Effective Inhibition of Candidiasis Using an Eco-Friendly Leaf Extract of Calotropis-gigantean-Mediated Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Collection
2.3. Test Organism and Growth Conditions
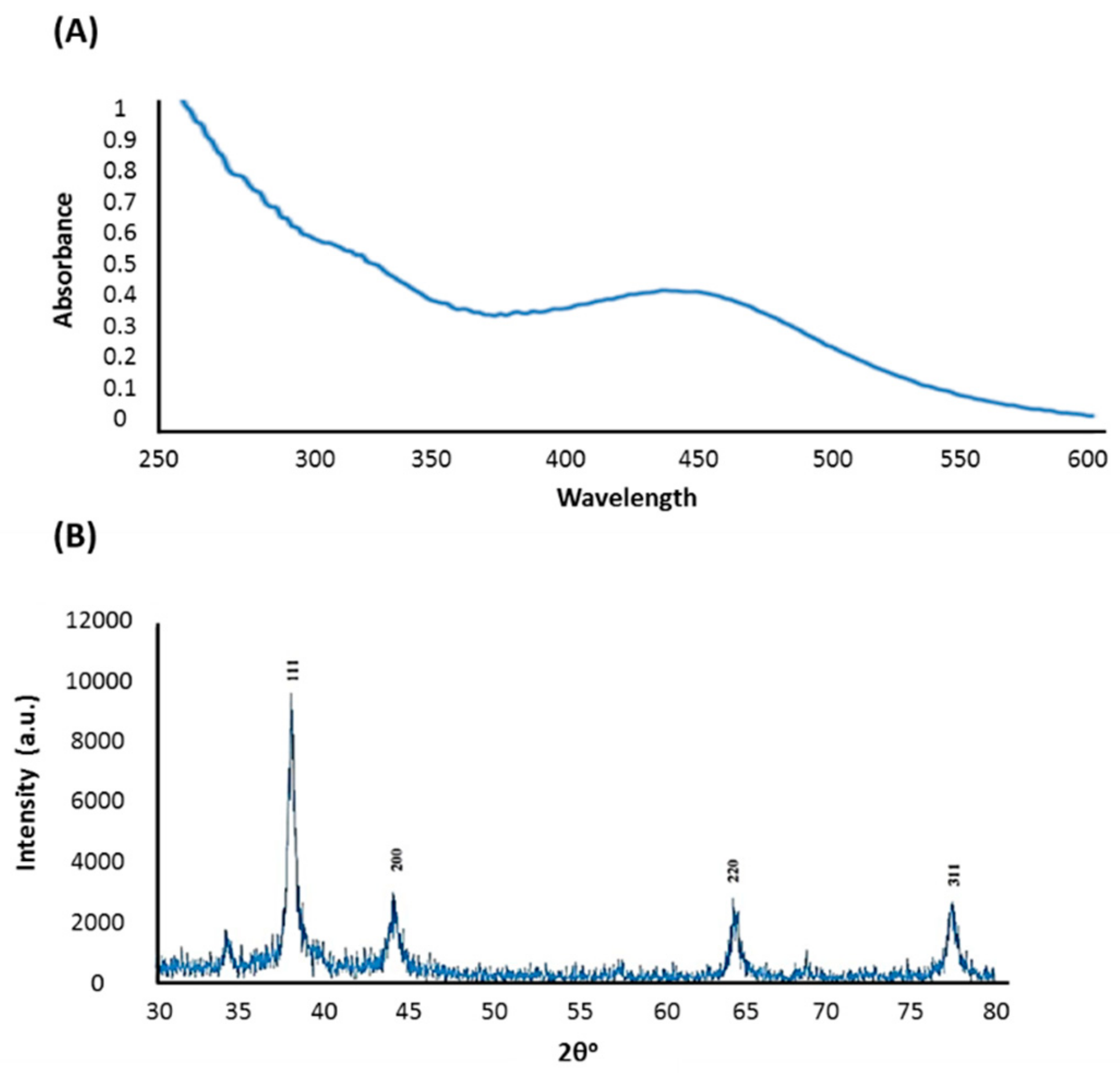
2.4. Preparation and Characterization of AgNPs
2.4.1. Preparation of the Leaf Extract
2.4.2. Biosynthesis of AgNPs
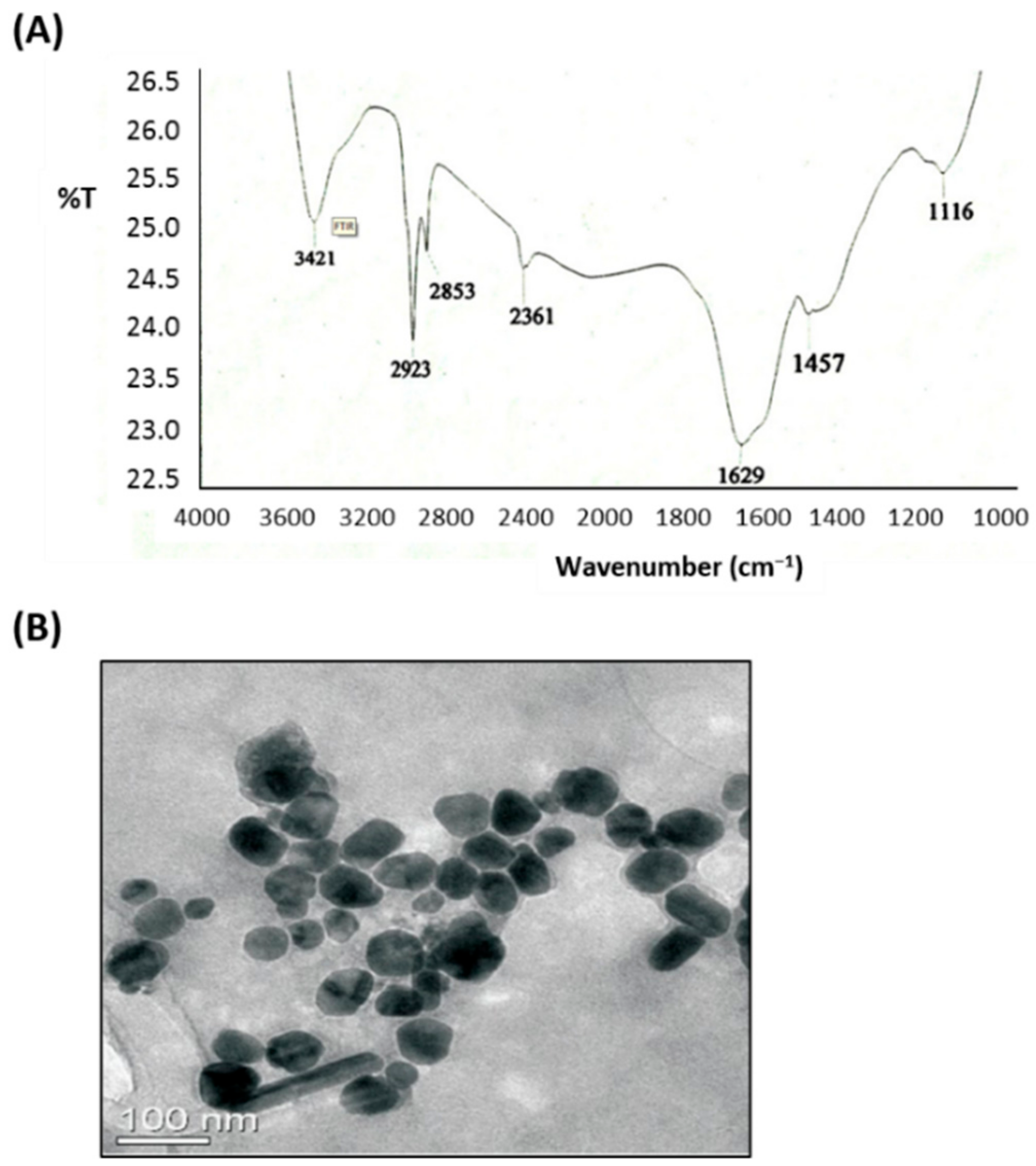
2.4.3. Transmission Electron Microscopy (TEM)
2.4.4. X-ray Diffraction Analysis (XRD)
2.4.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Anti-Candidal Activity
2.6. Time-Kill Assays
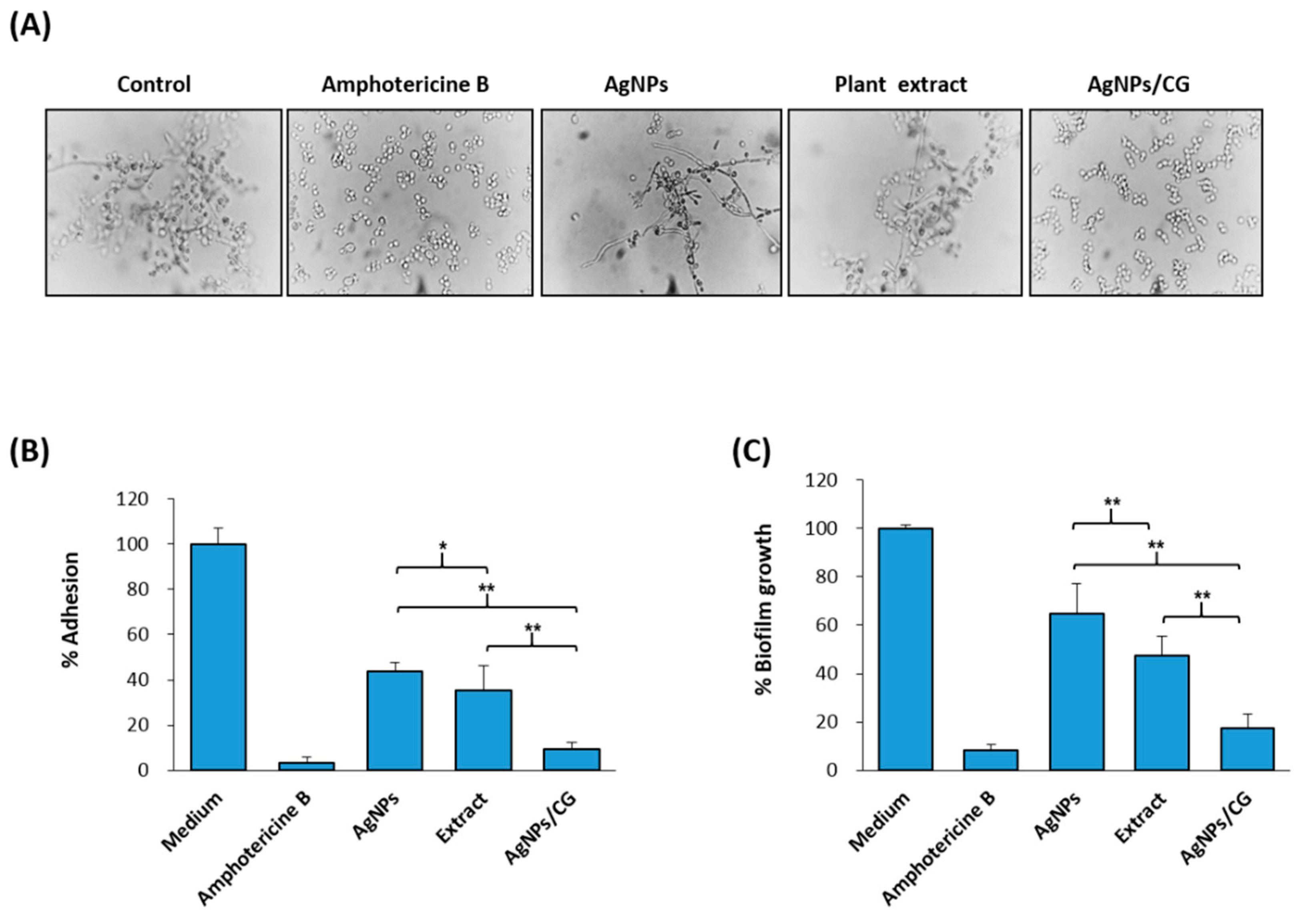
2.7. Assay of C. albicans Hyphal Development in Liquid Media
2.8. Adhesion and Biofilm Formation Assays
2.9. Determination of Antioxidant Enzymes
2.10. Transmission Electron Microscopy
2.11. Cell Culture
2.12. Cytotoxicity Assay
2.13. Statistical Analysis
3. Results
3.1. Preparation and Characterization of AgNPs
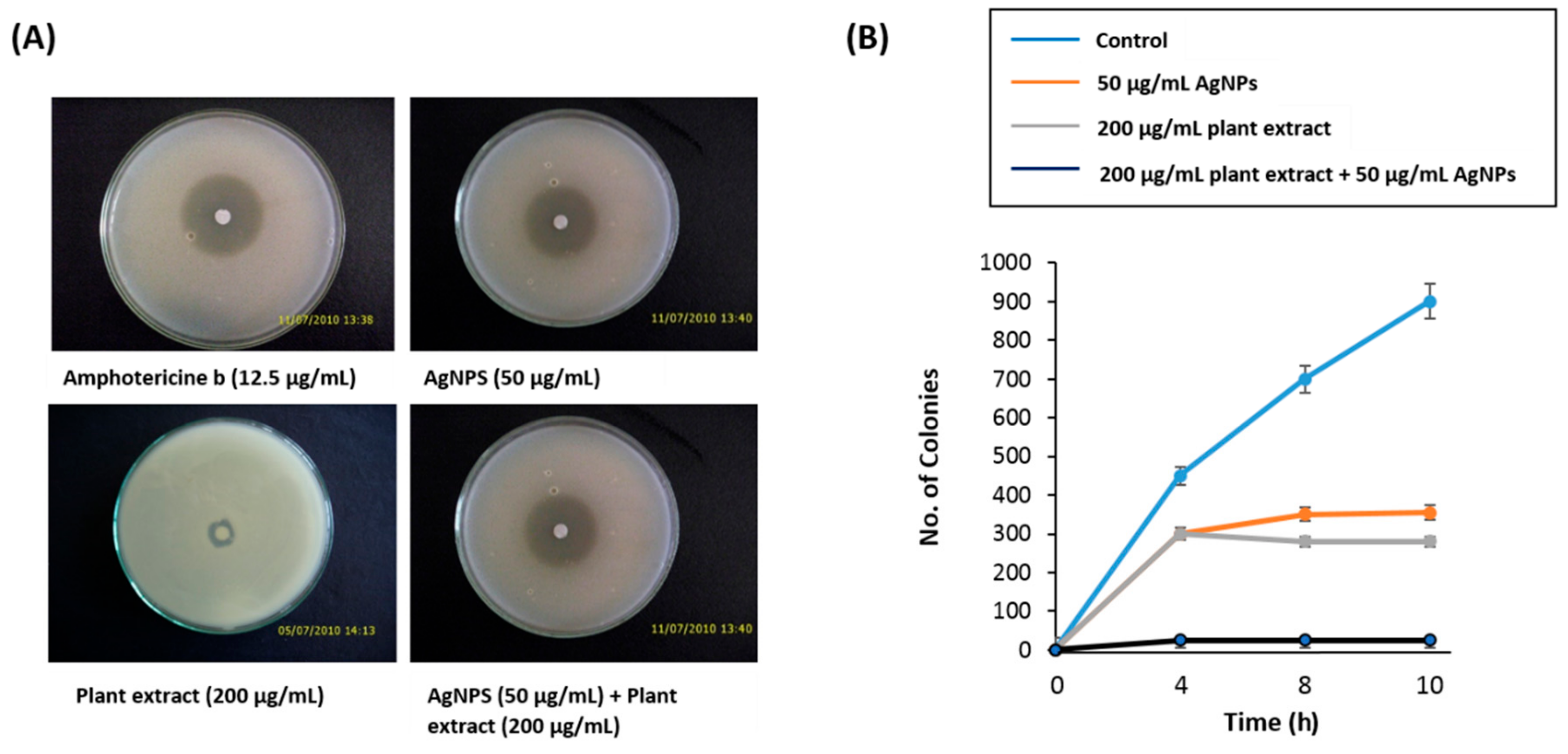
3.2. Anti-Candidal Activity of AgNPs
3.3. Synergistic Anti-Candidal Activity of AgNPs and Plant Extract
3.4. Effect of AgNPs/CG on the Virulence Factors of C. albicans
3.5. AgNPs/CG Suppressed the Production of Antioxidant Enzymes by C. albicans
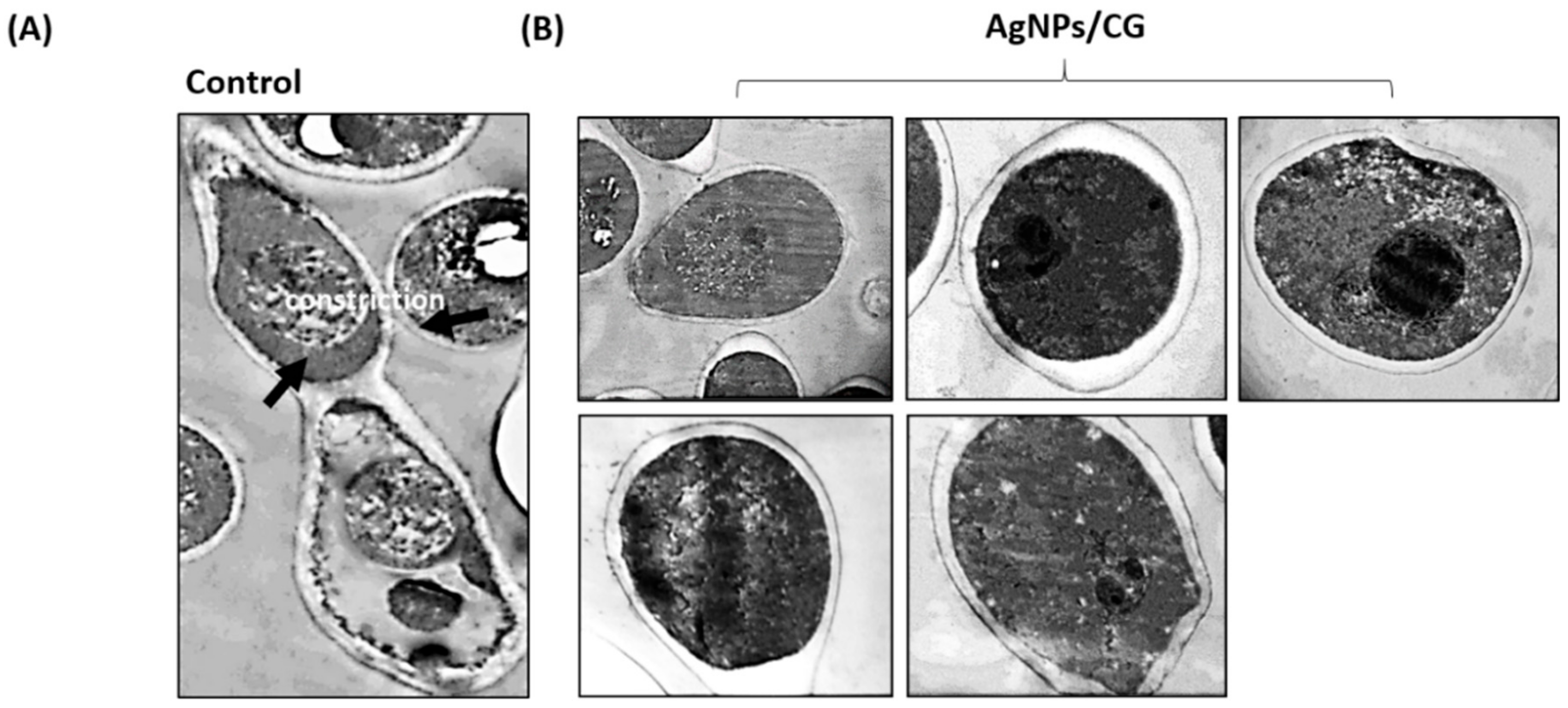
3.6. Morphological and Ultrastructural Alteration Caused by AgNPs/CG
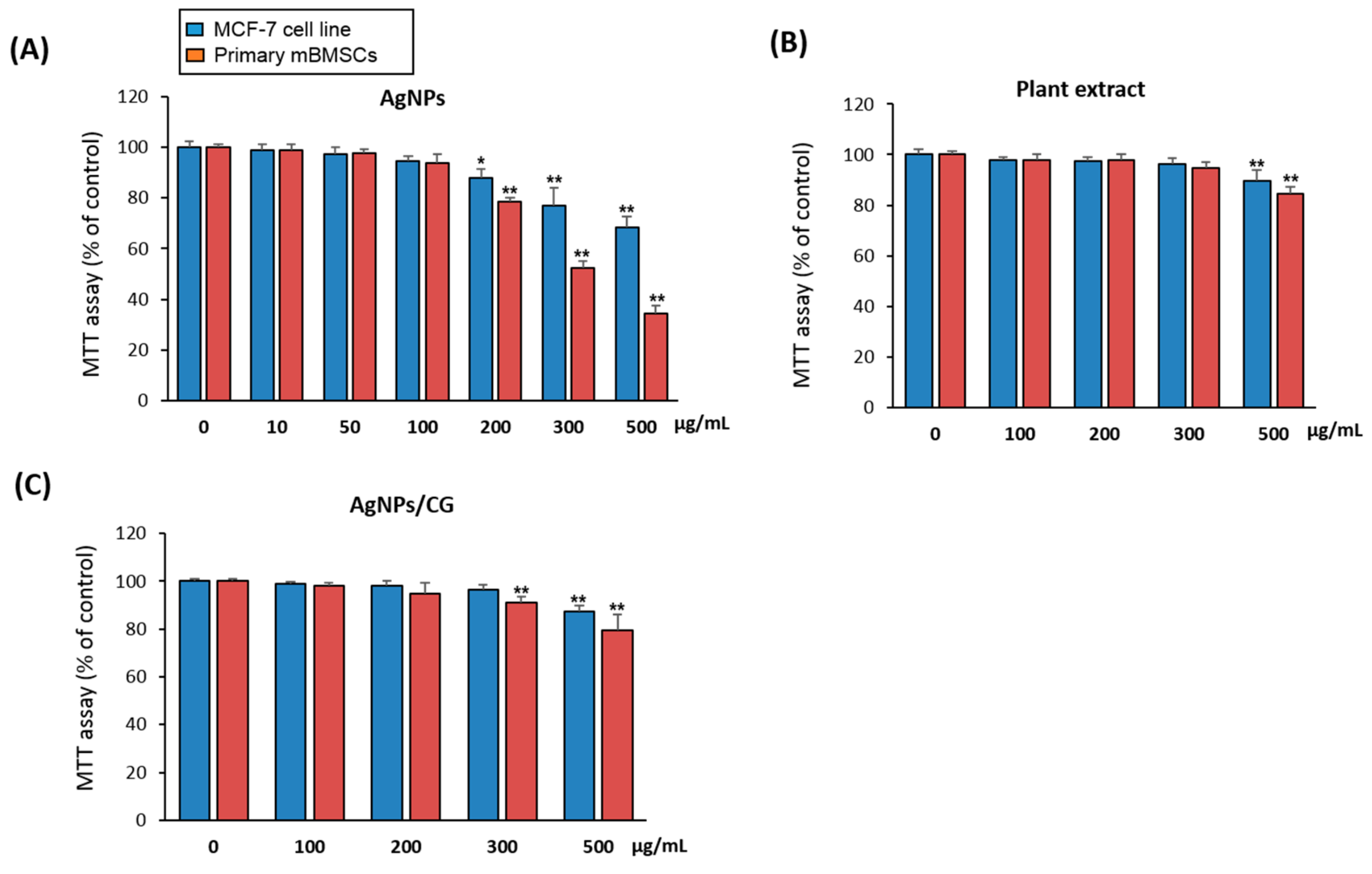
3.7. Cytotoxicity of AgNPs/CG
4. Discussion
5. Conclusions
Availability of Data and Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gow, N.A.R.; Yadav, B. Microbe Profile: Candida albicans: A shape-changing, opportunistic pathogenic fungus of humans. Microbiology 2017, 163, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Ferreira, I.C.; Barros, L.; Silva, S. Henriques M: Candidiasis: Predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 2014, 177, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Marak, M.B.; Dhanashree, B. Antifungal Susceptibility and Biofilm Production of Candida spp. Isolated from Clinical Samples. Int. J. Microbiol. 2018, 2018, 7495218. [Google Scholar] [CrossRef]
