Quantitative and Selective Surface Plasmon Resonance Response Based on a Reduced Graphene Oxide–Polyamidoamine Nanocomposite for Detection of Dengue Virus E-Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
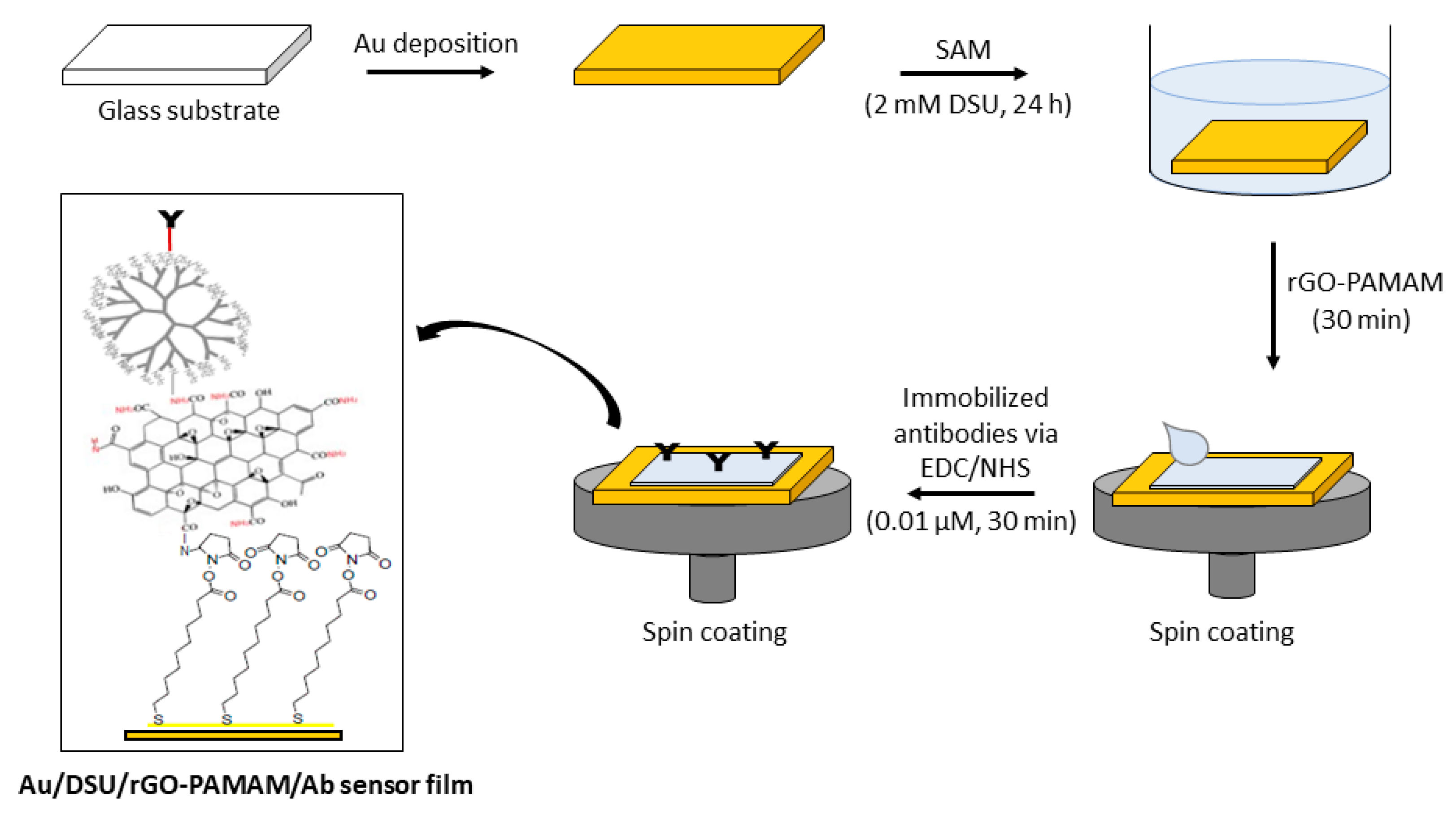
2.2. Sensor Surface Fabrication
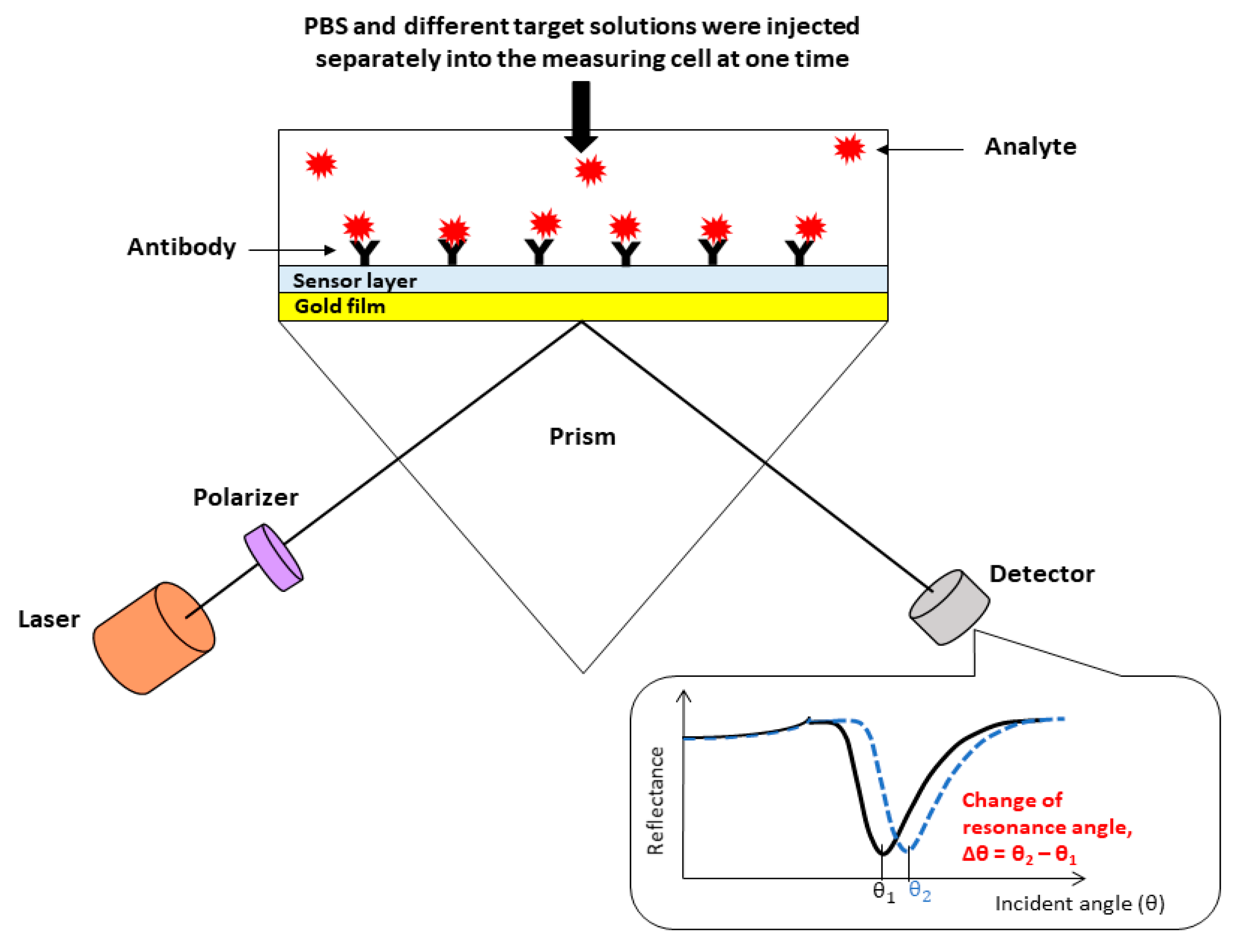
2.3. DENV E-Proteins Detection
2.4. SPR Performances
2.5. Sensor Surface Characterization
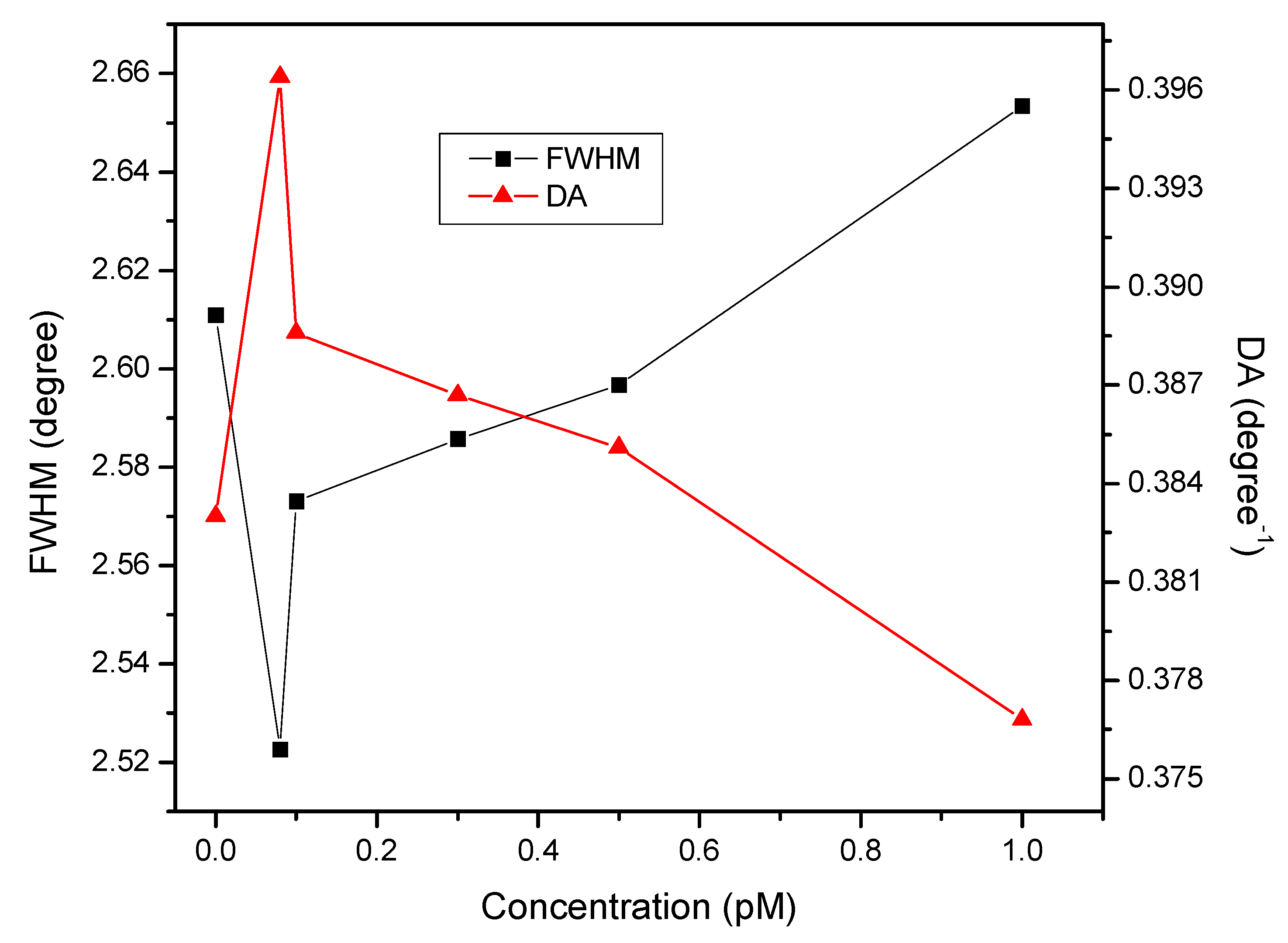
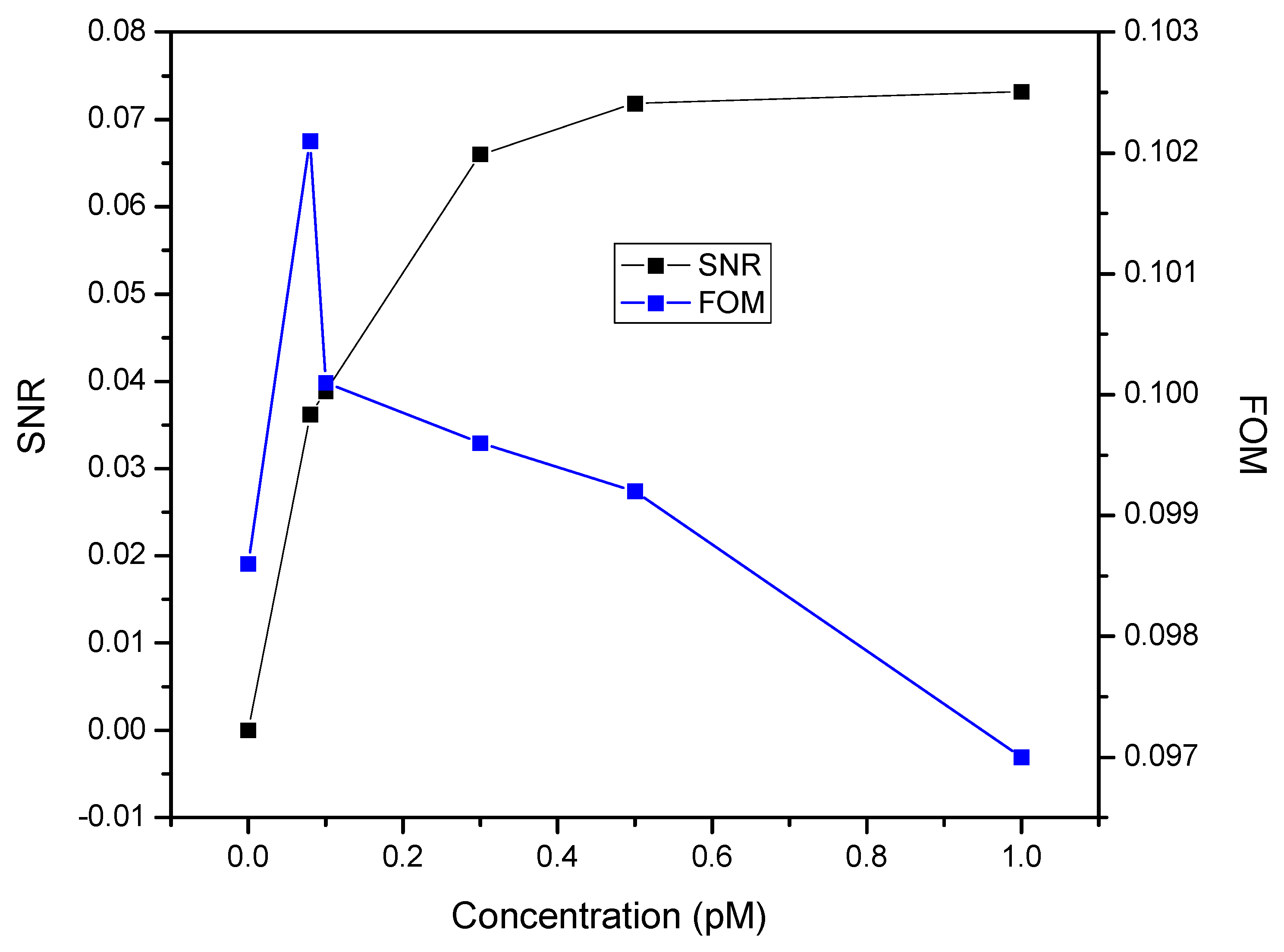
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shankaran, D.R.; Gobi, K.V.; Miura, N. Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food, and environmental interest. Sens. Actuators B Chem. 2007, 121, 158–177. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Al-Rekabi, S.H.; Mahdi, M.A.; Omar, N.A.S. Incorporation of surface plasmon resonance with novel valinomycin doped chitosan-graphene oxide thin film for sensing potassium ion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 191, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Rich, R.L.; Myszka, D.G. Survey of the year commercial optical biosensor literature. J. Mol. Recognit. 2008, 21, 355–400. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W. Recent development of SPR spectroscopy as potential method for diagnosis of dengue virus E-protein. Sens. Rev. 2017, 38, 106–116. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Label-free optical spectroscopy for characterizing binding properties of highly sensitive nanocrystalline cellulose-graphene oxide-based nanocomposite towards nickel ion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Abdullah, J.; Zaid, M.H.M. Structural and optical studies of cadmium sulfide quantum dot-graphene oxide-chitosan nanocomposite thin film as a novel SPR spectroscopy active layer. J. Nanomater. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Ahuja, D.; Parande, D. Optical sensors and their applications. J. Sci. Res. Rev. 2012, 1, 60–68. [Google Scholar]
- Homola, J.; Piliarik, M. Surface plasmon resonance (SPR) sensors. In Surface Plasmon Resonance-Based Sensors; Springer: Berlin/Heidelberg, Germany, 2006; pp. 45–67. [Google Scholar]
