Growth of Self-Catalyzed InAs/InSb Axial Heterostructured Nanowires: Experiment and Theory
Abstract
1. Introduction
2. Methods
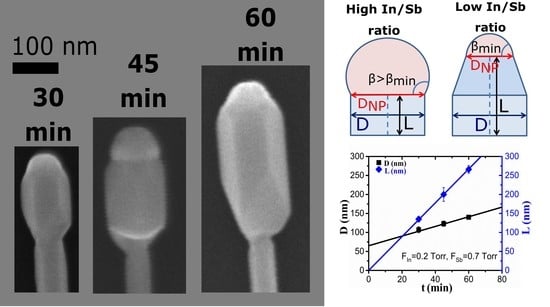
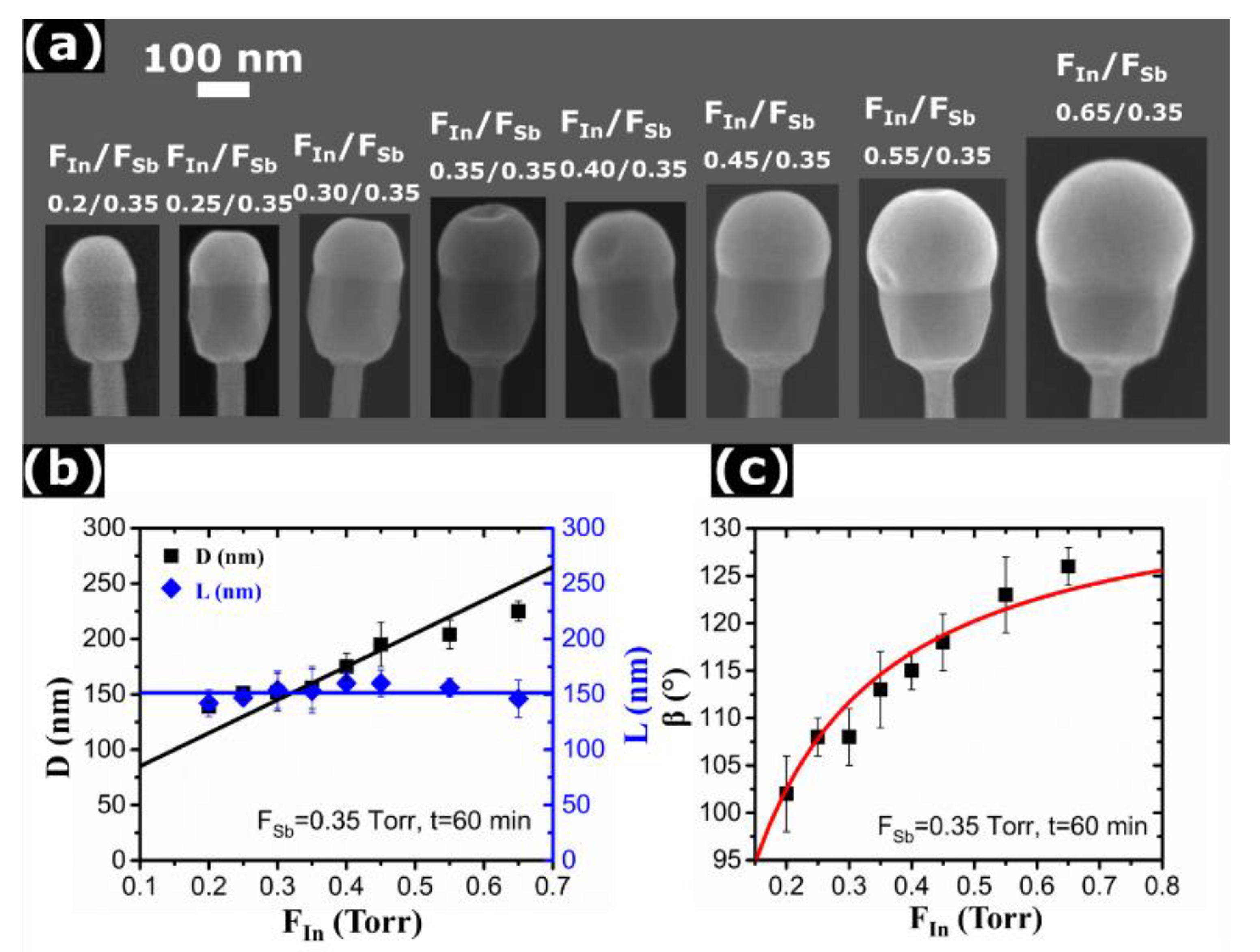
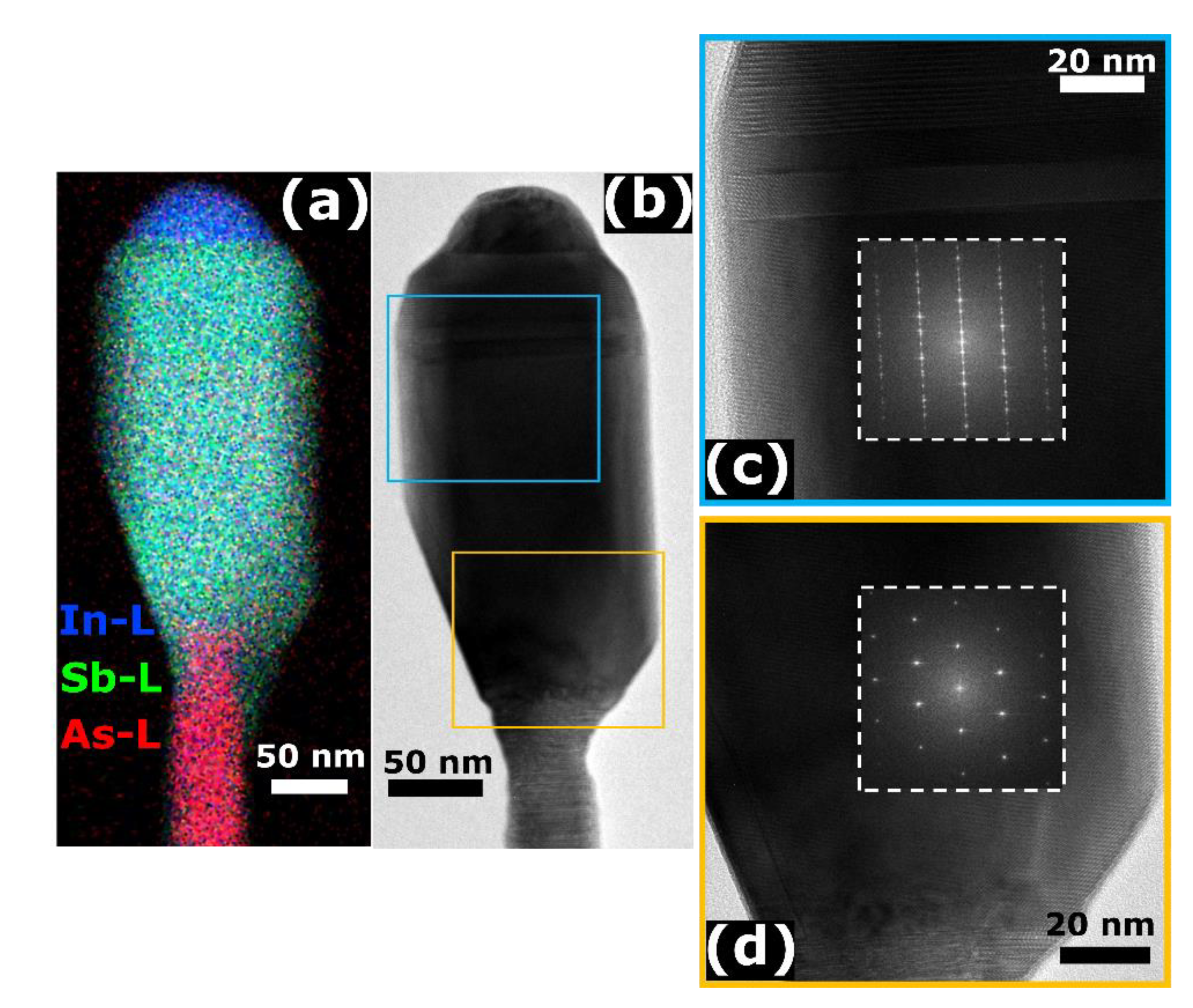
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vurgaftman, I.; Meyer, J.R.; Ram-Mohan, L.R. Band Parameters for III-V Compound Semiconductors and Their Alloys. J. Appl. Phys. 2001, 89, 5815–5875. [Google Scholar] [CrossRef]
- Caroff, P.; Wagner, J.B.; Dick, K.A.; Nilsson, H.A.; Jeppsson, M.; Deppert, K.; Samuelson, L.; Wallenberg, L.R.; Wernersson, L.E. High-Quality InAs/InSb Nanowire Heterostructures Grown by Metal-Organic Vapor-Phase Epitaxy. Small 2008, 4, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Capper, P.; Irvine, S.; Joyce, T. Epitaxial Crystal Growth: Methods and Materials. In Springer Handbook of Electronic and Photonic Materials; Kasap, S., Capper, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 309–341. [Google Scholar] [CrossRef]
- Kanisawa, K.; Yamaguchi, H.; Hirayama, Y. Two-Dimensional Growth of InSb Thin Films on GaAs(111)A Substrates. Appl. Phys. Lett. 2000, 76, 589–591. [Google Scholar] [CrossRef]
- Badawy, G.; Gazibegovic, S.; Borsoi, F.; Heedt, S.; Wang, C.; Koelling, S.; Verheijen, M.A.; Kouwenhoven, L.P.; Bakkers, E.P.A.M. High Mobility Stemless InSb Nanowires. Nano Lett. 2019, 19, 3575–3582. [Google Scholar] [CrossRef]
- Webb, J.L.; Knutsson, J.; Hjort, M.; Gorji Ghalamestani, S.; Dick, K.A.; Timm, R.; Mikkelsen, A. Electrical and Surface Properties of InAs/InSb Nanowires Cleaned by Atomic Hydrogen. Nano Lett. 2015, 15, 4865–4875. [Google Scholar] [CrossRef]
- Anandan, D.; Kakkerla, R.K.; Yu, H.W.; Ko, H.L.; Nagarajan, V.; Singh, S.K.; Lee, C.T.; Chang, E.Y. Growth of Foreign-Catalyst-Free Vertical InAs/InSb Heterostructure Nanowires on Si (111) Substrate by MOCVD. J. Cryst. Growth 2019, 506, 45–54. [Google Scholar] [CrossRef]
- Caroff, P.; Messing, M.E.; Mattias Borg, B.; Dick, K.A.; Deppert, K.; Wernersson, L.E. InSb Heterostructure Nanowires: MOVPE Growth under Extreme Lattice Mismatch. Nanotechnology 2009, 20, 495606–495612. [Google Scholar] [CrossRef]
