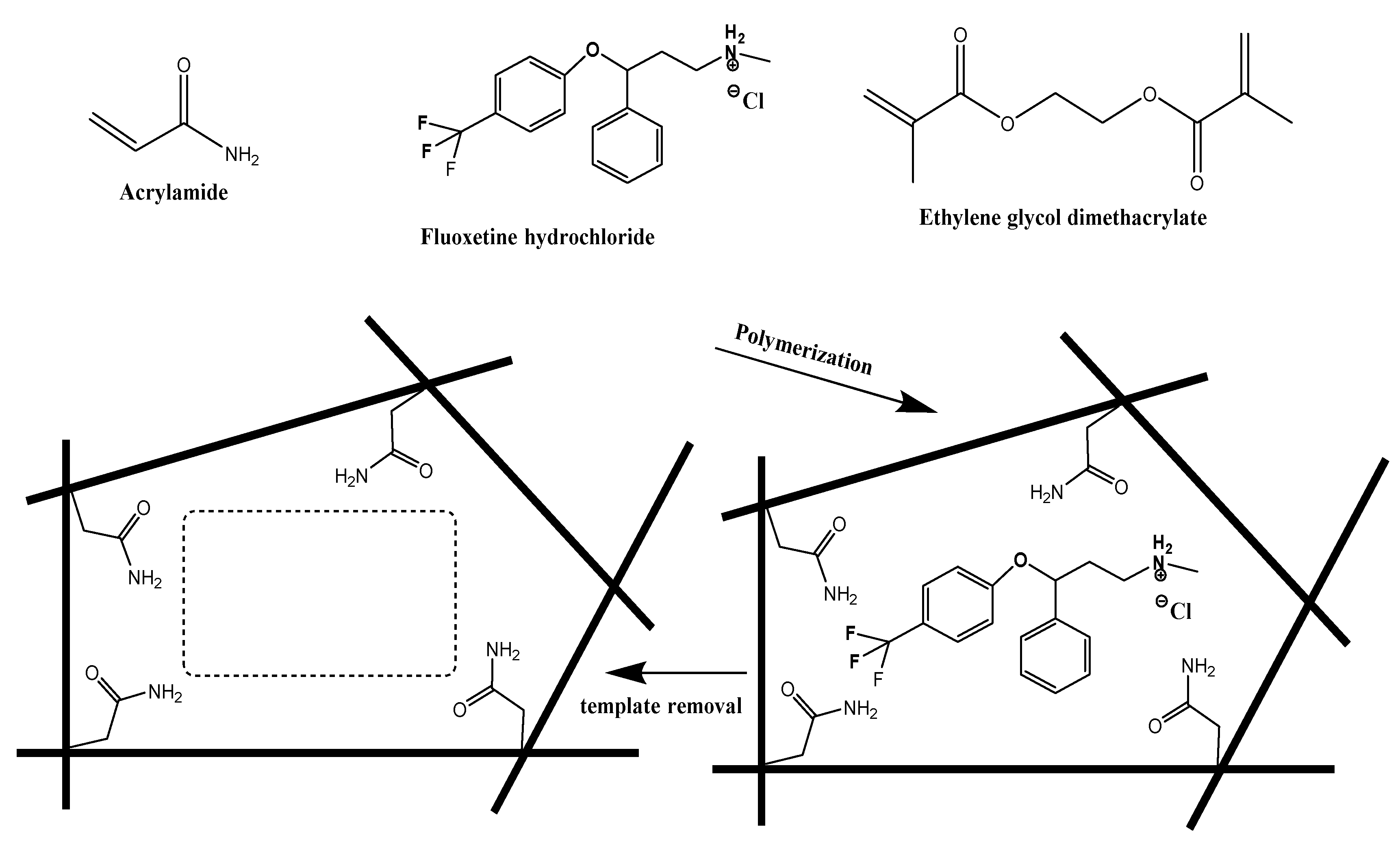
2.3. Synthesis of MIPs
2.4. Fabrication of the Sensors and EMF Measurements
2.5. Analytical Applications
3. Results and Discussion
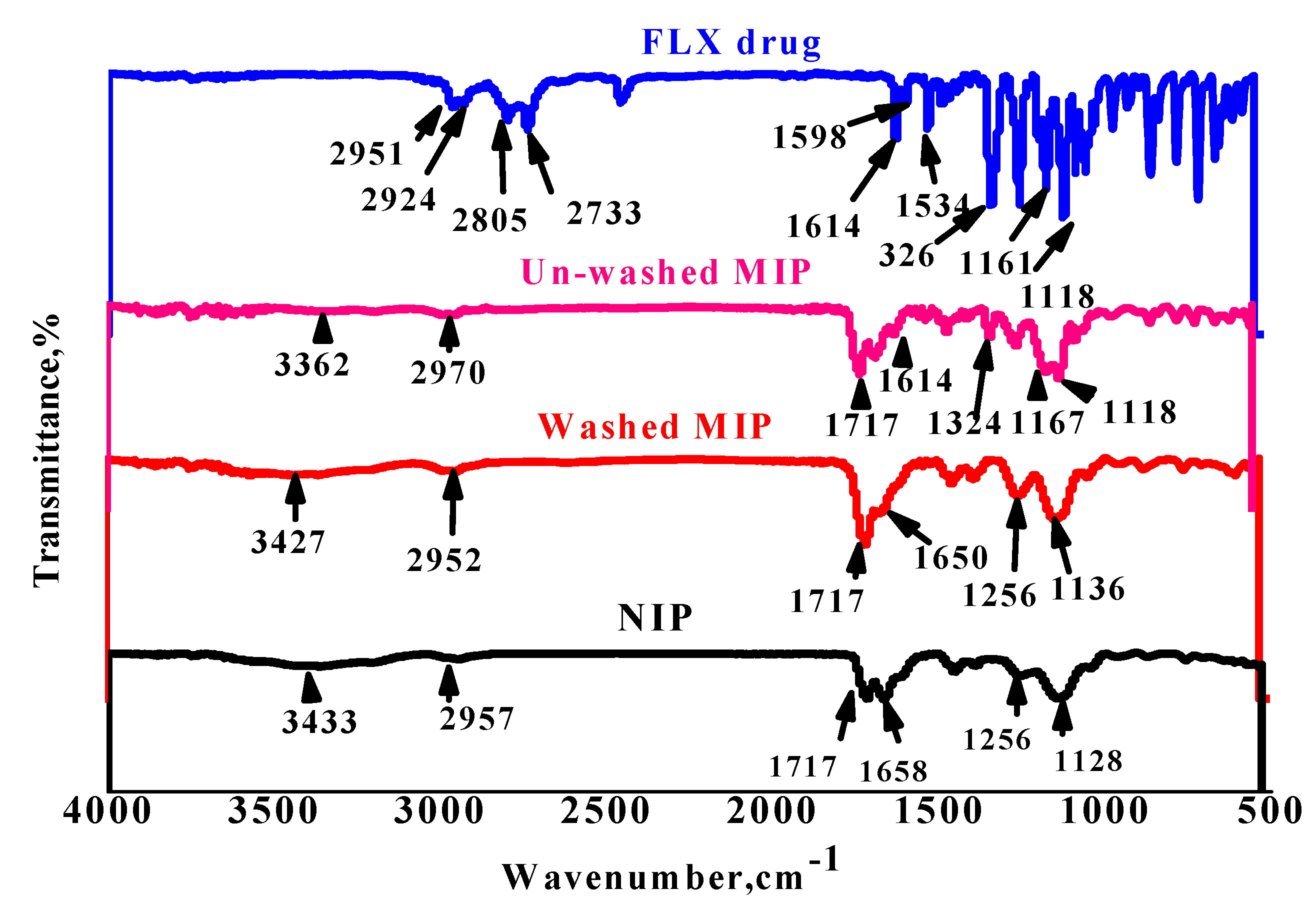
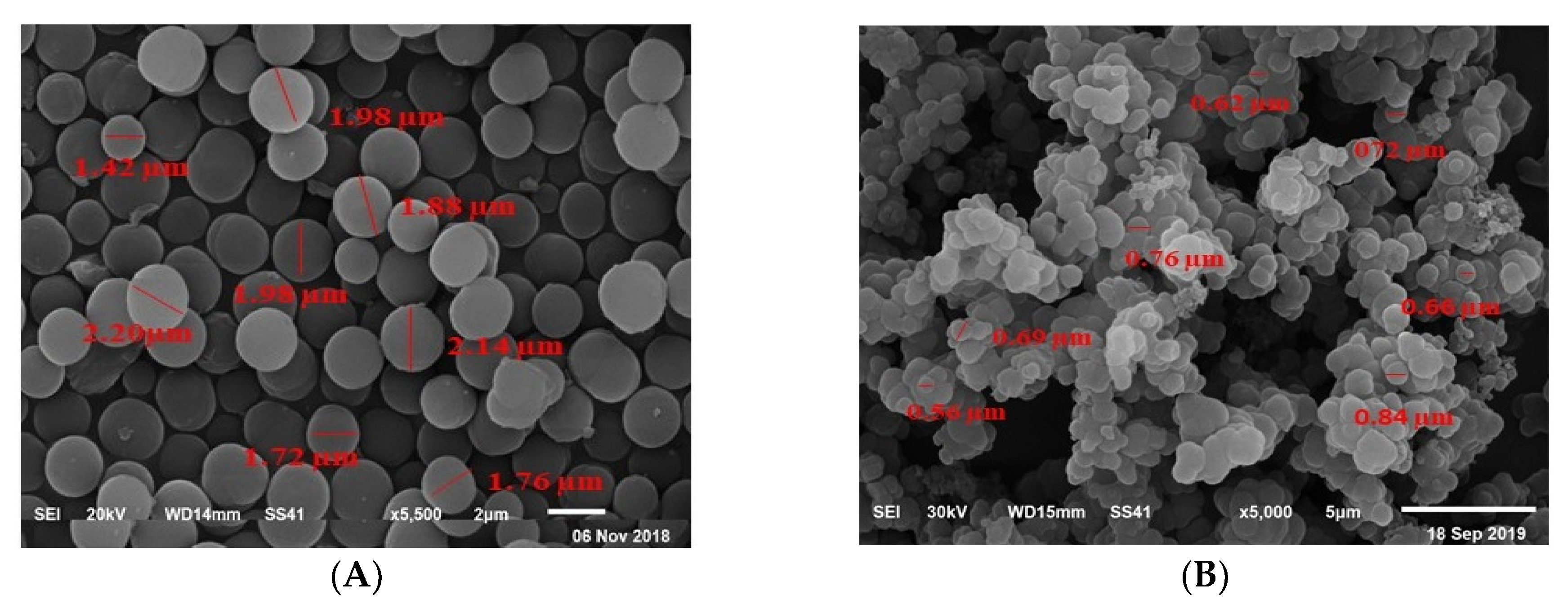
3.1. Characterization of the MIP Particles
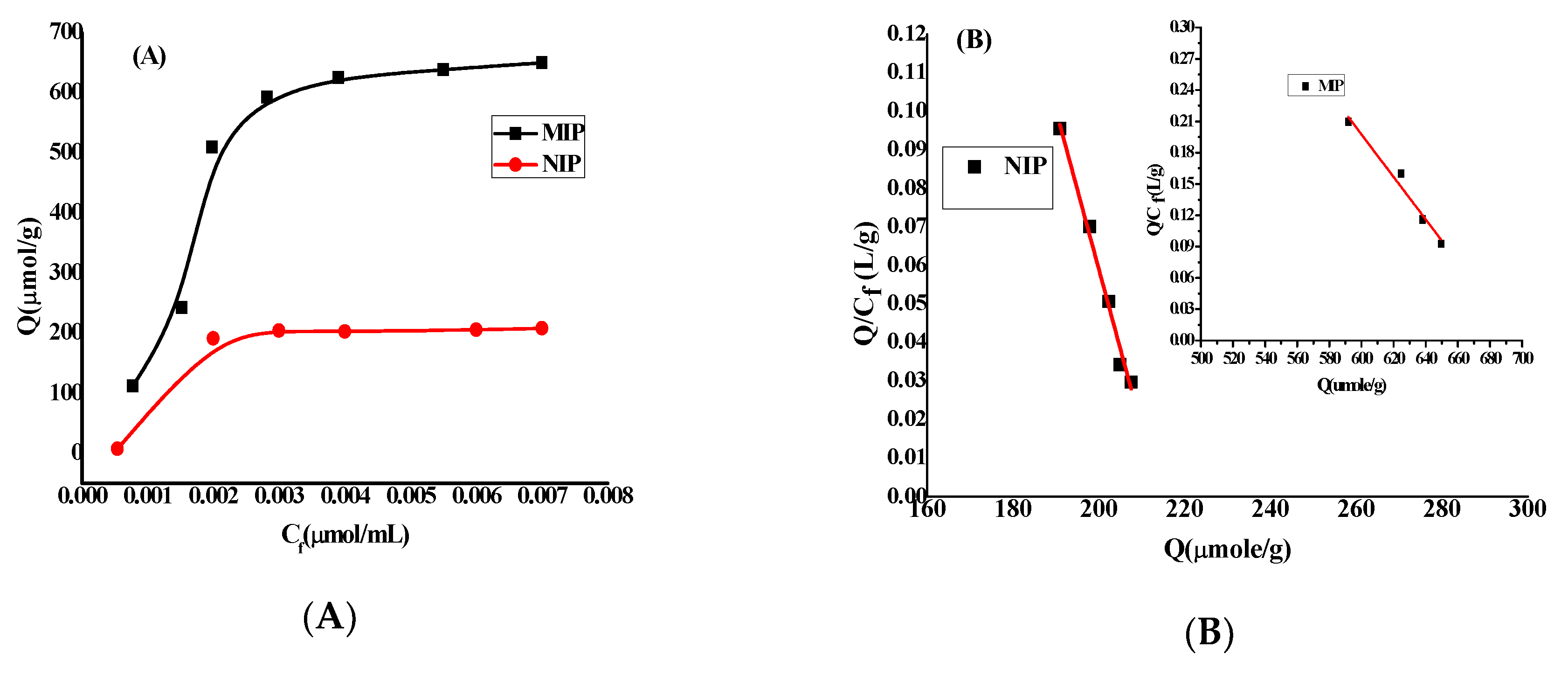
3.2. Sensor Analytical Features
3.3. Method Validation
3.3.1. Detection Limit and Method Linearity
3.3.2. Method for Accuracy and Precision
3.3.3. Method Ruggedness (Robustness)
3.3.4. Sensors’ Selectivity
3.4. Potential Stability
3.5. Electrochemical Impedance Spectrometry (EIS)
3.6. Analytical Applications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Depression: A Global Crisis. World Mental Health Day. Available online: http://www.emro.who.int/media/news/mental-health-day2012.html (accessed on 16 June 2012).
- Desouky, D.; Allam, H. Occupational stress, anxiety and depression among Egyptian teachers. J. Epidemiol. Glob. Health 2017, 7, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. The discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Pigott, T.A.; Seay, S.M. A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J. Clin. Psychiatry 1999, 60, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Amer, S.M.; Abdine, H.H.; Al-Rayes, L.I. New spectrophotometric and fluorimetric methods for determination of fluoxetine in pharmaceutical formulations. Int. J. Environ. Anal. Chem. 2009, 2009, 1–9. [Google Scholar] [CrossRef]
- Bigdelifam, D.; Mirzaei, M.; Hashemi, M.; Amoli-Diva, M.; Rahmani, O.; Zohrabi, P.; Taherimaslak, Z.; Turkjokar, M. Sensitive spectrophotometric determination of fluoxetine from urine samples using charge transfer complex formation after solid phase extraction by magnetic multiwalled carbon nanotubes. Anal. Methods 2014, 6, 8633–8639. [Google Scholar] [CrossRef]
