Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Scaffolds Preparation
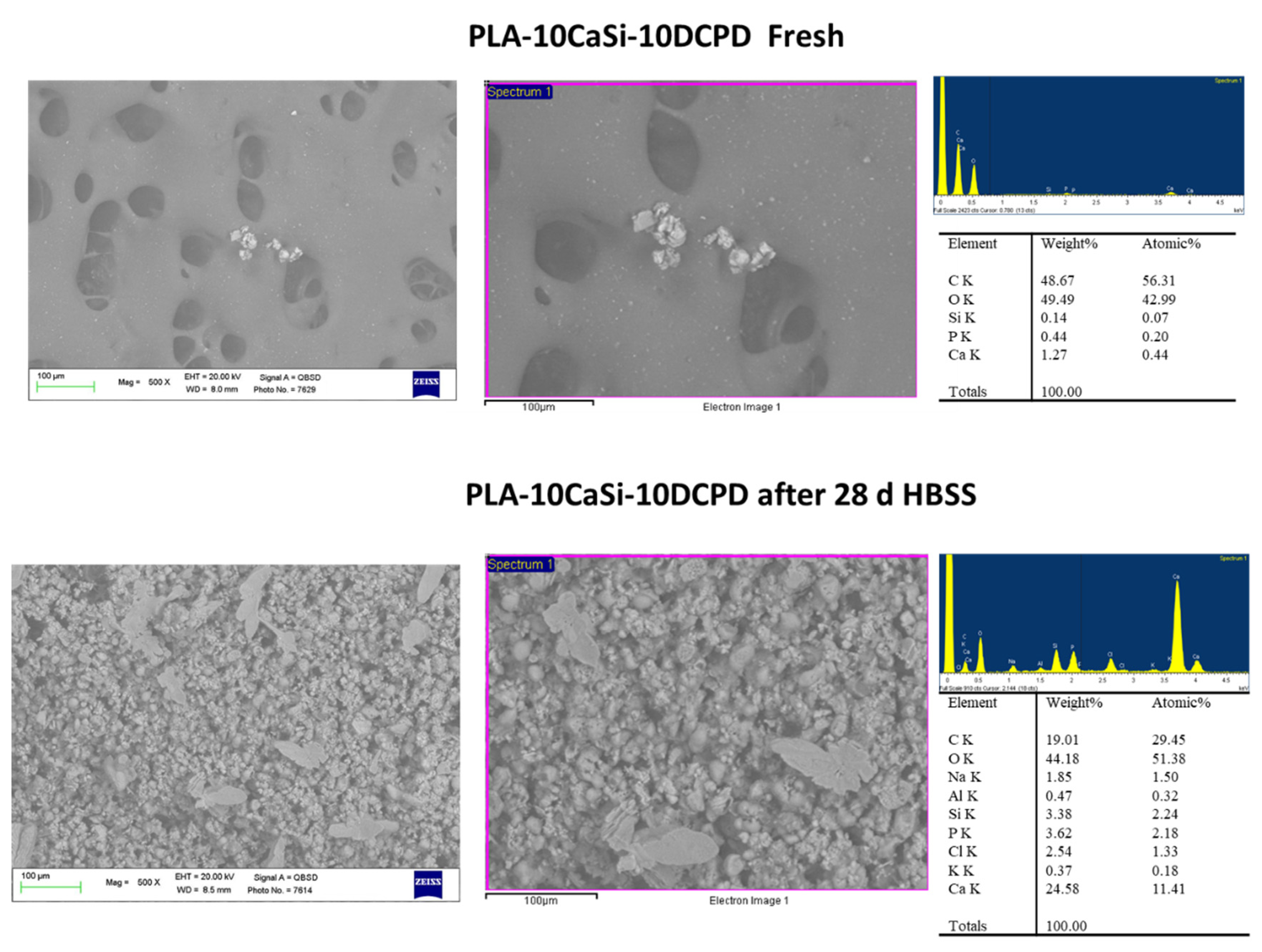
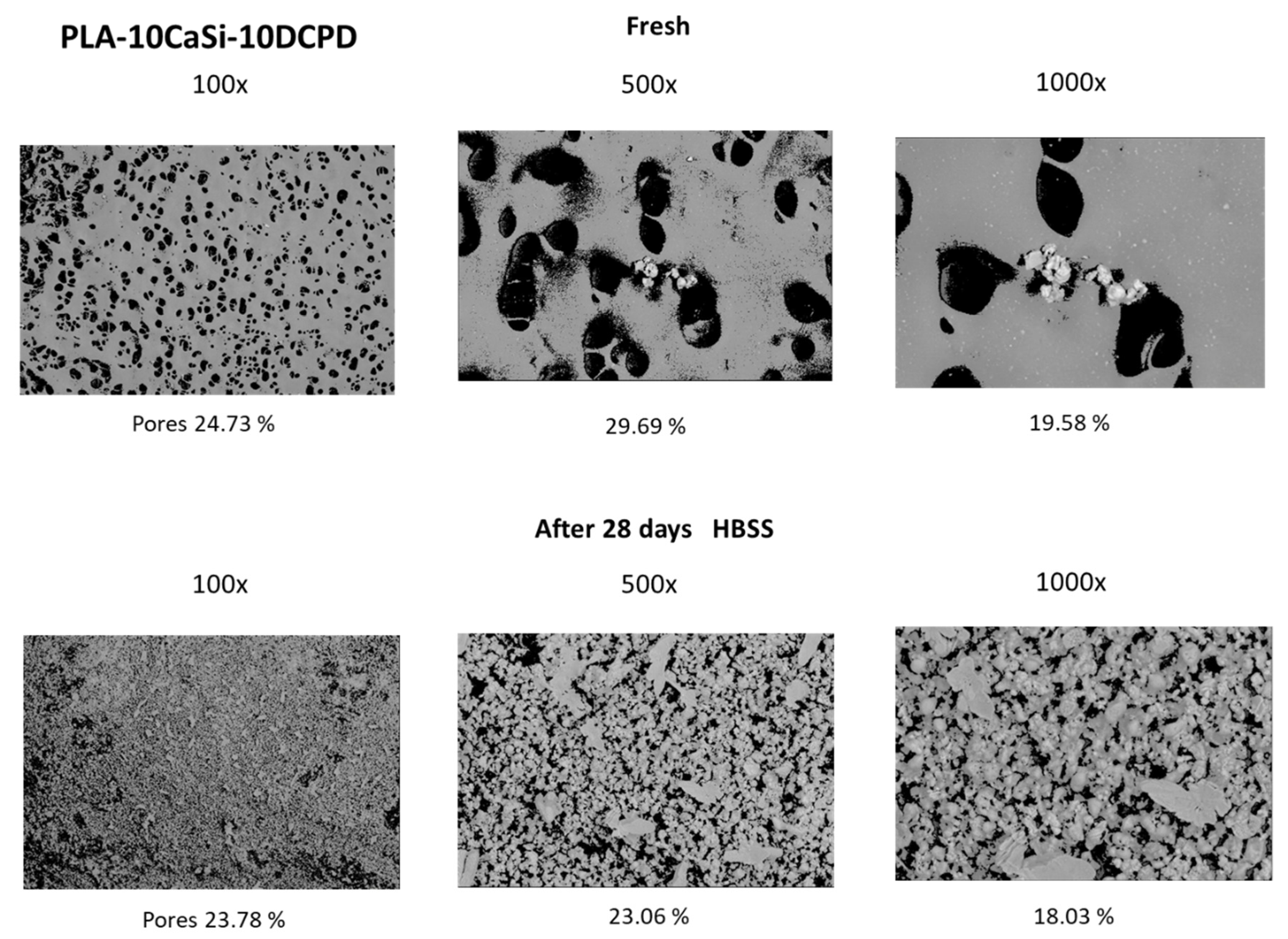
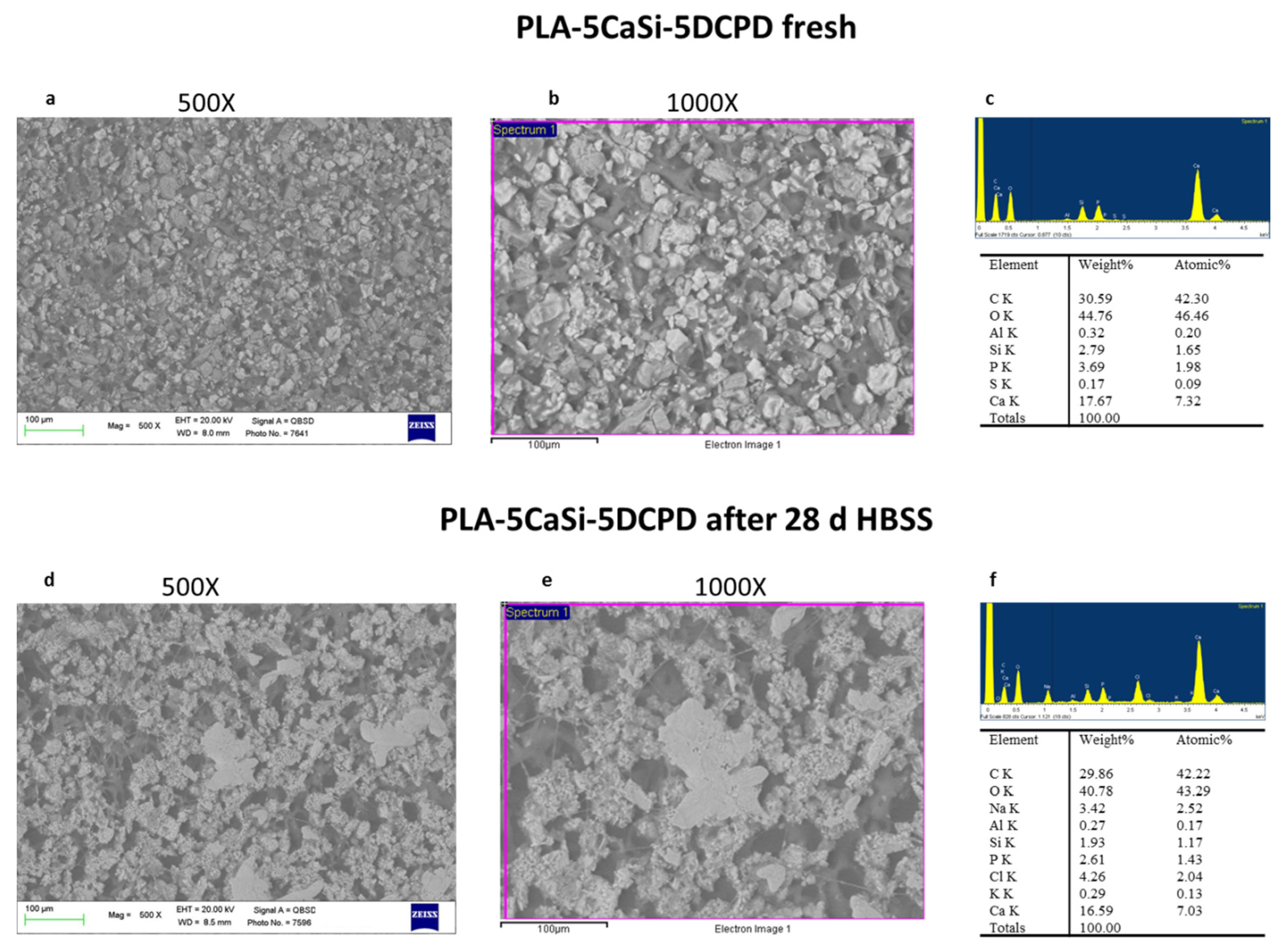
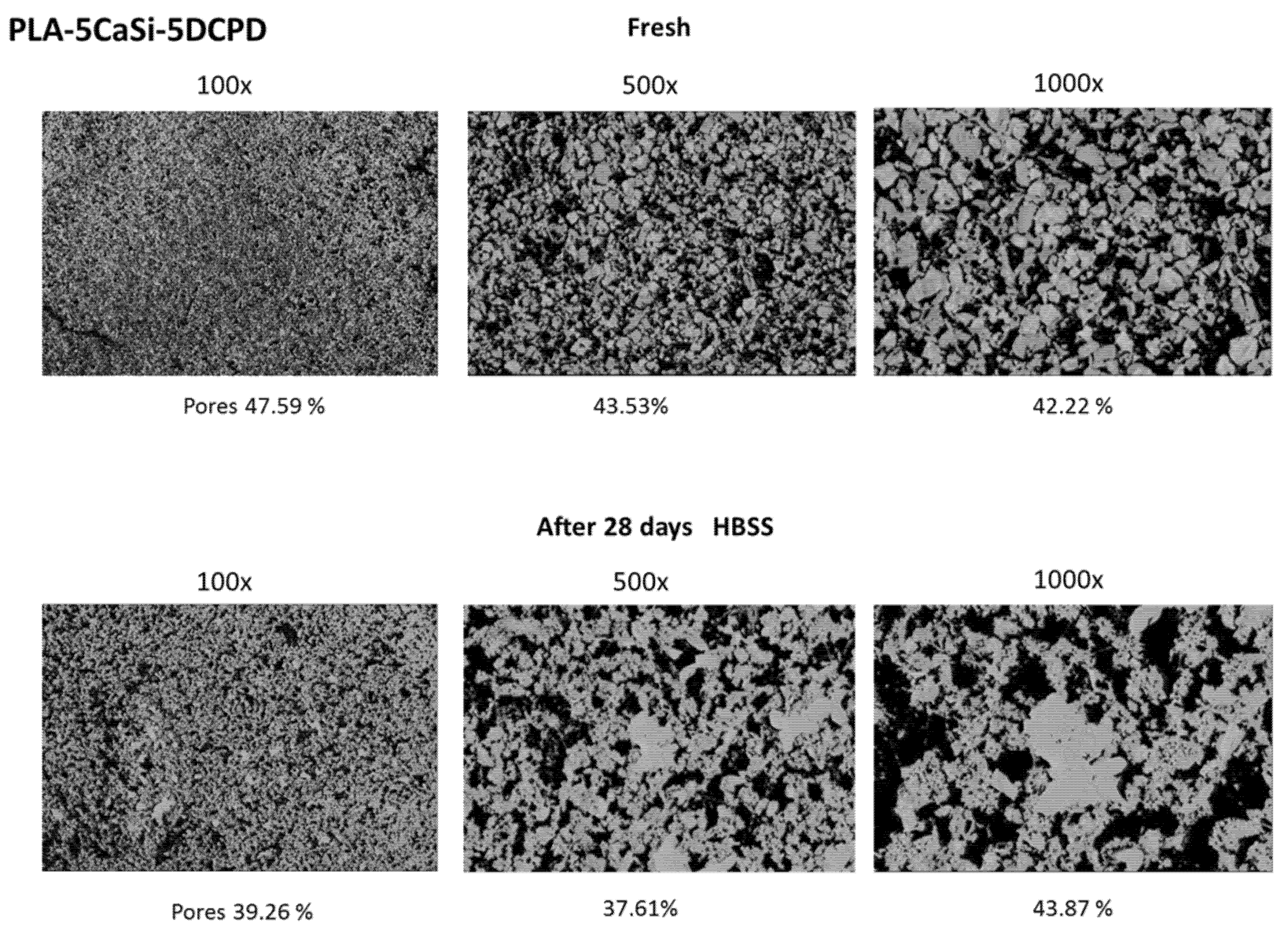
2.2. ESEM-EDX Analysis for Morphometric Analysis and Surface Porosity after 28 Days Soaking in Simulated Body Fluid
2.3. Cell Culture
2.4. hAD-MSCs Viability by Tetrazolium Salt (MTT) Assay
2.5. Exosomes Isolation from Stem Cells
2.6. Exosome Labeling with Red Fluorescent
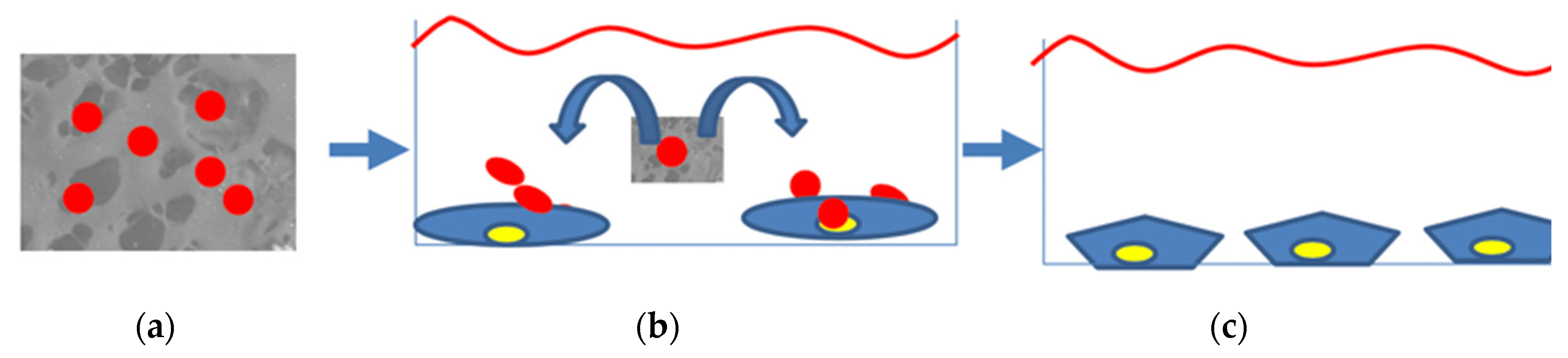
2.7. Seeding Exosomes on the Scaffolds and Cell Culture in Proximity of Exosomes-Enriched-Scaffolds
2.8. Osteogenic Commitment by Real-Time Polymerase Chain Reaction (RT-PCR)
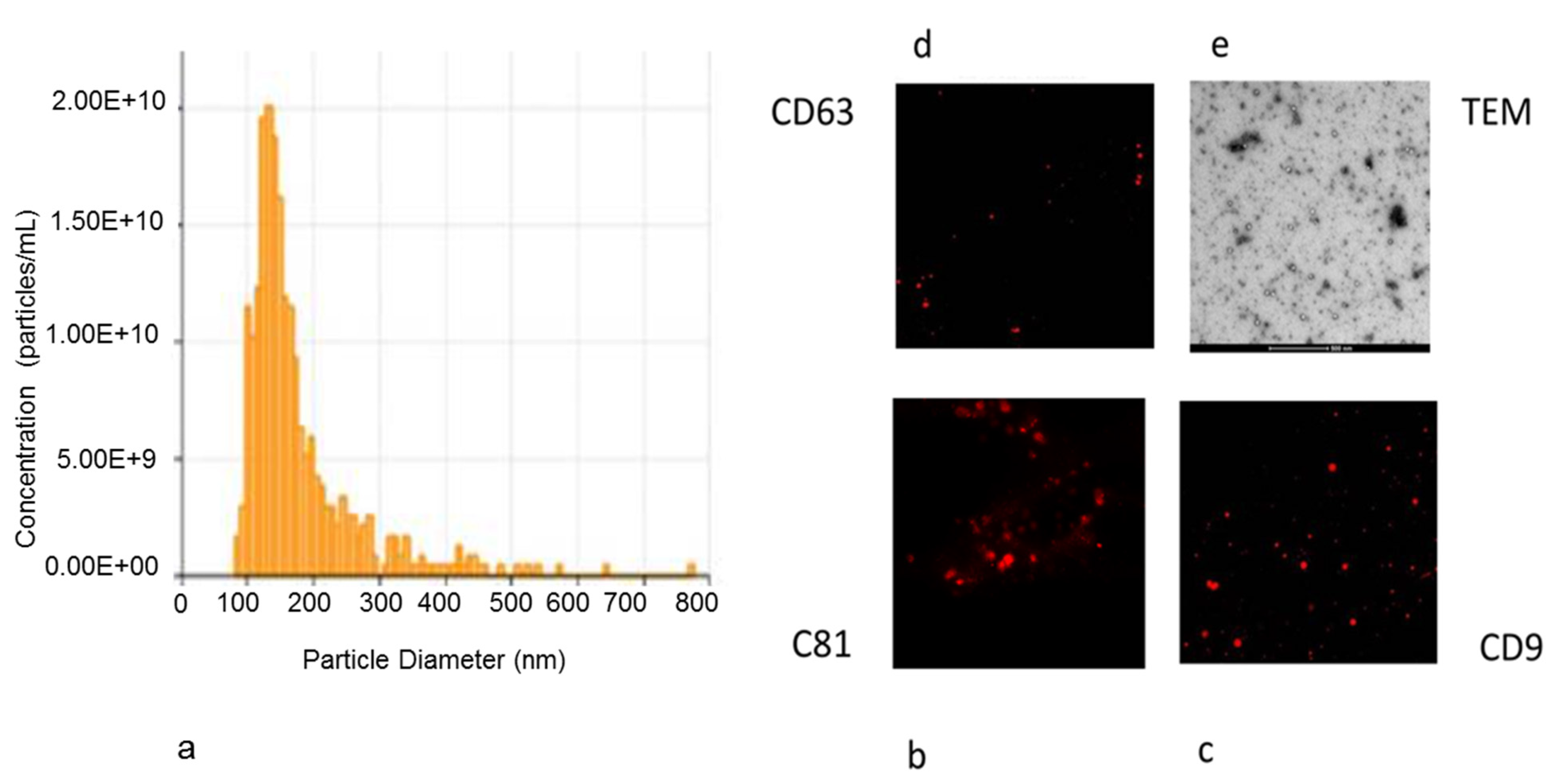
2.9. Nanoparticle Characterization of Exosomes Isolated from hAD-MSCs
2.10. Transmission Electron Microscopy (TEM) Observation of Released Exosomes
2.11. Immunofluorescence Staining of Exosomes
3. Results
3.1. PLA-10CaSi-10DCPD Scaffolds
3.2. PLA-5CaSi-5DCPD Scaffolds
3.3. In Vitro Biocompatibility of Scaffolds
3.4. Analysis of EVs Isolated from hAD-MSCs
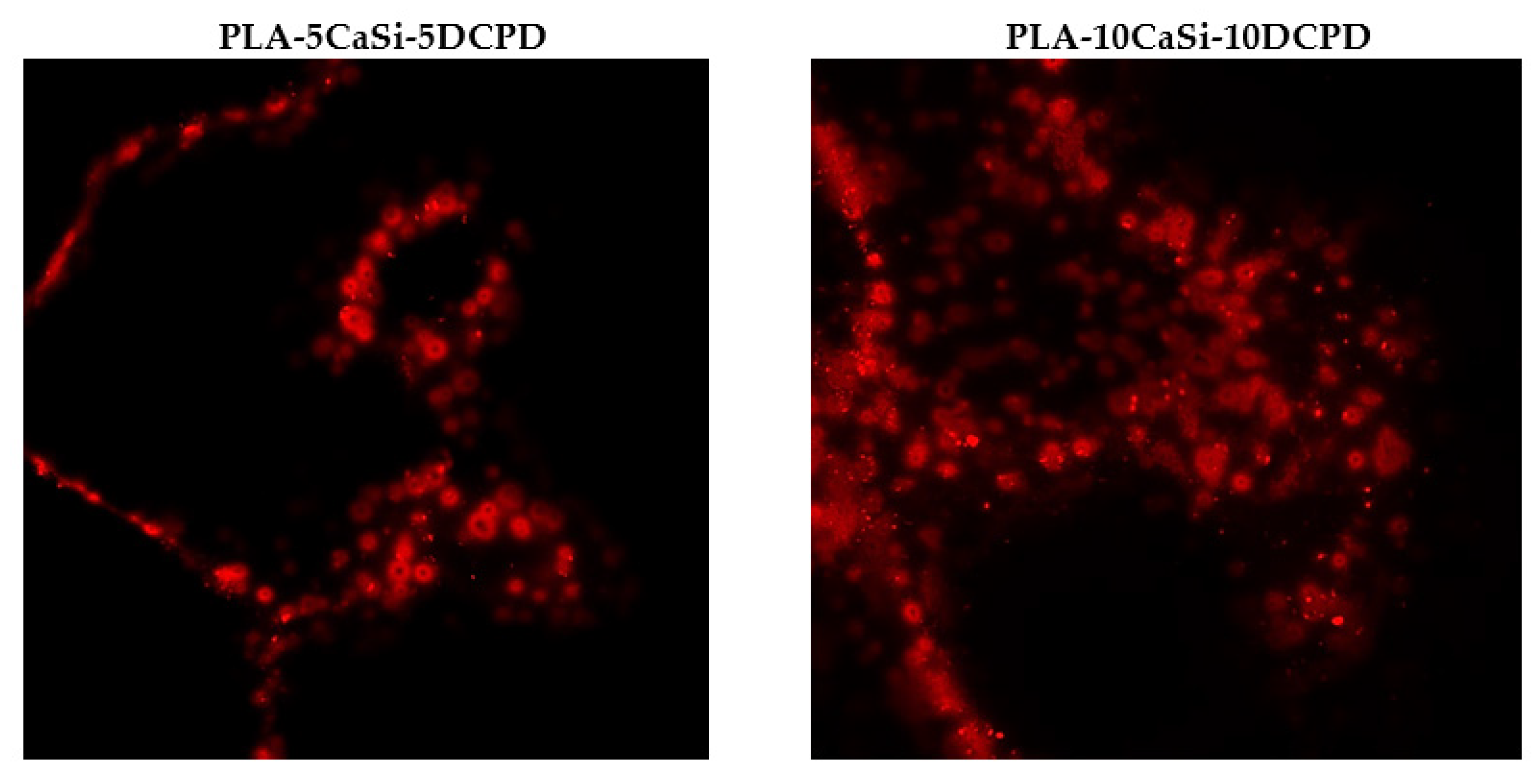
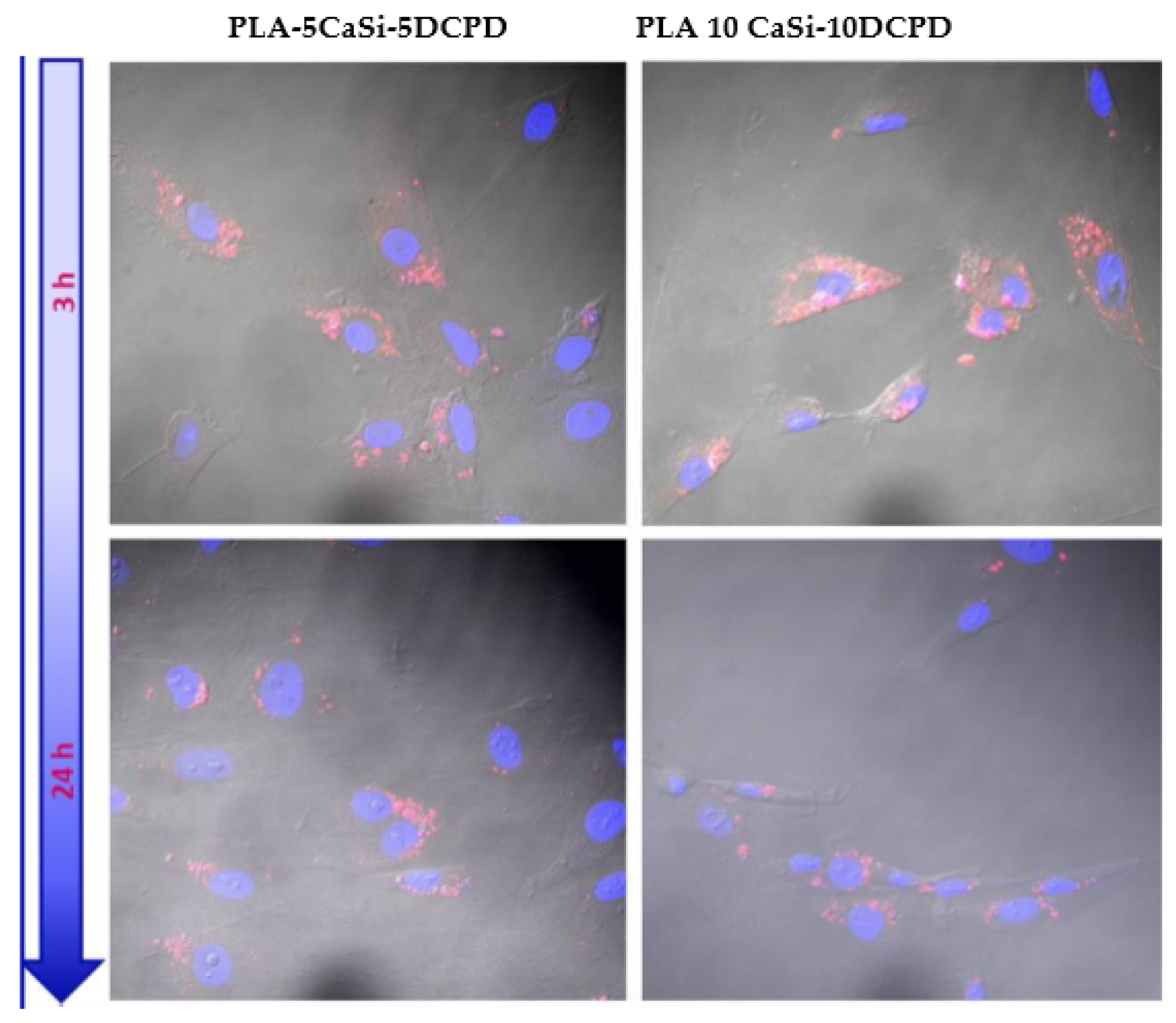
3.5. Exosome Adhesion on Scaffolds and Their Release
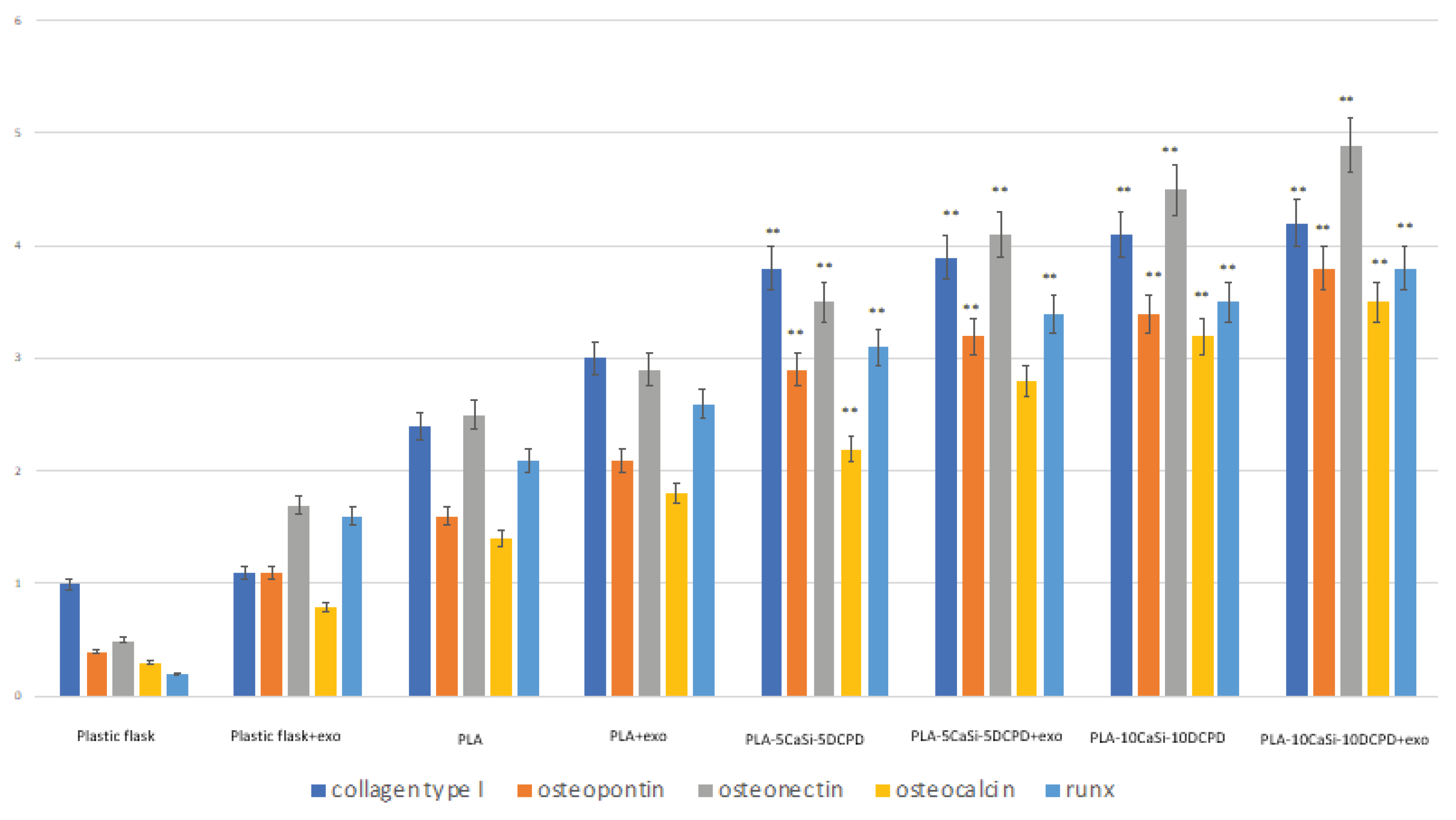
3.6. Effect of Exosomes on Osteogenic Commitment of hAD-MSCs
4. Discussion
5. Conclusions
- The experimental PLA-based scaffolds are able to adsorb, keep on their surface, and release exosomes secreted by hAD-MSCs.
- The exosome-enriched scaffolds have enhanced ability to trigger the osteogenic commitment of hAD-MSCs improving their osteogenic properties.
- Mineral-doped scaffolds, in particular the formulation with the highest amount of mineral fillers (PLA-10CaSi-10DCPD), showed a great potential in regenerative bone healing by stimulating the osteogenic commitment of hAD-MSCs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kriebel, K.; Hieke, C.; Müller-Hilke, B.; Nakata, M.; Kreikemeyer, B. Oral Biofilms from Symbiotic to Pathogenic Interactions and Associated Disease -Connection of Periodontitis and Rheumatic Arthritis by Peptidylarginine Deiminase. Front. Microbiol. 2018, 30, 9–53. [Google Scholar] [CrossRef]