- Li, A.; Sibirev, N.V.; Ercolani, D.; Dubrovskii, V.G.; Sorba, L. Readsorption Assisted Growth of InAs/InSb Heterostructured Nanowire Arrays. Cryst. Growth Des. 2013, 13, 878–882. [Google Scholar] [CrossRef]
- Nilsson, H.A.; Caroff, P.; Thelander, C.; Larsson, M.; Wagner, J.B.; Wernersson, L.E.; Samuelson, L.; Xu, H.Q. Giant, Level-Dependent g Factors in InSb Nanowire Quantum Dots. Nano Lett. 2009, 9, 3151–3156. [Google Scholar] [CrossRef]
- Lugani, L.; Ercolani, D.; Beltram, F.; Sorba, L. Growth Mechanism of InAs-InSb Heterostructured Nanowires Grown by Chemical Beam Epitaxy. J. Cryst. Growth 2011, 323, 304–306. [Google Scholar] [CrossRef]
- Li, T.; Gao, L.; Lei, W.; Guo, L.; Pan, H.; Yang, T.; Chen, Y.; Wang, Z. InAs-Mediated Growth of Vertical InSb Nanowires on Si Substrates. Nanoscale Res. Lett. 2013, 8, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Lugani, L.; Ercolani, D.; Sorba, L.; Sibirev, N.V.; Timofeeva, M.A.; Dubrovskii, V.G. Modeling of InAs-InSb Nanowires Grown by Au-Assisted Chemical Beam Epitaxy. Nanotechnology 2012, 23, 095602–095609. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Azizur-Rahman, K.M.; Parent, D.; Wojdylo, P.; Thompson, D.A.; Lapierre, R.R. Multispectral Absorptance from Large-Diameter InAsSb Nanowire Arrays in a Single Epitaxial Growth on Silicon. Nano Futur. 2017, 1, 035001–035007. [Google Scholar] [CrossRef]
- Sokolovskii, A.S.; Robson, M.T.; Lapierre, R.R.; Dubrovskii, V.G. Modeling Selective-Area Growth of InAsSb Nanowires. Nanotechnology 2019, 30, 285601–285607. [Google Scholar] [CrossRef]
- Perea, D.E.; Allen, J.E.; May, S.J.; Wessels, B.W.; Seidman, D.N.; Lauhon, L.J. Three-Dimensional Nanoscale Composition Mapping of Semiconductor Nanowires. Nano Lett. 2006, 6, 181–185. [Google Scholar] [CrossRef]
- Zhang, G.; Tateno, K.; Gotoh, H.; Sogawa, T. Vertically Aligned InP Nanowires Grown via the Self-Assisted Vapor-Liquid-Solid Mode. Appl. Phys. Express 2012, 5, 055201. [Google Scholar] [CrossRef]
- Gomes, U.P.; Ercolani, D.; Zannier, V.; David, J.; Gemmi, M.; Beltram, F.; Sorba, L. Nucleation and Growth Mechanism of Self-Catalyzed InAs Nanowires on Silicon. Nanotechnology 2016, 27, 255601–255607. [Google Scholar] [CrossRef]
