Manganese and Graphene Included Titanium Dioxide Composite Nanowires: Fabrication, Characterization and Enhanced Photocatalytic Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of G Included TiO2 Composite NWs
2.3. Preparation of Mn Included TiO2 Composite NWs
2.4. Characterization
2.5. Photocatalytic Experiments
3. Results and Discussion
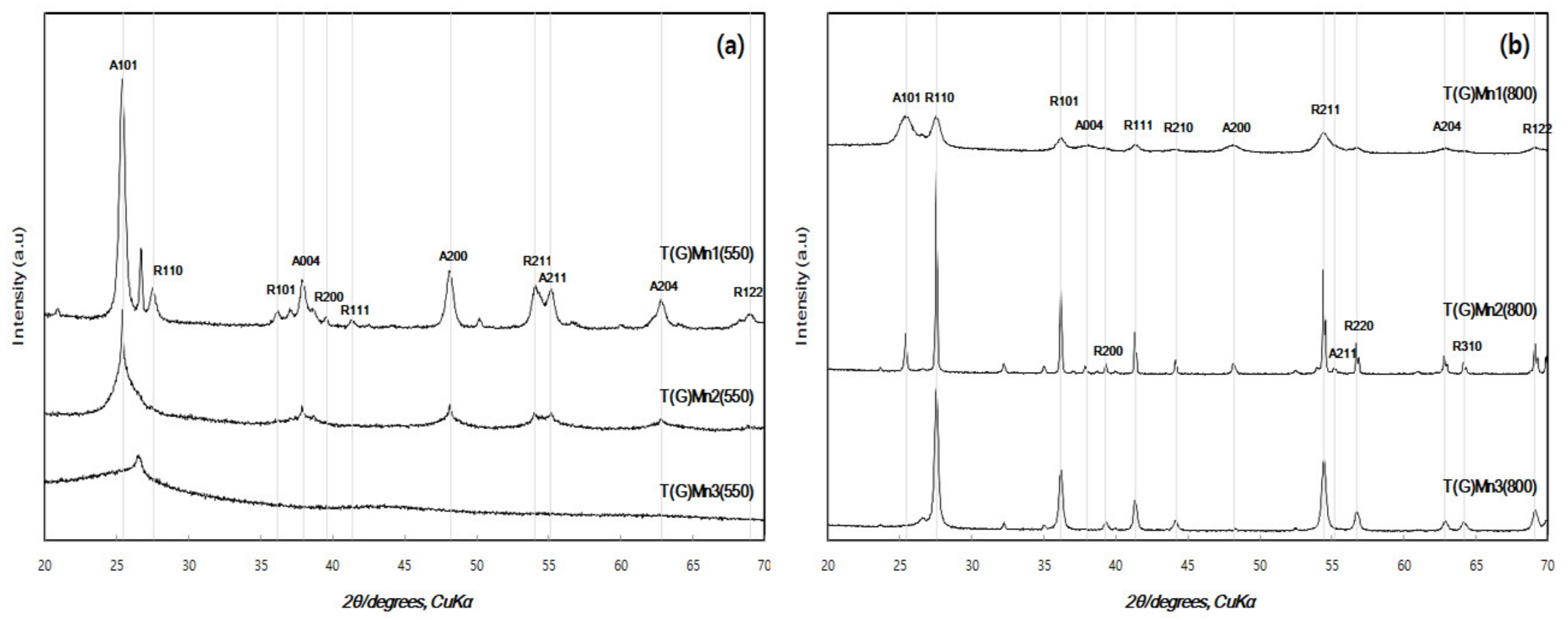
3.1. Phase Composition and Crystal Structure
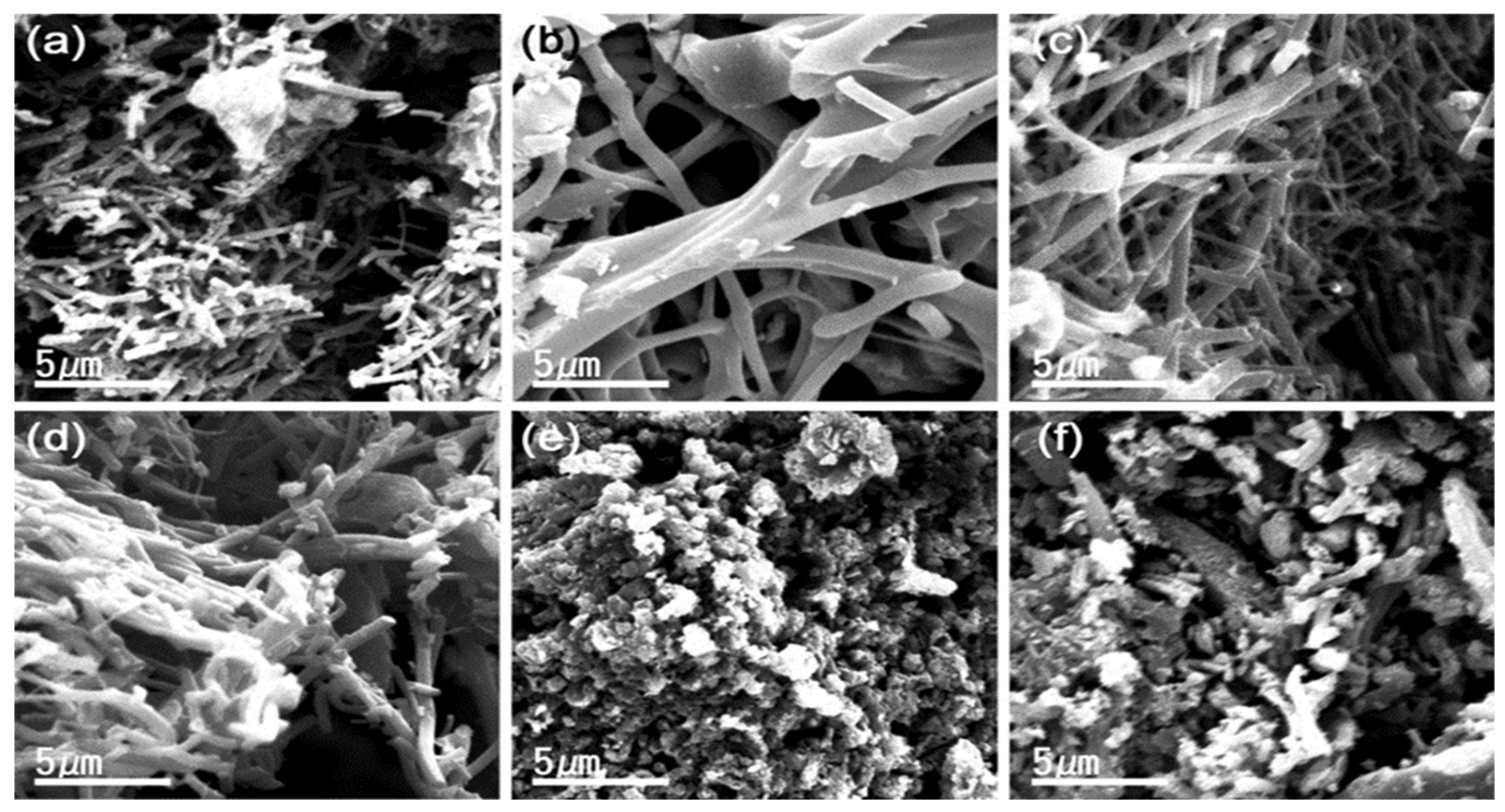
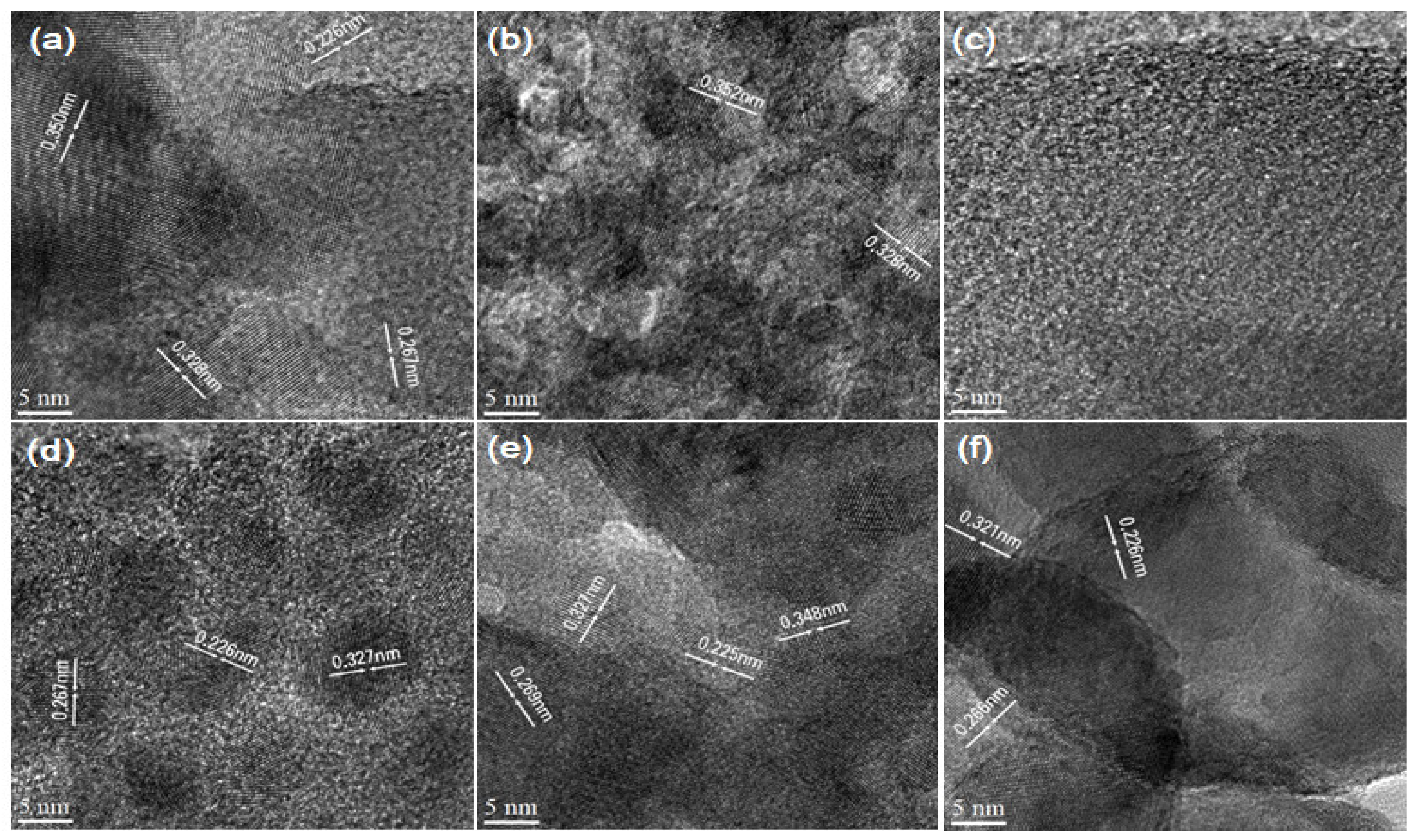
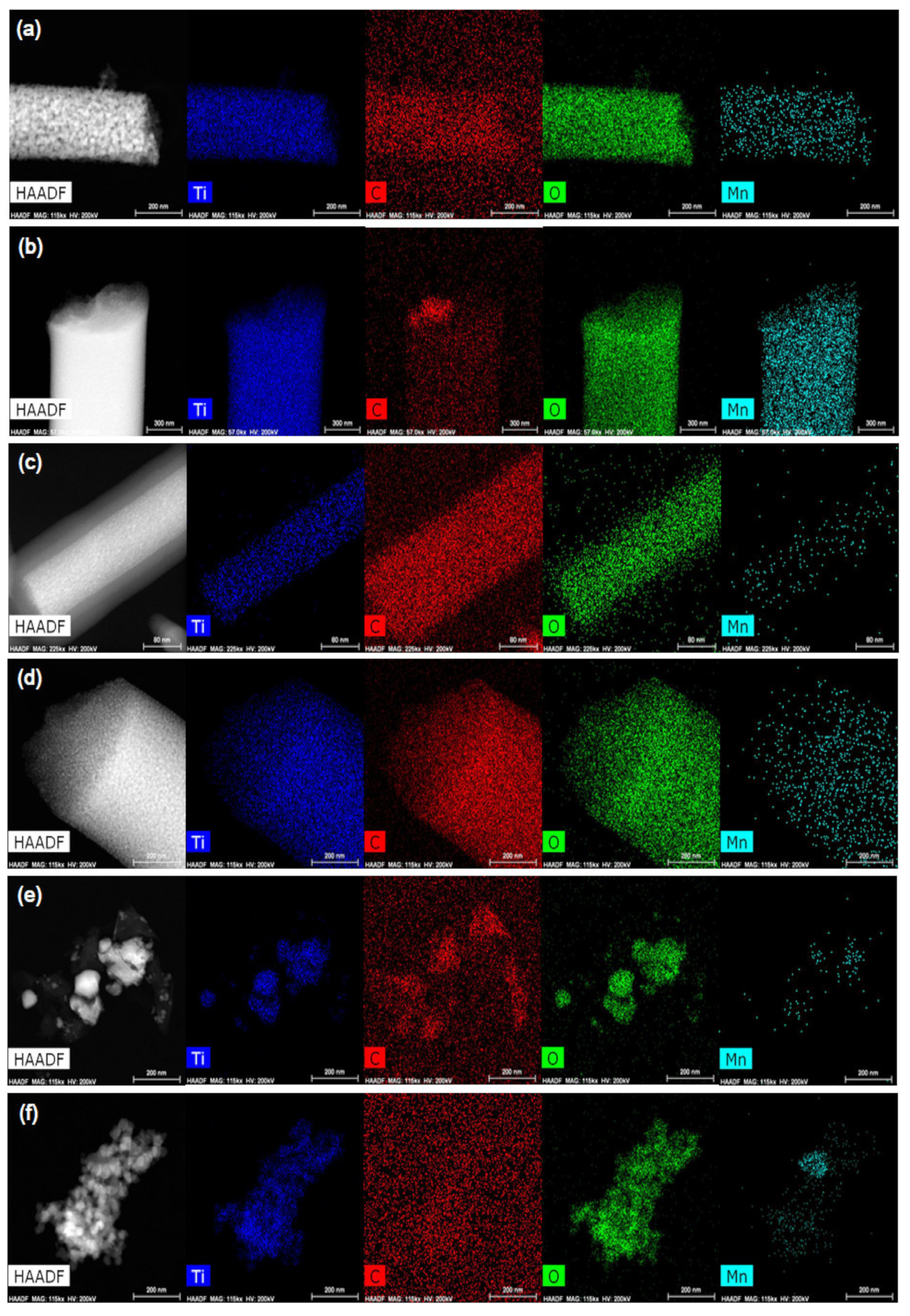
3.2. Probing Morphology Through SEM, TEM and HR-TEM
3.3. X-ray Photoelectron Spectroscopy (XPS), Raman and Diffuse Reflectance Spectra
3.4. Photocatalytic Performance
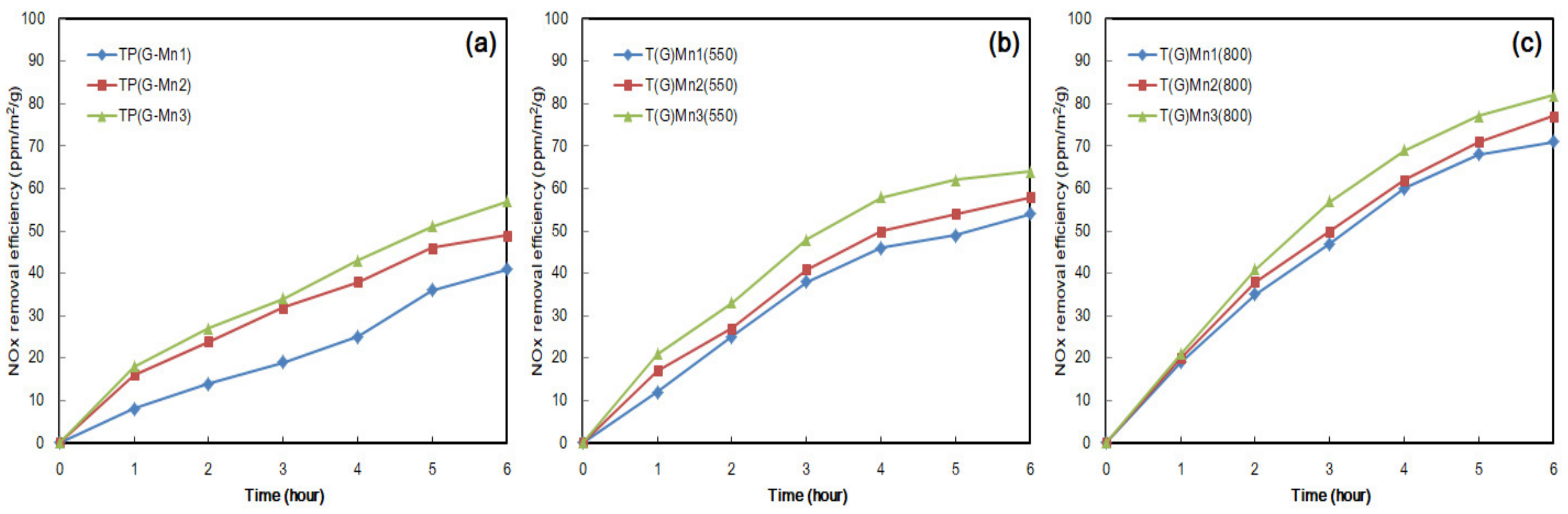
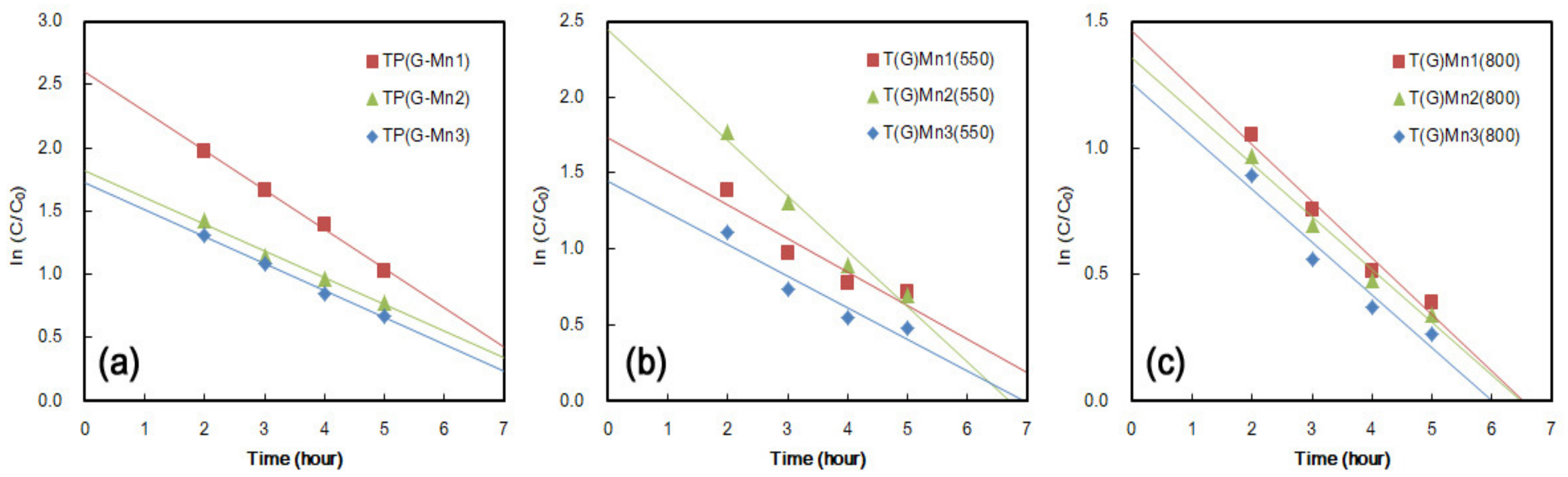
3.4.1. NOx Removal
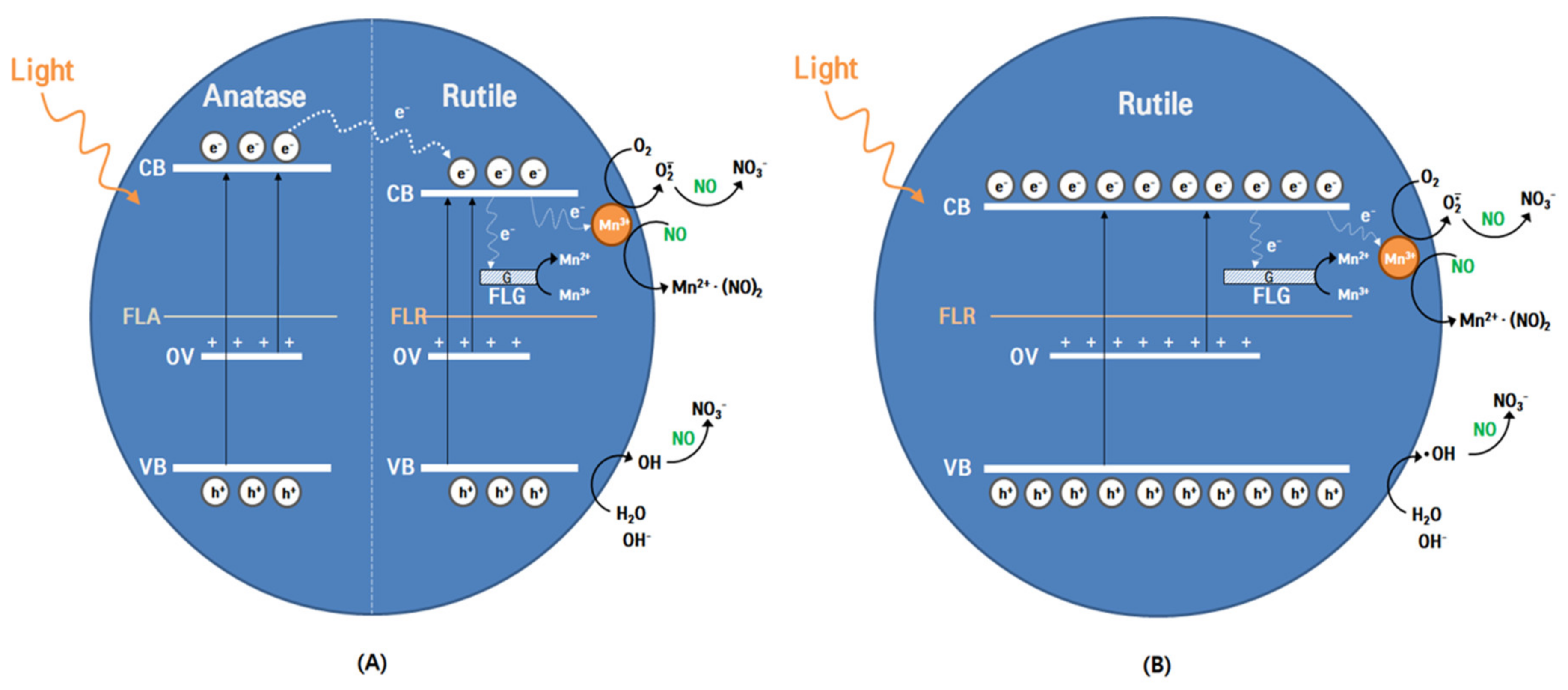
3.4.2. Plausible Mechanism
h+ + NO + 2H2O → NO3– + 4H+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, H.; Ahn, C.; Park, J.; Jeon, S. Strategies to improve the photocatalytic activity of TiO2: 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale. 2019, 11, 7025–7040. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Sridara, T.; Upan, J.; Saianand, G.; Tuantranont, A.; Karuwan, C.; Jakmunee, J. Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode. Sensors 2020, 20, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashmi, M.; Padmanaban, R.; Karthikeyan, V.; Roy, V.A.L.; Gopalan, A.-I.; Saianand, G.; Kim, W.; Kannan, V. A Comparative Evaluation of Physicochemical Properties and Photocatalytic Efficiencies of Cerium Oxide and Copper Oxide Nanofluids. Catalysts 2019, 10, 34. [Google Scholar]
- Bui, Q.-T.; Yu, I.-K.; Gopalan, A.I.; Saianand, G.; Kim, W.; Choi, S.-H. Facile Fabrication of Metal Oxide Based Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide. Catalysts 2019, 9, 888. [Google Scholar] [CrossRef] [Green Version]
- Saianand, G.; Gopalan, A.-I.; Lee, J.-C.; Sathish, C.I.; Gopalakrishnan, K.; Unni, G.E.; Shanbhag, D.; Dasireddy, V.D.B.C.; Yi, J.; Xi, S.; et al. Mixed Copper/Copper-Oxide Anchored Mesoporous Fullerene Nanohybrids as Superior Electrocatalysts toward Oxygen Reduction Reaction. Small 2019. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Saianand, G.; Gopalan, A.; Sarkar, S.; Srinivasan, S.; Park, D.H.; Kim, S.; Talapaneni, S.N.; Ramadass, K.; et al. Highly ordered iron oxide-mesoporous fullerene nanocomposites for oxygen reduction reaction and supercapacitor applications. Microporous Mesoporous Mater. 2019, 285, 21–31. [Google Scholar] [CrossRef]