- Papageorgiou, S.N.; Papageorgiou, P.N.; Deschner, J.; Götz, W. Comparative effectiveness of natural and synthetic bone grafts in oral and maxillofacial surgery prior to insertion of dental implants: Systematic review and network meta-analysis of parallel and cluster randomized controlled trials. J. Dent. 2016, 48, 1–8. [Google Scholar] [CrossRef]
- Forrestal, D.P.; Klein, T.J.; Woodruff, M.A. Challenges in engineering large customized bone constructs. Biotechnol. Bioeng. 2017, 114, 1129–1139. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.M.; Mundy, G.R. Eph receptors and ephrin signaling pathways: A role in bone homeostasis. Int. J. Med. Sci. 2008, 5, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Gooi, J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell Dev. Biol. 2008, 19, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Otaki, N. Bone cell interactions through Eph/ephrin: Bone modeling, remodeling and associated diseases. Cell Adh. Migr. 2012, 6, 148–156. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Conlan, R.S.; Pisano, S.; Oliveira, M.I.; Ferrari, M.; Mendes Pinto, I. Exosomes as Reconfigurable Therapeutic Systems. Trends Mol. Med. 2017, 23, 636–650. [Google Scholar] [CrossRef]
- Campanella, C.; Caruso Bavisotto, C.; Logozzi, M.; Marino Gammazza, A.; Mizzoni, D.; Cappello, F.; Fais, S. On the Choice of the Extracellular Vesicles for Therapeutic Purposes. Int. J. Mol. Sci. 2019, 9, 236. [Google Scholar] [CrossRef]
- Liu, W.C.; Chen, S.; Zheng, L.; Qin, L. Angiogenesis Assays for the Evaluation of Angiogenic Properties of Orthopaedic Biomaterials—A General Review. Adv. Health Mat. 2017, 6, 1600434. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Stein, G.S.; van Wijnen, A.J.; Stein, J.L.; Hassan, M.Q.; Gaur, T.; Zhang, Y. MicroRNA control of bone formation and homeostasis. Nat. Rev. Endocrinol. 2012, 31, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.C.; Tao, S.C.; Yin, W.J.; Qi, X.; Yuan, T.; Zhang, C.Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 17, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Erbe, E.M.; Marx, J.G.; Clineff, T.D.; Bellincampi, L.D. Potential of an ultraporous beta-tricalcium phosphate synthetic cancellous bone void filler and bone marrow aspirate composite graft. Eur. Spine J. 2001, 10, 141–146. [Google Scholar]