- Gauwerky, K.; Borelli, C.; Korting, H.C. Targeting virulence: A new paradigm for antifungals. Drug Discov. Today 2009, 14, 214–222. [Google Scholar] [CrossRef]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Siddiqui, M.I. Anticandidal activity of biosynthesized silver nanoparticles: Effect on growth, cell morphology, and key virulence attributes of Candida species. Int. J. Nanomed. 2019, 14, 4667–4679. [Google Scholar] [CrossRef]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef]
- El-Moslamy, S.H. Bioprocessing strategies for cost-effective large-scale biogenic synthesis of nano-MgO from endophytic Streptomyces coelicolor strain E72 as an anti-multidrug-resistant pathogens agent. Sci. Rep. 2018, 8, 3820. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 11, 3157–3177. [Google Scholar] [CrossRef] [PubMed]
- Kadiyala, M.; Ponnusankar, S.; Elango, K. Calotropis gigantiea (L.) R. Br (Apocynaceae): A phytochemical and pharmacological review. J. Ethnopharmacol. 2013, 150, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Rabiu Muhammad, A.; Mikaeel Bala, A.; Adamu Bello, K.; Yakubu Umar, D.; Nafiu, L.; Muhammad Bashir, B.; Aminu Yusuf, F. Efficacy and Phytochemical Analysis of Aqueous Extract of Calotropis procera against Selected Dermatophytes. J. Complement Med. Res. 2015, 4, 314. [Google Scholar]
- Kumar, G.; Loganathan, K.; Rao, B. Antimicrobial Activity of Latex of Calotropis gigantea Against Pathogenic Microorganisms—An In Vitro Study. Pharmacologyonline 2010, 3, 155–163. [Google Scholar]
- Singh, N.; Kannojia, P.; Garud, N.; Pathak, A.; Mehta, S. In vitro antioxidant activity of Calotropis gigantea hydroalcohlic leaves extract. Der Pharmacia Lettre 2010, 2, 95–100. [Google Scholar]
