Plasmon-Emitter Hybrid Nanostructures of Gold Nanorod-Quantum Dots with Regulated Energy Transfer as a Universal Nano-Sensor for One-step Biomarker Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Apparatus
2.3. Synthesis of Au NRs
2.4. Functionalization of Au NRs with Amino Groups
2.5. Preparation of Au NR-QDs Assemblies
2.6. Sensing Behavior of the Plasmon-regulated FRET Sensing System of Au NR-QDs
2.7. Reversible RI-Dependent Properties of Au NR-QDs
2.8. Fabrication of Au NR-QDs Based Nano-sensors
2.9. Detection of AFP Using the Au NR-QDs Apta-sensor
2.10. Detection of AFP in Spiked Serum Samples
2.11. Detection of cTnI Using the Antibodies Modified Au NR-QDs and Au NP-QDs Nano-sensors
3. Results and Discussion
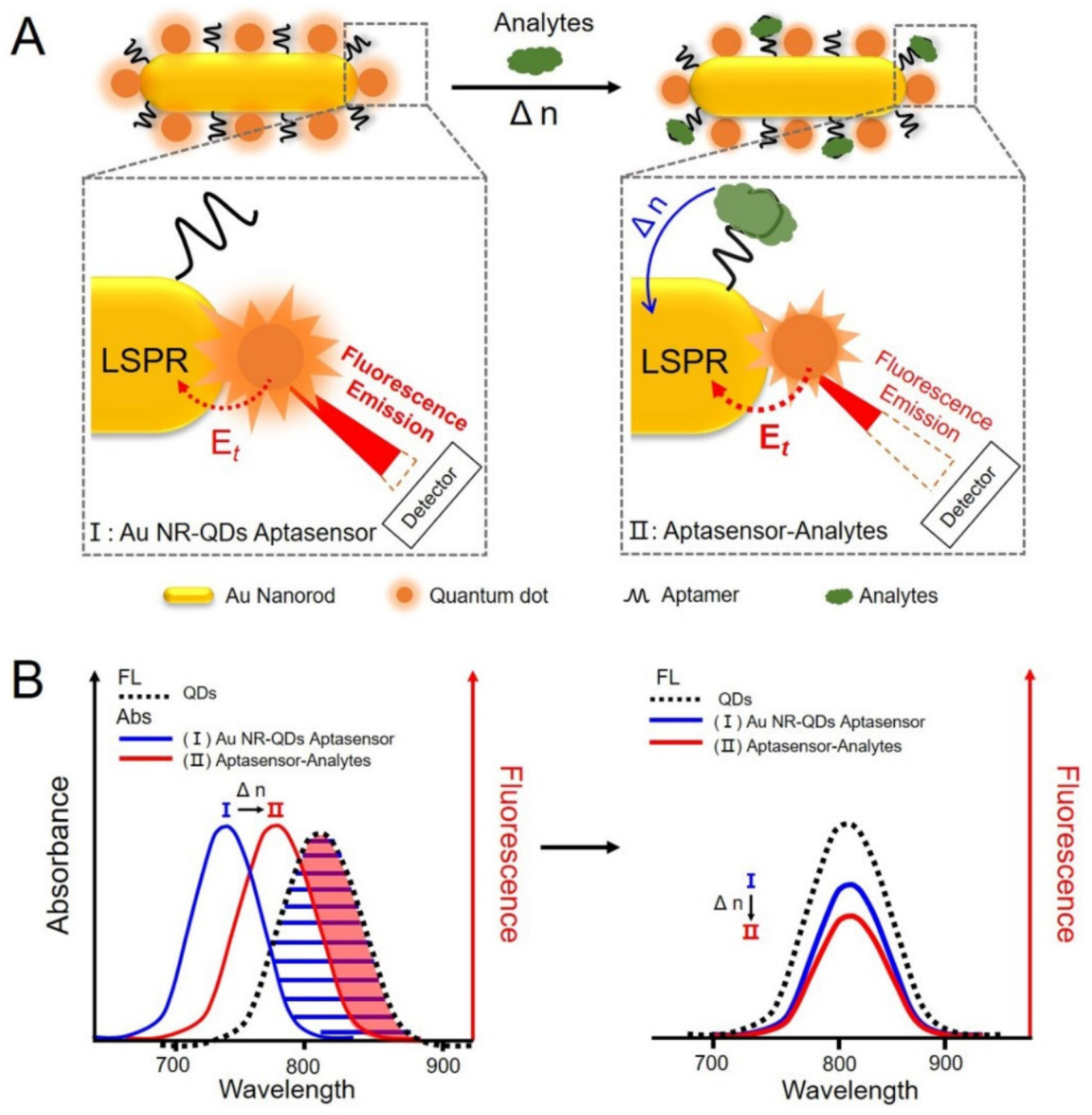
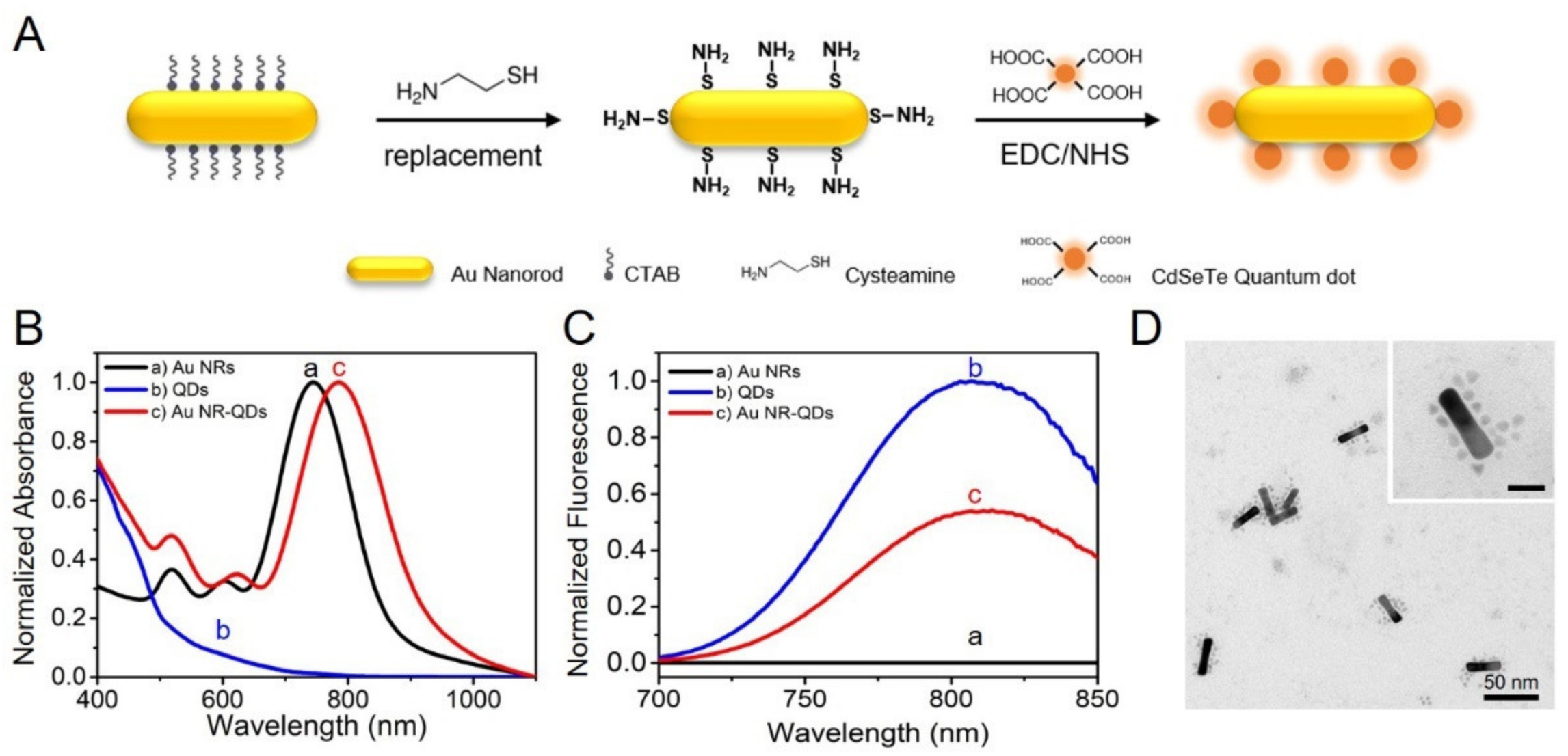
3.1. Fabrication and Characterization of the Plasmon-Emitter Hybrid Nanostructures of Au NR-QDs Assemblies
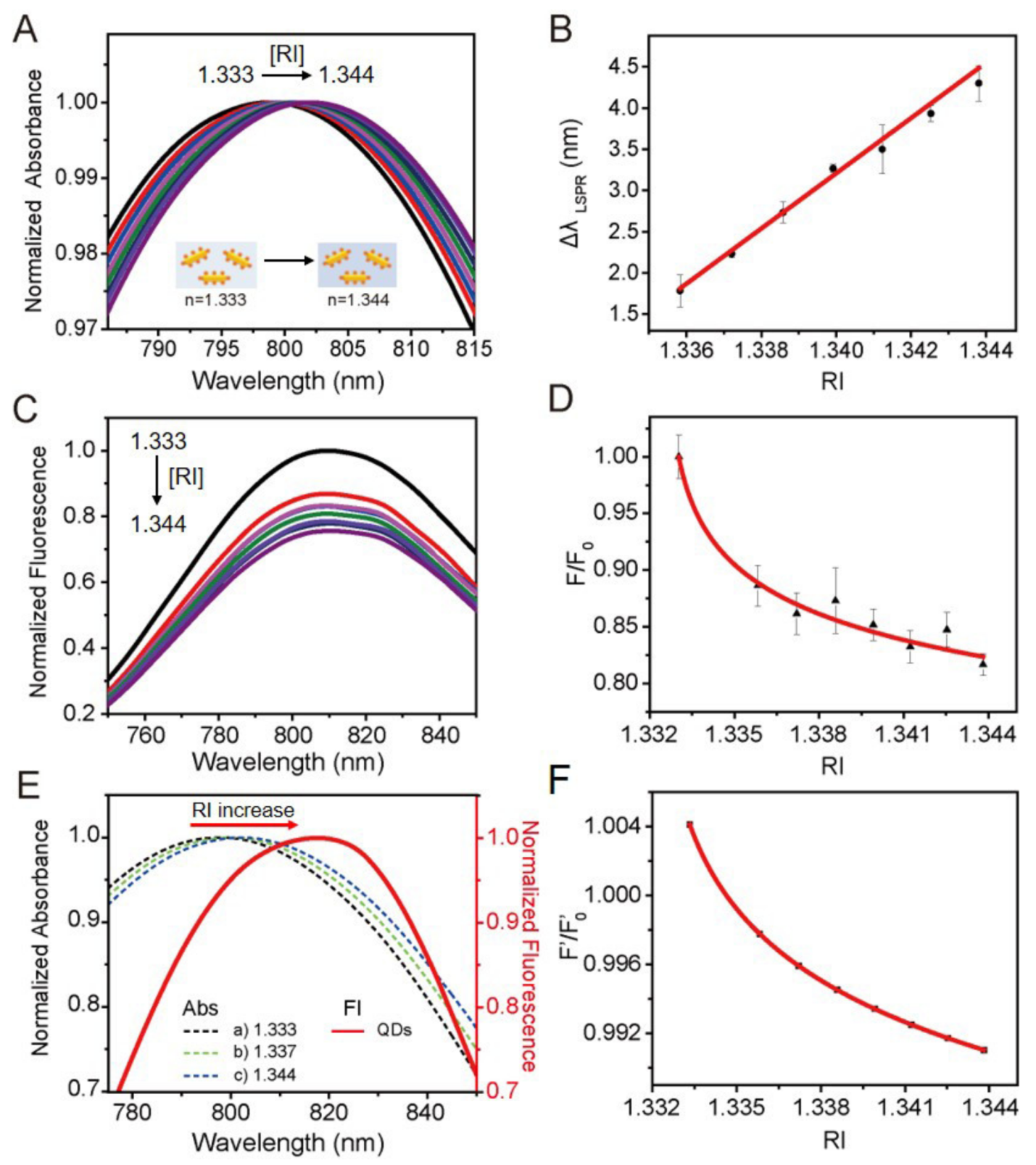
3.2. Sensing Behavior of the Plasmon-Regulated FRET Sensing System of Au NR-QDs
3.3. Construction of the Au NR-QDs Nano-sensor
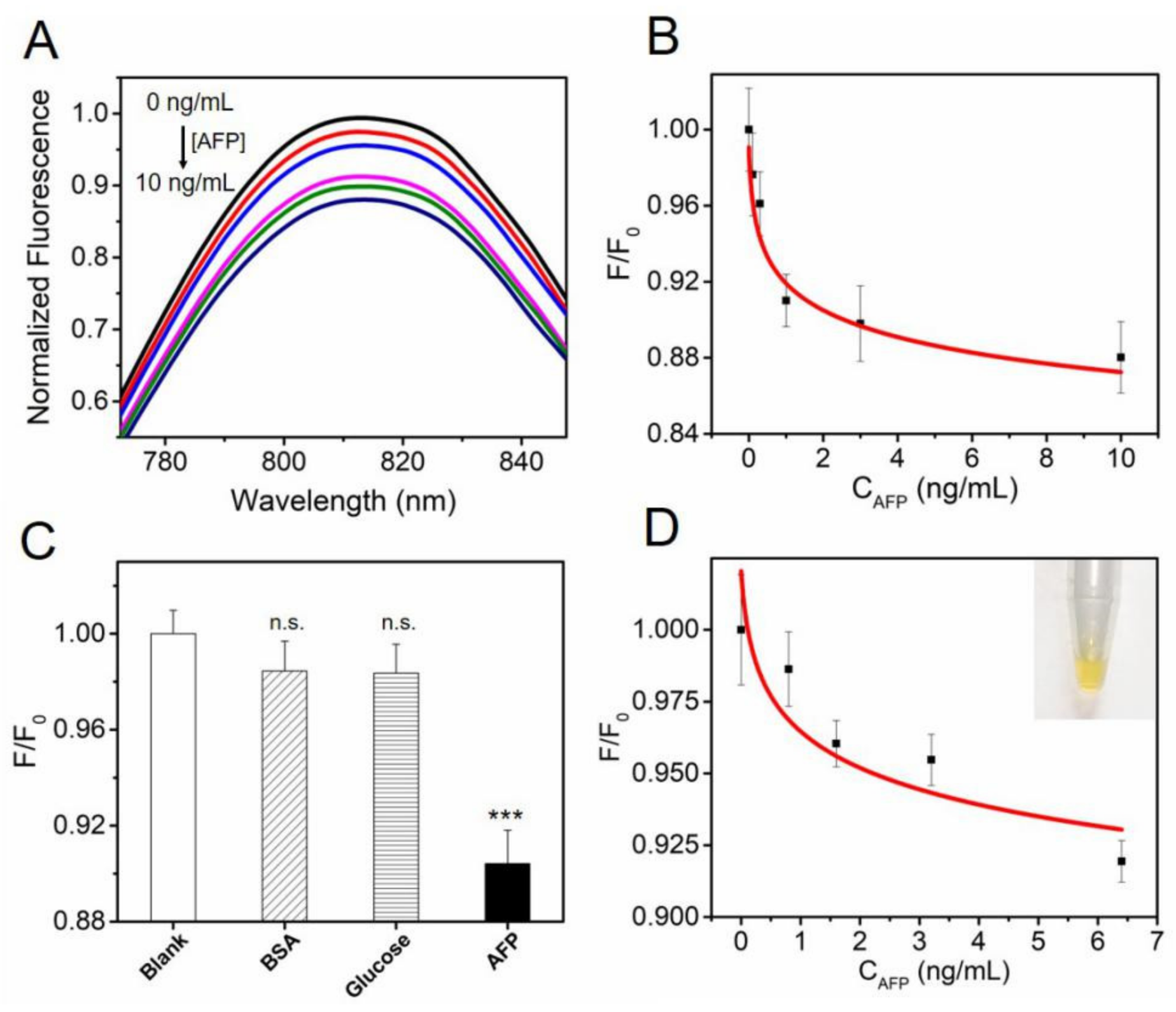
3.4. Au NR-QDs Apta-sensor for AFP Detection
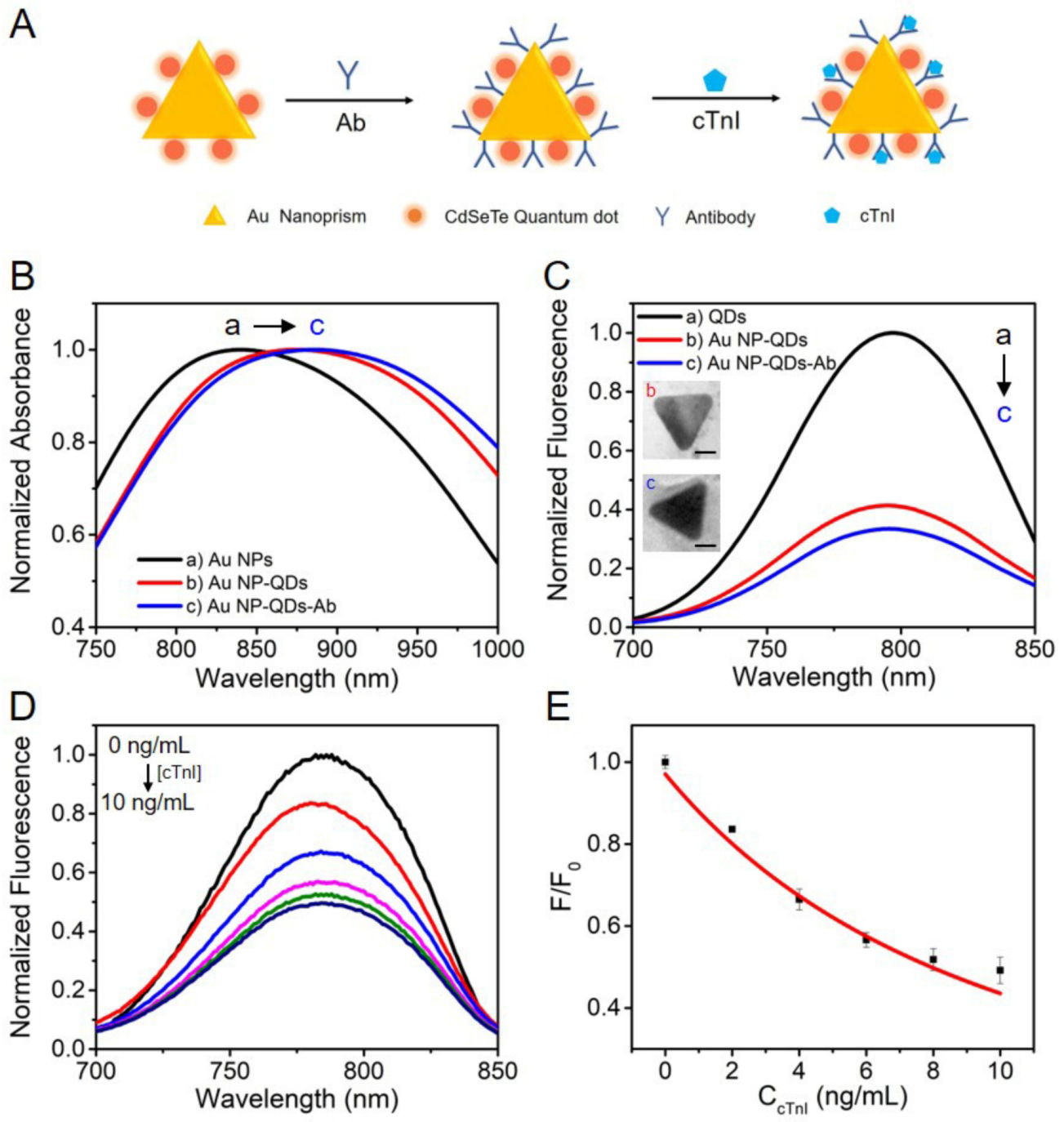
3.5. Construction of Au NR-QDs and Au NP-QDs Nano-sensors for cTnI Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maccaferri, N.; Zhao, Y.Q.; Isoniemi, T.; Iarossi, M.; Parracino, A.; Strangi, G.; De Angelis, F. Hyperbolic meta-antennas enable full control of scattering and absorption of light. Nano Lett. 2019, 19, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhou, J.H.; Liu, T.R.; Tao, Y.T.; Jiang, R.B.; Liu, M.X.; Xiao, G.H.; Zhu, J.H.; Zhou, Z.K.; Wang, X.H.; et al. Plasmonic gold mushroom arrays with refractive index sensing figures of merit approaching the theoretical limit. Nat. Commun. 2013, 4, 2381. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Li, J.H. Plasmon-based colorimetric nanosensors for ultrasensitive molecular diagnostics. ACS Sens. 2017, 2, 857–875. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Alapan, Y.; ElKabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; De Luca, A.; Strangi, G. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 2016, 15, 621–628. [Google Scholar] [CrossRef]