- Loh, X.J.; Kai, D. Special issue: Biomedical applications editorial. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 933–934. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef]
- Boyan, B.D.; Bonewald, L.F.; Paschalis, E.P.; Lohmann, C.H.; Rosser, J.; Cochran, D.L.; Dean, D.D.; Schwartz, Z.; Boskey, A.L. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif. Tissue Int. 2002, 71, 519–529. [Google Scholar] [CrossRef]
- Washburn, N.R.; Yamada, K.M.; Simon CGJr Kennedy, S.B.; Amis, E.J. High-throughput investigation of osteoblast response to polymer crystallinity: Influence of nanometer-scale roughness on proliferation. Biomaterials 2004, 25, 17–28. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Kawazoe, N.; Chen, G. Promoted Angiogenesis and Osteogenesis by Dexamethasone-loaded Calcium Phosphate Nanoparticles/Collagen Composite Scaffolds with Microgroove Networks. Sci. Rep. 2018, 20, 14143. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 922–939. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix Nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Mater 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen Med. 2019, 13, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 3, 161–176. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Mol. Sci. 2011, 19, 1–19. [Google Scholar] [CrossRef]
- Ginjupalli, K.; Shavi, G.V.; Averineni, R.K.; Bhat, M.; Udupa, N.; Nagaraja Upadhya, P. Poly(α-hydroxy acid) based polymers: A review on material and degradation aspects. Polym. Degrad. Stab. 2017, 144, 520–535. [Google Scholar] [CrossRef]
- Vert, M.; Chen, J.; Hellwich, K.H.; Hodge, P.; Nakano, T.; Scholz, C.; Slomkowski, S.; Vohlidal, J. Nomenclature and terminology for linear lactic acid-based polymers (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 92, 193–211. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Rábago, K.R.; Glassner DAGruber, P.R. Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab. 2005, 80, 403–419. [Google Scholar] [CrossRef]