- Nezhadali, A.; Motlagh, M.O.; Sadeghzadeh, S. Spectrophotometric determination of fluoxetine by molecularly imprinted polypyrrole and optimization by experimental design, artificial neural network and genetic algorithm. Spectrochim. Acta Part A Acta A Mol. Biomol. Spectrosc. 2018, 190, 181–187. [Google Scholar] [CrossRef]
- Parmar, V.K.; Patel, J.N.; Jani, G.K.; Prajapati, L.M.; Gagoria, J. FIRST derivative spectrophotometric determination of fluoxetine hydrochloride and olanzapine in tablets. Int. J. Pharm. Sci. Rev. Res. 2011, 2, 2996. [Google Scholar]
- Murtada, K.; de Andrés, F.; Ríos, A.; Zougagh, M. Determination of antidepressants in human urine extracted by magnetic multiwalled carbon nanotube poly (styrene-co-divinylbenzene) composites and separation by capillary electrophoresis. Electrophoresis 2018, 39, 1808–1815. [Google Scholar] [CrossRef]
- Catai, A.P.F.; Carrilho, E.; Lanças, F.M.; Queiroz, M.E.C. Fast separation of selective serotonin reuptake inhibitors antidepressants in plasma sample by nonaqueous capillary electrophoresis. J. Chromatogr. A 2009, 1216, 5779–5782. [Google Scholar] [CrossRef]
- Himmelsbach, M.; Buchberger, W.; Klampfl, C.W. Determination of antidepressants in surface and waste water samples by capillary electrophoresis with electrospray ionization mass spectrometric detection after preconcentration using off-line solid-phase extraction. Electrophoresis 2006, 27, 1220–1226. [Google Scholar] [CrossRef]