- Nguyen, H.; Park, J.; Kang, S.; Kim, M. Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef]
- Syahir, A.; Usui, K.; Tomizaki, K.Y.; Kajikawa, K.; Mihara, H. Label and label-free detection techniques for protein microarrays. Microarrays 2015, 4, 228–244. [Google Scholar] [CrossRef]
- Martins, T.D.; Ribeiro, A.C.C.; de Camargo, H.S.; da Costa Filho, P.A.; Cavalcante, H.P.M.; Dias, D.L. New insights on optical biosensors: Techniques, construction and application. In State of the Art in Biosensors—General Aspects; InTech Open: London, UK, 2013; pp. 112–139. [Google Scholar]
- Daaboul, G.G.; Lopez, C.A.; Yurt, A.; Goldberg, B.B.; Connor, J.H.; Ünlü, M.S. Label-free optical biosensors for virus detection and characterization. IEEE J. Sel. Top. Quantum Electron. 2011, 18, 1422–1433. [Google Scholar] [CrossRef]
- Peltomaa, R.; Glahn-Martínez, B.; Benito-Peña, E.; Moreno-Bondi, M. Optical biosensors for label-free detection of small molecules. Sensors 2018, 18, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.H.; Vishwanath, K.; Mycek, M.A. Optical methods for quantitative and label-free sensing in living human tissues: Principles, techniques, and applications. Adv. Phys. X 2016, 1, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Kamil, Y.M.; Bakar, M.H.A.; Yaacob, M.H.; Syahir, A.; Lim, H.N.; Mahdi, M.A. Dengue E protein detection using a graphene oxide integrated tapered optical fiber sensor. IEEE J. Sel. Top. Quantum Electron. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Sabri, N.; Aljunid, S.A.; Salim, M.S.; Fouad, S. Fiber optic sensors: Short review and applications. In Recent Trends in Physics of Material Science and Technology; Springer: Singapore, 2015; pp. 299–311. [Google Scholar]
