Pure Acetylene Semihydrogenation over Ni–Cu Bimetallic Catalysts: Effect of the Cu/Ni Ratio on Catalytic Performance
Abstract
1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalytic Performance Test
3. Results and Discussion
3.1. Catalyst Activity and Characterization
3.2. Optimization of Reaction Conditions
3.3. Stability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rahimpour, M.R.; Dehghani, O.; Gholipour, M.R.; Shokrollahi Yancheshmeh, M.S.; Seifzadeh Haghighi, S.; Shariati, A. A novel configuration for Pd/Ag/α-Al2O3 catalyst regeneration in the acetylene hydrogenation reactor of a multi feed cracker. Chem. Eng. J. 2012, 198, 491–502. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Wu, K.H.; Niu, Y.; Luo, Y.; Yang, X.; Zhang, B.; Su, D. Pd@C core-shell nanoparticles on carbon nanotube as highly stable and selective catalysts for hydrogenation of acetylene to ethylene. Nanoscale 2017, 9, 14317–14321. [Google Scholar] [CrossRef] [PubMed]
- Tysoe, W.T.; Nyberg, G.L.; Lambert, R.M. Low temperature catalytic chemistry of the Pd(111) surface: Benzene and ethylene from acetylene. J. Chem. Soc. Chem. Commun. 1983, 623–625. [Google Scholar] [CrossRef]
- Mccue, A.J.; Shepherd, A.M.; Anderson, J.A. Optimisation of preparation method for Pd doped Cu/Al2O3 catalysts for selective acetylene hydrogenation. Catal. Sci. Technol. 2015, 5, 2880–2890. [Google Scholar] [CrossRef]