- Ghorbani, M.; Esmaelnia, M.; Aghamohammadhasan, M.; Akhlaghi, H.; Seyedin, O.; Azari, Z.A. Preconcentration and Determination Of Fluoxetine and Norfluoxetine in Biological and Water Samples with β-cyclodextrin Multi-walled Carbon Nanotubes as a Suitable Hollow Fiber Solid phase Microextraction Sorbent and High Performance Liquid Chromatography. J. Anal. Chem. 2019, 74, 540–549. [Google Scholar] [CrossRef]
- Wróblewski, K.; Petruczynik, A.; Waksmundzka-Hajnos, M. Separation and determination of selected psychotropic drugs in human serum by SPE/HPLC/DAD on C18 and Polar-RP columns. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 75–82. [Google Scholar] [CrossRef]
- Song, L.; Zheng, Z.; Liang, C.; Chen, X.; Zhang, R.; Hong, Z.; Chai, Y. Rapid solid-phase extraction coupled with GC–MS method for the determination of venlafaxine in rat plasma: Application to the drug–drug pharmacokinetic interaction study of venlafaxine combined with fluoxetine. J. Sep. Sci. 2017, 40, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Erarpat, S.; Cağlak, A.; Bodur, S.; Chormey, S.D.; Engin, Ö.G.; Bakırdere, S. Simultaneous Determination of Fluoxetine, Estrone, Pesticides, and Endocrine Disruptors in Wastewater by Gas Chromatography–Mass Spectrometry (GC–MS) Following Switchable Solvent–Liquid Phase Microextraction (SS–LPME). Anal. Lett. 2019, 52, 869–878. [Google Scholar] [CrossRef]
- González Martín, M.; González Pérez, C. Batch and flow injection fluorimetric determination of fluoxetine. Anal. Lett. 1997, 30, 2493–2502. [Google Scholar] [CrossRef]
- Lencastre, R.P.; Delerue-Matos, C.; Garrido, J.; Borges, F.; Garrido, E.M. Voltammetric quantification of fluoxetine: Application to quality control and quality assurance processes. J. Food Drug Anal. 2006, 14, 242–246. [Google Scholar]
- Da Silva, A.R.; Lima, J.; Teles, M.O.; Brett, A.O. Electrochemical studies and square wave adsorptive stripping voltammetry of the antidepressant fluoxetine. Talanta 1999, 49, 611–617. [Google Scholar] [CrossRef]