- Perrotton, C.; Westerwaal, R.J.; Javahiraly, N.; Slaman, M.; Schreuders, H.; Dam, B.; Meyrueis, P. A reliable, sensitive and fast optical fiber hydrogen sensor based on surface plasmon resonance. Opt. Express 2013, 21, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, B.; Purwidyantri, A.; Liu, K.C. Surface plasmon resonance optical sensor: A review on light source technology. Biosensors 2018, 8, 1–27. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, H.; Hu, S.; Xia, K.; Xiong, X.; Huang, W.; Lu, H.; Yu, J.; Guan, H.; et al. Titanium dioxide nanoparticle modified plasmonic interface for enhanced refractometric and biomolecular sensing. Opt. Express 2018, 26, 33226–33237. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Chik, C.E.N.C.E.; Mahdi, M.A. Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E-protein. Sens. Biosens. Res. 2018, 20, 16–21. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: A review. Sens. Rev. 2013, 33, 304–314. [Google Scholar]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene-based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S. Structural, optical and sensing properties of ionophore doped graphene based bionanocomposite thin film. Optik 2017, 144, 308–315. [Google Scholar] [CrossRef]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-based materials for biosensors: A review. Sensors 2017, 17, 1–24. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic transport properties of individual chemically reduced graphene oxide sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef]
- Gilje, S.; Han, S.; Wang, M.; Wang, K.L.; Kaner, R.B. A chemical route to graphene for device applications. Nano Lett. 2007, 7, 3394–3398. [Google Scholar] [CrossRef]
- Sinitskii, A.; Fursina, A.A.; Kosynkin, D.V.; Higginbotham, A.L.; Natelson, D.; Tour, J.M. Electronic transport in monolayer graphene nanoribbons produced by chemical unzipping of carbon nanotubes. Appl. Phys. Lett. 2009, 95, 253108. [Google Scholar] [CrossRef]
- Lu, C.; Huang, P.J.J.; Liu, B.; Ying, Y.; Liu, J. Comparison of graphene oxide and reduced graphene oxide for DNA adsorption and sensing. Langmuir 2016, 32, 10776–10783. [Google Scholar] [CrossRef]
