Acid Dye Removal from Aqueous Solution by Using Neodymium(III) Oxide Nanoadsorbents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nd2O3 Nanoadsorbent
2.3. Characterizations
2.4. Adsorption/Desorption Experiments
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Techniques of Characterization
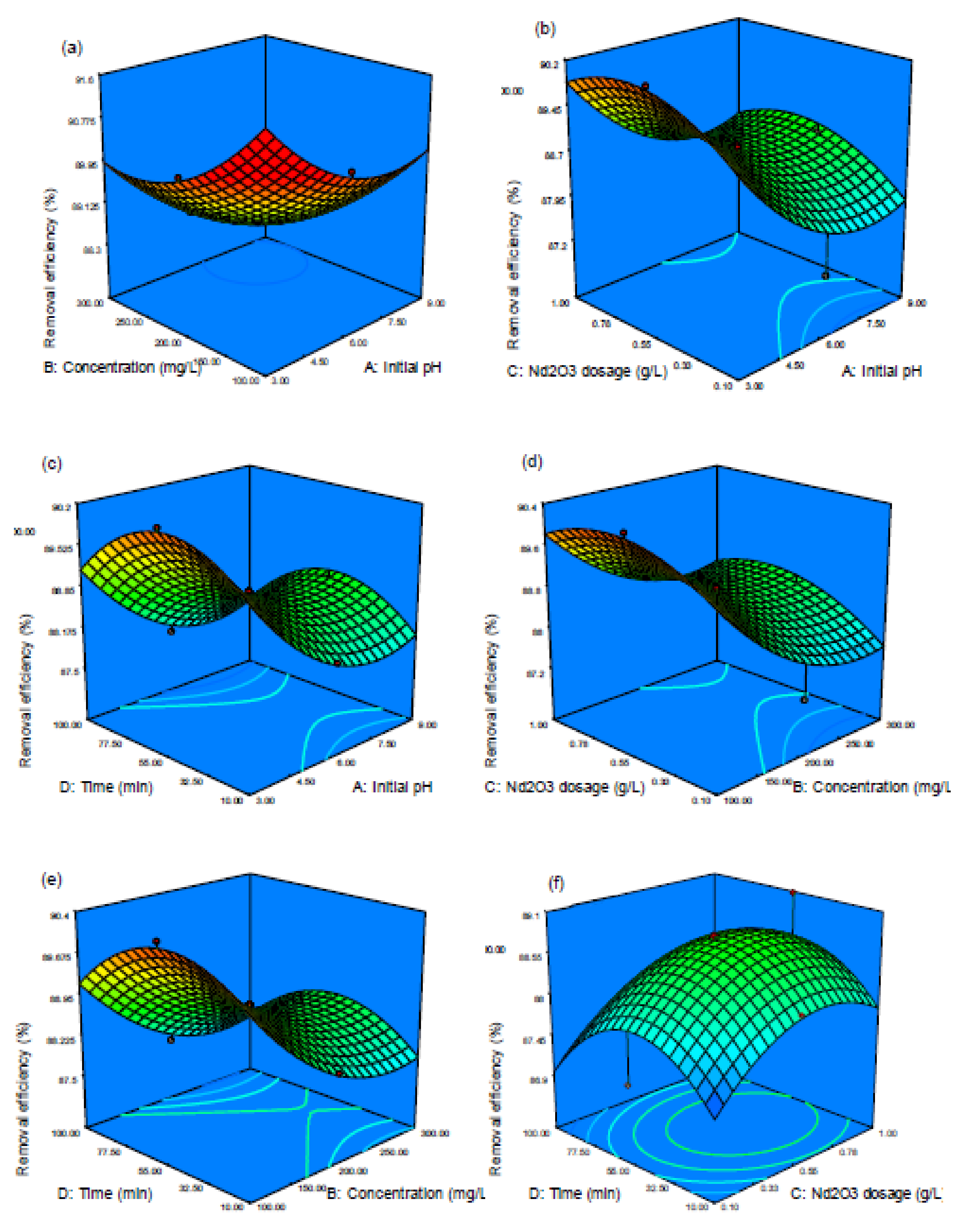
3.2. Model Fitting—Statistical Analysis—Adsorption Optimization
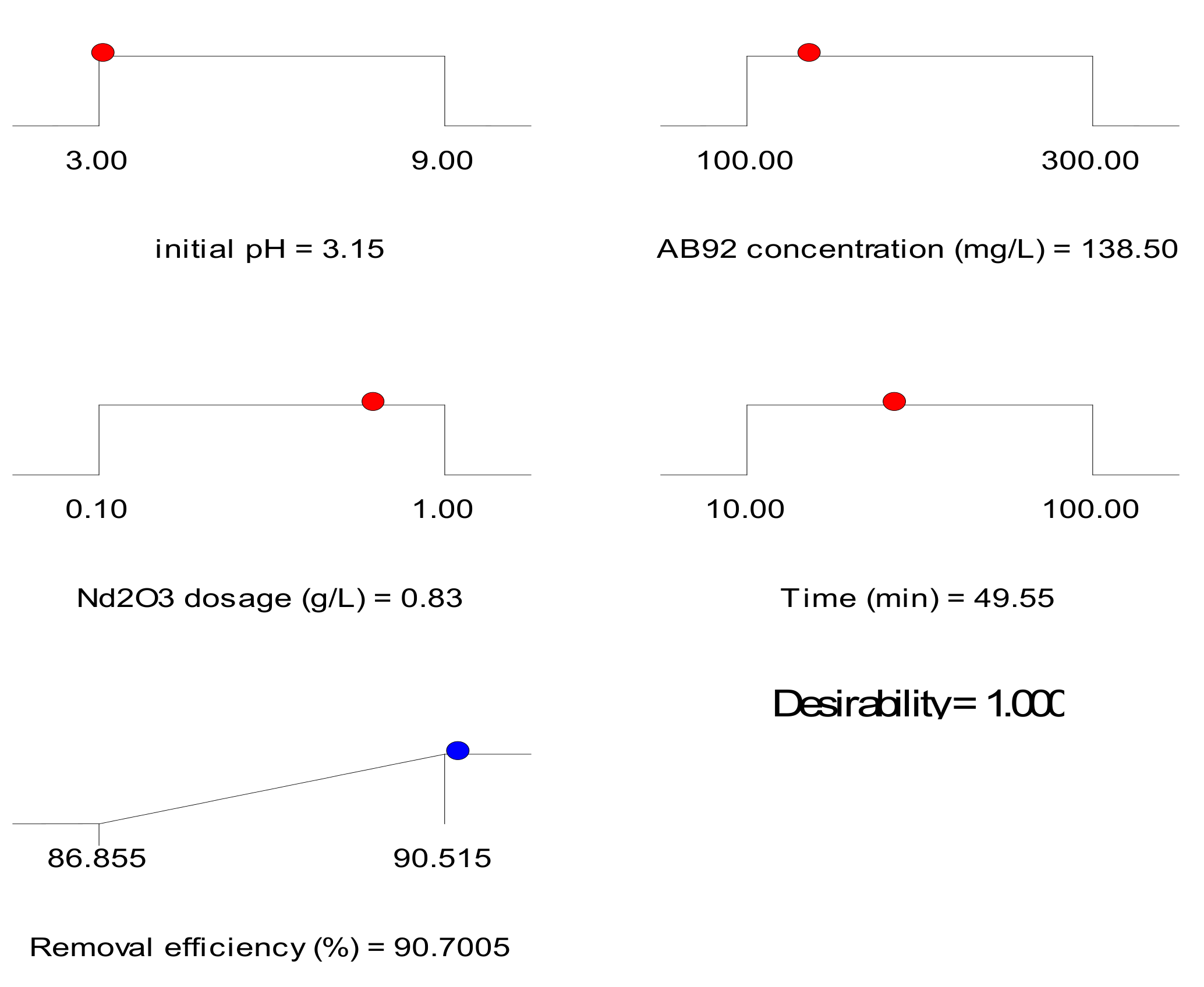
3.3. Optimization of Acid Blue 92 (AB92) Decolorization
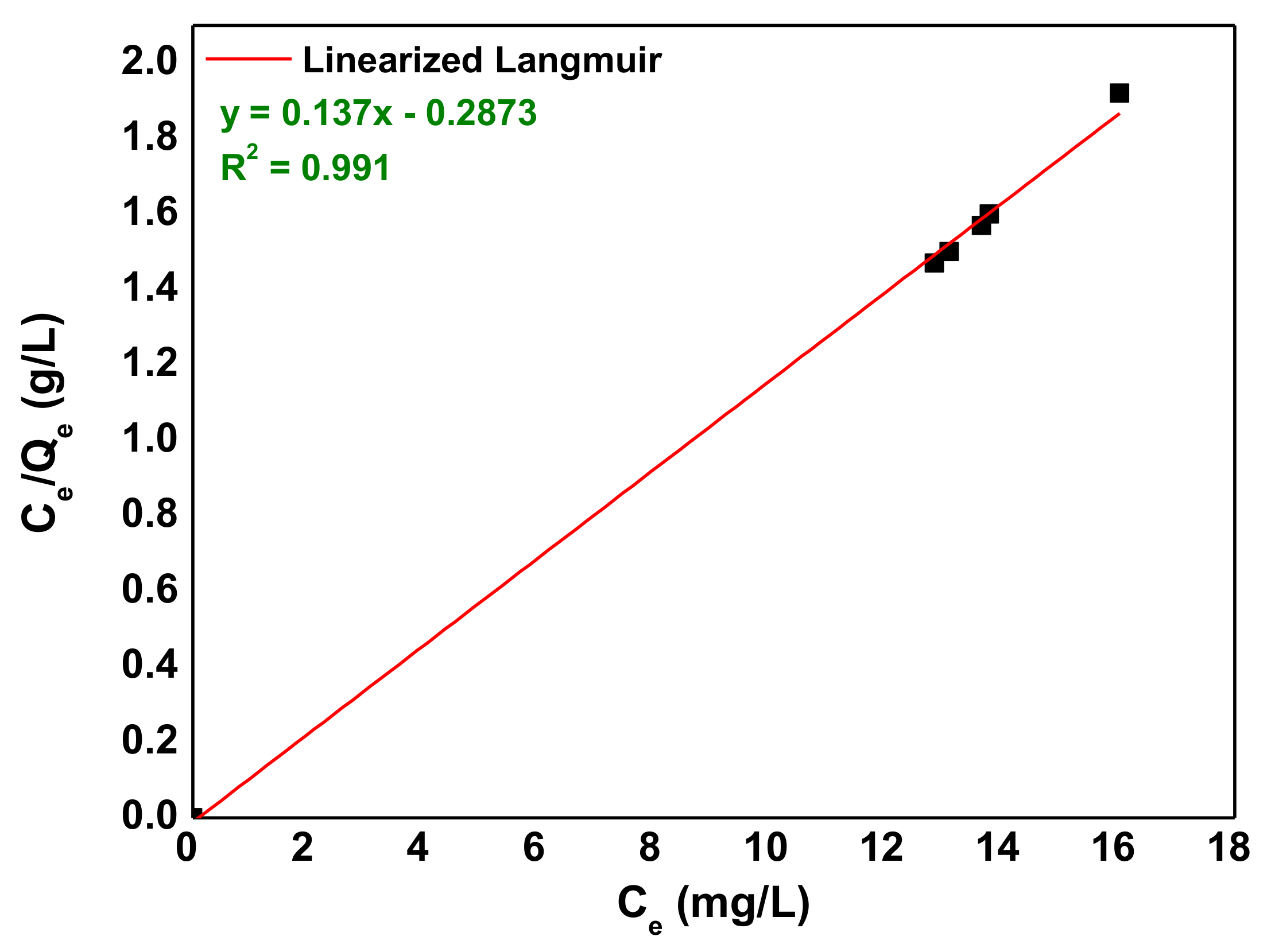
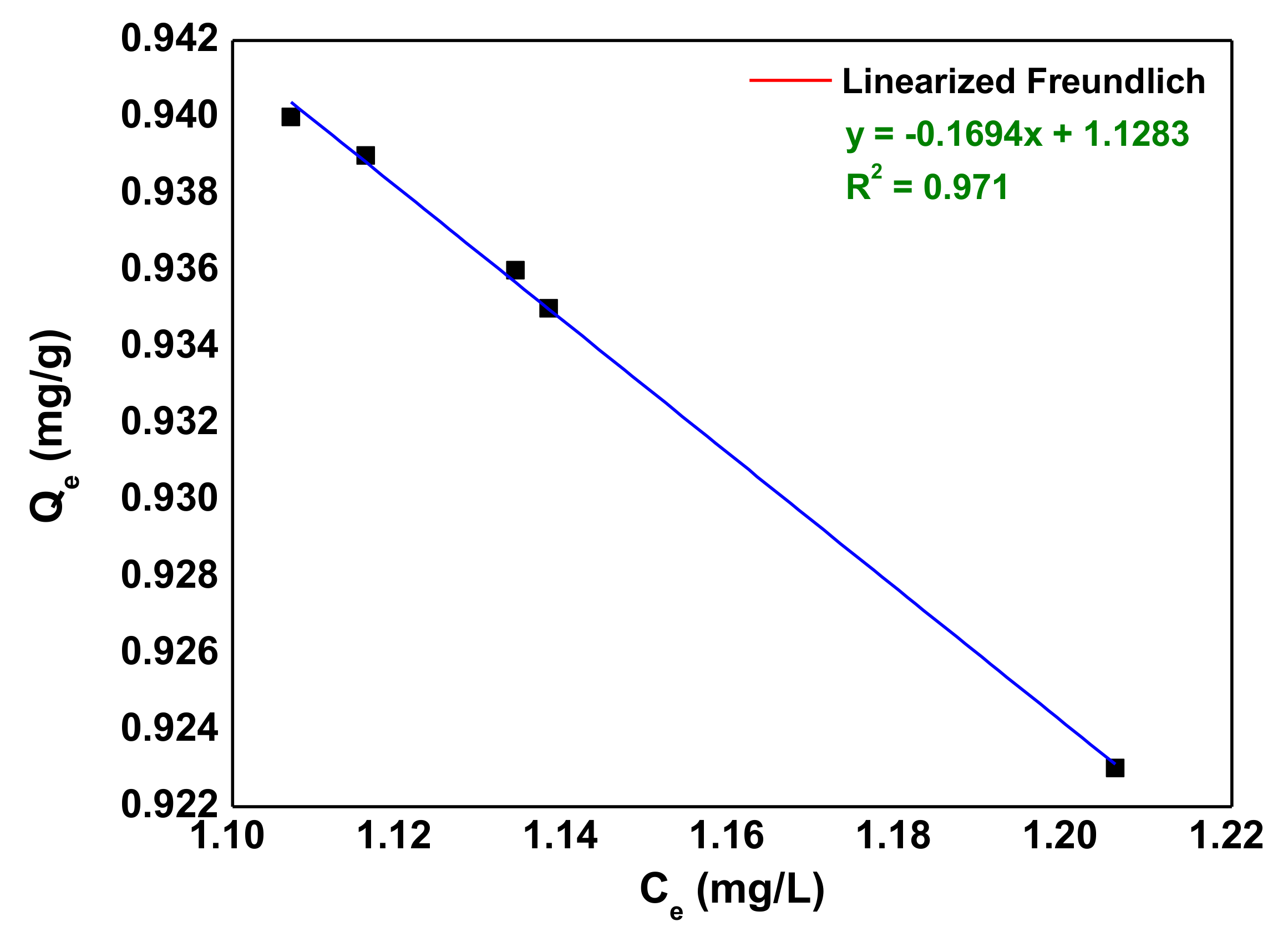
3.4. Adsorption Isotherms
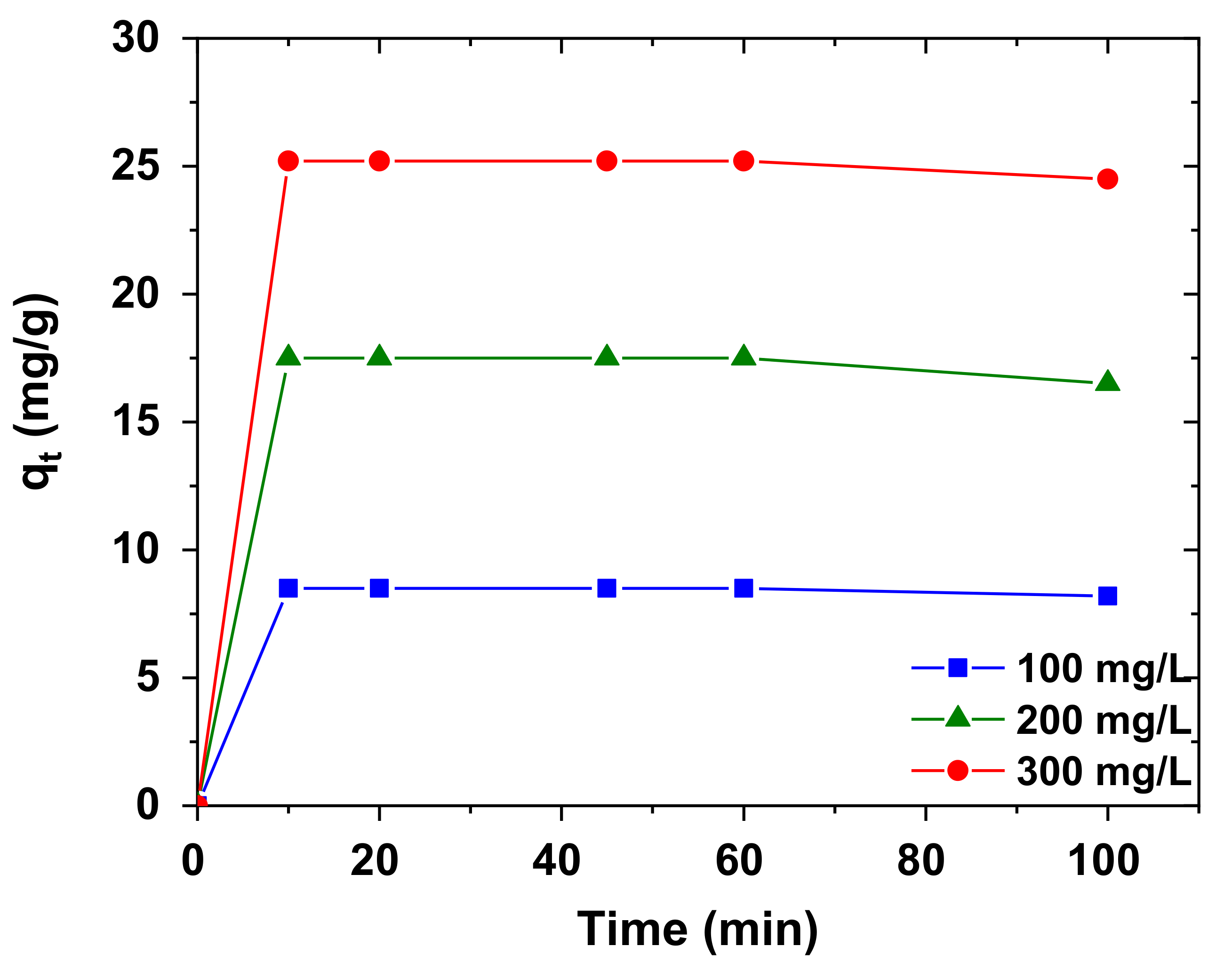
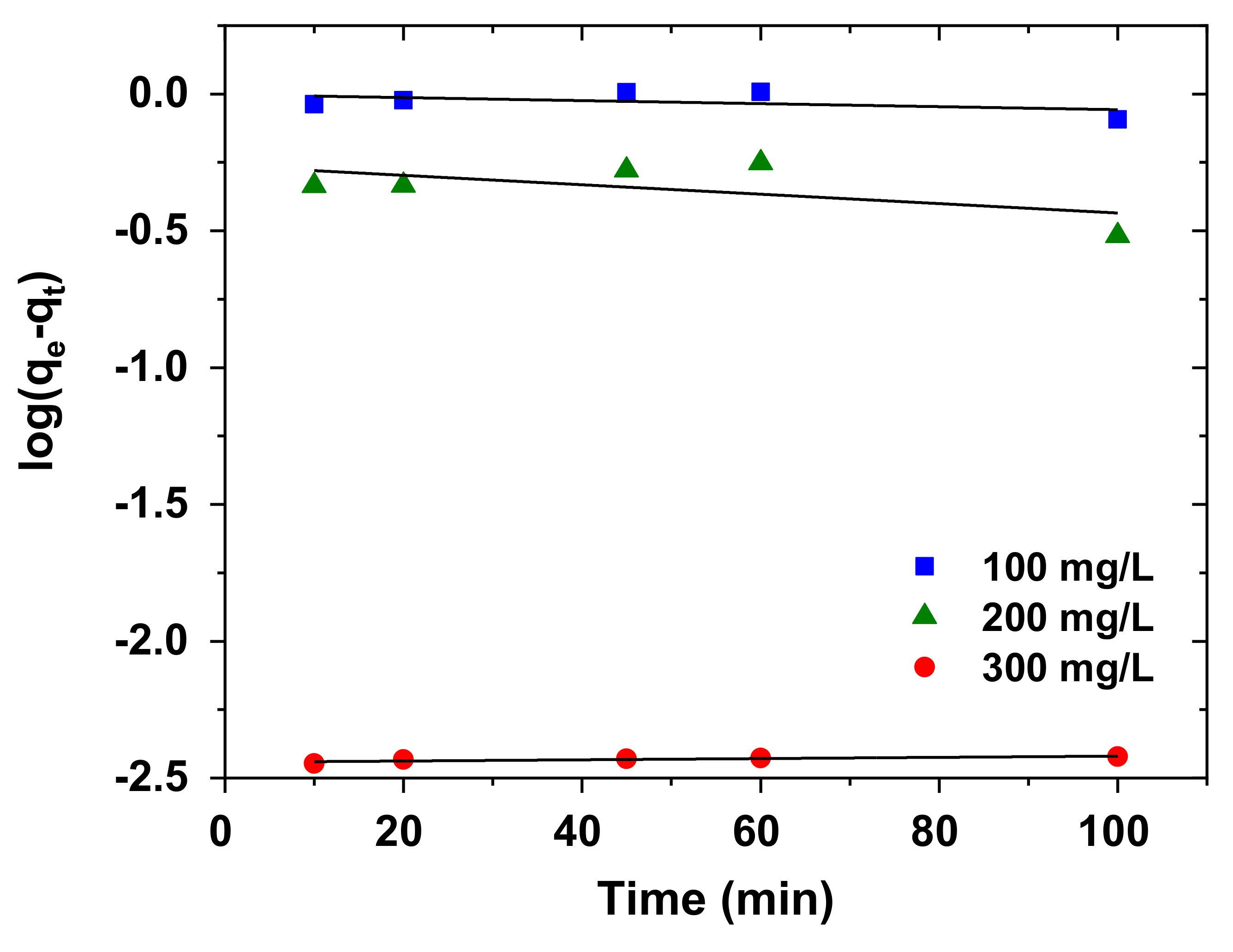
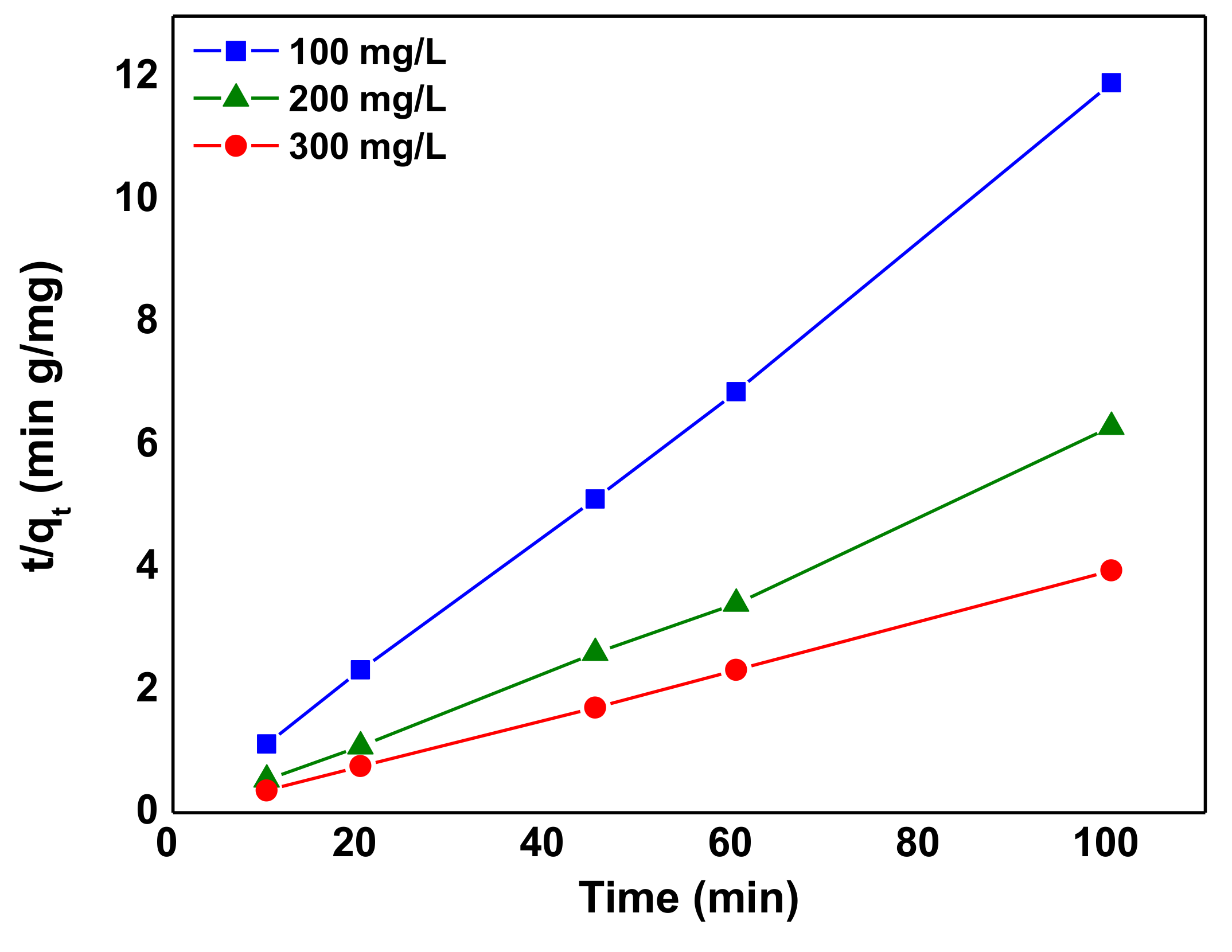
3.5. Kinetics of Adsorption
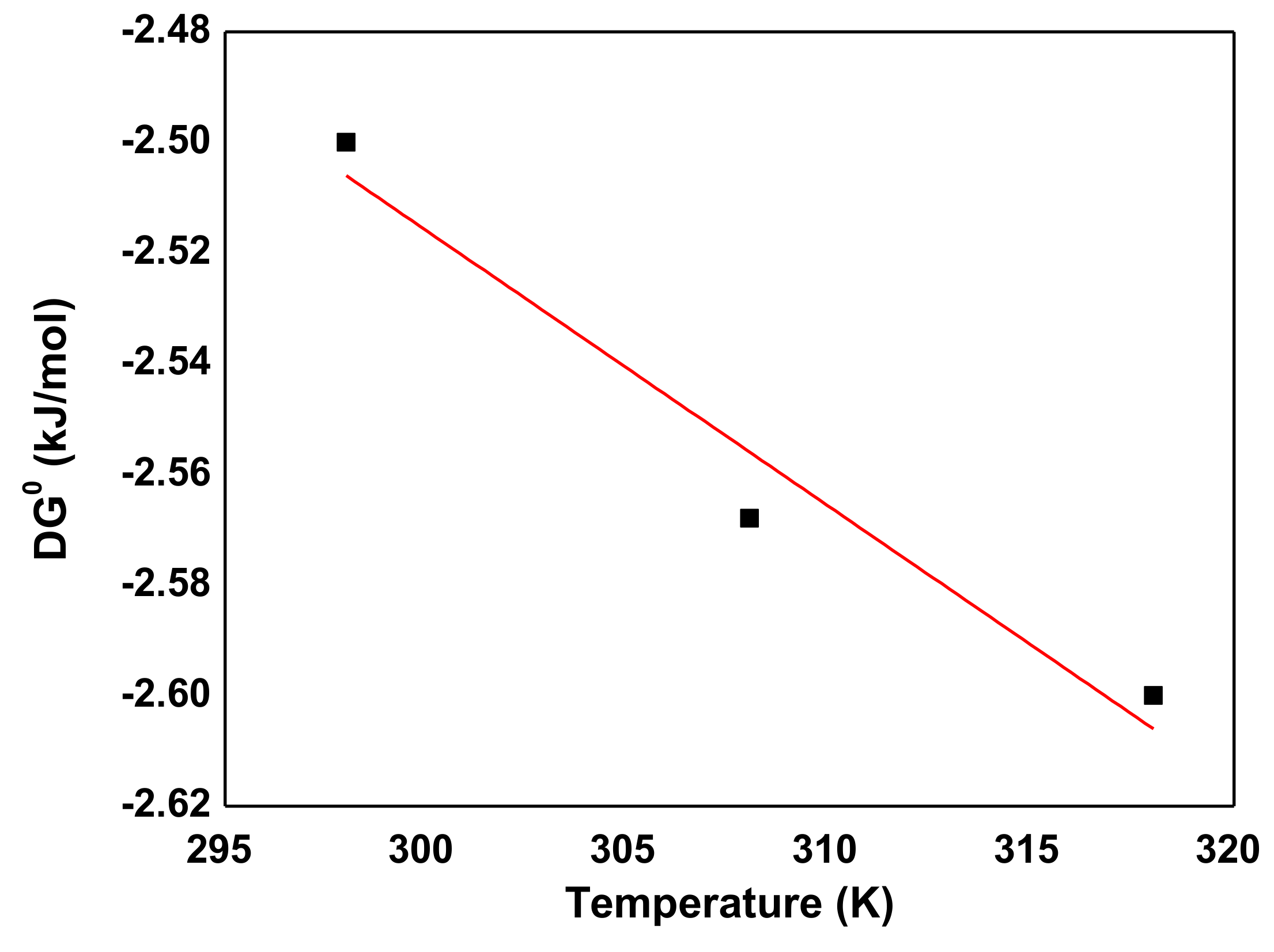
3.6. Thermodynamic Studies
3.7. Reuse
4. Brief Cost Analysis
4.1. Theoretical Approach
4.2. Application to the Present Nanomaterial
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, T.L. Decolourization of reactive azo dyes by transformation with Pseudomonas luteola. Bioresour. Technol. 1994, 49, 47–51. [Google Scholar] [CrossRef]
- Rahman, F. The treatment of industrial effluents for the discharge of textile dyes using by techniques and adsorbents. J. Text. Sci. Eng. 2016, 6, 1000242. [Google Scholar]
- Igwegbe, C.A.; Banach, A.M.; Ahmadi, S. Adsorption of Reactive Blue 19 from aqueous environment on magnesium oxide nanoparticles: Kinetic, isotherm and thermodynamic studies. Pharm. Chem. J. 2018, 5, 111–121. [Google Scholar]
- Jung, K.-W.; Choi, B.H.; Ahn, K.-H.; Lee, S.-H. Synthesis of a novel magnetic Fe3O4/γ-Al2O3 hybrid composite using electrode-alternation technique for the removal of an azo dye. Appl. Surf. Sci. 2017, 423, 383–393. [Google Scholar] [CrossRef]
- Ertugay, N.; Acar, F.N. Removal of COD and color from Direct Blue 71 azo dye wastewater by Fenton’s oxidation: Kinetic study. Arab. J. Chem. 2017, 10, S1158–S1163. [Google Scholar] [CrossRef]
- Sohrabi, M.R.; Khavaran, A.; Shariati, S.; Shariati, S. Removal of Carmoisine edible dye by Fenton and photo Fenton processes using Taguchi orthogonal array design. Arab. J. Chem. 2017, 10, S3523–S3531. [Google Scholar] [CrossRef]
- Cui, D.; Guo, Y.-Q.; Cheng, H.-Y.; Liang, B.; Kong, F.-Y.; Lee, H.-S.; Wang, A.-J. Azo dye removal in a membrane-free up-flow biocatalyzed electrolysis reactor coupled with an aerobic bio-contact oxidation reactor. J. Hazard. Mater. 2012, 239–240, 257–264. [Google Scholar] [CrossRef]
- Uzoh, C.F.; Onukwuli, O.D.; Nwabanne, J.T. Characterization, kinetics and statistical screening analysis of gmelina seed oil extraction process. Mater. Renew. Sustain. Energy 2014, 3, 38. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kamel, A.H.; Hassan, A.A.; Amr, A.E.G.E.; El-Naby, H.A.; Elsayed, E.A. A sno2/ceo2 nano-composite catalyst for alizarin dye removal from aqueous solutions. Nanomaterials 2020, 10, 254. [Google Scholar] [CrossRef]