- Cao, X.; Mirjalili, A.; Wheeler, J.; Wheeler, J.; Xie, W.; Jang, B. Investigation of the preparation methodologies of Pd-Cu single atom alloy catalysts for selective hydrogenation of acetylene. Front. Chem. Sci. Eng. 2015, 9, 442–449. [Google Scholar] [CrossRef]
- Borodziński, A.; Bond, G.C. Selective Hydrogenation of Ethyne in Ethene Inich Streams on Palladium Catalysts, Part 2: Steady logtate Kinetics and Effects of Palladium Particle Size, Carbon Monoxide, and Promoters. Catal. Rev. 2008, 50, 379–469. [Google Scholar] [CrossRef]
- Trimm, D.L.; Liu, I.O.Y.; Cant, N.W. The oligomerization of acetylene in hydrogen over Ni/SiO2 catalysts: Product distribution and pathways. J. Mol. Catal. A Chem. 2008, 288, 63–74. [Google Scholar] [CrossRef]
- Molnár, Á.; Sárkány, A.; Varga, M. Hydrogenation of Carbon-Carbon Multiple Bonds: Chemo-, Regio- and Stereo-Selectivity. J. Mol. Catal. A Chem. 2001, 173, 185–221. [Google Scholar] [CrossRef]
- Ahn, I.Y.; Lee, J.H.; Kum, S.S.; Moon, S.H. Formation of C4 species in the deactivation of a Pd/SiO2 catalyst during the selective hydrogenation of acetylene. Catal. Today 2007, 123, 151–157. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S.; Ismagilov, Z.R. Properties of Pd-Ag/C catalysts in the reaction of selective hydrogenation of acetylene. Kinet. Catal. 2017, 58, 649–654. [Google Scholar] [CrossRef]