- Xing, F.; Liu, Z.B.; Deng, Z.C.; Kong, X.T.; Yan, X.Q.; Chen, X.D.; Ye, Q.; Zhang, C.P.; Chen, Y.S.; Tian, J.G. Sensitive real-time monitoring of refractive indexes using a novel graphene-based optical sensor. Sci. Rep. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Navaee, A.; Salimi, A. Efficient amine functionalization of graphene oxide through the Bucherer reaction: An extraordinary metal-free electrocatalyst for the oxygen reduction reaction. RSC Adv. 2015, 5, 59874–59880. [Google Scholar] [CrossRef]
- Guo, L.; Yan, H.; Chen, Z.; Liu, Q.; Feng, Y.; Ding, F.; Nie, Y. Amino functionalization of reduced graphene oxide/tungsten disulfide hybrids and their bismaleimide composites with enhanced mechanical properties. Polymers 2018, 10, 1–13. [Google Scholar] [CrossRef]
- Hidayah, N.M.S.; Liu, W.W.; Lai, C.W.; Noriman, N.Z.; Khe, C.S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. In Proceedings of the AIP Conference Proceedings, Penang, Malaysia, 8–9 August 2017; AIP Publishing: Melville, NY, USA, 2017; Volume 1892, p. 150002. [Google Scholar]
- Zhu, J.; Ye, Z.; Fan, X.; Wang, H.; Wang, Z.; Chen, B. A highly sensitive biosensor based on au NPs/rGo-PaMaM-Fc nanomaterials for detection of cholesterol. Int. J. Nanomed. 2019, 14, 835–849. [Google Scholar] [CrossRef]
- Bharti, J.P.; Prajapati, S.K.; Jaiswal, M.K.; Yadav, R.D. Dendrimer multifunctional nano-device: A review. Int. J. Pharm. Sci. Res. 2011, 2, 1947–1960. [Google Scholar]
- Calabretta, M.K.; Kumar, A.; McDermott, A.M.; Cai, C. Antibacterial activities of poly (amidoamine) dendrimers terminated with amino and poly (ethylene glycol) groups. Biomacromolecules 2007, 8, 1807–1811. [Google Scholar] [CrossRef]
- Tripathy, S.; Das, M.K. Dendrimers and their applications as novel drug delivery carriers. J. Appl. Pharm. Sci. 2013, 3, 142–149. [Google Scholar]
- Navakul, K.; Warakulwit, C.; Yenchitsomanus, P.; Panya, A.; Lieberzeit, P.; Sangma, C. A novel method for dengue virus detection and antibody screening using a graphene-polymer based electrochemical biosensor. Nanomedicine 2017, 13, 549–557. [Google Scholar] [CrossRef]
- Rice, C.M.; Lenches, E.M.; Eddy, S.R.; Shin, S.J.; Sheets, R.L.; Strauss, J.H. Nucleotide sequence of yellow fever virus: Implications for flavivirus gene expression and evolution. Science 1985, 229, 726–733. [Google Scholar] [CrossRef]