- Gruber, P.; O’Brien, M. Polylactides: NatureWorks™ PLA. In Biopolymers in 10 volumes, Volume 4, Polyesters III, Applications and Commercial Products; Doi, Y., Steinbüchel, A., Eds.; Wiley-VCH: Weinheim, Germany, 2002; pp. 235–249. [Google Scholar]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-lactic acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Tomlin, E.M.; Nelson, S.J.; Rossmann, J.A. Ridge preservation for implant therapy: A review of the literature. Open Dent. J. 2014, 8, 1874–2106. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yang, H.; Yang, D.; Yu, Z.Z. Polylactic Acid Nanofiber Scaffold Decorated with Chitosan Islandlike Topography for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 21094–21104. [Google Scholar] [CrossRef]
- Holmes, B.; Bulusu, K.; Plesniak, M.; Zhang, L.G. A synergistic approach to the design, fabrication and evaluation of 3D printed micro and nano featured scaffolds for vascularized bone tissue repair. Nanotechnology 2016, 27, 064001. [Google Scholar] [CrossRef]
- Jaidev, L.R.J. Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mat. Des. 2019, 161, 44–54. [Google Scholar] [CrossRef]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, B.S.; Ozcopur, B.; Gandolfi, M.G.; Prati, C.; Belli, S. The response of cementoblasts to calcium phosphate resin-based and calcium silicate-based commercial sealers. Int. Endod. J. 2013, 46, 242–252. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Spagnuolo, G.; Siboni, F.; Procino, A.; Rivieccio, V.; Pelliccioni, G.A.; Prati, C.; Rengo, S. Calcium silicate/calcium phosphate biphasic cements for vital pulp therapy: Chemical-physical properties and human pulp cells response. Clin. Oral. Investig. 2015, 19, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Shah, S.N.; Feng, R.; Prati, C.; Akintoye, S.O. Biomimetic calcium-silicate cements support differentiation of human orofacial mesenchymal stem cells. J. Endod. 2011, 37, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Ciapetti, G.; Taddei, P.; Perut, F.; Tinti, A.; Cardoso, M.V.; Van Meerbeck, B.; Prati, C. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent. Mater. 2010, 26, 974–992. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Spagnuolo, G.; Codispoti, B.; Zamparini, F.; Zhang, A.; Degli Esposti, M.; Aparicio, C.; Rengo, C.; Nuzzolese, M.; Manzoli, L.; et al. PLA-based mineral-doped scaffolds seeded with human periapical cyst-derived MSCs: A promising tool for regenerative healing in dentistry. Materials 2019, 12, 597. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Siboni, F.; Botero, T.; Bossù, M.; Riccitiello, F.; Prati, C. Calcium silicate and calcium hydroxide materials for pulp capping: Biointeractivity, porosity, solubility and bioactivity of current formulations. J. Appl. Biomater. Funct Mater. 2015, 13, 43–60. [Google Scholar] [CrossRef]
- Sun, J.; Wei, L.; Liu, X.; Li, J.B.; Wang, G.; Meng, F. Influences of ionic dissolution products of dicalcium silicate coating on osteoblastic proliferation, differentiation and gene expression. Acta Biomater. 2009, 5, 1284–1293. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Modena, E.; Siboni, F.; Prati, C. Biointeractivity-related versus chemi/physisorption related apatite precursor-forming ability of current root end filling materials. J. Biomed. Mater. Res. B 2013, 101, 1107–1123. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; Ciapetti, G.; Prati, C. Development of the foremost light-curable calcium-silicate mta cement as root-end in oral surgery. Chemical-physical properties, bioactivity and biological behaviour. Dent. Mater. 2011, 27, 134–157. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Tinti, A.; Dorigo, D.E.; Stefano, E.; Rossi, P.L.; Prati, C. Kinetics of apatite formation on a calcium silicate cement for root-end filling during ageing in physiological like phosphate solutions. Clin. Oral Investig. 2010, 14, 659–668. [Google Scholar] [CrossRef]