- Pongthawornsakun, B.; Mekasuwandumrong, O.; Santos Aires, F.J.C.; Büchel, B.; Baiker, A.; Pratsinis, S.E.; Panpranot, J. Variability of particle configurations achievable by 2-nozzle flame syntheses of the Au-Pd-TiO2, system and their catalytic behaviors in the selective hydrogenation of acetylene. Appl. Catal. A Gen. 2018, 549, 1–7. [Google Scholar] [CrossRef]
- Studt, F.; Abild-Pedersen, F.; Bligaard, T.; Sørensen, R.Z.; Christensen, C.H.; Nørskov, J.K. Identification of Non-Precious Metal Alloy Catalysts for Selective Hydrogenation of Acetylene. Science 2008, 320, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Spanjers, C.S.; Held, J.T.; Jones, M.J.; Stanley, D.D.; Sim, R.S.; Janik, M.J.; Rioux, R.M. Zinc inclusion to heterogeneous nickel catalysts reduces oligomerization during the semi-hydrogenation of acetylene. J. Catal. 2014, 316, 164–173. [Google Scholar] [CrossRef]
- Wang, L.; Li, F.; Chen, Y.; Chen, J. Selective hydrogenation of acetylene on SiO2 -supported Ni-Ga alloy and intermetallic compound. J. Energy Chem. 2019, 29, 40–49. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J. Selective hydrogenation of acetylene on SiO2 supported Ni-In bimetallic catalysts: Promotional effect of In. Appl. Surf. Sci. 2016, 387, 16–27. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Wang, A.; Su, Y.; Li, L.; Zhang, T. Selective hydrogenation of acetylene in an ethylene-rich stream over silica supported Ag-Ni bimetallic catalysts. Appl. Catal. A Gen. 2017, 545, 90–96. [Google Scholar] [CrossRef]