- Barrero, P.R.; Mistchenko, A.S. Complete genome sequencing of dengue virus type 1 isolated in Buenos Aires, Argentina. Virus Res. 2004, 101, 135–145. [Google Scholar] [CrossRef]
- Guzmán, M.G.; Kouri, G. Dengue diagnosis, advances and challenges. Int. J. Infect. Dis. 2004, 8, 69–80. [Google Scholar] [CrossRef]
- Chawla, P.; Yadav, A.; Chawla, V. Clinical implications and treatment of dengue. Asian Pac. J. Trop. Med. 2014, 7, 169–178. [Google Scholar] [CrossRef]
- Dhanoa, A.; Hassan, S.S.; Ngim, C.F.; Lau, C.F.; Chan, T.S.; Adnan, N.A.A.; Eng, W.W.H.; Gan, H.M.; Rajasekram, G. Impact of dengue virus (DENV) co-infection on clinical manifestations, disease severity and laboratory parameters. BMC Infect. Dis. 2016, 16, 1–14. [Google Scholar] [CrossRef]
- Gelanew, T.; Hunsperger, E. Development and characterization of serotype-specific monoclonal antibodies against the dengue virus-4 (DENV-4) non-structural protein (NS1). Virol. J. 2018, 15, 1–12. [Google Scholar] [CrossRef]
- Fry, S.R.; Meyer, M.; Semple, M.G.; Simmons, C.P.; Sekaran, S.D.; Huang, J.X.; McElnea, C.; Huang, C.; Valks, A.; Young, P.R.; et al. The diagnostic sensitivity of dengue rapid test assays is significantly enhanced by using a combined antigen and antibody testing approach. PLoS Negl. Trop. Dis. 2011, 5, 1–8. [Google Scholar] [CrossRef]
- Wong, W.R.; Sekaran, S.D.; Adikan, F.R.M.; Berini, P. Detection of dengue NS1 antigen using long-range surface plasmon waveguides. Biosens. Bioelectron. 2016, 78, 132–139. [Google Scholar] [CrossRef]
- Kumarasamy, V.; Chua, S.K.; Hassan, Z.; Wahab, A.H.A.; Chem, Y.K.; Mohamad, M.; Chua, K.B. Evaluating the sensitivity of a commercial dengue NSI antigen—Capture ELISA for early diagnosis of acute dengue virus infection. Singap. Med. J. 2007, 48, 669–673. [Google Scholar]
- Bessoff, K.; Delorey, M.; Sun, W.; Hunsperger, E. Comparison of two commercially available dengue virus (DENV) NS1 capture enzyme-linked immunosorbent assays using a single clinical sample for diagnosis of acute DENV infection. Clin. Vaccine Immunol. 2008, 15, 1513–1518. [Google Scholar] [CrossRef]