- Qin, Q.; Sun, T.; Wang, H.; Brault, P.; An, H.; Xie, L.; Peng, Q. Adsorption and diffusion of hydrogen in carbon honeycomb. Nanomaterials 2020, 10, 344. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Bi, J.; Huang, X.; Mao, Y.; Luo, L.; Hao, H. Partial oxidation strategy to synthesize WS2/WO3 heterostructure with enhanced adsorption performance for organic dyes: Synthesis, modelling, and mechanism. Nanomaterials 2020, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, H.; Ma, C.; Han, Q.; Li, M.; Liu, H. Preparation of Fe3O4@polyoxometalates nanocomposites and their efficient adsorption of cationic dyes from aqueous solution. Nanomaterials 2019, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Hu, S.; Liu, J.; Zhu, Q.; Zhang, S. Synthesis of hierarchical porous carbon in molten salt and its application for dye adsorption. Nanomaterials 2019, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Lazzara, G.; Merli, M.; Milioto, S.; Princivalle, F.; Sciascia, L. Simultaneous removal and recovery of metal ions and dyes from wastewater through montmorillonite clay mineral. Nanomaterials 2019, 9, 1699. [Google Scholar] [CrossRef]
- Rupa, E.J.; Kaliraj, L.; Abid, S.; Yang, D.C.; Jung, S.K. Synthesis of a zinc oxide nanoflower photocatalyst from sea buckthorn fruit for degradation of industrial dyes in wastewater treatment. Nanomaterials 2019, 9, 1692. [Google Scholar] [CrossRef]
- Sabeela, N.I.; Almutairi, T.M.; Al-Lohedan, H.A.; Ezzat, A.O.; Atta, A.M. Reactive mesoporous ph-sensitive amino-functionalized silica nanoparticles for efficient removal of coomassie blue dye. Nanomaterials 2019, 9, 1721. [Google Scholar] [CrossRef]
- Wang, C.; Yin, J.; Wang, R.; Jiao, T.; Huang, H.; Zhou, J.; Zhang, L.; Peng, Q. Facile preparation of self-assembled polydopamine-modified electrospun fibers for highly effective removal of organic dyes. Nanomaterials 2019, 9, 116. [Google Scholar] [CrossRef]
- Zhan, S.; Li, C.; Tian, H.; Ma, C.; Liu, H.; Luo, J.; Li, M. Synthesis, characterization and dye removal behavior of core–shell–shell Fe3O4/Ag/polyoxometalates ternary nanocomposites. Nanomaterials 2019, 9, 1255. [Google Scholar] [CrossRef]
- Shu, H.-Y.; Huang, C.-R.; Chang, M.-C. Decolorization of mono-azo dyes in wastewater by advanced oxidation process: A case study of acid red 1 and acid yellow 23. Chemosphere 1994, 29, 2597–2607. [Google Scholar] [CrossRef]
- Elisangela, F.; Andrea, Z.; Fabio, D.G.; de Menezes Cristiano, R.; Regina, D.L.; Artur, C.-P. Biodegradation of textile azo dyes by a facultative Staphylococcus arlettae strain VN-11 using a sequential microaerophilic/aerobic process. Int. Biodeterior. Biodegrad. 2009, 63, 280–288. [Google Scholar] [CrossRef]
- Rahdar, S.; Shikh, L.; Ahmadi, S. Removal of Reactive Blue 19 Dye Using a Combined Sonochemical and Modified Pistachio Shell Adsorption Processes from Aqueous Solutions. Iran. J. Health Sci. 2018, 6, 8–20. [Google Scholar] [CrossRef]
- Obiora-Okafo, I.A.; Onukwuli, O.D. Characterization and optimization of spectrophotometric colour removal from dye containing wastewater by Coagulation-Flocculation. Pol. J. Chem. Technol. 2018, 20, 49–59. [Google Scholar] [CrossRef]