- Sai-Anand, G.; Dubey, A.; Gopalan, A.-I.; Venkatesan, S.; Ruban, S.; Reza, K.M.; Choi, J.; Lakhi, K.S.; Xu, B.; Qiao, Q.; et al. Additive assisted morphological optimization of photoactive layer in polymer solar cells. Sol. Energy Mater. Sol. Cells 2018, 182, 246–254. [Google Scholar] [CrossRef]
- Xu, B.; Sai-Anand, G.; Jeong, H.-M.; Kim, S.W.; Kim, J.S.; Kwon, J.B.; Kang, S.W. Improving Air-Stability and Performance of Bulk Heterojunction Polymer Solar Cells Using Solvent Engineered Hole Selective Interlayer. Materials 2018, 11, 1143. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Sai-Anand, G.; Gopalan, A.-I.; Qiao, Q.; Kang, S.-W. Improving Photovoltaic Properties of P3HT:IC60BA through the Incorporation of Small Molecules. Polymers 2018, 10, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Sai-Anand, G.; Xu, B.; Jae-Sung, L.; Sae-Wan, K.; Se-Hyuk, Y.; Jin-Hyuk, B.; Shin-Won, K. Enhancing the Photovoltaic Performance of Polymer Solar Cells by Manipulating Photoactive/Metal Interface. J. Nanosci. Nanotechnol. 2017, 17, 8024–8030. [Google Scholar] [CrossRef]
- Xu, B.; Gopalan, S.; Gopalan, A.; Muthuchamy, N.; Lee, K.; Lee, J.; Jiang, Y.; Lee, S.; Kim, S.; Kim, J.; et al. Functional solid additive modified PEDOT:PSS as an anode buffer layer for enhanced photovoltaic performance and stability in polymer solar cells. Sci. Rep. 2017, 7, 45079. [Google Scholar] [CrossRef]
- Lee, S.; Choi, K.; Kang, B.; Lee, J.; Kim, S.; Kwon, J.; Gopalan, S.; Bae, J.; Kim, E.; Kwon, D.; et al. Low dark current and improved detectivity of hybrid ultraviolet photodetector based on carbon-quantum-dots/zinc-oxide-nanorod composites. Org. Electron. 2016, 39, 250–257. [Google Scholar] [CrossRef]
- Lee, S.; Cha, S.; Choi, K.; Kang, B.; Lee, J.; Kim, S.; Kim, J.; Jeong, H.; Gopalan, S.; Kwon, D.; et al. Low Dark-Current, High Current-Gain of PVK/ZnO Nanoparticles Composite-Based UV Photodetector by PN-Heterojunction Control. Sensors 2016, 16, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, B.H.; Kim, J.S.; Lee, J.S.; Lee, S.W.; Sai-Anand, G.; Jeong, H.M.; Lee, S.H.; Kwon, D.H.; Kang, S.W. Solution Processable CdSe/ZnS Quantum Dots Light-Emitting Diodes Using ZnO Nanocrystal as Electron Transport Layer. J. Nanosci. Nanotechnol. 2015, 15, 7416–7420. [Google Scholar] [CrossRef] [PubMed]
- Sai-Anand, G.; Sivanesan, A.; Benzigar, M.R.; Singh, G.; Gopalan, A.-I.; Baskar, A.V.; Ilbeygi, H.; Ramadass, K.; Kambala, V.; Vinu, A. Recent Progress on the Sensing of Pathogenic Bacteria Using Advanced Nanostructures. Bull. Chem. Soc. Jpn. 2019, 92, 216–244. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, X.; Liu, H.; Wang, C.; Liu, S.; Sun, P.; Wang, L.; Liu, Y. Heterostructured TiO2/WO3 porous microspheres: Preparation, characterization and photocatalytic properties. Catal. Today 2013, 201, 195–202. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, H.; Humayun, M.; Qu, Y.; Fan, N.; Sun, X.; Jing, L. Exceptional performance of photoelectrochemical water oxidation of single-crystal rutile TiO2 nanorods dependent on the hole trapping of modified chloride. Sci. Rep. 2016, 6, 21430. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, B.; Mabrouk, S.; Adhikari, N.; Elbohy, H.; Gurung, A.; Reza, K.M.; Pathak, R.; Chowdhury, A.H.; Saianand, G.; Yue, W.; et al. Nanoscale control of grain boundary potential barrier, dopant density and filled trap state density for higher efficiency perovskite solar cells. InfoMat 2019. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Mao, S.S. Synthesis of Titanium Dioxide (TiO2) Nanomaterials. J. Nanosci. Nanotechnol. 2006, 6, 906–925. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, Y.K.; Seok, J.; Lee, N.S.; Son, B.; Lee, J.W.; Baik, J.M.; Lee, C.; Lee, Y.; Kim, M.H. Highly Branched RuO2 Nanoneedles on Electrospun TiO2 Nanofibers as an Efficient Electrocatalytic Platform. Acs Appl. Mater. Interfaces 2015, 7, 15321–15330. [Google Scholar] [CrossRef]
- Lee, J.-C.; Gopalan, A.-I.; Sai-Anand, G.; Lee, K.-P.; Kim, W.-J. Preparation of Visible Light Photocatalytic Graphene Embedded Rutile Titanium(IV) Oxide Composite Nanowires and Enhanced NOx Removal. Catalysts 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-G.; Sai-Anand, G.; Komathi, S.; Gopalan, A.-I.; Kang, S.-W.; Lee, K.-P. Efficient visible-light-driven photocatalytic degradation of nitrophenol by using graphene-encapsulated TiO2 nanowires. J. Hazard. Mater. 2015, 283, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-G.; Gopalan, A.-I.; Sai-Anand, G.; Kang, S.-W.; Lee, K.-P. New Heterojunction Titanium Dioxide Nanowire as Photocatalyst. J. Nanosci. Nanotechnol. 2015, 15, 7421–7425. [Google Scholar] [CrossRef]
- Lee, H.-G.; Gopalan, A.-I.; Sai-Anand, G.; Lee, B.-C.; Kang, S.-W.; Lee, K.-P. Facile synthesis of functionalized graphene-palladium nanoparticle incorporated multicomponent TiO2 composite nanofibers. Mater. Chem. Phys. 2015, 154, 125–136. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Sai-Anand, G.; Gopalan, A.-I.; Lee, Hy.; Yeo, H.K.; Kang, S.-W.; Lee, K.-P. Direct electrochemistry of cytochrome c with three-dimensional nanoarchitectured multicomponent composite electrode and nitrite biosensing. Sens. Actuators B Chem. 2016, 228, 737–747. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Bessegato, G.G.; Cardoso, J.C.; Zanoni, M.V.B. Enhanced photoelectrocatalytic degradation of an acid dye with boron-doped TiO2 nanotube anodes. Catal. Today 2015, 240, 100–106. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; Dantas, R.F.; Bayarri, B.; González, O.; Giménez, J.; Esplugas, S.; Juniora, A.M. Synthesis and characterization of B-doped TiO2 and their performance for the degradation of metoprolol. Catal. Today 2015, 252, 27–34. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Burda, C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Batzill, M.; Morales, E.H.; Diebold, U. Influence of Nitrogen Doping on the Defect Formation and Surface Properties of ${\mathrm{TiO}}_{2}$ Rutile and Anatase. Phys. Rev. Lett. 2006, 96, 026103. [Google Scholar] [CrossRef] [PubMed]
- Thunkhamrak, C.; Chuntib, P.; Ounnunkad, K.; Banet, P.; Aubert, P.-H.; Saianand, G.; Gopalan, A.-I.; Jakmunee, J. Highly sensitive voltammetric immunosensor for the detection of prostate specific antigen based on silver nanoprobe assisted graphene oxide modified screen printed carbon electrode. Talanta 2020, 208, 120389. [Google Scholar] [CrossRef]
- Gopalan, A.; Komathi, S.; Muthuchamy, N.; Lee, K.; Whitcombe, M.J.; Dhana, L.; Sai-Anan, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar]
- Bhatia, V.; Malekshoar, G.; Dhir, A.; Ray, A.K. Enhanced photocatalytic degradation of atenolol using graphene TiO2 composite. J. Photochem. Photobiol. A Chem. 2017, 332, 182–187. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, Z.; Chen, C.; Zhu, L.; Chang, Q.; Wang, N.; Wei, W.; Tang, H. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 2011, 49, 2693–2701. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z. Comparing Graphene-TiO2 Nanowire and Graphene-TiO2 Nanoparticle Composite Photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 3944–3950. [Google Scholar] [CrossRef]
- Nallal, M.; Anantha Iyengar, G.; Pill-Lee, K. New Titanium Dioxide-Based Heterojunction Nanohybrid for Highly Selective Photoelectrochemical–Electrochemical Dual-Mode Sensors. ACS Appl. Mater. Interfaces 2017, 9, 37166–37183. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Effective photocatalytic degradation of anthropogenic dyes using graphene oxide grafting titanium dioxide nanoparticles under UV-light irradiation. J. Photochem. Photobiol. A Chem. 2017, 333, 92–104. [Google Scholar] [CrossRef]
- Xing, M.; Li, X.; Zhang, J. Synergistic effect on the visible light activity of Ti3+ doped TiO2 nanorods/boron doped graphene composite. Sci. Rep. 2014, 4, 5493. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene Modified TiO2 Composite Photocatalysts: Mechanism, Progress and Perspective. Nanomaterials 2018, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.B.; Basavarajappa, P.S.; Ganganagappa, N.; Jyothi, M.S.; Raghu, A.V.; Reddy, K.R. Recent advances in non-metals-doped TiO2 nanostructured photocatalysts for visible-light driven hydrogen production, CO2 reduction and air purification. Int. J. Hydrog. Energy 2019, 44, 13022–13039. [Google Scholar] [CrossRef]