- Torabinejad, M.; Chiavian, N. Clinical application of mineral trioxide aggregate. J. Endod. 1993, 25, 197–205. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Aparicio, C.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Polylactic acid-based porous scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for biomedical application. Mater. Sci. Eng. C 2018, 82, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, P.; Cannillo, V.; Sola, A.A.; Chiellini, F. Highly porous polycaprolactone-45S5 Bioglass® scaffolds for bone tissue engineering. Compos. Sci. Technol. 2010, 70, 1869–1878. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Castilla Bolaños, M.A.; Buttigieg, J.; Briceño Triana, J.C. Development and characterization of a novel porous small intestine submucosa-hydroxyapatite scaffold for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 519–525. [Google Scholar] [CrossRef]
- Eyckmans, J.; Roberts, S.J.; Bolander, J.; Schrooten, J. Mapping calcium phosphate activated gene networks as a strategy for targeted osteoinduction of human progenitors. Biomaterials 2013, 34, 4612–4621. [Google Scholar] [CrossRef]
- Ma, S.; Yang, Y.; Carnes, D.L.; Kim, K.; Park, S.; Oh., S.H.; Ong, J.L. Effects of dissolved calcium and phosphorous on osteoblast responses. J. Oral Implantol. 2005, 31, 61–67. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblastlike cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef]
- An, S.; Gao, Y.; Ling, J.; Wei, X.Y. Calcium ions promote osteogenic differentiation and mineralization of human dental pulp cells: Implications for pulp capping materials. J. Mater. Sci. Mater. Med. 2012, 23, 789–795. [Google Scholar] [CrossRef]
- Forni, M.; Bernardini, C.; Zamparini, F.; Zannoni, A.; Salaroli, R.; Ventrella, D.; Parchi, G.; Degli Esposti, M.; Polimeni, A.; Fabbri, P.; et al. Vascular Wall—Mesenchymal Stem Cells differentiation on 3D biodegradable highly porous CaSi-DCPD doped Poly (α- hydroxy) acids scaffolds for bone regeneration. Nanomaterials 2020, 10, 243. [Google Scholar] [CrossRef]
- Huang, C.C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef]
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu, J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104 . [Google Scholar] [CrossRef]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016, 2016, 7653489. [Google Scholar] [CrossRef] [PubMed]