- Nouws, H.P.; Delerue-Matos, C.; Barros, A.A.; Rodrigues, J.A.; Santos-Silva, A.; Borges, F. Square-wave adsorptive-stripping voltammetric detection in the quality control of fluoxetine. Anal. Lett. 2007, 40, 1131–1146. [Google Scholar] [CrossRef]
- Alizadeh, T.; Azizi, S. Graphene/graphite paste electrode incorporated with molecularly imprinted polymer nanoparticles as a novel sensor for differential pulse voltammetry determination of fluoxetine. Biosens. Bioelectron. 2016, 81, 198–206. [Google Scholar] [CrossRef]
- Mostafa, G.; Hefnawy, M.; El-Majed, A. PVC membrane sensor for potentiometric determination of fluoxetine in some pharmaceutical formulations. Instrum. Sci. Technol. 2008, 36, 279–290. [Google Scholar] [CrossRef]
- Hussien, E.; Abdel-Gawad, F.; Issa, Y. Ion-selective electrodes for determination of fluoxetine in capsules and in biological fluids. Biochem. Eng. J. 2011, 53, 210–215. [Google Scholar] [CrossRef]
- Arvand, M.; Rad, N.A. Determination of fluoxetine in pharmaceutical preparations and biological samples using potentiometric sensors based on polymeric membranes. J. Anal. Chem. 2013, 68, 183–188. [Google Scholar] [CrossRef]
- Abdalla, N.S.; Amr, A.E.G.E.; El-Tantawy, A.S.M.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Tailor-made specific recognition of cyromazine pesticide integrated in a potentiometric strip cell for environmental and food analysis. Polymers 2019, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.A.; Ahmed, M.; Amr, E.; El-Galil, A.; AAl-Omar, M.H.; Kamel, A.; Khalifa, N.M. Single-Piece All-Solid-State Potential Ion-Selective Electrodes Integrated with Molecularly Imprinted Polymers (MIPs) for Neutral 2, 4-Dichlorophenol Assessment. Materials 2019, 12, 2924. [Google Scholar] [CrossRef] [PubMed]