- Ismael, M. Highly effective ruthenium-doped TiO2 nanoparticles photocatalyst for visible-light-driven photocatalytic hydrogen production. New J. Chem. 2019, 43, 9596–9605. [Google Scholar] [CrossRef]
- Devi, L.G.; Kottam, N.; Murthy, B.N.; Kumar, S.G. Enhanced photocatalytic activity of transition metal ions Mn2+, Ni2+ and Zn2+ doped polycrystalline titania for the degradation of Aniline Blue under UV/solar light. J. Mol. Catal. A Chem. 2010, 328, 44–52. [Google Scholar] [CrossRef]
- Shao, G. Electronic Structures of Manganese-Doped Rutile TiO2 from First Principles. J. Phys. Chem. C 2008, 112, 18677–18685. [Google Scholar] [CrossRef]
- Wei, F.; Ni, L.; Cui, P. Preparation and characterization of N–S-codoped TiO2 photocatalyst and its photocatalytic activity. J. Hazard. Mater. 2008, 156, 135–140. [Google Scholar] [CrossRef]
- Zhang, S.; Song, L. Preparation of visible-light-active carbon and nitrogen codoped titanium dioxide photocatalysts with the assistance of aniline. Catal. Commun. 2009, 10, 1725–1729. [Google Scholar] [CrossRef]
- Song, K.; Zhou, J.; Bao, J.; Feng, Y. Photocatalytic Activity of (Copper, Nitrogen)-Codoped Titanium Dioxide Nanoparticles. J. Am. Ceram. Soc. 2008, 91, 1369–1371. [Google Scholar] [CrossRef]
- Sun, X.; Liu, H.; Dong, J.; Wei, J.; Zhang, Y. Preparation and Characterization of Ce/N-Codoped TiO2 Particles for Production of H2 by Photocatalytic Splitting Water Under Visible Light. Catal. Lett. 2010, 135, 219–225. [Google Scholar] [CrossRef]
- Biswas, S.K.; Pathak, A.; Pramanik, N.K.; Dhak, D.; Pramanik, P. Codoped Cr and W rutile nanosized powders obtained by pyrolysis of triethanolamine complexes. Ceram. Int. 2008, 34, 1875–1883. [Google Scholar] [CrossRef]
- Feng, Z.; Kaiming, L. Preparation of Ce4+, Sb3+ -Codoped TiO2 Films in Electric Field Heating-Treatment. J. Rare Earths 2006, 24, 68–70. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Visible-Light-Response and Photocatalytic Activities of TiO2 and SrTiO3 Photocatalysts Codoped with Antimony and Chromium. J. Phys. Chem. B 2002, 106, 5029–5034. [Google Scholar] [CrossRef]
- Niishiro, R.; Kato, H.; Kudo, A. Nickel and either tantalum or niobium-codoped TiO2 and SrTiO3 photocatalysts with visible-light response for H2 or O2 evolution from aqueous solutions. Phys. Chem. Chem. Phys. 2005, 7, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Birajdar, B. Effective La-Na Co-Doped TiO2 Nano-Particles for Dye Adsorption: Synthesis, Characterization and Study on Adsorption Kinetics. Nanomaterials 2019, 9, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Xie, Y.; Lin, Q.; Zheng, R.; Diao, Y. Preparation of C, N and P co-doped TiO2 and its photocatalytic activity under visible light. Funct. Mater. Lett. 2019, 12, 1950045. [Google Scholar] [CrossRef]