- Hynes, D.; Menicanin, J.; Han, V.; Marino, K.; Mrozik, S.; Gronthos, P.M. Bartold, Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J. Dent. Res. 2013, 92, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, M.; Crawford, R.Y.; Xiao, Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019, 86, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.P.; Tekale, S.U.; Shisodia, S.U.; Totre, J.T.; Domb, A.J. Biomedical Applications of Poly(Lactic Acid). Recent Pat. Regen Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Lin, A.S.; Barrows, T.H.; Cartmell, S.H.; Guldberg, R.E. Microarchitectural and mechanical characterization of oriented porous polymer scaffolds. Biomaterials 2003, 24, 481–489. [Google Scholar] [CrossRef]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2010, 28, 1–10. [Google Scholar] [CrossRef]
- Fernández, E.; Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Calcium phosphate bone cements for clinical applications. Part. I Solut. Chem. J. Mater. Sci. Mater. Med. 1999, 10, 169–176. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, S.K.; Lee, S.I.; Park, J.H.; Jang, J.H.; Kim, H.W.; Kim, E.C. Effect of calcium phosphate cements on growth and odontoblastic differentiation in human dental pulp cells. J. Endod. 2010, 36, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Taddei, P.; Tinti, A.; De Stefano Dorigo, E.; Prati, C. Alpha-TCP improves the apatite-formation ability of calcium-silicate hydraulic cement soaked in phosphate solutions. Mat. Sci. Eng. C 2011, 31, 1412–1422. [Google Scholar] [CrossRef]
- Driessens, F.C.; Planell, J.A.; Boltong, M.G.; Khairoun, I.; Ginebra, M.P. Osteotransductive bone cements. Proc Inst. Mech. Eng. H. J. Eng. Med. 1998, 212, 427–435. [Google Scholar] [CrossRef]
- Takagi, S.; Chow, L.C.K. Formation of hydroxyapatite in new calcium phosphate cements. Biomaterials 1998, 19, 1593–1599. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Highly porous polycaprolactone scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.C.; Huang, S.C.; Ding, S.J. Comparative osteogenesis of radiopaque dicalcium silicate cement and white-colored mineral trioxide aggregate in a rabbit femur model. Materials 2013, 6, 5675–5689. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Kim, H.I.; Park, H.J.; Pi, S.H.; Hong, C.U.; Kim, E.C. Human pulp cells response to Portland cement in vitro. J. Endod. 2007, 33, 163–166 71. [Google Scholar] [CrossRef]