- Galal Eldin, A.; Amr, E.; El-Galil, A.; HKamel, A.S.M.; Hassan, S. Screen-printed Microsensors Using Polyoctyl-thiophene (POT) Conducting Polymer As Solid Transducer for Ultratrace Determination of Azides. Molecules 2019, 24, 1392. [Google Scholar] [CrossRef]
- El-Naby, E.H.; Kamel, A.H. Potential transducers based man-tailored biomimetic sensors for selective recognition of dextromethorphan as an antitussive drug. Mater. Sci. Eng. C 2015, 54, 217–224. [Google Scholar] [CrossRef]
- El-Kosasy, A.; Kamel, A.H.; Hussin, L.; Ayad, M.F.; Fares, N. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2, 4-dichlorophenol as a food Taint. Food Chem. 2018, 250, 188–196. [Google Scholar] [CrossRef]
- Abdalla, N.S.; Youssef, M.A.; Algarni, H.; Awwad, N.S.; Kamel, A.H. All solid-state poly (vinyl chloride) membrane potentiometric sensor integrated with nano-beads imprinted polymers for sensitive and rapid detection of bispyribac herbicide as organic pollutant. Molecules 2019, 24, 712. [Google Scholar] [CrossRef]
- Elbehery, N.H.A.; Amr, A.E.G.E.; Kamel, A.H.; Elsayed, E.A.; Hassan, S.S.M. Novel potentiometric 2,6-Dichlorophenolindophenolate (DCPIP) membrane-based sensors: Assessment of their input in the determination of total phenolics and ascorbic acid in beverages. Sensors 2019, 19, 2058. [Google Scholar] [CrossRef]
- Hassan SS, M.; Amr AE, G.E.; El-Naby, H.A.; El-Naggar, M.; Kamel, A.H.; Khalifa, N.M. Novel aminoacridine sensors based on molecularly imprinted hybrid polymeric membranes for static and hydrodynamic drug quality control monitoring. Materials 2019, 12, 3327. [Google Scholar] [CrossRef]