- Zhu, W.; Qiu, X.; Iancu, V.; Chen, X.-Q.; Pan, H.; Wang, W.; Dimitrijevic, N.M.; Rajh, T.; Meyer, H.M., III; Paranthaman, M.P.; et al. Band Gap Narrowing of Titanium Oxide Semiconductors by Noncompensated Anion-Cation Codoping for Enhanced Visible-Light Photoactivity. Phys. Rev. Lett. 2009, 103, 226401. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cheng, Z.; Tan, S.; Shao, X.; Wang, B.; Hou, J.G. Characterization of Cr–N codoped anatase TiO2(001) thin films epitaxially grown on SrTiO3(001) substrate. Surf. Sci. 2013, 616, 93–99. [Google Scholar] [CrossRef]
- Zhengwang, C.; Lili, Z.; Shihui, D.; Xiaochuan, M.; Huanxin, J.; Junfa, Z.; Xuefeng, C.; Jin, Z.; Bing, W. Electronic properties of Cr-N codoped rutile TiO2(110) thin films. Surf. Sci. 2017, 666, 84–89. [Google Scholar]
- Li, H.; Yu, Q.; Huang, Y.; Yu, C.; Li, R.; Wang, J.; Guo, F.; Jiao, S.; Gao, S.; Zhang, Y.; et al. Ultralong Rutile TiO2 Nanowire Arrays for Highly Efficient Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 13384–13391. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Reduced and n-Type Doped TiO2: Nature of Ti3+ Species. J. Phys. Chem. C 2009, 113, 20543–20552. [Google Scholar] [CrossRef]
- Wetchakun, N.; Incessungvorn, B.; Wetchakun, K.; Phanichphant, S. Influence of calcination temperature on anatase to rutile phase transformation in TiO2 nanoparticles synthesized by the modified sol–gel method. Mater. Lett. 2012, 82, 195–198. [Google Scholar] [CrossRef]
- Pal, M.; García Serrano, J.; Santiago, P.; Pal, U. Size-Controlled Synthesis of Spherical TiO2 Nanoparticles: Morphology, Crystallization, and Phase Transition. J. Phys. Chem. C 2007, 111, 96–102. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Lin, C.Y.W.; Channei, D.; Koshy, P.; Nakaruk, A.; Sorrell, C.C. Multivalent Mn-doped TiO2 thin films. Phys. E Low-Dimens. Syst. Nanostructures 2012, 44, 1969–1972. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Iida, Y.; Ozaki, S. Grain Growth and Phase Transformation of Titanium Oxide During Calcination. J. Am. Ceram. Soc. 1961, 44, 120–127. [Google Scholar] [CrossRef]
- Reidy, D.J.; Holmes, J.D.; Nagle, C.; Morris, M.A. A highly thermally stable anatase phase prepared by doping with zirconia and silica coupled to a mesoporous type synthesis technique. J. Mater. Chem. 2005, 15, 3494–3500. [Google Scholar] [CrossRef]