- Liu, B.W.; Chen, S.; Zhang, J.C.; Yao, X.; Zhong, J.H.; Lin, H.X.; Huang, T.X.; Yang, Z.L.; Zhu, J.F.; Liu, S.; et al. A plasmonic sensor array with ultrahigh figures of merit and resonance linewidths down to 3 nm. Adv. Mater. 2018, 30, 1706031. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhou, J.H.; Li, J.H. Construction of plasmonic nano-biosensor-based devices for point-of-care testing. Small Methods 2017, 1, 1700197. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Sensing using localised surface plasmon resonance sensors. Chem. Commun. 2012, 48, 8999–9010. [Google Scholar] [CrossRef]
- Culver, H.R.; Wechsler, M.E.; Peppas, N.A. Label-free detection of tear biomarkers using hydrogel-coated gold nanoshells in a localized surface plasmon resonance-based biosensor. ACS Nano 2018, 12, 9342–9354. [Google Scholar] [CrossRef]
- Li, W.; Jiang, X.; Xue, J.; Zhou, Z.; Zhou, J. Antibody modified gold nano-mushroom arrays for rapid detection of alpha-fetoprotein. Biosens. Bioelectron. 2015, 68, 468–474. [Google Scholar] [CrossRef]
- Sabela, M.; Balme, S.; Bechelany, M.; Janot, J.M.; Bisetty, K. A review of gold and silver nanoparticle-based colorimetric sensing assays. Adv. Eng. Mater. 2017, 19, 1700270. [Google Scholar] [CrossRef]
- Coglitore, D.; Janot, J.M.; Balme, S. Protein at liquid solid interfaces: Toward a new paradigm to change the approach to design hybrid protein/solid-state materials. Adv. Colloid Interface Sci. 2019, 270, 278–292. [Google Scholar] [CrossRef]
- Li, J.F.; Li, C.Y.; Aroca, R.F. Plasmon-enhanced fluorescence spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, J.H.; Colas, M.; Kim, D.H.; Lee, H. Gold-based hybrid nanomaterials for biosensing and molecular diagnostic applications. Biosens. Bioelectron. 2016, 80, 543–559. [Google Scholar] [CrossRef]