- Rajkuberan, C.; Sudha, K.; Sathishkumar, G.; Sivaramakrishnan, S. Antibacterial and cytotoxic potential of silver nanoparticles synthesized using latex of Calotropis gigantea L. Spectrochim. Acta A 2015, 136, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.K.; Akhtar, M.S.; Ameen, S.; Srivastava, P.; Singh, G. Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloys Compd. 2015, 632, 321–325. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Sargunar, C.G.; Lin, Y.C.; Chen, J.C. Green synthesis of Silver nanoparticles through Calotropis gigantea leaf extracts and evaluation of antibacterial activity against Vibrio alginolyticus. Nanotechnol. Dev. 2012, 2, e3. [Google Scholar]
- Ali, E.M. Contributions of some biological activities of honey bee venom. J. Apic. Res. 2014, 53, 441–451. [Google Scholar] [CrossRef]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Shukla, A.K.; Ali, S.G.; Khan Haris, M.; Pal, R.; Alam, J.; Cameotra, S.S. Green synthesis and antifungal activity of Al2O3 NPs against fluconazole-resistant Candida spp isolated from a tertiary care hospital. RSC Adv. 2016, 6, 107577–107590. [Google Scholar] [CrossRef]
- Bahrami-Teimoori, B.; Nikparast, Y.; Hojatianfar, M.; Akhlaghi, M.; Ghorbani, R.; Pourianfar, H.R. Characterisation and antifungal activity of silver nanoparticles biologically synthesised by Amaranthus retroflexus leaf extract. J. Exp. Nanosci. 2017, 12, 129–139. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Antibacterial Activity and Synergistic Antibacterial Potential of Biosynthesized Silver Nanoparticles against Foodborne Pathogenic Bacteria along with its Anticandidal and Antioxidant Effects. Front. Microbiol. 2017, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fujita, K.; Kubo, A.; Nihei, K.; Ogura, T. Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis. J. Agric. Food Chem. 2004, 52, 3329–3332. [Google Scholar] [CrossRef] [PubMed]