- Saber, A.L.; Elmosallamy, M.A.; Killa, H.M.; Ghoneim, M.M. Selective potentiometric method for determination of flucloxacillin antibiotic. J. Taibah Univ. Med. Sci. 2013, 7, 195–201. [Google Scholar] [CrossRef][Green Version]
- Khaled, E.; Hassan, H.; Mohamed, G.G.; Seleim, A. Carbon paste and PVC electrodes for the flow injection potentiometric determination of dextromethorphan. Talanta 2010, 81, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y. Potentiometric Determination of Ion-Pair Formation Constants of Crown Ether-Complex Ions with Some Pairing Anions in Water Using Commercial Ion-Selective Electrodes. In Electrochemistry; Intech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Othman, A.; Rizka, N.; El-Shahawi, M. Potentiometric determination of dopamine in pharmaceutical preparations by crown ether-PVC membrane sensors. Anal. Sci. 2004, 20, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Attiyat, A.S.; Christian, G.D.; Bartsch, R.A. Potentiometric selectivity study of crown ethers containing four ring oxygen atoms and benzoxymethyl or carboxylic acid side chains as ionophores for lithium and potassium. Electroanalysis 1989, 1, 63–67. [Google Scholar] [CrossRef]
- Arada Pérez, M.A.; Cortés Nodarse, I.; Yazdani-Pedram Zobeiri, M.; Pérez Saavedra, J.J. Effect of Plasticizer Type on the Potentiometric Selectivity Coefficient (KAPot) of Electrodes for Nitrate Ion Determination Constructed by Using PVC as Polymeric Membrane. Revista Cubana de Química 2003, 15, 36–43. [Google Scholar]