- Lee, B.I.; Wang, X.; Bhave, R.; Hu, M. Synthesis of brookite TiO2 nanoparticles by ambient condition sol process. Mater. Lett. 2006, 60, 1179–1183. [Google Scholar] [CrossRef]
- Jing, L.; Li, S.; Song, S.; Xue, L.; Fu, H. Investigation on the electron transfer between anatase and rutile in nano-sized TiO2 by means of surface photovoltage technique and its effects on the photocatalytic activity. Sol. Energy Mater. Sol. Cells 2008, 92, 1030–1036. [Google Scholar] [CrossRef]
- Bersani, D.; Antonioli, G.; Lottici, P.P.; Lopez, T. Raman study of nanosized titania prepared by sol–gel route. J. Non-Cryst. Solids 1998, 232, 175–181. [Google Scholar] [CrossRef]
- Kominami, H.; Kato, J.; Murakami, S.; Kera, Y.; Inoue, M.; Inui, T.; Ohtani, B. Synthesis of titanium(IV) oxide of ultra-high photocatalytic activity: High-temperature hydrolysis of titanium alkoxides with water liberated homogeneously from solvent alcohols. J. Mol. Catal. A Chem. 1999, 144, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Bacsa, R.R.; Kiwi, J. Effect of rutile phase on the photocatalytic properties of nanocrystalline titania during the degradation of p-coumaric acid. Appl. Catal. B Environ. 1998, 16, 19–29. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Deng, K.; Chen, X.; Zou, Z. Low Temperature Synthesis and Photocatalytic Activity of Rutile TiO2 Nanorod Superstructures. J. Phys. Chem. C 2007, 111, 2709–2714. [Google Scholar] [CrossRef]
- Beck, D.D.; Siegel, R.W. The dissociative adsorption of hydrogen sulfide over nanophase titanium dioxide. J. Mater. Res. 1992, 7, 2840–2845. [Google Scholar] [CrossRef]
- Ohno, T.; Haga, D.; Fujihara, K.; Kaizaki, K.; Matsumura, M. Unique Effects of Iron(III) Ions on Photocatalytic and Photoelectrochemical Properties of Titanium Dioxide. J. Phys. Chem. B 1997, 101, 6415–6419. [Google Scholar] [CrossRef]
- Bointon, T.H.; Jones, G.F.; De Sanctis, A.; Hill-Pearce, R.; Craciun, M.F.; Russo, S. Large-area functionalized CVD graphene for work function matched transparent electrodes. Sci. Rep. 2015, 5, 16464. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhang, D.; Yu, J.C.; Leung, M.K.H. An Efficient Bismuth Tungstate Visible-Light-Driven Photocatalyst for Breaking Down Nitric Oxide. Environ. Sci. Technol. 2010, 44, 4276–4281. [Google Scholar] [CrossRef]
- Amore Bonapasta, A.; Filippone, F.; Mattioli, G.; Alippi, P. Oxygen vacancies and OH species in rutile and anatase TiO2 polymorphs. Catal. Today 2009, 144, 177–182. [Google Scholar] [CrossRef]
- Liu, R.; Fang, L.; Hao, Y.; Chi, Y. Influence of Oxygen Vacancy Density on the Polaronic Configuration in Rutile. Materials 2018, 11, 2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Titanium Precursor | Polymer (PVP) (g) | Doping Precursors | Mn | Electrospun Products | Calcination Temperature | ||