- Wang, M.S.; Krasnok, A.; Zhang, T.Y.; Scarabelli, L.; Liu, H.; Wu, Z.L.; Liz-Marzan, L.M.; Terrones, M.; Alu, A.; Zheng, Y.B. Tunable Fano resonance and plasmon-exciton coupling in single Au nanotriangles on monolayer WS2 at room temperature. Adv. Mater. 2018, 30, 1705779. [Google Scholar] [CrossRef]
- Luk’yanchuk, B.; Zheludev, N.I.; Maier, S.A.; Halas, N.J.; Nordlander, P.; Giessen, H.; Chong, C.T. The Fano resonance in plasmonic nanostructures and metamaterials. Nat. Mater. 2010, 9, 707–715. [Google Scholar] [CrossRef]
- Ramirez, M.O.; Molina, P.; Gomez-Tornero, A.; Hernandez-Pinilla, D.; Sanchez-Garcia, L.; Carretero-Palacios, S.; Bausa, L.E. Hybrid plasmonic-ferroelectric architectures for lasing and SHG processes at the nanoscale. Adv. Mater. 2019, 31, 1901428. [Google Scholar] [CrossRef]
- Rakovich, A.; Albella, P.; Maier, S.A. Plasmonic control of radiative properties of semiconductor quantum dots coupled to plasmonic ring cavities. ACS Nano 2015, 9, 2648–2658. [Google Scholar] [CrossRef]
- Werschler, F.; Lindner, B.; Hinz, C.; Conradt, F.; Gumbsheimer, P.; Behovits, Y.; Negele, C.; de Roo, T.; Tzang, O.; Mecking, S.; et al. Efficient emission enhancement of single CdSe/CdS/PMMA quantum dots through controlled near-field coupling to plasmonic bullseye resonators. Nano Lett. 2018, 18, 5396–5400. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Yang, X.; Tang, Y.; Han, S.; Kang, A.; Deng, H.; Chi, Y.; Zhu, D.; Lu, Y. Forster resonance energy transfer (FRET)-based biosensors for biological applications. Biosens. Bioelectron. 2019, 138, 111314. [Google Scholar] [CrossRef]
- Ling, J.A.; Huang, C.Z. Energy transfer with gold nanoparticles for analytical applications in the fields of biochemical and pharmaceutical sciences. Anal. Methods 2010, 2, 1439–1447. [Google Scholar] [CrossRef]