- Obiora-Okafo, I.A.; Onukwuli, O.D. Optimization of coagulation-flocculation process for colour removal from azo dye using natural polymers: Response surface methodological approach. Niger. J. Technol. 2017, 36, 482–495. [Google Scholar] [CrossRef]
- Homaeigohar, S. The Nanosized Dye Adsorbents for Water Treatment. Nanomaterials 2020, 10, 295. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Botcha, N.K.; Zarie, E.S.; Elbahri, M. Ups and Downs of Water Photodecolorization by Nanocomposite Polymer Nanofibers. Nanomaterials 2019, 9, 250. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. An Amphiphilic, Graphitic Buckypaper Capturing Enzyme Biomolecules from Water. Water 2018, 11, 2. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Strunskus, T.; Strobel, J.; Kienle, L.; Elbahri, M. A Flexible Oxygenated Carbographite Nanofilamentous Buckypaper as an Amphiphilic Membrane. Adv. Mater. Interfaces 2018, 5, 1800001. [Google Scholar] [CrossRef]
- Ahmadi, S.; Igwegbe, C.A. Adsorptive removal of phenol and aniline by modified bentonite: Adsorption isotherm and kinetics study. Appl. Water Sci. 2018, 8, 170. [Google Scholar] [CrossRef]
- Zafar, M.N.; Dar, Q.; Nawaz, F.; Zafar, M.N.; Iqbal, M.; Nazar, M.F. Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 2019, 8, 713–725. [Google Scholar] [CrossRef]
- Guo, R.; Wang, R.; Yin, J.; Jiao, T.; Huang, H.; Zhao, X.; Zhang, L.; Li, Q.; Zhou, J.; Peng, Q. Fabrication and highly efficient dye removal characterization of beta-cyclodextrin-based composite polymer fibers by electrospinning. Nanomaterials 2019, 9, 127. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Xiao, A.; Qiu, H.; Liu, L. Preparation of magnetic Fe3O4/MIL-88A nanocomposite and its adsorption properties for bromophenol blue dye in aqueous solution. Nanomaterials 2019, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, S.; Chen, X.; Mijowska, E. Evaluation of nanoporous carbon synthesized from direct carbonization of a metal-organic complex as a highly effective dye adsorbent and supercapacitor. Nanomaterials 2019, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, L.; Sun, M.; Paul Chen, J. Facile synthesis of highly active hydrated yttrium oxide towards arsenate adsorption. J. Colloid Interface Sci. 2016, 474, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Xiong, G.; Sheng, S.; Cui, W.; Xu, L.; Guo, X. Partial oxidation of methane to syngas over nickel-based catalysts modified by alkali metal oxide and rare earth metal oxide. Appl. Catal. A Gen. 1997, 154, 17–27. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Schreiner, B. Separation Hydrometallurgy of Rare Earth Elements; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Zinatloo-Ajabshir, S.; Mortazavi-Derazkola, S.; Salavati-Niasari, M. Schiff-base hydrothermal synthesis and characterization of Nd2O3 nanostructures for effective photocatalytic degradation of eriochrome black T dye as water contaminant. J. Mater. Sci. Mater. Electron. 2017, 28, 17849–17859. [Google Scholar] [CrossRef]
- Sime, M.; Das, A.; Galt, G.; Hope, G.; Young, C. Studies on the nature of salicyl hydroxamate adsorption at the surface of neodymium oxide. J. Dispers. Sci. Technol. 2019, 40, 1488–1498. [Google Scholar] [CrossRef]