- Mangoyi, R.; Midiwo, J.; Mukanganyama, S. Isolation and characterization of an antifungal compound 5-hydroxy-7,4′-dimethoxyflavone from Combretum zeyheri. BMC Complement. Altern. Med. 2015, 15, 405. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.K.; Lee, J.-H.; Kim, Y.-G.; Lee, J. Alizarin and Chrysazin Inhibit Biofilm and Hyphal Formation by Candida albicans. Front. Cell. Infect. Microbiol. 2017, 7, 447. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLoS ONE 2014, 9, e108876. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S. Catalase Activities of Hydrocarbon-utilizing Candida Yeasts. Agric. Biol. Chem. 1974, 38, 1213–1220. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [PubMed]
- Mohandas, J.; Marshall, J.J.; Duggin, G.G.; Horvath, J.S.; Tiller, D.J. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer research 1984, 44, 5086–5091. [Google Scholar]
- Zaheer, N.; Tewari, K.K.; Krishnan, P.S. Mitochondrial forms of glucose 6-phosphate dehydrogenase and 6-phosphogluconic acid dehydrogenase in rat liver. Arch. Biochem. Biophys. 1967, 120, 22–34. [Google Scholar] [CrossRef]
- Shinobu-Mesquita, C.S.; Bonfim-Mendonca, P.S.; Moreira, A.L.; Ferreira, I.C.; Donatti, L.; Fiorini, A.; Svidzinski, T.I. Cellular Structural Changes in Candida albicans Caused by the Hydroalcoholic Extract from Sapindus saponaria L. Molecules 2015, 20, 9405–9418. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.M.; Alzahrani, A.M.; Abdel-Moneim, A.M.; Ditzel, N.; Kassem, M. A simple and reliable protocol for long-term culture of murine bone marrow stromal (mesenchymal) stem cells that retained their in vitro and in vivo stemness in long-term culture. Biol. Proced. Online 2019, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Kazemi Noureini, S.; Fatemi, L.; Wink, M. Telomere shortening in breast cancer cells (MCF7) under treatment with low doses of the benzylisoquinoline alkaloid chelidonine. PLoS ONE 2018, 13, e0204901. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.M.; Ali, E.M. 5’-hydroxy Auraptene stimulates osteoblast differentiation of bone marrow-derived mesenchymal stem cells via a BMP-dependent mechanism. J. Biomed. Sci. 2019, 26, 51. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Chitme, H.R.; Chandra, R.; Kaushik, S. Studies on anti-inflammatory activity of Calotropis gigantea in experimental animals. Asia Pac. J. Pharmacol. 2006, 16, 163. [Google Scholar]
- Lhinhatrakool, T.; Sutthivaiyakit, S. 19-Nor- and 18,20-Epoxy-cardenolides from the Leaves of Calotropis gigantea. J. Nat. Prod. 2006, 69, 1249–1251. [Google Scholar] [CrossRef]
- Tenpe, C.R.; Upaganlawar, A.B.; Dongre, P.A.; Yeole, P.G. Screening of methanolic extract of Calotropis gigantea leaves for hepatoprotective activity. Indian Drugs 2007, 44, 874–875. [Google Scholar]
- Gupta, J.; Sanjrani, M. Rare chemical constituents from Calotropis gigantea roots. Indian J. Pharm. Sci. 2000, 136, 29–32. [Google Scholar]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Fu, L.; Su, M.; Aslam, M.; Wong, K.C.; Dravid, V.P. Interaction of Fatty Acid Monolayers with Cobalt Nanoparticles. Nano Lett. 2004, 4, 383–386. [Google Scholar] [CrossRef]
- Vilchis-Nestor, A.R.; Sánchez-Mendieta, V.; Camacho-López, M.A.; Gómez-Espinosa, R.M.; Camacho-López, M.A.; Arenas-Alatorre, J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008, 62, 3103–3105. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, X.; Chen, J.; Wang, H. Preparation of silver nanoparticles in water-in-oil AOT reverse micelles. J. Colloid Interface Sci. 2006, 302, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Nazeruddin, G.M.; Prasad, N.R.; Prasad, S.R.; Shaikh, Y.I.; Waghmare, S.R.; Adhyapak, P. Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Ind. Crops Prod. 2014, 60, 212–216. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Geffers, C.; Gastmeier, P. Nosocomial infections and multidrug-resistant organisms in Germany: Epidemiological data from KISS (the Hospital Infection Surveillance System). Dtsch. Arzteblatt Online 2011, 108, 87–93. [Google Scholar]