- Bitton, O.; Gupta, S.N.; Haran, G. Quantum dot plasmonics: From weak to strong coupling. Nanophotonics 2019, 8, 559–575. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.Y.; Choi, H.K.; Lee, J.Y.; Choi, J.W. Application of gold nanoparticle to plasmonic biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef]
- Jeong, Y.; Kook, Y.M.; Lee, K.; Koh, W.G. Metal enhanced fluorescence (MEF) for biosensors: General approaches and a review of recent developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Brennan, M.R.; Wilson, W.L.; Murphy, C.J. Distance and plasmon wavelength dependent fluorescence of molecules bound to silica-coated gold nanorods. ACS Nano 2014, 8, 8392–8406. [Google Scholar] [CrossRef]
- Aissaoui, N.; Moth Poulsen, K.; Kall, M.; Johansson, P.; Wilhelmsson, L.M.; Albinsson, B. FRET enhancement close to gold nanoparticles positioned in DNA origami constructs. Nanoscale 2017, 9, 673–683. [Google Scholar] [CrossRef]
- Fang, A.J.; Chen, H.Y.; Li, H.T.; Liu, M.L.; Zhang, Y.Y.; Yao, S.Z. Glutathione regulation-based dual-functional upconversion sensing-platform for acetylcholinesterase activity and cadmium ions. Biosens. Bioelectron. 2017, 87, 545–551. [Google Scholar] [CrossRef]
- Xia, Y.; Song, L.; Zhu, C. Turn-on and near-infrared fluorescent sensing for 2,4,6-trinitrotoluene based on hybrid (gold nanorod)-(quantum dots) assembly. Anal. Chem. 2011, 83, 1401–1407. [Google Scholar] [CrossRef]
- Qu, A.H.; Xu, L.G.; Sun, M.Z.; Liu, L.Q.; Kuang, H.; Xu, C.L. Photoactive hybrid AuNR-Pt@Ag2S core-satellite nanostructures for near-infrared quantitive cell imaging. Adv. Funct. Mater. 2017, 27, 1703408. [Google Scholar] [CrossRef]
- He, W.; Sun, X.; Liu, B.; Shen, J. A label-free “SEF-FRET” fluorescent sensing platform for ultrasensitive DNA detection based on AgNPs SAMs. Talanta 2019, 205, 120072. [Google Scholar] [CrossRef]
- Zhou, L.; Ji, F.; Zhang, T.; Wang, F.; Li, Y.; Yu, Z.; Jin, X.; Ruan, B. An fluorescent aptasensor for sensitive detection of tumor marker based on the FRET of a sandwich structured QDs-AFP-AuNPs. Talanta 2019, 197, 444–450. [Google Scholar] [CrossRef]