- Ahmad, Z.U.; Yao, L.; Wang, J.; Gang, D.D.; Islam, F.; Lian, Q.; Zappi, M.E. Neodymium embedded ordered mesoporous carbon (OMC) for enhanced adsorption of sunset yellow: Characterizations, adsorption study and adsorption mechanism. Chem. Eng. J. 2019, 359, 814–826. [Google Scholar] [CrossRef]
- Hafshejani, M.K.; Ogugbue, C.J.; Morad, N. Application of response surface methodology for optimization of decolorization and mineralization of triazo dye Direct Blue 71 by Pseudomonas aeruginosa. 3 Biotech 2014, 4, 605–619. [Google Scholar] [CrossRef]
- Beyki, M.H.; Alijani, H.; Fazli, Y. Optimization using response surface methodology for fast removal of hazardous azo dye by γ-Fe2O3@ CuO nanohybrid synthesized by sol-gel combustion. Res. Chem. Intermed. 2017, 43, 6245–6257. [Google Scholar] [CrossRef]
- Goodman, S.M.; Bura, R.; Dichiara, A.B. Facile Impregnation of Graphene into Porous Wood Filters for the Dynamic Removal and Recovery of Dyes from Aqueous Solutions. ACS Appl. Nano Mater. 2018, 1, 5682–5690. [Google Scholar] [CrossRef]
- Mortazavi-Derazkola, S.; Zinatloo-Ajabshir, S.; Salavati-Niasari, M. Preparation and characterization of Nd2O3 nanostructures via a new facile solvent-less route. J. Mater. Sci. Mater. Electron. 2015, 26, 5658–5667. [Google Scholar] [CrossRef]
- Azami, M.; Bahram, M.; Nouri, S. Central composite design for the optimization of removal of the azo dye, Methyl red, from waste water using Fenton reaction. Curr. Chem. Lett. 2013, 2, 57–68. [Google Scholar] [CrossRef]
- Vaez, M.; Zarringhalam Moghaddam, A.; Alijani, S. Optimization and Modeling of Photocatalytic Degradation of Azo Dye Using a Response Surface Methodology (RSM) Based on the Central Composite Design with Immobilized Titania Nanoparticles. Ind. Eng. Chem. Res. 2012, 51, 4199–4207. [Google Scholar] [CrossRef]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Isotherms, kinetics and thermodynamic studies of adsorption of Cu2+ from aqueous solutions by Mg2+/K+ type orange peel adsorbents. J. Hazard. Mater. 2010, 174, 756–762. [Google Scholar] [CrossRef]
- Asaithambi, P.; Beyene, D.; Aziz, A.R.A.; Alemayehu, E. Removal of pollutants with determination of power consumption from landfill leachate wastewater using an electrocoagulation process: Optimization using response surface methodology (RSM). Appl. Water Sci. 2018, 8, 69. [Google Scholar] [CrossRef]
- Ponnusamy, S.K.; Subramaniam, R. Process optimization studies of Congo red dye adsorption onto cashew nut shell using response surface methodology. Int. J. Ind. Chem. 2013, 4, 17. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mohammadi, L.; Igwegbe, C.A.; Rahdar, S.; Banach, A.M. Application of response surface methodology in the degradation of Reactive Blue 19 using H2O2/MgO nanoparticles advanced oxidation process. Int. J. Ind. Chem. 2018, 9, 241–253. [Google Scholar] [CrossRef]
- Tetteh, E.; Rathilal, S.; Chollom, M. Treatment of industrial mineral oil wastewater-optimisation of coagulation flotation process using response surface methodology (RSM). Int. J. Appl. Eng. Res. 2017, 12, 13084–13091. [Google Scholar]