- Yang, B.; Burch, R.; Hardacre, C.; Headdock, G.; Hu, P. Origin of the Increase of Activity and Selectivity of Nickel Doped by Au, Ag, and Cu for Acetylene Hydrogenation. ACS Catal. 2012, 2, 1027–1032. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Feng, J.; He, Y.; Du, Y.; Li, D. Layered double hydroxide-derived Ni-Cu nanoalloy catalysts for semi-hydrogenation of alkynes: Improvement of selectivity and anti-coking ability via alloying of Ni and Cu. J. Catal. 2018, 359, 251–260. [Google Scholar] [CrossRef]
- Dai, B.; Wen, B.; Zhu, M.; Kang, L.; Yu, F. Nickel catalysts supported on amino-functionalized MCM–41 for syngas methanation. RSC Adv. 2016, 6, 66957–66962. [Google Scholar] [CrossRef]
- Luo, G.; Kang, L.; Zhu, M.; Dai, B. Highly active phosphotungstic acid immobilized on amino functionalized MCM-41 for the oxidesulfurization of dibenzothiophene. Fuel Process. Technol. 2014, 118, 20–27. [Google Scholar] [CrossRef]
- Chai, M.; Liu, X.; Li, L.; Pei, G.; Ren, Y.; Su, Y.; Cheng, H.; Wang, A.; Zhang, T. SiO2 -supported Au-Ni bimetallic catalyst for the selective hydrogenation of acetylene. Chin. J. Catal. 2017, 38, 1338–1346. [Google Scholar] [CrossRef]