- Monteiro, D.R.; Silva, S.; Negri, M.; Gorup, L.F.; de Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver colloidal nanoparticles: Effect on matrix composition and structure of Candida albicans and Candida glabrata biofilms. J. Appl. Microbiol. 2013, 114, 1175–1183. [Google Scholar] [CrossRef]
- Muthamil, S.; Devi, V.A.; Balasubramaniam, B.; Balamurugan, K.; Pandian, S.K. Green synthesized silver nanoparticles demonstrating enhanced in vitro and in vivo antibiofilm activity against Candida spp. J. Basic Microbiol. 2018, 58, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Cho, M.H.; Kim, J.A.; Lee, J. 7-fluoroindole as an antivirulence compound against Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2012, 329, 36–44. [Google Scholar] [CrossRef]
- Khatoon, N.; Sharma, Y.; Sardar, M.; Manzoor, N. Mode of action and anti-Candida activity of Artemisia annua mediated-synthesized silver nanoparticles. J. Mycol. Méd. 2019, 29, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Sung, W.S.; Suh, B.K.; Moon, S.-K.; Choi, J.-S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. BioMetals 2009, 22, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Dantas Ada, S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Karim, M. Antimicrobial and Cytotoxic Activity of Di-(2-ethylhexyl) Phthalate and Anhydrosophoradiol-3-acetate Isolated from Calotropis gigantea (Linn.) Flower. Mycobiology 2009, 37, 31–36. [Google Scholar] [CrossRef]
- Pattnaik, P.K.; Kar, D.; Chhatoi, H.; Shahbazi, S.; Ghosh, G.; Kuanar, A. Chemometric profile & antimicrobial activities of leaf extract of Calotropis procera and Calotropis gigantea. Nat. Prod. Res. 2017, 31, 1954–1957. [Google Scholar]
- Peters, B.M.; Palmer, G.E.; Nash, A.K.; Lilly, E.A.; Fidel, P.L.; Noverr, M.C., Jr. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect. Immun. 2014, 82, 532–543. [Google Scholar] [CrossRef]