- Xu, D.D.; Liu, C.; Li, C.Y.; Song, C.Y.; Kang, Y.F.; Qi, C.B.; Lin, Y.; Pang, D.W.; Tang, H.W. Dual amplification fluorescence assay for alpha fetal protein utilizing immunohybridization chain reaction and metal-enhanced fluorescence of carbon nanodots. ACS Appl. Mater. Interfaces 2017, 9, 37606–37614. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, E.B.; Lee, S.W.; Cheon, S.A.; Kim, H.J.; Lee, J.; Lee, M.K.; Ko, S.; Park, T.J. An easy and sensitive sandwich assay for detection of Mycobacterium tuberculosis Ag85B antigen using quantum dots and gold nanorods. Biosens. Bioelectron. 2017, 87, 150–156. [Google Scholar] [CrossRef]
- Yang, S.H.; Zhang, F.F.; Wang, Z.H.; Liang, Q.L. A graphene oxide-based label-free electrochemical aptasensor for the detection of alpha-fetoprotein. Biosens. Bioelectron. 2018, 112, 186–192. [Google Scholar] [CrossRef]
- Haran, G.; Chuntonov, L. Artificial plasmonic molecules and their interaction with real molecules. Chem. Rev. 2018, 118, 5539–5580. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wang, Q.Y.; Shi, X.D.; Hornak, L.A.; Hong, Z.L.; Wu, N.Q. Size-dependent energy transfer between CdSe/ZnS quantum dots and gold nanoparticles. J. Phys. Chem. Lett. 2011, 2, 2125–2129. [Google Scholar] [CrossRef]
- Bujak, L.; Ishii, T.; Sharma, D.K.; Hirata, S.; Vacha, M. Selective turn-on and modulation of resonant energy transfer in single plasmonic hybrid nanostructures. Nanoscale 2017, 9, 1511–1519. [Google Scholar] [CrossRef]
- Yu, M.Q.; Wang, H.; Fu, F.; Li, L.Y.; Li, J.; Li, G.; Song, Y.; Swihart, M.T.; Song, E.Q. Dual-recognition Forster resonance energy transfer based platform for one-step sensitive detection of pathogenic bacteria using fluorescent vancomycin-gold nanoclusters and aptamer-gold nanoparticles. Anal. Chem. 2017, 89, 4085–4090. [Google Scholar] [CrossRef]