- Bayraktar, O.; Kugler, E.L. Temperature-programmed reduction of metal-contaminated fluid catalytic cracking (FCC) catalysts. Appl. Catal. A Gen. 2004, 260, 125–132. [Google Scholar] [CrossRef]
- Nima, B.; Mehran, R.; Fereshteh, M. Methane dissociation to COx-free hydrogen and carbon nanofiber over Ni-Cu/Al2O3 catalysts. Fuel 2017, 195, 8–96. [Google Scholar]
- Wang, X.; Zhu, M.; Dai, B. Effect of Phosphorus Ligand on Cu-based Catalysts for Acetylene Hydrochlorination. ACS Sustain. Chem. Eng. 2019, 7, 6170–6177. [Google Scholar] [CrossRef]
- Balaraju, M.; Rekha, V.; Prasad, P.S.S.; Prasad, R.B.N.; Lingaiah, N. Selective Hydrogenolysis of Glycerol to 1, 2 Propanediol Over Cu–ZnO Catalysts. Catal. Lett. 2008, 126, 119–124. [Google Scholar] [CrossRef]
- Naresh, G.; Velisoju, V.K.; Chatla, A.; Venu, B.; Tardio, J.; Patel, J.; Akula, V. Promotional effect of Cu and influence of surface Ni-Cu alloy for enhanced H2 yields from CH4 decomposition over Cu modified Ni supported on MCM-41 catalyst. Energy Fuels 2018, 32, 4008–4015. [Google Scholar]
- Yang, Z.; Liu, Y.; Liu, D.; Meng, X.; Liu, C. Hydroisomerization of, n -octane over bimetallic Ni-Cu/SAPO-11 catalysts. Appl. Catal. A Gen. 2017, 543, 274–282. [Google Scholar] [CrossRef]
- Liu, Z.D.; Yin, Z.Y.; Du, Z.H.; Yang, Y.; Zhu, M.M.; Xie, L.H.; Huang, W. Low temperature growth of graphene on Cu-Ni alloy nanofibers for stable, flexible electrodes. Nanoscale 2014, 6, 5110–5115. [Google Scholar] [CrossRef]