- Chávez, J.H.; Silva, J.R.; Amarilla, A.A.; Figueiredo, L.T.M. Domain III peptides from flavivirus envelope protein are useful antigens for serologic diagnosis and targets for immunization. Biologicals 2010, 38, 613–618. [Google Scholar] [CrossRef]
- Sarma, S.; Lee, J.H. Developing efficient thin film temperature sensors utilizing layered carbon nanotube films. Sensors 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Green, T.A. Gold electrodeposition for microelectronic, optoelectronic and microsystem applications. Gold Bull. 2007, 40, 105–114. [Google Scholar] [CrossRef]
- Jilani, A.; Abdel-Wahab, M.S.; Hammad, A.H. Advance deposition techniques for thin film and coating. In Modern Technologies for Creating the Thin-Film Systems and Coatings; IntechOpen: London, UK, 2017; Volume 2, pp. 137–149. [Google Scholar]
- Jha, R.; Sharma, A.K. High-performance sensor based on surface plasmon resonance with chalcogenide prism and aluminum for detection in infrared. Opt. Lett. 2009, 34, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Maharana, P.K.; Jha, R. Chalcogenide prism and graphene multilayer based surface plasmon resonance affinity biosensor for high performance. Sens Actuators B Chem. 2012, 169, 161–166. [Google Scholar] [CrossRef]
- Yoon, S.J.; Kim, D. Thin-film-based field penetration engineering for surface plasmon resonance biosensing. JOSA A 2007, 24, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.S.; Kumar, A.; Kumar, R.; Srivastava, M.; Srivastava, S.K. Zinc oxide, gold and graphene-based surface plasmon resonance (SPR) biosensor for detection of pseudomonas like bacteria: A comparative study. Optik 2018, 172, 697–707. [Google Scholar] [CrossRef]
- Han, X.; Liu, K.; Sun, C. Plasmonics for biosensing. Materials 2019, 12, 1–24. [Google Scholar] [CrossRef]