- Carradori, S.; Chimenti, P.; Fazzari, M.; Granese, A.; Angiolella, L. Antimicrobial activity, synergism and inhibition of germ tube formation by Crocus sativus-derived compounds against Candida spp. J. Enzyme Inhib. Med. Chem. 2016, 31, 189–193. [Google Scholar] [CrossRef]
- Modrzewska, B.; Kurnatowski, P. Adherence of Candida sp. to host tissues and cells as one of its pathogenicity features. Ann. Parasitol. 2015, 61, 3–9. [Google Scholar]
- Rozalska, B.; Sadowska, B.; Budzynska, A.; Bernat, P.; Rozalska, S. Biogenic nanosilver synthesized in Metarhizium robertsii waste mycelium extract—As a modulator of Candida albicans morphogenesis, membrane lipidome and biofilm. PLoS ONE 2018, 13, e0194254. [Google Scholar] [CrossRef] [PubMed]
- Halbandge, S.D.; Jadhav, A.K.; Jangid, P.M.; Shelar, A.V.; Patil, R.H.; Karuppayil, S.M. Molecular targets of biofabricated silver nanoparticles in Candida albicans. J. Antibiot. 2019, 72, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.D.; Wilson, D.; Wachtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti-Infect. Ther. 2012, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Vila, T.; Romo, J.A.; Pierce, C.G.; McHardy, S.F.; Saville, S.P.; Lopez-Ribot, J.L. Targeting Candida albicans filamentation for antifungal drug development. Virulence 2017, 8, 150–158. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jimenez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnol. 2015, 13, 91. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef]
- Sardi, J.C.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Patel, H.; Patel, J.; Patel, B. Comparative efficacy of phytochemical analysis and antioxidant activity of methanolic extract of calotropis gigantea and calotropis procera. Int. J. Biol. Pharm. Res. 2014, 5, 107–113. [Google Scholar]
- Rathod, N.R.; Raghuveer, I.; Chitme, H.R.; Chandra, R. Free Radical Scavenging Activity of Calotropis gigantea on Streptozotocin-Induced Diabetic Rats. Indian J. Pharm. Sci. 2009, 71, 615–621. [Google Scholar] [CrossRef]