- Ambrosini, S.; Fanetti, M.; Grillo, V.; Franciosi, A.; Rubini, S. Self-Catalyzed GaAs Nanowire Growth on Si-Treated GaAs(100) Substrates. J. Appl. Phys. 2011, 109, 094306–094312. [Google Scholar] [CrossRef]
- Munshi, A.M.; Dheeraj, D.L.; Todorovic, J.; Van Helvoort, A.T.J.; Weman, H.; Fimland, B.O. Crystal Phase Engineering in Self-Catalyzed GaAs and GaAs/GaAsSb Nanowires Grown on Si(111). J. Cryst. Growth 2013, 372, 163–169. [Google Scholar] [CrossRef]
- Gomes, U.P.; Ercolani, D.; Sibirev, N.V.; Gemmi, M.; Dubrovskii, V.G.; Beltram, F.; Sorba, L. Catalyst-Free Growth of InAs Nanowires on Si (111) by CBE. Nanotechnology 2015, 26, 415604–415614. [Google Scholar] [CrossRef]
- Soo, M.T.; Zheng, K.; Gao, Q.; Tan, H.H.; Jagadish, C.; Zou, J. Growth of Catalyst-Free Epitaxial InAs Nanowires on Si Wafers Using Metallic Masks. Nano Lett. 2016, 16, 4189–4193. [Google Scholar] [CrossRef] [PubMed]
- Dimakis, E.; Lähnemann, J.; Jahn, U.; Breuer, S.; Hilse, M.; Geelhaar, L.; Riechert, H. Self-Assisted Nucleation and Vapor-Solid Growth of InAs Nanowires on Bare Si(111). Cryst. Growth Des. 2011, 11, 4001–4008. [Google Scholar] [CrossRef]
- Yip, S.; Shen, L.; Ho, J.C. Recent Advances in III-Sb Nanowires: From Synthesis to Applications. Nanotechnology 2019, 30, 202003–202027. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Spirkoska, D.; Frimmer, M.; Abstreiter, G.; Fontcuberta, I.; Morral, A. Ga-Assisted Catalyst-Free Growth Mechanism of GaAs Nanowires by Molecular Beam Epitaxy. Phys. Rev. B 2008, 77, 155326–155330. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Theory of VLS growth of compound semiconductors. In Semiconductors and Semimetals; Fontcuberta i Morral, A., Dayeh, S.A., Jagadish, C., Eds.; Academic Press: Burlington, MA, USA, 2015; Volume 93, pp. 1–78. [Google Scholar]
- So, H.; Pan, D.; Li, L.; Zhao, J. Foreign-Catalyst-Free Growth of InAs/InSb Axial Heterostructure Nanowires on Si (111) by Molecular-Beam Epitaxy. Nanotechnology 2017, 28, 135704–135712. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo, M.; Zhou, H.; Lin, S.; Lipman, S.A.; Goorsky, M.S.; Hicks, R.F.; Kodambaka, S. Self-Catalyzed Growth of InP/InSb Axial Nanowire Heterostructures. J. Cryst. Growth 2011, 329, 6–11. [Google Scholar] [CrossRef]