|---|---|---|---|---|---|---|---|
| TIIP (g) | Molecular Weight (40,000) | Molecular Weight (1,300,000) | Graphene (g) | Designation | 550 °C | 800 °C | |
| 1.5 | 2.4 | 0.40 | 0 | 0 | TP | T(550) | T(800) |
| 0.080 | 0 | TP(G) | T(G)(550) | T(G)(800) | |||
| 0.080 | 0.04 | TP(G-Mn1) | T(G)Mn1(550) | T(G)Mn1 (800) | |||
| 0.080 | 0.08 | TP(G-Mn2) | T(G)Mn1(550) | T(G)Mn2 (800) | |||
| 0.080 | 0.12 | TP(G-Mn3) | T(G)Mn3(550) | T(G)Mn3 (880) | |||
| Lattice Parameters | Mass Fraction (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample Designation | d-spacing (A) | a (Å) | b (Å) | c (Å) | Unit Cell Volume (a3) | Crystallite Size (t) (nm) | Anatase XA | Rutile XR |
| T(G)Mn1(550) | 3.531 | 3.780 | 3.780 | 9.867 | 140.98 | 18.7 | 79.16 | 20.84 |
| T(G)Mn2(550) | 3.531 | 3.780 | 3.780 | 9.867 | 140.98 | 24.1 | 72.70 | 27.30 |
| T(G)Mn3(550) | - | - | - | - | - | 2.58 | - | - |
| T(550) | 3.515 | 3.782 | 3.782 | 9.499 | 136.78 | 21.0 | 90.14 | 9.86 |
| T(G)(550) | 3.518 | 3.781 | 3.781 | 9.495 | 136.43 | 15.6 | 93.76 | 6.24 |
| T(G)Mn1(800) | 3.246 | 4.600 | 4.600 | 4.564 | 96.574 | 8.12 | 0.87 | 99.13 |
| T(G)Mn2(800) | 3.251 | 4.600 | 4.600 | 4.564 | 96.574 | 66.8 | Trace | ~100 |
| T(G)Mn3(880) | 3.251 | 4.600 | 4.600 | 4.564 | 96.574 | 29.9 | - | 100 |
| T(800) | 3.250 | 4.594 | 4.594 | 2.959 | 62.450 | 49.5 | 1.00 | 99.0 |
| T(G)(800) | 3.507 | 4.593 | 4.593 | 2.959 | 62.42 | 31.9 | Trace | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-C.; Gopalan, A.-I.; Saianand, G.; Lee, K.-P.; Kim, W.-J. Manganese and Graphene Included Titanium Dioxide Composite Nanowires: Fabrication, Characterization and Enhanced Photocatalytic Activities. Nanomaterials 2020, 10, 456. https://doi.org/10.3390/nano10030456
Lee J-C, Gopalan A-I, Saianand G, Lee K-P, Kim W-J. Manganese and Graphene Included Titanium Dioxide Composite Nanowires: Fabrication, Characterization and Enhanced Photocatalytic Activities. Nanomaterials. 2020; 10(3):456. https://doi.org/10.3390/nano10030456
Chicago/Turabian StyleLee, Jun-Cheol, Anantha-Iyengar Gopalan, Gopalan Saianand, Kwang-Pill Lee, and Wha-Jung Kim. 2020. "Manganese and Graphene Included Titanium Dioxide Composite Nanowires: Fabrication, Characterization and Enhanced Photocatalytic Activities" Nanomaterials 10, no. 3: 456. https://doi.org/10.3390/nano10030456
APA StyleLee, J.-C., Gopalan, A.-I., Saianand, G., Lee, K.-P., & Kim, W.-J. (2020). Manganese and Graphene Included Titanium Dioxide Composite Nanowires: Fabrication, Characterization and Enhanced Photocatalytic Activities. Nanomaterials, 10(3), 456. https://doi.org/10.3390/nano10030456