- Yin, L.Y.; Huang, Y.H.; Wang, X.; Ning, S.T.; Liu, S.D. Double Fano resonances in nanoring cavity dimers: The effect of plasmon hybridization between dark subradiant modes. Aip Adv. 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.M.; Daniyal, W.M.E.M.M.; Mahdi, M.A. Sensitive surface plasmon resonance performance of cadmium sulphide quantum dots-amine functionalized graphene oxide based thin film towards dengue virus E-proteins. Opt. Laser Technol. 2019, 114, 204–208. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.M.; Mahdi, M.A. Structural, optical and sensing properties of CdS-NH2GO thin film as a dengue virus E-protein sensing material. Optik 2018, 171, 934–940. [Google Scholar] [CrossRef]
- Hoet, M.; Cohen, E.H.; Kent, R.B.; Rookey, K.; Schoonbroodt, S.; Hogan, S.; Rem, L.; Frans, N.; Daukandt, M.; Pieters, H.; et al. Generation of high-affinity human antibodies by combining donor-derived and synthetic complementarity-determining-region diversity. Nat. Biotechnol. 2005, 23, 344–348. [Google Scholar] [CrossRef]
- Salahudeen, M.S.; Nishtala, P.S. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi Pharm. J. 2017, 25, 165–175. [Google Scholar] [CrossRef]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef]
- Camara, A.R.; Gouvêa, P.M.; Dias, A.C.M.; Braga, A.M.; Dutra, R.F.; de Araujo, R.E.; Carvalho, I.C. Dengue immunoassay with an LSPR fiber optic sensor. Opt. Express 2013, 21, 27023–27031. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, I.T.; Silva, B.V.M.; Peres, N.G.; Moura, P.; Sotomayor, M.D.P.T.; Guedes, M.I.F.; Dutra, R.F. A disposable chitosan-modified carbon fiber electrode for dengue virus envelope protein detection. Talanta 2012, 91, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kamil, Y.M.; Bakar, M.A.; Mustapa, M.A.; Yaacob, M.H.; Abidin, N.H.Z.; Syahir, A.; Lee, H.J.; Mahdi, M.A. Label-free Dengue E protein detection using a functionalized tapered optical fiber sensor. Sens. Actuators B Chem. 2018, 257, 820–828. [Google Scholar] [CrossRef]
- Jahanshahi, P.; Wei, Q.; Jie, Z.; Ghomeishi, M.; Sekaran, S.D.; Mahamd Adikan, F.R. Kinetic analysis of IgM monoclonal antibodies for determination of dengue sample concentration using SPR technique. Bioengineered 2017, 8, 239–247. [Google Scholar] [CrossRef]

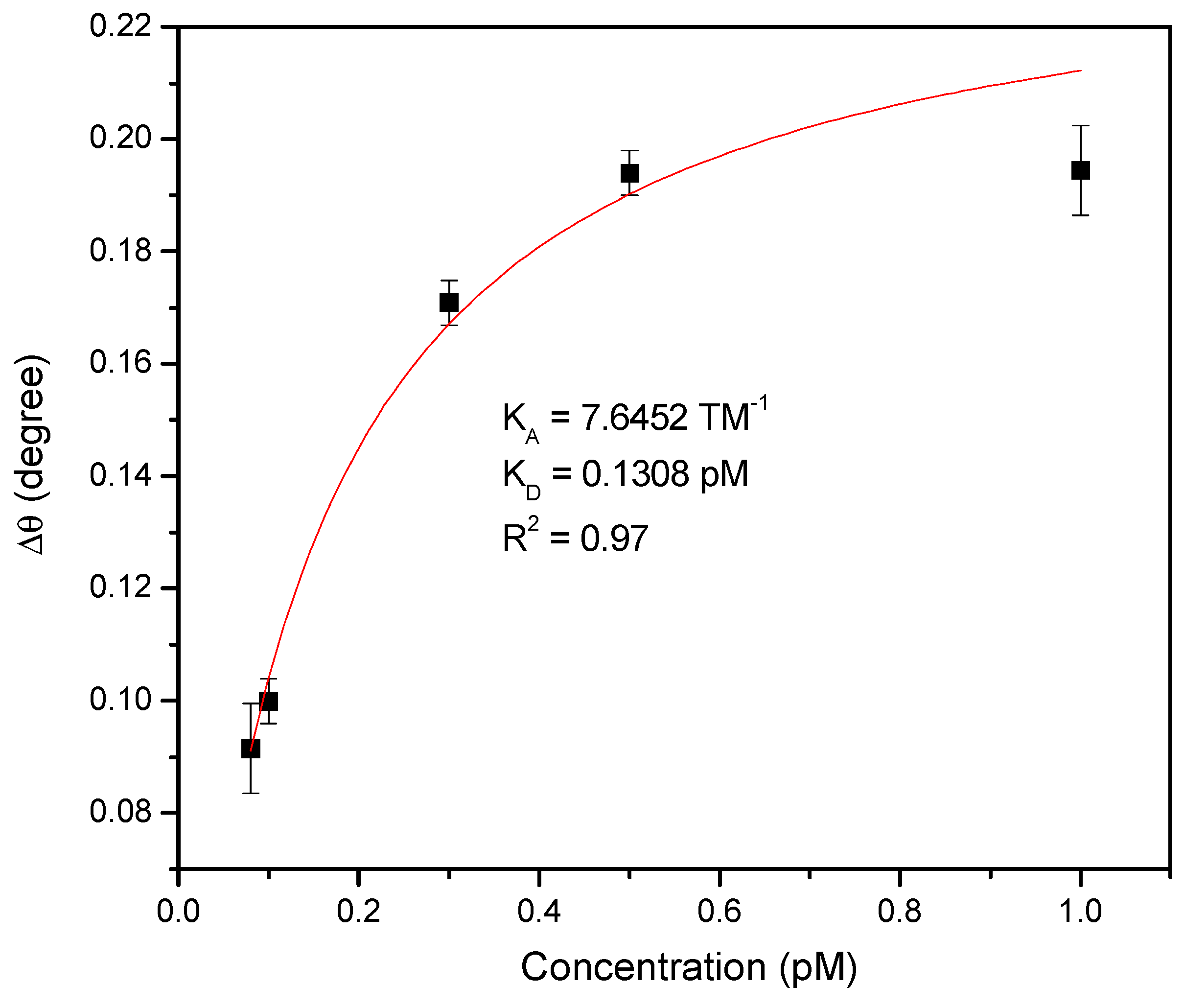