- Mihali, C.; Vaum, N. Use of plasticizers for electrochemical sensors. In Recent Advances of Plasticizer; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Procedures, A. Methods Validation: Chemistry, Manufacturing, and Controls. Fed. Regist. 2000, 65, 52776–52777. [Google Scholar]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ionselective electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Electrochemistry, P.; Elements, C.; Equivalent, C.; Models, C. Basics of electrochemical impedance spectroscopy. Appl. Note AC 2010, 286, R491–R497. [Google Scholar]
- U.P. USP30-NF25; US Pharmacopoeial Convention, Inc.: Rockville, MD, USA, 2007.
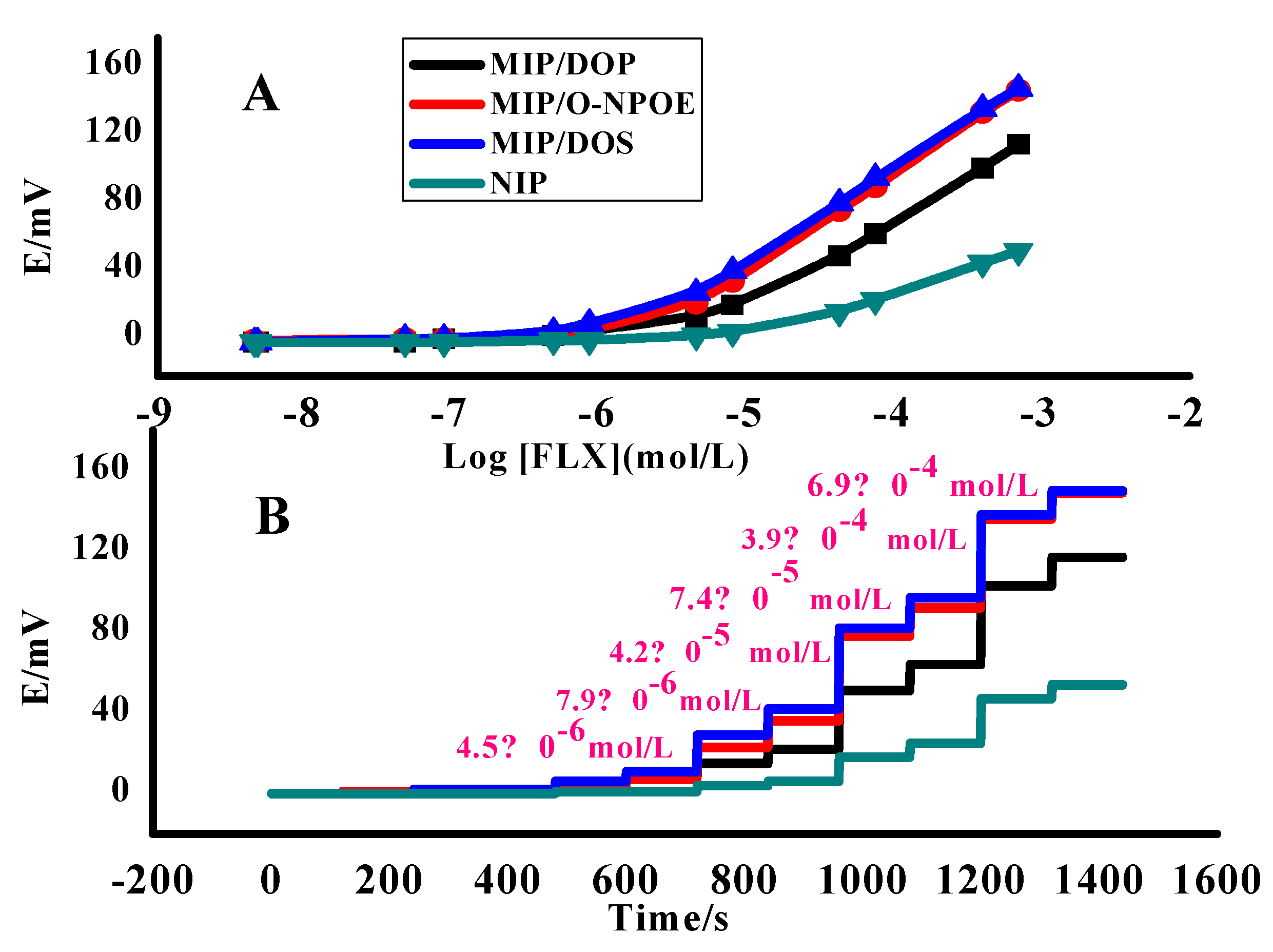

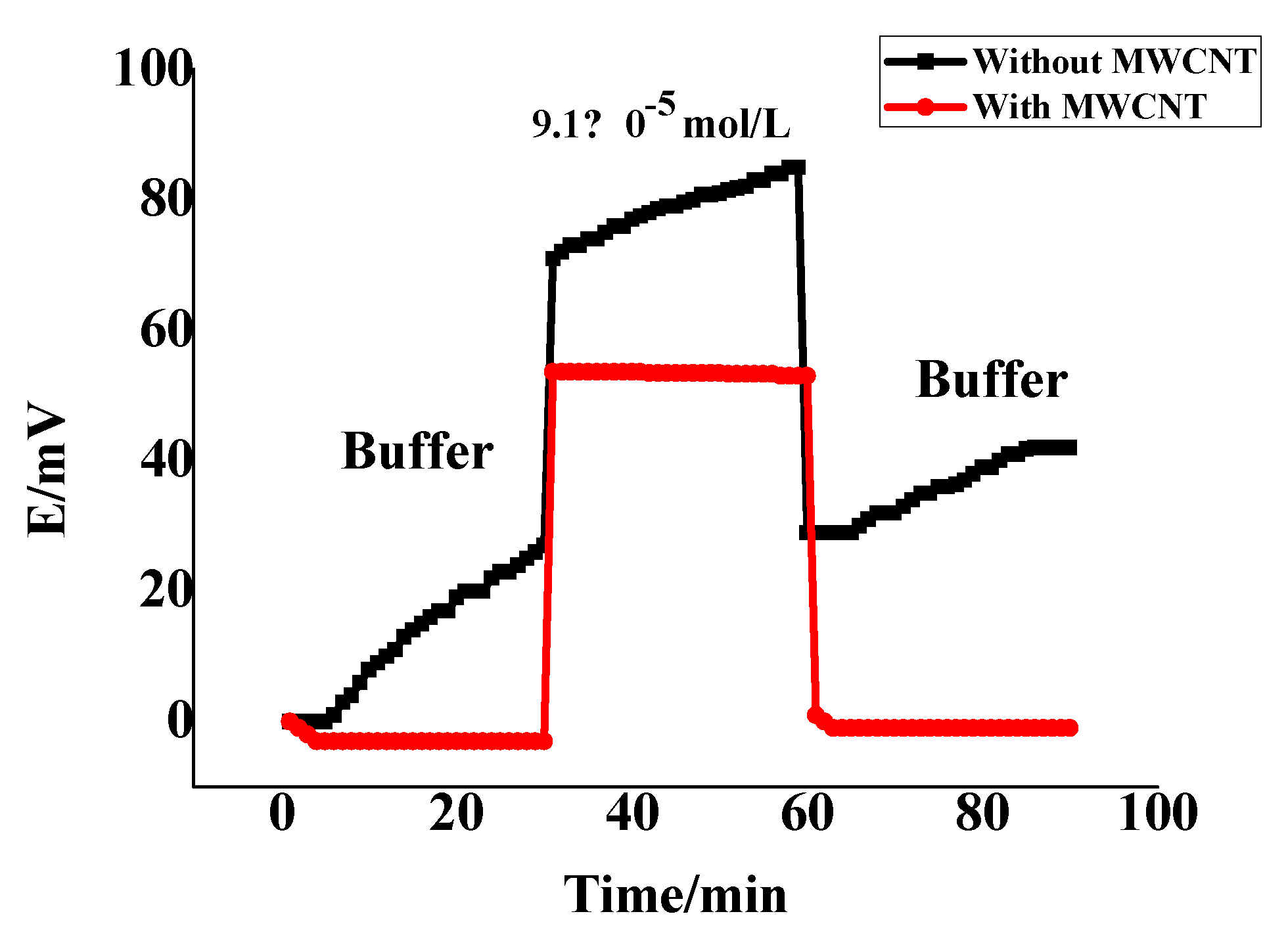
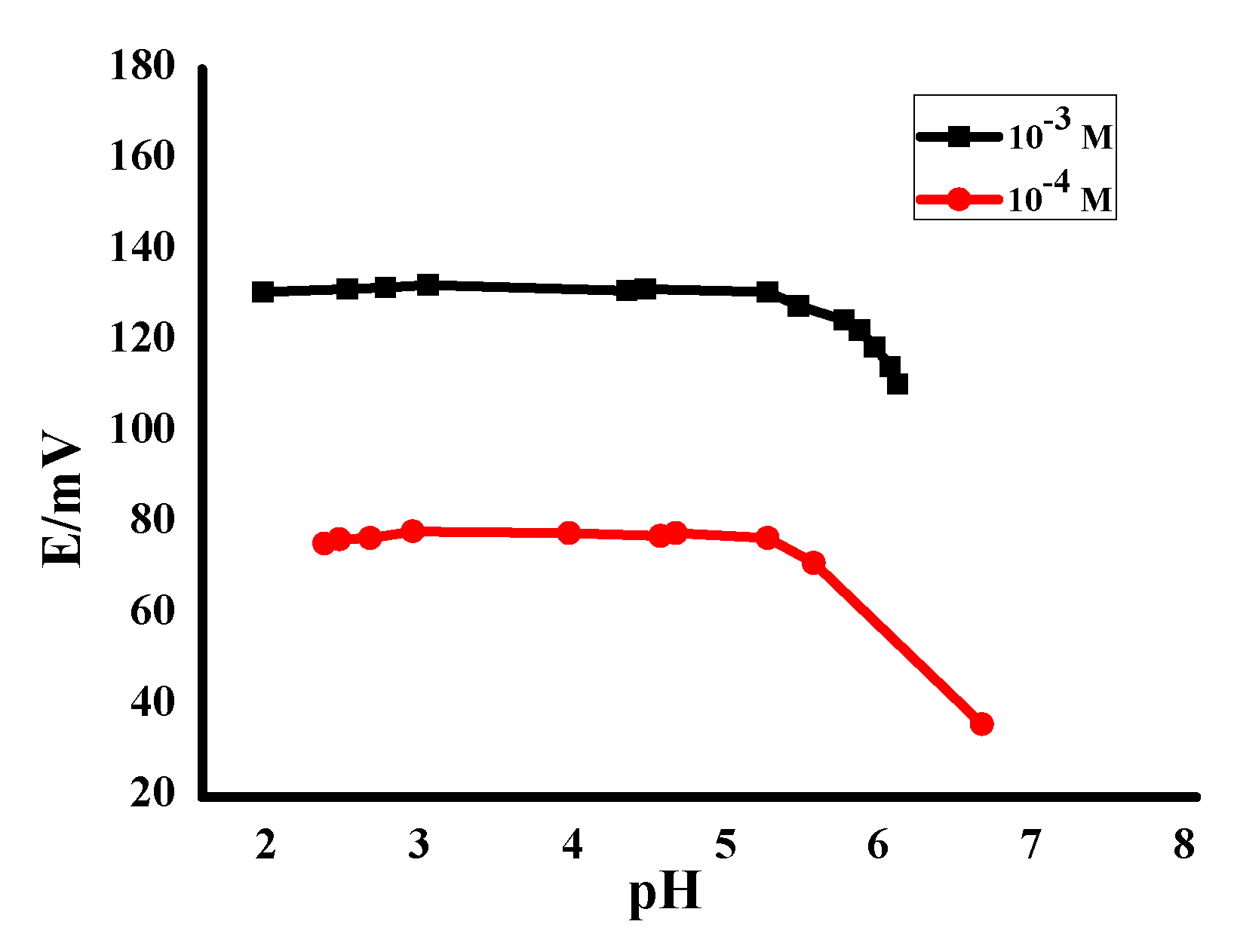
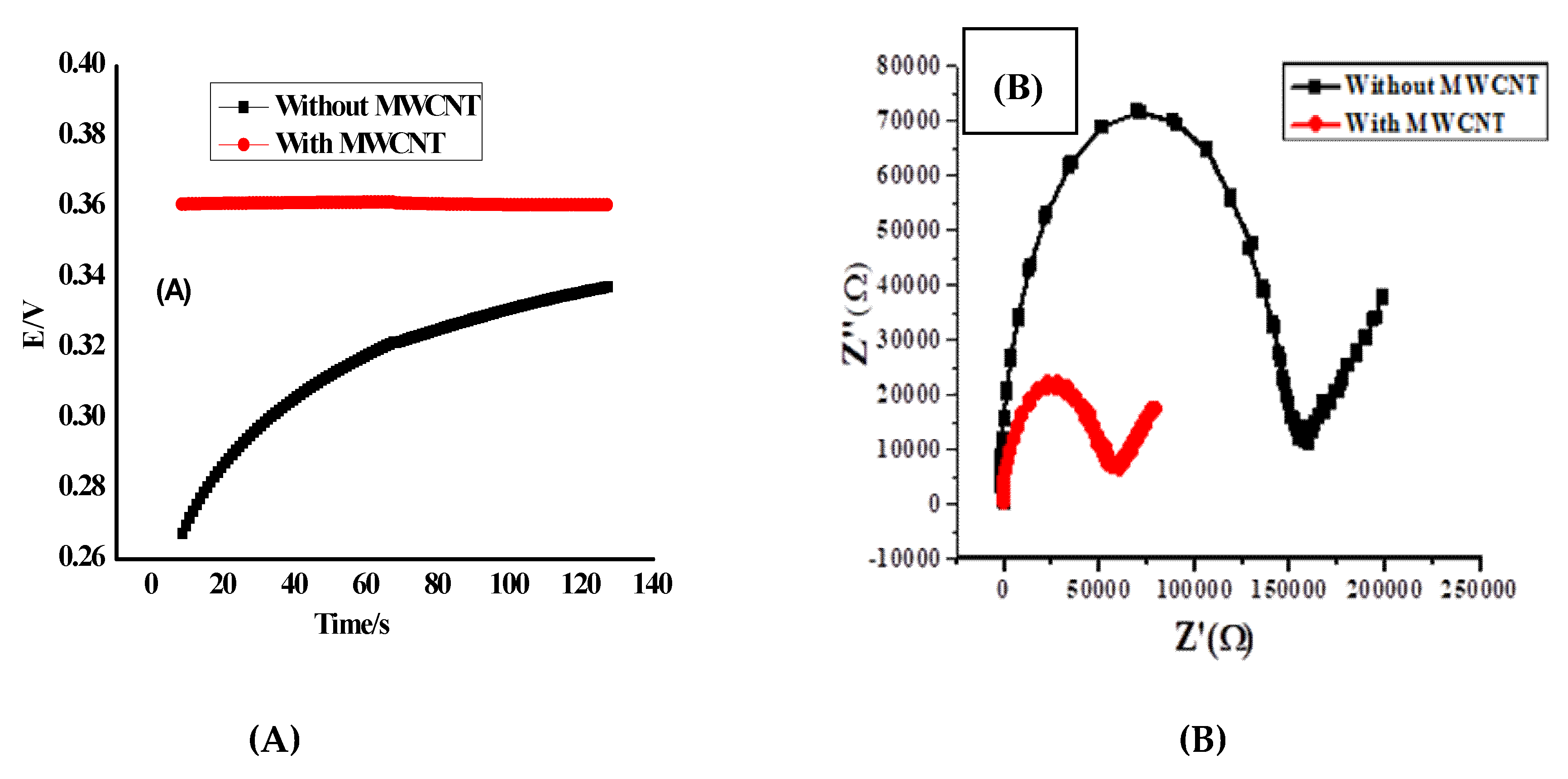
| No | MIP, mg | NIP, mg | TPB, mg | Plasticizer, mg | PVC, mg | * Slope, (mV/Decade) | Detection Limit, (mol/L) | r2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| DOP | o-NPOE | DOS | ||||||||
| 1 | 12.00 | - | 2.00 | 118.00 | - | - | 68.00 | 57.1 ± 0.3 | 4.9 × 10−6 | 0.999 |
| 2 | 12.00 | - | 2.00 | - | 118.00 | - | 68.00 | 58.9 ± 0.2 | 2.1 × 10−6 | 0.999 |
| 3 | 12.00 | - | 2.00 | - | - | 118.00 | 68.00 | 56.0 ± 0.6 | 1.4 × 10−6 | 0.999 |
| 4 | 12.00 | - | - | 118.00 | - | - | 68.00 | 48.8 ± 0.5 | 8.5 ×10−6 | 0.999 |
| 5 | - | 12.00 | - | 118.00 | - | - | 68.00 | 29.8 ± 1.3 | 1.2 ×10−5 | 0.999 |
| 6 | - | - | 2.00 | 118.00 | - | - | 68.00 | 50.9 ± 0.3 | 7.1 × 10−6 | 0.999 |
| Interfering Ion | −log K potFLX, J | ||
|---|---|---|---|
| DOP | o-NPOE | DOS | |
| Na+ | 5.0 | 5.0 | 5.7 |
| K+ | 4.7 | 5.0 | 5.3 |
| Mg2+ | 5.0 | 4.9 | 5.4 |
| Ca2+ | 5.0 | 4.9 | 5.6 |
| Ba2+ | 4.7 | 4.9 | 5.6 |
| Alanine | 4.9 | 4.7 | 5.6 |
| Arginine | 7.1 | 6.1 | 7.1 |
| Glucose | 5.0 | 5.0 | 5.7 |
| Lactose | 4.7 | 5.0 | 5.7 |
| Caffeine | 5.0 | 5.0 | 5.7 |
| Sildenafil | 3.2 | 2.8 | 3.5 |
| Pharmaceutical Product and Source | Nominal Content Taken, mg tablet−1 | Found, mg tablet−1 | t-Student Test | F-Test | |||