- Khromova, S.A.; Smirnov, A.A.; Bulavchenko, O.A.; Saraev, A.A.; Kaichev, V.V.; Reshetnikov, S.I.; Yakovlev, V.A. Anisole hydrodeoxygenation over Ni-Cu bimetallic catalysts: The effect of Ni/Cu ratio on selectivity. Appl. Catal. A Gen. 2014, 470, 261–270. [Google Scholar] [CrossRef]
- Zhang, M.J.; Li, P.P.; Zhu, M.Y.; Tian, Z.Q.; Dan, J.M.; Li, J.B.; Dai, B.; Yu, F. Ultralow-weight loading Ni catalyst supported on two-dimensional vermiculite for carbon monoxide methanation. Chin. J. Chem. Eng. 2018, 26, 107–112. [Google Scholar] [CrossRef]
- Hsieh, H.H.; Chang, Y.K.; Pong, W.F.; Pieh, J.Y.; Tseng, P.K.; Sham, T.K.; Coulthard, I.; Naftel, S.J.; Lee, J.F.; Chung, S.C.; et al. Electronic structure of Ni-Cu alloys: The d-electron charge distribution. Phys. Rev. B 1998, 57, 15204–15210. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kang, J.H.; Ahn, I.Y.; Moon, S.H. Deactivation behavior of a TiO2-added Pd catalyst in acetylene hydrogenation. J. Catal. 2004, 226, 226–229. [Google Scholar] [CrossRef]
- Kim, W.J.; Moon, S.H. Modified Pd catalysts for the selective hydrogenation of acetylene. Catal. Today 2012, 185, 2–16. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Sahebdelfar, S. Pd-Ag/Al2O3 Catalyst: Stages of Deactivation in Tail-End Acetylene Selective Hydrogenation. Appl. Catal. A Gen. 2016, 525, 197–203. [Google Scholar] [CrossRef]