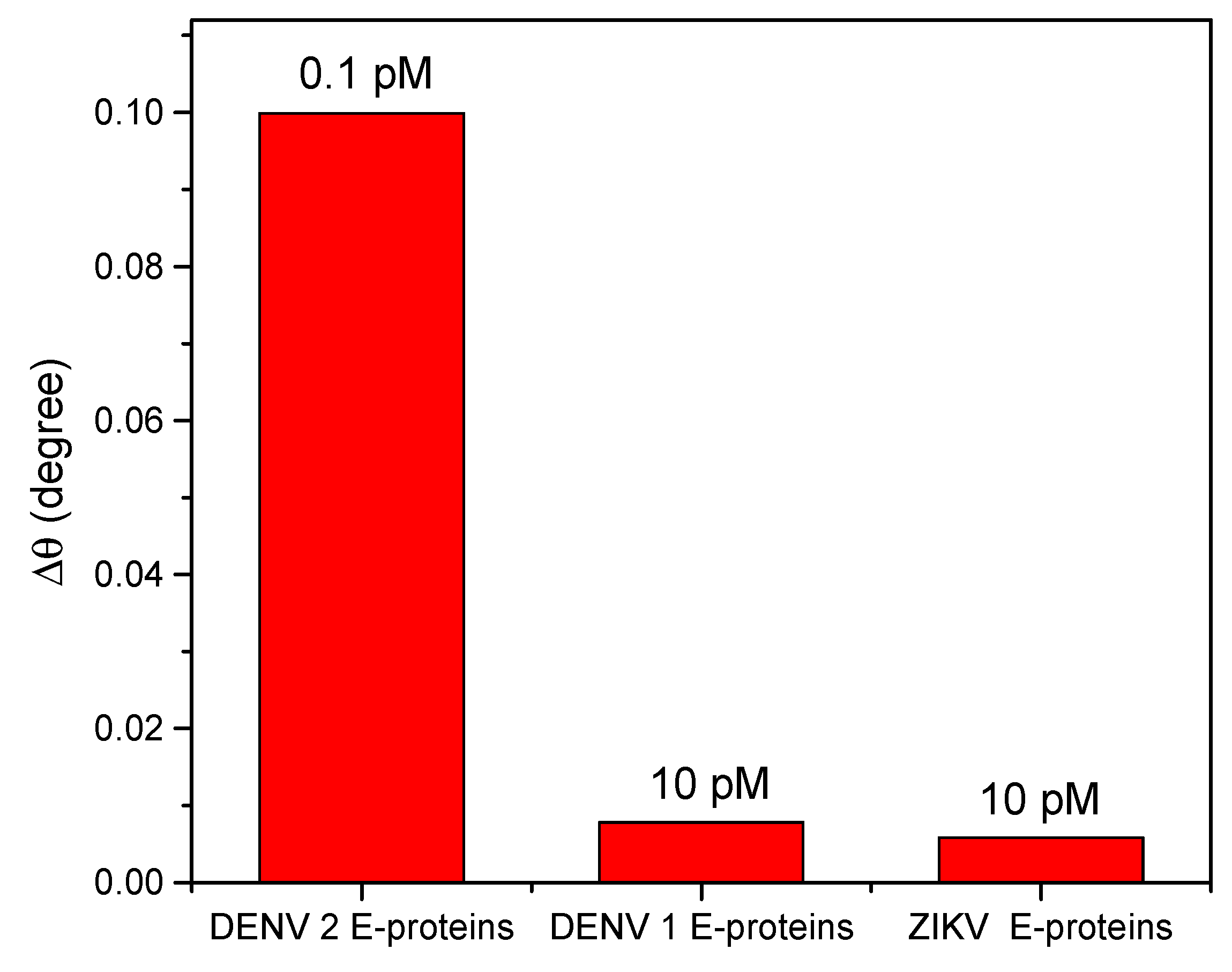
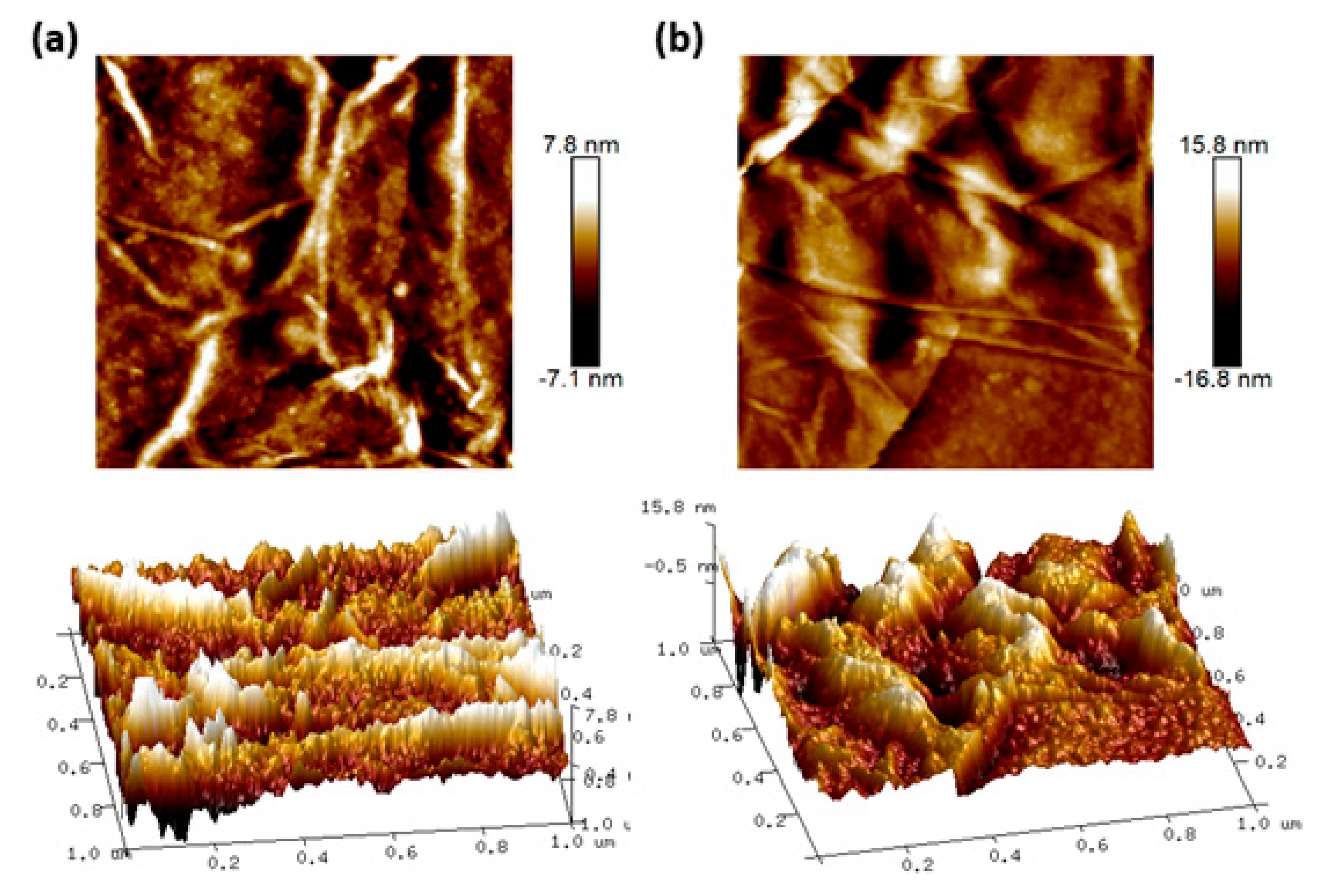
| Technique | Target | Sensitivity | Limit of Detection | Reference |
|---|---|---|---|---|
| ELISA | NS1 | - | 0.02 nM | 67 |
| LSPR | NS1 | 43 nm/(ng/mm2) | 1.54 nM | 68 |
| Rapid kits | NS1 | - | 0.1 nM | 49 |
| Electronic biosensor | E-proteins | - | 2.11 pM | 69 |
| Tapered fiber sensor | E-proteins | 5.02 nm/nM | 1 pM | 70 |
| SPR Biacore 3000 (automated) | IgM antibodies | 0.0132 pM/sec.−1 | 2.125 pM | 71 |
| Modified SPR sensor (custom-made) | E-proteins | 0.2576°/pM | 0.08 pM | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Mustapha Kamil, Y.; Fauzi, N.‘I.M.; Hashim, H.S.; Mahdi, M.A. Quantitative and Selective Surface Plasmon Resonance Response Based on a Reduced Graphene Oxide–Polyamidoamine Nanocomposite for Detection of Dengue Virus E-Proteins. Nanomaterials 2020, 10, 569. https://doi.org/10.3390/nano10030569
Omar NAS, Fen YW, Abdullah J, Sadrolhosseini AR, Mustapha Kamil Y, Fauzi N‘IM, Hashim HS, Mahdi MA. Quantitative and Selective Surface Plasmon Resonance Response Based on a Reduced Graphene Oxide–Polyamidoamine Nanocomposite for Detection of Dengue Virus E-Proteins. Nanomaterials. 2020; 10(3):569. https://doi.org/10.3390/nano10030569
Chicago/Turabian StyleOmar, Nur Alia Sheh, Yap Wing Fen, Jaafar Abdullah, Amir Reza Sadrolhosseini, Yasmin Mustapha Kamil, Nurul ‘Illya Muhamad Fauzi, Hazwani Suhaila Hashim, and Mohd Adzir Mahdi. 2020. "Quantitative and Selective Surface Plasmon Resonance Response Based on a Reduced Graphene Oxide–Polyamidoamine Nanocomposite for Detection of Dengue Virus E-Proteins" Nanomaterials 10, no. 3: 569. https://doi.org/10.3390/nano10030569
APA StyleOmar, N. A. S., Fen, Y. W., Abdullah, J., Sadrolhosseini, A. R., Mustapha Kamil, Y., Fauzi, N. ‘I. M., Hashim, H. S., & Mahdi, M. A. (2020). Quantitative and Selective Surface Plasmon Resonance Response Based on a Reduced Graphene Oxide–Polyamidoamine Nanocomposite for Detection of Dengue Virus E-Proteins. Nanomaterials, 10(3), 569. https://doi.org/10.3390/nano10030569