- Kyzas, G.Z.; Deliyanni, E.A.; Bikiaris, D.N.; Mitropoulos, A.C. Graphene composites as dye adsorbents: Review. Chem. Eng. Res. Des. 2018, 129, 75–88. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.C.; Kyzas, G.Z. A review for coffee adsorbents. J. Mol. Liq. 2017, 229, 555–565. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Kyzas, G.Z.; Triantafyllidis, K.S.; Matis, K.A. Activated carbons for the removal of heavy metal ions: A systematic review of recent literature focused on lead and arsenic ions. Open Chem. 2015, 13, 699–708. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mostafapour, F.K. Adsorptive removal of aniline from aqueous solutions by Pistacia atlantica (Baneh) shells: Isotherm and kinetic studies. J. Sci. Technol. Environ. Inform. 2017, 5, 327–335. [Google Scholar] [CrossRef]
- Rahdar, S. Removal of phenol and aniline from aqueous solutions by using adsorption on to pistacia terebinthus: Study of adsorption isotherm and kinetics. J. Health Res. Community Winter 2017, 2, 35–45. [Google Scholar]
- Anastopoulos, I.; Kyzas, G.Z. Composts as biosorbents for decontamination of various pollutants: A review. Water Air Soil Pollut. 2015, 226, 61. [Google Scholar] [CrossRef]
- Godini, H.; Dargahi, A.; Mohammadi, M.; Hosseini Ahagh, M.; Mohammadi, S.; Jalilian, Z. Application of Response Surface Methodology for Optimization of Ammonia Nitrogen Removal from Aqueous Solutions Using Powdered Activated Carbon. Res. J. Environ. Sci. 2017, 11, 36–47. [Google Scholar]
- Roy, A.; Chakraborty, S.; Kundu, S.P.; Adhikari, B.; Majumder, S.B. Adsorption of Anionic-Azo Dye from Aqueous Solution by Lignocellulose-Biomass Jute Fiber: Equilibrium, Kinetics, and Thermodynamics Study. Ind. Eng. Chem. Res. 2012, 51, 12095–12106. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1916, 40, 1361–1368. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- LeVan, M.D.; Vermeulen, T. Binary Langmuir and Freundlich isotherms for ideal adsorbed solutions. J. Phys. Chem. 1981, 85, 3247–3250. [Google Scholar] [CrossRef]
- Sheindorf, C.; Rebhun, M.; Sheintuch, M. A Freundlich-type multicomponent isotherm. J. Colloid Interface Sci. 1981, 79, 136–142. [Google Scholar] [CrossRef]
- Malik, P.K. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: A case study of Acid Yellow 36. Dye. Pigm. 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Wang, C.C. Pseudo-isotherms for the sorption of cadmium ion onto tree fern. Process Biochem. 2004, 39, 761–765. [Google Scholar] [CrossRef]
- Igwegbe, C.; Onukwuli, O.; Nwabanne, J. Adsorptive Removal of Vat Yellow 4 on Activated Mucuna pruriens (Velvet Bean) Seed Shells Carbon. Asian J. Chem. Sci. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sharma, A. Kinetics and thermodynamics of Methylene Blue adsorption on Neem (Azadirachta indica) leaf powder. Dye. Pigm. 2005, 65, 51–59. [Google Scholar] [CrossRef]
- Giles, C.H.; McIver, N. Adsorption of dyes and the gibbs equation. An investigation by the monolayer technique. J. Colloid Interface Sci. 1977, 62, 329–335. [Google Scholar] [CrossRef]