- Priante, G.; Ambrosini, S.; Dubrovskii, V.G.; Franciosi, A.; Rubini, S. Stopping and Resuming at Will the Growth of GaAs Nanowires. Cryst. Growth Des. 2013, 13, 3976–3984. [Google Scholar] [CrossRef]
- Yuan, Y.; Randall Lee, T. Contact angle and wetting properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer Series in Surface Sciences; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. [Google Scholar] [CrossRef]
- Panciera, F.; Baraissov, Z.; Patriarche, G.; Dubrovskii, V.G.; Glas, F.; Travers, L.; Mirsaidov, U.; Harmand, J.C. Phase selection in self-catalyzed GaAs nanowires. Nano Lett. 2020. [Google Scholar] [CrossRef]
- Glas, F.; Ramdani, M.R.; Patriarche, G.; Harmand, J.C. Predictive Modeling of Self-Catalyzed III-V Nanowire Growth. Phys. Rev. B 2013, 88, 195304–195317. [Google Scholar] [CrossRef]
- Kim, W.; Dubrovskii, V.G.; Vukajlovic-Plestina, J.; Tütüncüoglu, G.; Francaviglia, L.; Güniat, L.; Potts, H.; Friedl, M.; Leran, J.B.; Fontcuberta, I.; et al. Bistability of Contact Angle and Its Role in Achieving Quantum-Thin Self-Assisted GaAs Nanowires. Nano Lett. 2018, 18, 49–57. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Xu, T.; Álvarez, A.D.; Plissard, S.R.; Caroff, P.; Glas, F.; Grandidier, B. Self-Equilibration of the Diameter of Ga-Catalyzed GaAs Nanowires. Nano Lett. 2015, 15, 5580–5584. [Google Scholar] [CrossRef] [PubMed]
- Leshchenko, E.D.; Kuyanov, P.; LaPierre, R.R.; Dubrovskii, V.G. Tuning the morphology of self-assisted GaP nanowires. Nanotechnology 2018, 29, 225603–225609. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskii, V.G. Nucleation Theory and Growth of Nanostructures. In NanoScience and Technology; Avouris, P., Bhushan, B., Klitzing, K.V., Sakaki, H., Wiesendanger, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Sibirev, N.V.; Berdnikov, Y.; Gomes, U.P.; Ercolani, D.; Zannier, V.; Sorba, L. Length Distributions of Au-Catalyzed and In-Catalyzed InAs Nanowires. Nanotechnology 2016, 27, 375602–375610. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskii, V.G.; Berdnikov, Y.; Schmidtbauer, J.; Borg, M.; Storm, K.; Deppert, K.; Johansson, J. Length distributions of nanowires growing by surface diffusion. Cryst. Growth Des. 2016, 16, 2167–2172. [Google Scholar] [CrossRef]