- Cao, Y.; Sui, Z.J.; Zhu, Y.; Zhou, X.; Chen, D. Selective Hydrogenation of Acetylene over Pd-In/Al2O3 Catalyst: Promotional Effect of Indium and Composition-dependent Performance. ACS Catal. 2017, 226, 226–229. [Google Scholar] [CrossRef]
- Dong, M.; Pan, Z.; Peng, Y.; Meng, X.; Mu, X.; Zong, B.; Zhang, J. Selective acetylene hydrogenation over core–shell magnetic Pd-supported catalysts in a magnetically stabilized bed. AIChE J. 2008, 54, 1358–1364. [Google Scholar] [CrossRef]
- Hou, R.; Wang, T.; Lan, X. Enhanced Selectivity in the Hydrogenation of Acetylene due to the Addition of a Liquid Phase as a Selective Solvent. Ind. Eng. Chem. Res. 2013, 52, 13305–13312. [Google Scholar] [CrossRef]
- García-Mota, M.; Bridier, B.; Pérez-Ramírez, J.; López, N. Interplay between carbon monoxide, hydrides, and carbides in selective alkyne hydrogenation on palladium. J. Catal. 2010, 273, 92–102. [Google Scholar] [CrossRef]

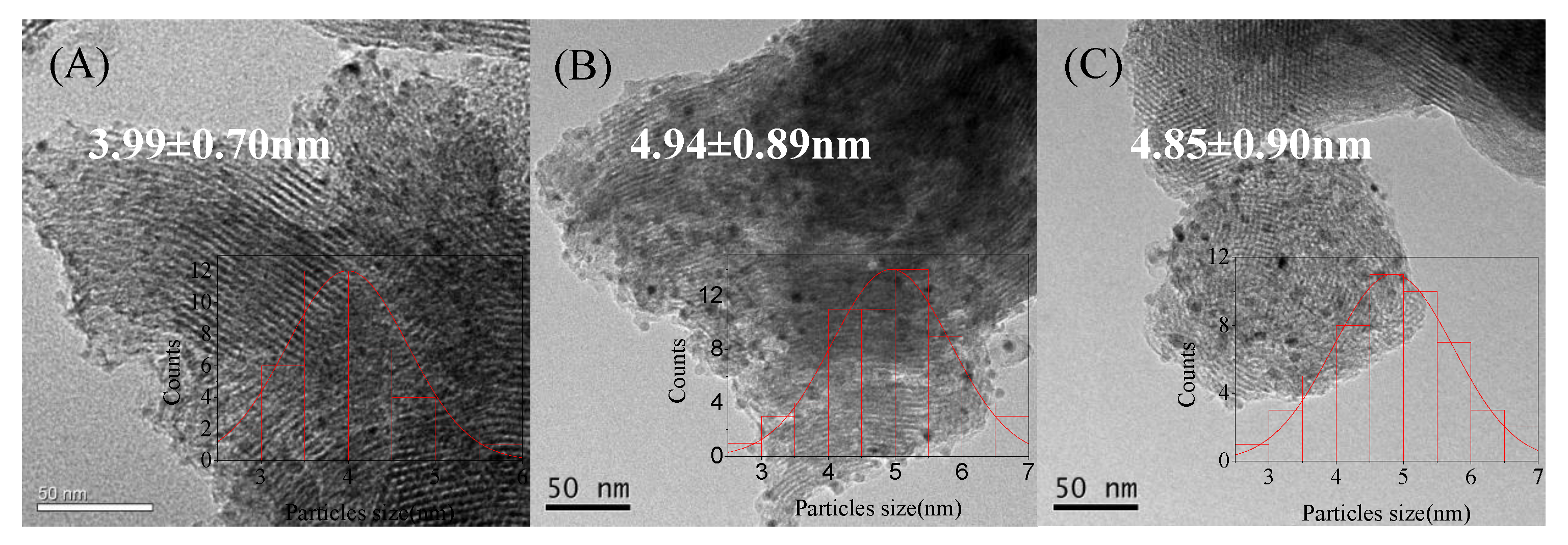
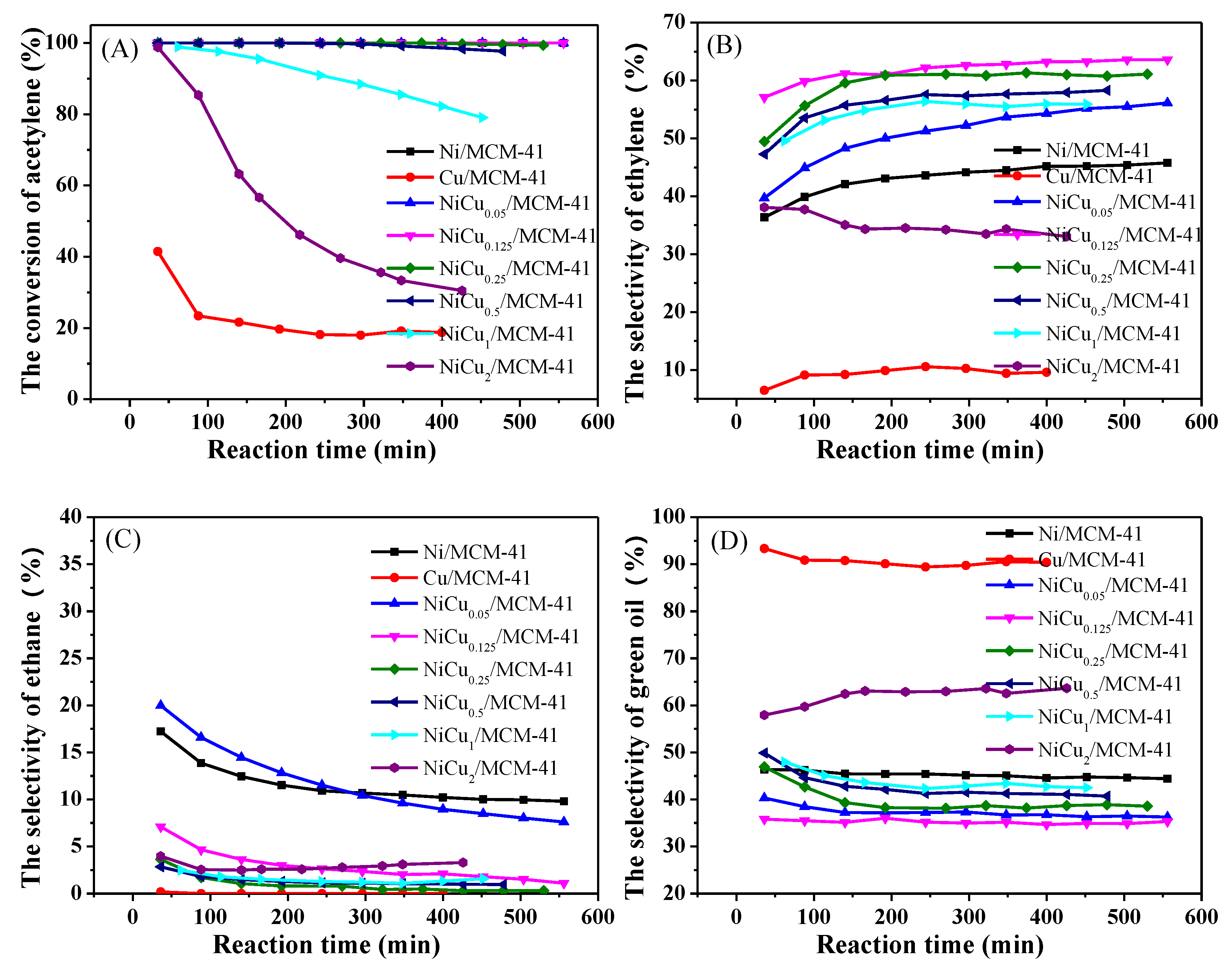
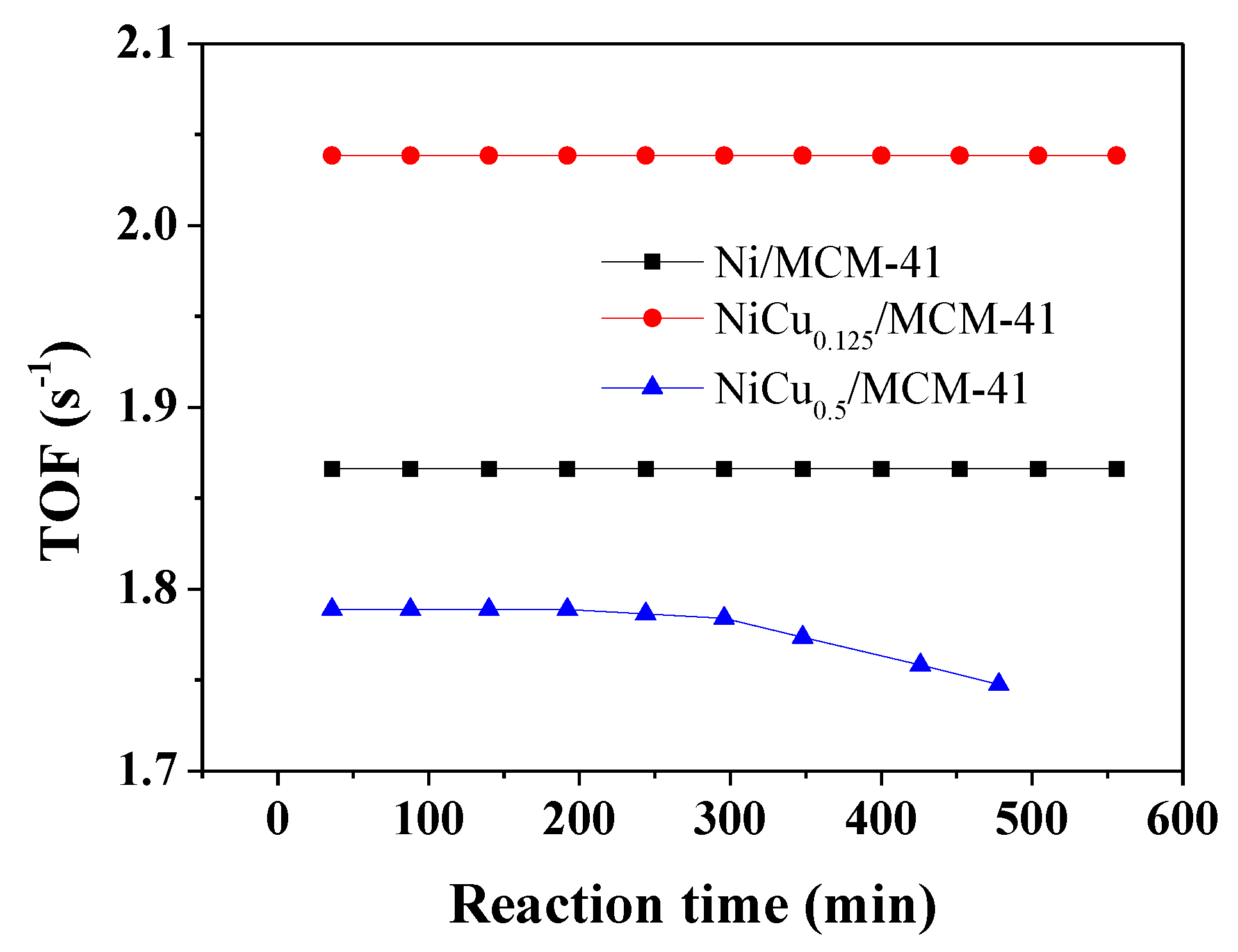
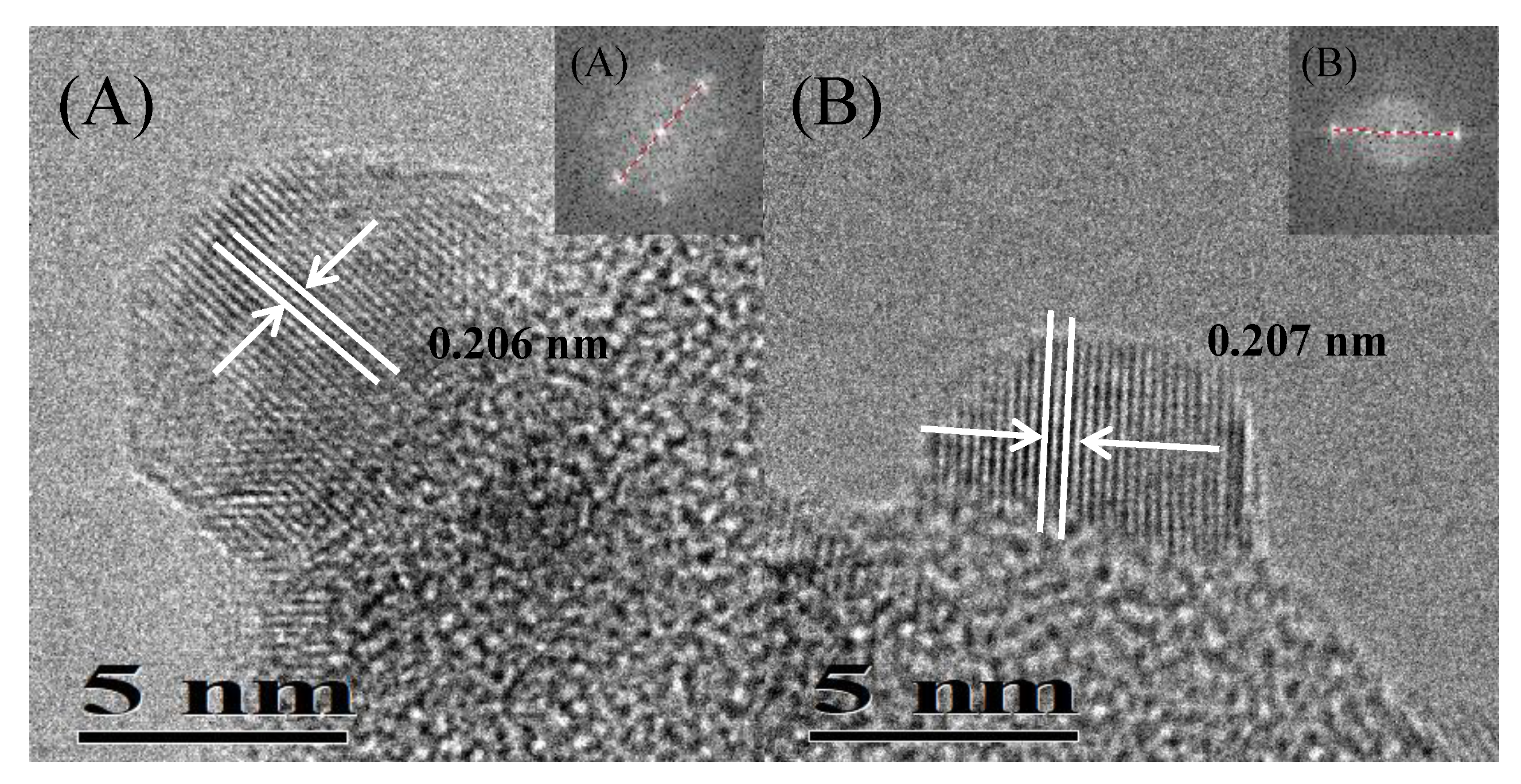

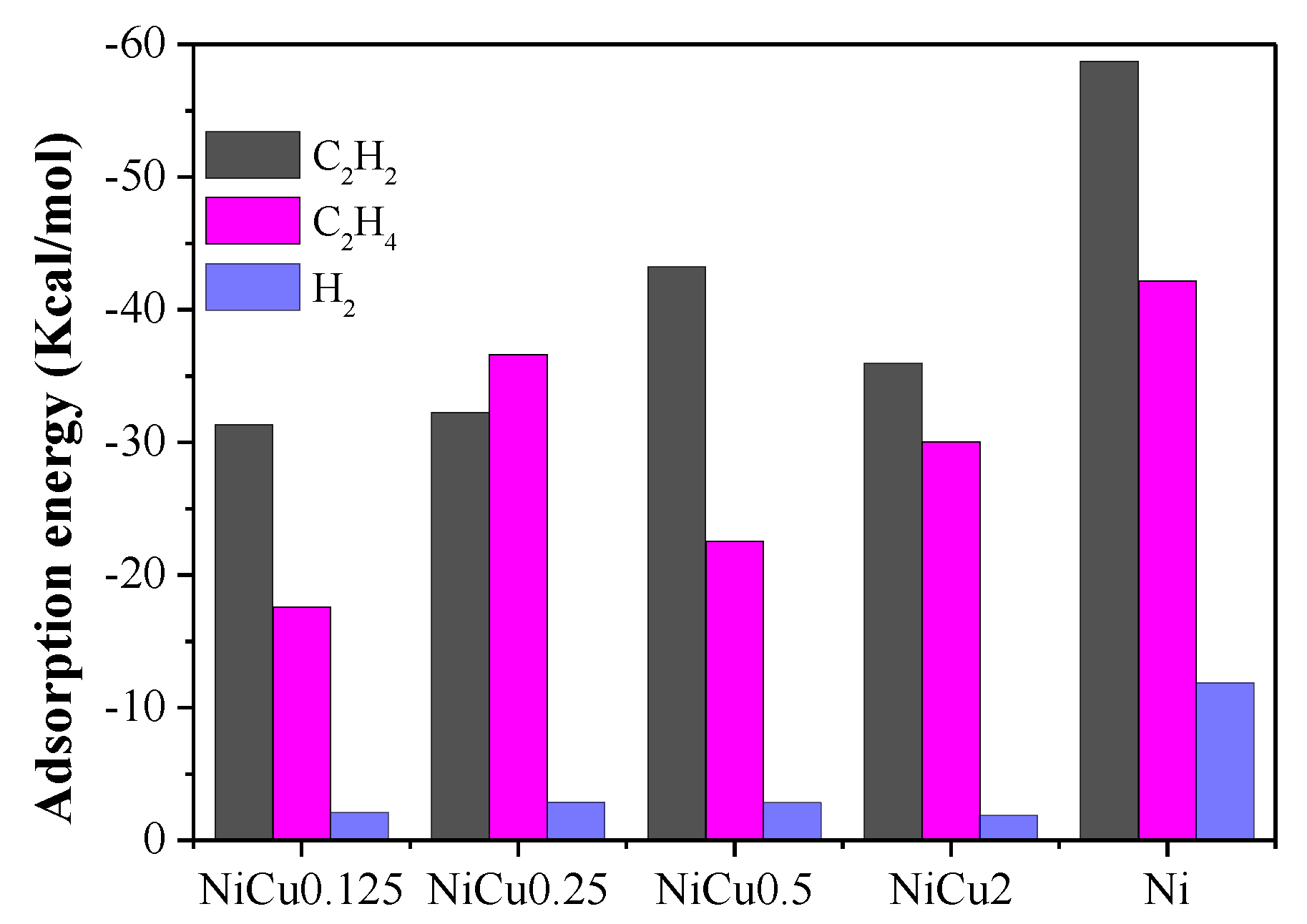
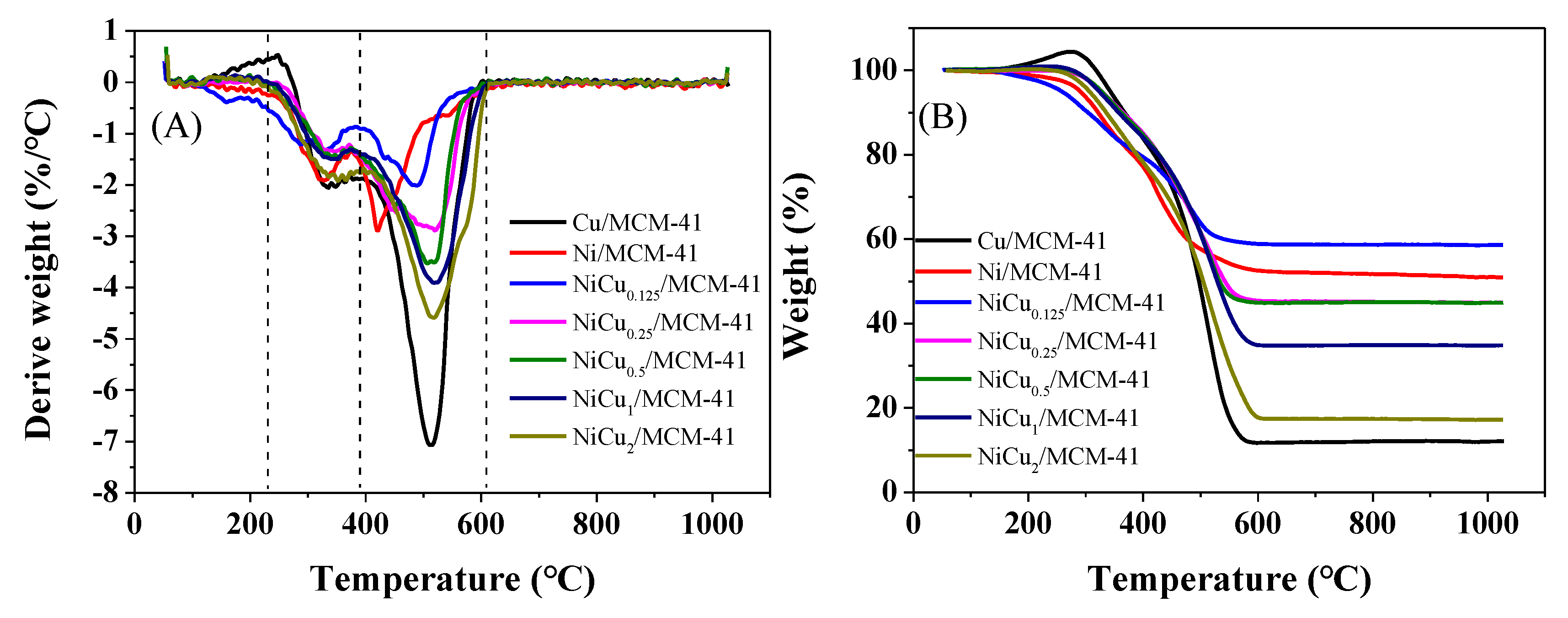

| Catalyst | Theoretical Loading (wt%) | Actual Loading a (wt%) | Actual Cu:Ni Molar Ratio | Dispersion b | ||
|---|---|---|---|---|---|---|
| Ni | Cu | Ni | Cu | |||
| Ni/MCM-41 | 1 | / | 1.12 | / | / | 0.25 |