- Ooi, J.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Lim, S.S.; Gan, S. Assessment of fish scales waste as a low cost and eco-friendly adsorbent for removal of an azo dye: Equilibrium, kinetic and thermodynamic studies. Bioresour. Technol. 2017, 245, 656–664. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Miyake, Y. Adsorption of PCE from soil vapors extraction on activated carbon fiber. J. Resour. Envrion. 1997, 33, 395–401. [Google Scholar]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A review. J. Environ. Manag. 2010, 91, 1915–1929. [Google Scholar] [CrossRef] [PubMed]
- Tsezos, M. Biosorption of metals. The experience accumulated and the outlook for technology development. Hydrometallurgy 2001, 59, 241–243. [Google Scholar] [CrossRef]
- Garnham, G.W. The Use of Algae as Metal Biosorbents. In Biosorbents for metal ions; Wase, J., Forster, C., Eds.; CRC Press: London, UK, 1997; pp. 11–37. [Google Scholar]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, F.; Erto, A.; Lancia, A.; Musmarra, D. Mercury adsorption on granular activated carbon in aqueous solutions containing nitrates and chlorides. J. Hazard. Mater. 2011, 192, 1842–1850. [Google Scholar] [CrossRef]
- Vreysen, S.; Maes, A.; Wullaert, H. Removal of organotin compounds, Cu and Zn from shipyard wastewaters by adsorption–flocculation: A technical and economical analysis. Mar. Pollut. Bull. 2008, 56, 106–115. [Google Scholar] [CrossRef]
- Kyzas, G.Z. A decolorization technique with spent "Greek coffee" grounds as zero-cost adsorbents for industrial textile wastewaters. Materials 2012, 5, 2069–2087. [Google Scholar] [CrossRef]
- Kyzas, G.Z. Commercial coffee wastes as materials for adsorption of heavy metals from aqueous solutions. Materials 2012, 5, 1826–1840. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K.; Mitropoulos, A.C. Removal of dyes from aqueous solutions with untreated coffee residues as potential low-cost adsorbents: Equilibrium, reuse and thermodynamic approach. Chem. Eng. J. 2012, 189–190, 148–159. [Google Scholar] [CrossRef]
- O’Neill, C.; Hawkes, F.R.; Hawkes, D.L.; Lourenco, N.D.; Pinheiro, H.M.; Delee, W. Colour in textile effluents—Sources, measurement, discharge consents and simulation: A review. J. Chem. Technol. Biotechnol. 1999, 74, 1009–1018. [Google Scholar] [CrossRef]
- Patterson, J.W. Industrial Wastewater Treatment Technology, 2nd ed.; Butterworth-Heinemann: London, UK, 1985. [Google Scholar]
- Gkika, A.D.; Liakos, V.E.; Vordos, N.; Kontogoulidou, C.; Magafas, L.; Bikiaris, N.D.; Bandekas, V.D.; Mitropoulos, C.A.; Kyzas, Z.G. Cost Estimation of Polymeric Adsorbents. Polymers 2019, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Public Power Corporation S.A.-Hellas. Available online: https://www.dei.gr/en (accessed on 1 January 2020).
- Glassdoor S.A. Available online: https://www.glassdoor.co.uk/ (accessed on 1 January 2020).


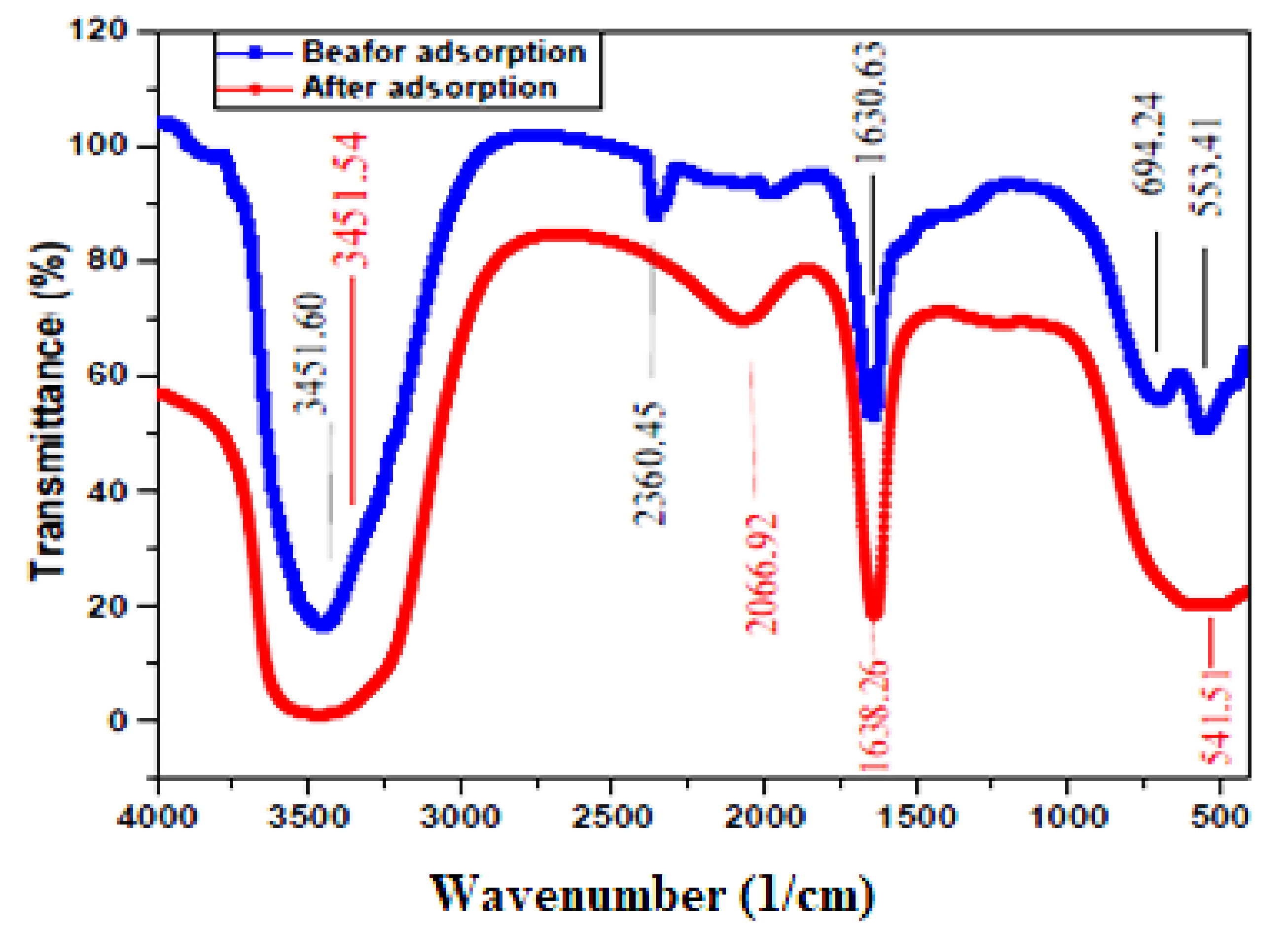
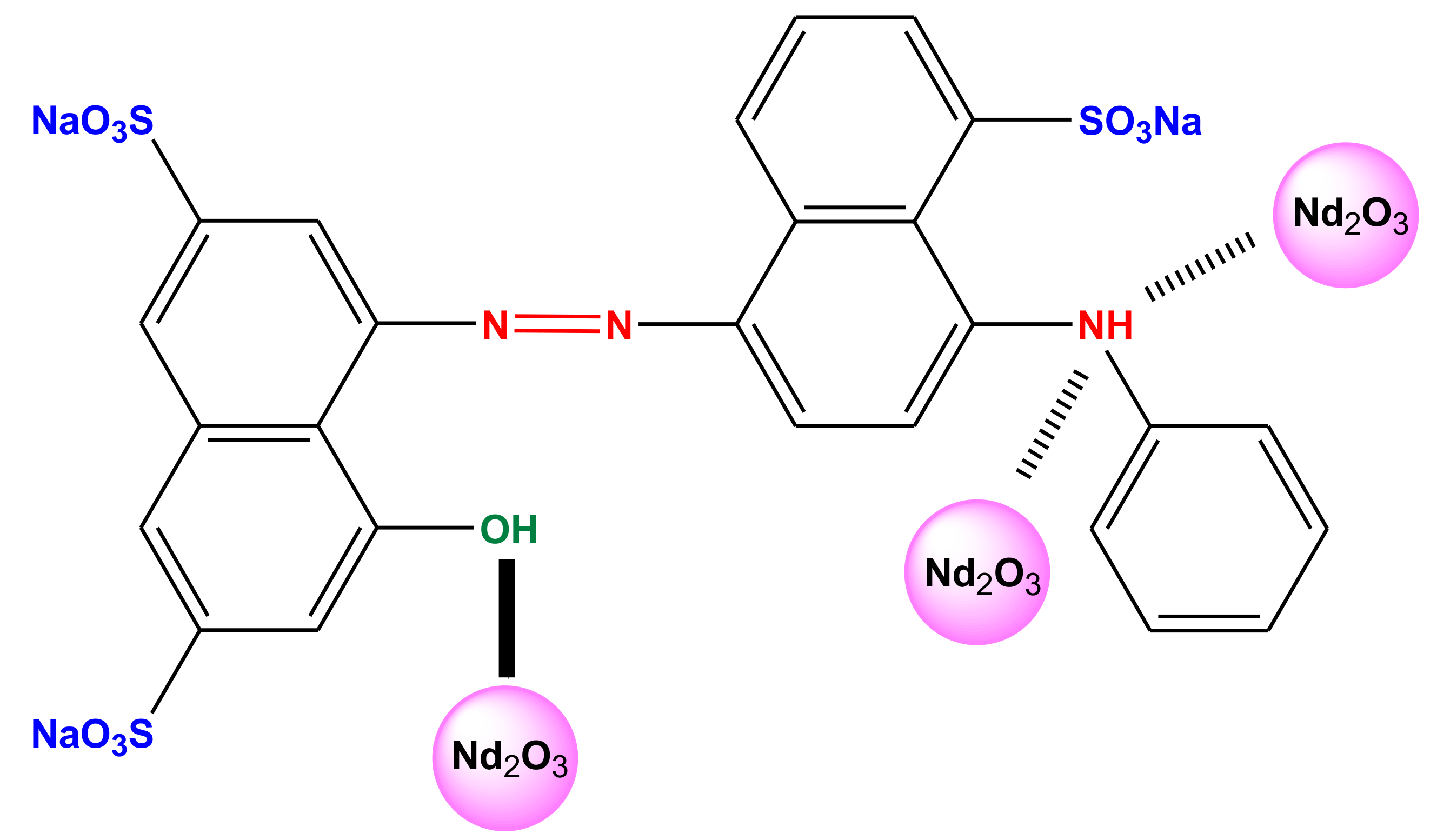
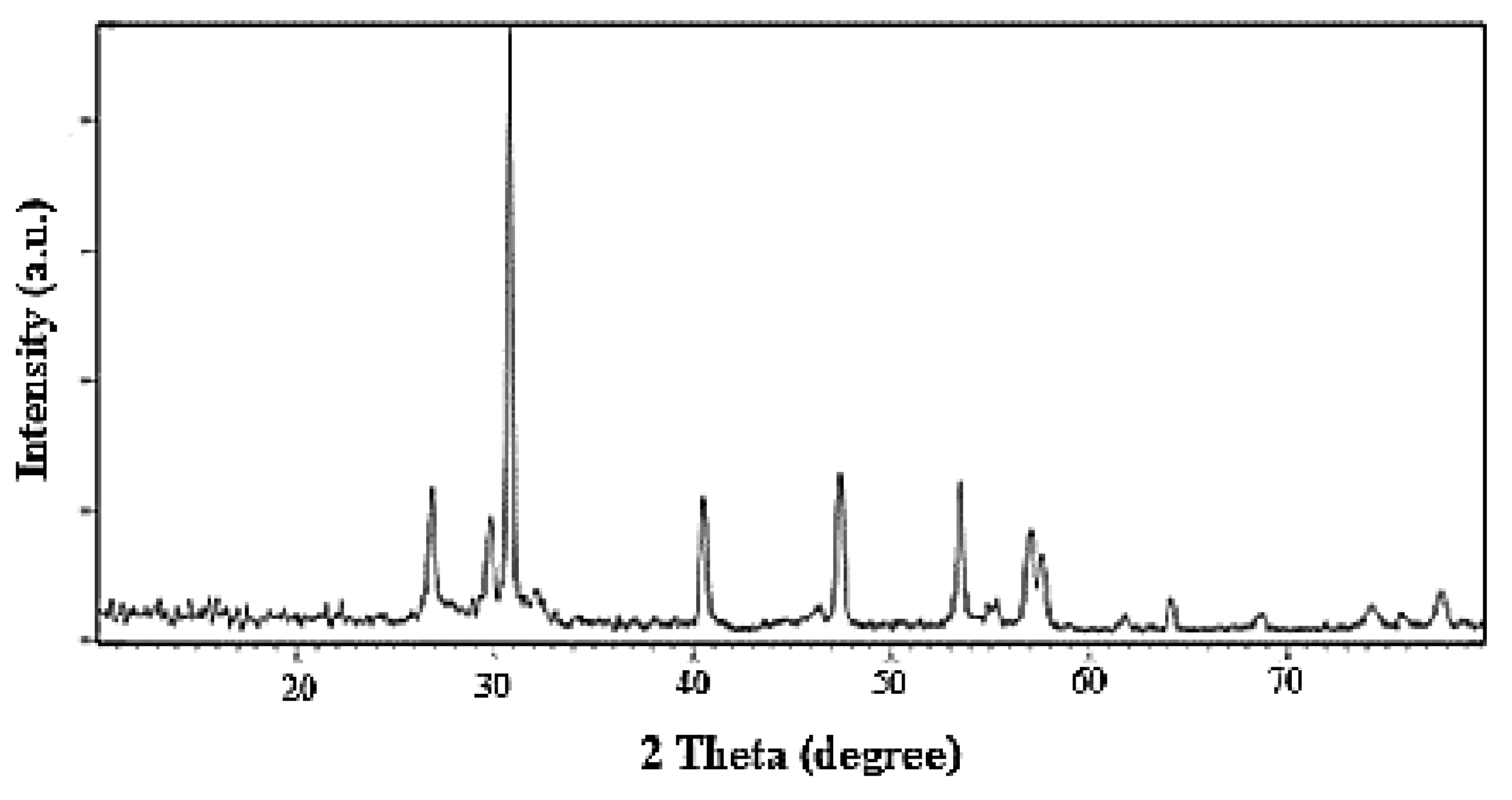
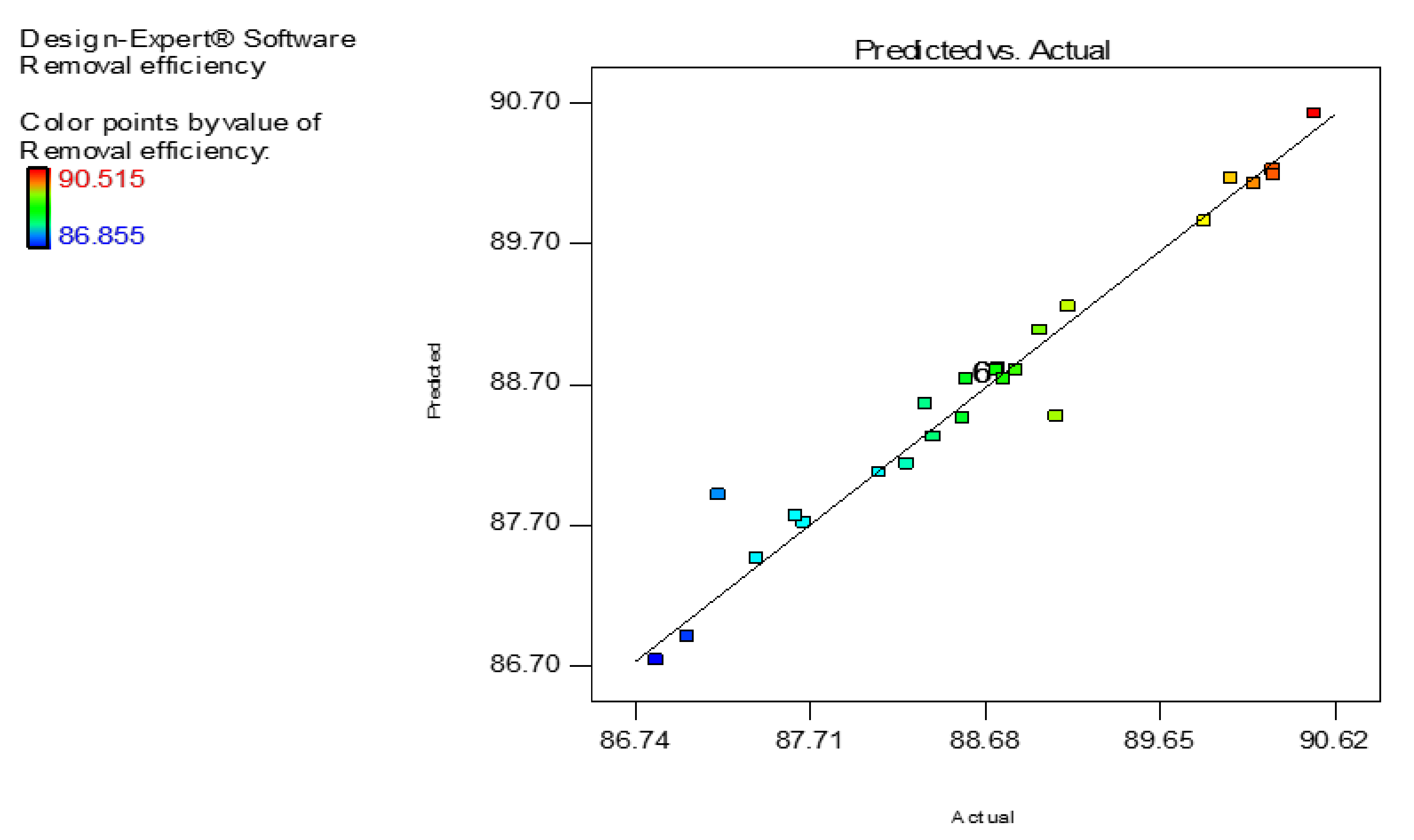


| Factor | Independent Variables | Unit | Range and Level of Actual and Coded Values | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| A (x1) | Initial pH | 3 | 6 | 9 | |