- Tersoff, J. Stable Self-Catalyzed Growth of III-V Nanowires. Nano Lett. 2015, 15, 6609–6613. [Google Scholar] [CrossRef] [PubMed]
- Glas, F. Vapor Fluxes on the Apical Droplet during Nanowire Growth by Molecular Beam Epitaxy. Phys. Status Solidi B 2010, 247, 254–258. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Development of Growth Theory for Vapor-Liquid-Solid Nanowires: Contact Angle, Truncated Facets, and Crystal Phase. Cryst. Growth Des. 2017, 17, 2544–2548. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Stabilization of the Morphology and Crystal Phase in Ensembles of Self-Catalyzed GaAs Nanowires. Phys. Status Solidi Rapid Res. Lett. 2019, 13, 1900301–1900305. [Google Scholar] [CrossRef]



| Degree | Torr | Torr | nm/min | nm/min | nm/min | ||
| 102 ± 2 | 0.2 | 0.35 | 0.57 ± 0.07 | 2.4 ± 0.12 | 6.18 ± 0.05 | 0.384 | 0.534 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, O.; Zannier, V.; Dubrovskii, V.G.; Shtrom, I.V.; Rossi, F.; Beltram, F.; Sorba, L. Growth of Self-Catalyzed InAs/InSb Axial Heterostructured Nanowires: Experiment and Theory. Nanomaterials 2020, 10, 494. https://doi.org/10.3390/nano10030494
Arif O, Zannier V, Dubrovskii VG, Shtrom IV, Rossi F, Beltram F, Sorba L. Growth of Self-Catalyzed InAs/InSb Axial Heterostructured Nanowires: Experiment and Theory. Nanomaterials. 2020; 10(3):494. https://doi.org/10.3390/nano10030494
Chicago/Turabian StyleArif, Omer, Valentina Zannier, Vladimir G. Dubrovskii, Igor V. Shtrom, Francesca Rossi, Fabio Beltram, and Lucia Sorba. 2020. "Growth of Self-Catalyzed InAs/InSb Axial Heterostructured Nanowires: Experiment and Theory" Nanomaterials 10, no. 3: 494. https://doi.org/10.3390/nano10030494
APA StyleArif, O., Zannier, V., Dubrovskii, V. G., Shtrom, I. V., Rossi, F., Beltram, F., & Sorba, L. (2020). Growth of Self-Catalyzed InAs/InSb Axial Heterostructured Nanowires: Experiment and Theory. Nanomaterials, 10(3), 494. https://doi.org/10.3390/nano10030494