|---|---|---|---|---|---|---|---|
| Proposed Method | Mean a (%) ± SD | Reference Method [42] | Mean a (%) ± SD | ||||
| Prozac (Lilly, France) | 20 | 20.2 | 101.0 ± 0.3 | 20.1 | 100.8 ± 0.6 | 0.4 | 4.4 |
| Philozac (Amoun, Egypt) | 20 | 19.8 | 99.2 ± 0.6 | 19.9 | 99.1 ± 1.7 | 0.2 | 8.7 |
| Flutin (Eipico, Egypt) | 20 | 20.3 | 101.1 ± 0.6 | 19.8 | 99.4 ± 0.9 | 2.3 | 2.6 |
| Depreban (Amirya, Egypt) | 20 | 19.7 | 98.6 ± 0.4 | 19.4 | 97.2 ± 0.8 | 1.7 | 2.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.S.M.; H. Kamel, A.; Amr, A.E.-G.E.; Hashem, H.M.; Bary, E.M.A. Imprinted Polymeric Beads-Based Screen-Printed Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) for Selective Recognition of Fluoxetine. Nanomaterials 2020, 10, 572. https://doi.org/10.3390/nano10030572
Hassan SSM, H. Kamel A, Amr AE-GE, Hashem HM, Bary EMA. Imprinted Polymeric Beads-Based Screen-Printed Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) for Selective Recognition of Fluoxetine. Nanomaterials. 2020; 10(3):572. https://doi.org/10.3390/nano10030572
Chicago/Turabian StyleHassan, Saad S.M., Ayman H. Kamel, Abd El-Galil E. Amr, Heba M. Hashem, and E.M. Abdel Bary. 2020. "Imprinted Polymeric Beads-Based Screen-Printed Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) for Selective Recognition of Fluoxetine" Nanomaterials 10, no. 3: 572. https://doi.org/10.3390/nano10030572
APA StyleHassan, S. S. M., H. Kamel, A., Amr, A. E.-G. E., Hashem, H. M., & Bary, E. M. A. (2020). Imprinted Polymeric Beads-Based Screen-Printed Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) for Selective Recognition of Fluoxetine. Nanomaterials, 10(3), 572. https://doi.org/10.3390/nano10030572