| B (x2) | Initial concentration | mg/L | 100 | 200 | 300 |
| C (x3) | Nd2O3 dosage | g/L | 0.1 | 0.55 | 1 |
| D (x4) | Time | min | 10 | 55 | 100 |
| Run | Initial pH (-) | C0 (mg/L) | Nd2O3 Dosage (g/L) | Time (min) | Experimental Adsorption (%) | Predicted Adsorption (%) |
|---|---|---|---|---|---|---|
| (A) | (B) | (C) | (D) | |||
| 1 | 6 | 200 | 0.55 | 55 | 88.78 | 88.73 |
| 2 | 9 | 300 | 0.1 | 100 | 86.85 | 86.74 |
| 3 | 6 | 200 | 0.55 | 55 | 88.78 | 88.73 |
| 4 | 9 | 300 | 1 | 10 | 87.68 | 87.72 |
| 5 | 3 | 200 | 0.55 | 55 | 90.18 | 88.73 |
| 6 | 3 | 100 | 0.1 | 100 | 89.90 | 89.85 |
| 7 | 9 | 100 | 1 | 100 | 88.76 | 88.82 |
| 8 | 9 | 100 | 0.1 | 100 | 88.56 | 88.45 |
| 9 | 3 | 300 | 0.1 | 10 | 88.40 | 88.32 |
| 10 | 3 | 300 | 0.1 | 100 | 88.25 | 88.13 |
| 11 | 9 | 300 | 0.1 | 10 | 87.03 | 86.90 |
| 12 | 6 | 200 | 0.55 | 55 | 88.78 | 88.73 |
| 13 | 6 | 200 | 1 | 55 | 89.08 | 88.47 |
| 14 | 6 | 200 | 0.55 | 100 | 87.63 | 87.76 |
| 15 | 3 | 100 | 1 | 10 | 90.51 | 90.62 |
| 16 | 9 | 200 | 0.55 | 55 | 88.58 | 88.74 |
| 17 | 6 | 200 | 0.55 | 55 | 88.78 | 88.73 |
| 18 | 9 | 300 | 1 | 100 | 87.41 | 87.46 |
| 19 | 9 | 100 | 1 | 10 | 89.15 | 89.25 |
| 20 | 6 | 200 | 0.55 | 55 | 88.78 | 88.73 |
| 21 | 9 | 100 | 0.1 | 10 | 88.85 | 88.79 |
| 22 | 3 | 100 | 1 | 100 | 90.05 | 90.16 |
| 23 | 6 | 200 | 0.55 | 10 | 88.10 | 88.07 |
| 24 | 3 | 100 | 0.1 | 10 | 90.28 | 90.18 |
| 25 | 6 | 100 | 0.55 | 55 | 90.28 | 90.22 |
| 26 | 6 | 200 | 0.1 | 55 | 87.20 | 87.91 |
| 27 | 3 | 300 | 1 | 10 | 88.99 | 89.08 |
| 28 | 6 | 200 | 0.55 | 55 | 88.78 | 88.73 |
| 29 | 6 | 300 | 0.55 | 55 | 88.35 | 88.56 |
| 30 | 3 | 300 | 1 | 100 | 88.75 | 88.80 |
| Sequential Model Sum of Squares | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F Value | P Value | Prob > F |
| Mean vs. Total | 236,119 | 1 | 236119 | |||
| Linear vs. Mean | 22.3634 | 4 | 5.5909 | 28.0665 | < 0.0001 | |
| 2FI vs. Linear | 0.1724 | 6 | 0.0287 | 0.1135 | 0.9937 | |
| Quadratic vs. 2FI | 3.7038 | 4 | 0.9259 | 12.5822 | 0.0001 | Suggested |
| Cubic vs. Quadratic | 1.0665 | 8 | 0.1333 | 24.9416 | 0.0002 | Aliased |
| Residual | 0.0374 | 7 | 0.0053 | |||
| Total | 236,146.4 | 30 | 7871.546 | |||
| Model Summary Statistics | ||||||
| Source | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | PRESS | |
| Linear | 0.4463 | 0.8179 | 0.7887 | 0.7743 | 6.1716 | |
| 2FI | 0.5030 | 0.8242 | 0.7316 | 0.7612 | 6.5289 | |
| Quadratic | 0.2713 | 0.9596 | 0.9220 | 0.8277 | 4.7118 | Suggested |
| Cubic | 0.0731 | 0.9986 | 0.9943 | 0.8374 | 4.4467 | Aliased |
| Source | Sum of Squares | df | Mean Square | F Value | p-value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 26.2396 | 14 | 1.8743 | 25.4683 | <0.0001 | significant |
| A-initial pH | 8.6015 | 1 | 8.6015 | 116.8807 | <0.0001 | |
| B-AB92 concentration | 11.9066 | 1 | 11.9066 | 161.7921 | <0.0001 | |
| C-Nd2O3 dosage | 1.4137 | 1 | 1.4137 | 19.2103 | 0.0005 | |
| D-Time | 0.4417 | 1 | 0.4417 | 6.0018 | 0.0270 | |
| AB | 3.26E-05 | 1 | 3.26E-05 | 0.0004 | 0.9835 | |
| AC | 0.0033 | 1 | 0.0033 | 0.0443 | 0.8362 | |