- Huang, C.J.; Lin, H.I.; Shiesh, S.C.; Lee, G.B. An integrated microfluidic system for rapid screening of alpha-fetoprotein-specific aptamers. Biosens. Bioelectron. 2012, 35, 50–55. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Wang, C.; Irudayaraj, J. Gold nanorod probes for the detection of multiple pathogens. Small 2008, 4, 2204–2208. [Google Scholar] [CrossRef]
- Maity, A.R.; Stepensky, D. Efficient subcellular targeting to the cell nucleus of quantum dots densely decorated with a nuclear localization sequence peptide. ACS Appl. Mater. Interfaces 2016, 8, 2001–2009. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Yue, Z.; Zhang, Z.; Teng, K.S.; Li, M.J.; Yi, C.; Yang, M. Coupling gold nanoparticles to silica nanoparticles through disulfide bonds for glutathione detection. Nanotechnology 2013, 24, 375501. [Google Scholar] [CrossRef]
- Wang, C.; Qian, J.; Wang, K.; Yang, X.; Liu, Q.; Hao, N.; Wang, C.; Dong, X.; Huang, X. Colorimetric aptasensing of ochratoxin A using Au@Fe3O4 nanoparticles as signal indicator and magnetic separator. Biosens. Bioelectron. 2016, 77, 1183–1191. [Google Scholar] [CrossRef]
- Wu, H.Y.; Huang, W.L.; Huang, M.H. Direct high-yield synthesis of high aspect ratio gold nanorods. Cryst. Growth Des. 2007, 7, 831–835. [Google Scholar] [CrossRef]
- Haldar, K.K.; Sen, T.; Patra, A. Metal conjugated semiconductor hybrid nanoparticle-based fluorescence resonance energy transfer. J. Phys. Chem. C 2010, 114, 4869–4874. [Google Scholar] [CrossRef]
- Stanisavljevic, M.; Krizkova, S.; Vaculovicova, M.; Kizek, R.; Adam, V. Quantum dots-fluorescence resonance energy transfer-based nanosensors and their application. Biosens. Bioelectron. 2015, 74, 562–574. [Google Scholar] [CrossRef]
- Gaur, G.; Koktysh, D.S.; Weiss, S.M. Immobilization of quantum dots in nanostructured porous silicon films: Characterizations and signal amplification for dual-mode optical biosensing. Adv. Funct. Mater. 2013, 23, 3604–3614. [Google Scholar] [CrossRef]