| NiCu0.05/MCM-41 | 1 | 0.05 | 1.13 | 0.04 | 0.034 | / |
| NiCu0.125/MCM-41 | 1 | 0.13 | 1.18 | 0.11 | 0.085 | 0.20 |
| NiCu0.25/MCM-41 | 1 | 0.27 | 1.16 | 0.21 | 0.166 | / |
| NiCu0.5/MCM-41 | 1 | 0.54 | 1.04 | 0.38 | 0.337 | 0.21 |
| NiCu1/MCM-41 | 1 | 1.07 | 0.97 | 0.80 | 0.739 | / |
| NiCu2/MCM-41 | 1 | 2.12 | 1.06 | 1.61 | 1.398 | / |
| Cu/MCM-41 | / | 1.0 | / | 0.71 | / | / |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Kang, L.; Zhou, X.; Xu, Z.; Zhu, M. Pure Acetylene Semihydrogenation over Ni–Cu Bimetallic Catalysts: Effect of the Cu/Ni Ratio on Catalytic Performance. Nanomaterials 2020, 10, 509. https://doi.org/10.3390/nano10030509
Zhou S, Kang L, Zhou X, Xu Z, Zhu M. Pure Acetylene Semihydrogenation over Ni–Cu Bimetallic Catalysts: Effect of the Cu/Ni Ratio on Catalytic Performance. Nanomaterials. 2020; 10(3):509. https://doi.org/10.3390/nano10030509
Chicago/Turabian StyleZhou, Shuzhen, Lihua Kang, Xuening Zhou, Zhu Xu, and Mingyuan Zhu. 2020. "Pure Acetylene Semihydrogenation over Ni–Cu Bimetallic Catalysts: Effect of the Cu/Ni Ratio on Catalytic Performance" Nanomaterials 10, no. 3: 509. https://doi.org/10.3390/nano10030509
APA StyleZhou, S., Kang, L., Zhou, X., Xu, Z., & Zhu, M. (2020). Pure Acetylene Semihydrogenation over Ni–Cu Bimetallic Catalysts: Effect of the Cu/Ni Ratio on Catalytic Performance. Nanomaterials, 10(3), 509. https://doi.org/10.3390/nano10030509