| AD | 0.0008 | 1 | 0.0008 | 0.0111 | 0.9176 | |
| BC | 0.1286 | 1 | 0.1286 | 1.7476 | 0.2060 | |
| BD | 0.0313 | 1 | 0.0313 | 0.4254 | 0.5241 | |
| CD | 0.0083 | 1 | 0.0083 | 0.1133 | 0.7410 | |
| A2 | 1.2554 | 1 | 1.2554 | 17.0588 | 0.0009 | |
| B2 | 1.0524 | 1 | 1.0524 | 14.3002 | 0.0018 | |
| C2 | 0.7592 | 1 | 0.7592 | 10.3157 | 0.0058 | |
| D2 | 1.7272 | 1 | 1.7272 | 23.4695 | 0.0002 | |
| Residual | 1.1039 | 15 | 0.0736 | |||
| Lack of Fit | 1.1039 | 10 | 0.1104 | |||
| Pure Error | 0 | 5 | 0 | |||
| Cor. Total | 27.3435 | 29 |
| Langmuir | Freundlich | |||
|---|---|---|---|---|
| Qmax | KL | KF | 1/n | R2 |
| 7.3 | 0.497 | 13.436 | 0.1694 | 0.9989 |
| Lagergren Isotherm | Ho Isotherm | |||||
|---|---|---|---|---|---|---|
| C0 | qe | k1 | R2 | qe | k2 | R2 |
| 100 | 0.95 | 0.0020 | 0.3547 | 15.847 | 0.027 | 0.9962 |
| 200 | 1.77 | 0.0048 | 0.5070 | 25.25 | 0.135 | 1.0000 |
| 300 | 262.30 | 1.1000 | 0.7862 | 8.39 | 0.129 | 0.9993 |
| Temperature (K) | C0 (mg/L) | ΔS0 (J/mol K) | ΔH0 (J/mol) | lnKa | ΔG0 (kJ/mol) |
|---|---|---|---|---|---|
| 298 | −1.01119 | −2.500 | |||
| 308 | 100 mg/L | −1.2 | 38.073 | −1.00307 | −2.568 |
| 318 | −1.00307 | −2.596 |
| Parameter | Green Adsorbents | Non Green Adsorbent | |
|---|---|---|---|
| Adsorbent | Agricultural wastes (AW) | Activated carbon (AC) | Activated carbon (ACM) |
| Pollutants used | Dyes, Metals, Others | Dyes, Metals, Others | Dyes, Metals, Others |
| Modification | No | No | Yes |
| Adsorption capacity | 100 mg/g | 200 mg/g | 300 mg/g |
| Mass of pollutant for removal | 1 kg | 1 kg | 1 kg |
| Adsorption-desorption cycles | 20 | 20 | 20 |
| Loss of capacity after cycles | 20% | 20% | 20% |
| Estimated cost for the adsorbent production* | 0.5 | 2 | 3 |
| Mass of adsorbent required | 10 kg | 5 kg | 3.3 kg |
| Order of profitability | 1 | 2 | 3 |
| Method | Duration (h) | Instrumentation |
|---|---|---|
| Soxhlet | 12 | Soxhlet Electrothermal (580 W) |
| Oven Drying | 3 | Oven Thermofisher (1450 W) |
| Stirring | 3 (total) | Stirrer CAT M 6,1 (580 W) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, S.; Mohammadi, L.; Rahdar, A.; Rahdar, S.; Dehghani, R.; Adaobi Igwegbe, C.; Kyzas, G.Z. Acid Dye Removal from Aqueous Solution by Using Neodymium(III) Oxide Nanoadsorbents. Nanomaterials 2020, 10, 556. https://doi.org/10.3390/nano10030556
Ahmadi S, Mohammadi L, Rahdar A, Rahdar S, Dehghani R, Adaobi Igwegbe C, Kyzas GZ. Acid Dye Removal from Aqueous Solution by Using Neodymium(III) Oxide Nanoadsorbents. Nanomaterials. 2020; 10(3):556. https://doi.org/10.3390/nano10030556
Chicago/Turabian StyleAhmadi, Shahin, Leili Mohammadi, Abbas Rahdar, Somayeh Rahdar, Ramin Dehghani, Chinenye Adaobi Igwegbe, and George Z. Kyzas. 2020. "Acid Dye Removal from Aqueous Solution by Using Neodymium(III) Oxide Nanoadsorbents" Nanomaterials 10, no. 3: 556. https://doi.org/10.3390/nano10030556
APA StyleAhmadi, S., Mohammadi, L., Rahdar, A., Rahdar, S., Dehghani, R., Adaobi Igwegbe, C., & Kyzas, G. Z. (2020). Acid Dye Removal from Aqueous Solution by Using Neodymium(III) Oxide Nanoadsorbents. Nanomaterials, 10(3), 556. https://doi.org/10.3390/nano10030556