- Link, S.; Mohamed, M.B.; El-Sayed, M.A. Simulation of the optical absorption spectra of gold nanorods as a function of their aspect ratio and the effect of the medium dielectric constant. J. Phys. Chem. B 1999, 103, 3073–3077. [Google Scholar] [CrossRef]
- Cui, M.; Wang, Y.; Jiao, M.; Jayachandran, S.; Wu, Y.; Fan, X.; Luo, X. Mixed self-assembled aptamer and newly designed zwitterionic peptide as antifouling biosensing interface for electrochemical detection of alpha-fetoprotein. ACS Sens. 2017, 2, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Breaker, R.R. Natural and engineered nucleic acids as tools to explore biology. Nature 2004, 432, 838–845. [Google Scholar] [CrossRef]
- Song, S.P.; Wang, L.H.; Li, J.; Zhao, J.L.; Fan, C.H. Aptamer-based biosensors. Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Wang, W.T.; Wang, W.; Davis, J.J.; Luo, X.L. Ultrasensitive and selective voltammetric aptasensor for dopamine based on a conducting polymer nanocomposite doped with graphene oxide. Microchim. Acta 2015, 182, 1123–1129. [Google Scholar] [CrossRef]
- Coglitore, D.; Giamblanco, N.; Kizalaite, A.; Coulon, P.E.; Charlot, B.; Janot, J.M.; Balme, S. Unexpected hard protein behavior of BSA on gold nanoparticle caused by resveratrol. Langmuir 2018, 34, 8866–8874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Qi, S.J.; Liu, Z.G.; Shi, Y.P.; Yue, W.Q.; Yi, C.Q. Rapid determination of dopamine in human plasma using a gold nanoparticle-based dual-mode sensing system. Mater. Sci. Eng. C 2016, 61, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Chen, Q.S.; Li, H.H.; Ouyang, Q.; Zhao, J.W. Fabricating a novel label-free aptasensor for acetamiprid by fluorescence resonance energy transfer between NH2-NaYF4: Yb, Ho@SiO2 and Au nanoparticles. Biosens. Bioelectron. 2016, 80, 398–404. [Google Scholar] [CrossRef]
- Mock, J.J.; Hill, R.T.; Tsai, Y.J.; Chilkoti, A.; Smith, D.R. Probing dynamically tunable localized surface plasmon resonances of film-coupled nanoparticles by evanescent wave excitation. Nano Lett. 2012, 12, 1757–1764. [Google Scholar] [CrossRef]