- Shen, Q.; Sun, J.; Wu, J.; Liu, C.; Chen, F. An in vitro investigation of the mechanical-chemical and biological properties of calcium phosphate/calcium silicate/bismutite cement for dental pulp capping. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 141–148. [Google Scholar]
- Quao, Q.; Ruixin, S.; Chuanlong, W. Exosome: A Novel Approach to Stimulate Bone Regeneration through Regulation of Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2016, 17, 712. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Lakhal, S.; Mäger, I.; Wood, M.J. Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug Deliv. Rev. 2013, 65, 391–397. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem. Cell Res. 2016, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Fleury, A.; Martinez, M.C.; Le Lay, S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front. Immunol. 2014, 5, 370. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Esposti, M.D.; Parchi, G.; Ercan, B.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials 2020, 10, 432. https://doi.org/10.3390/nano10030432
Gandolfi MG, Gardin C, Zamparini F, Ferroni L, Esposti MD, Parchi G, Ercan B, Manzoli L, Fava F, Fabbri P, et al. Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials. 2020; 10(3):432. https://doi.org/10.3390/nano10030432
Chicago/Turabian StyleGandolfi, Maria Giovanna, Chiara Gardin, Fausto Zamparini, Letizia Ferroni, Micaela Degli Esposti, Greta Parchi, Batur Ercan, Lucia Manzoli, Fabio Fava, Paola Fabbri, and et al. 2020. "Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells" Nanomaterials 10, no. 3: 432. https://doi.org/10.3390/nano10030432
APA StyleGandolfi, M. G., Gardin, C., Zamparini, F., Ferroni, L., Esposti, M. D., Parchi, G., Ercan, B., Manzoli, L., Fava, F., Fabbri, P., Prati, C., & Zavan, B. (2020). Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials, 10(3), 432. https://doi.org/10.3390/nano10030432