- Liu, Z.; Lee, H.; Xiong, Y.; Sun, C.; Zhang, X. Far-field optical hyperlens magnifying sub-diffraction-limited objects. Science 2007, 315, 1686. [Google Scholar] [CrossRef]
- Han, X.; Li, S.H.; Peng, Z.L.; Othman, A.M.; Leblanc, R. Recent development of cardiac troponin I detection. ACS Sens. 2016, 1, 106–114. [Google Scholar] [CrossRef]

| Sample | Concentration of AFP Spiked (ng/mL) | Concentration of AFP Found (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| diluted serum 1 | 1.6 | 1.9 | 118 | 9.48 |
| diluted serum 2 | 6.4 | 7.3 | 114 | 7.18 |
| diluted serum 3 | 10 | 10.40 | 104 | 2.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Y.; Fu, Q.; Wang, Y.; Ma, D.; Zhou, B.; Zhou, J. Plasmon-Emitter Hybrid Nanostructures of Gold Nanorod-Quantum Dots with Regulated Energy Transfer as a Universal Nano-Sensor for One-step Biomarker Detection. Nanomaterials 2020, 10, 444. https://doi.org/10.3390/nano10030444
Li X, Wang Y, Fu Q, Wang Y, Ma D, Zhou B, Zhou J. Plasmon-Emitter Hybrid Nanostructures of Gold Nanorod-Quantum Dots with Regulated Energy Transfer as a Universal Nano-Sensor for One-step Biomarker Detection. Nanomaterials. 2020; 10(3):444. https://doi.org/10.3390/nano10030444
Chicago/Turabian StyleLi, Xuemeng, Yingshuting Wang, Quanying Fu, Yangyang Wang, Dongxu Ma, Bin Zhou, and Jianhua Zhou. 2020. "Plasmon-Emitter Hybrid Nanostructures of Gold Nanorod-Quantum Dots with Regulated Energy Transfer as a Universal Nano-Sensor for One-step Biomarker Detection" Nanomaterials 10, no. 3: 444. https://doi.org/10.3390/nano10030444
APA StyleLi, X., Wang, Y., Fu, Q., Wang, Y., Ma, D., Zhou, B., & Zhou, J. (2020). Plasmon-Emitter Hybrid Nanostructures of Gold Nanorod-Quantum Dots with Regulated Energy Transfer as a Universal Nano-Sensor for One-step Biomarker Detection. Nanomaterials, 10(3), 444. https://doi.org/10.3390/nano10030444