- Singh, N.; Pathak, A.K.; Tailang, M. Phytochemistry and evaluation of antioxidant activity of whole plant of Calotropis gigantea Linn. Int. J. Res. Ayurveda Pharm. 2010, 1, 120–125. [Google Scholar]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Jain, J.; Rajwade, J.M.; Paknikar, K.M. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol. Lett. 2008, 179, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lian Wu, Y.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef]

| Concentration (µg/mL) | Antifungal Agents | ||
|---|---|---|---|
| Amphotericine B | AgNPs | Plant Extract | |
| IZD (mm) | |||
| 0 | 0 a ± 0.2 | 0 a ± 1.0 | 0 a ± 0.2 |
| 6.25 | 19 c ± 0.2 | 6 b ± 1.2 | 3.6 a ± 0.8 |
| 12.5 | No growth | 8.22 b ± 0.5 | 4.1 a ± 1.0 |
| 25 | 11.33 b ± 0.9 | 4.9 a ± 1.5 | |
| 50 | No growth | 5.2 a ± 1.5 | |
| 100 | 6.1 a ± 0.9 | ||
| 200 | No growth | ||
| 400 | |||
| Enzyme | Substrate | Specific Activity (U/mg Protein) | |
|---|---|---|---|
| Control | AgNPs/CG | ||
| Glutathone-S transferase | CDNB | 0.422 ± 0.11 | 0.0602 |
| Catalase | H2O2 | 2.3 ± 0.33 | 0.75 |
| Superoxide dismutase | Epinephrine | 0.405 ± 0.06 | 0.0675 |
| Glucose 6 phosphate dehydrogenase | NADP | 425.22 ± 5.11 | 53.15 |
| Glutathione reductase | NADPH | 45.23 ± 2.13 | 6.46 |
| Glutathione peroxidase | NADPH | 0.00055 ± 0.0001 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, E.M.; Abdallah, B.M. Effective Inhibition of Candidiasis Using an Eco-Friendly Leaf Extract of Calotropis-gigantean-Mediated Silver Nanoparticles. Nanomaterials 2020, 10, 422. https://doi.org/10.3390/nano10030422
Ali EM, Abdallah BM. Effective Inhibition of Candidiasis Using an Eco-Friendly Leaf Extract of Calotropis-gigantean-Mediated Silver Nanoparticles. Nanomaterials. 2020; 10(3):422. https://doi.org/10.3390/nano10030422
Chicago/Turabian StyleAli, Enas M., and Basem M. Abdallah. 2020. "Effective Inhibition of Candidiasis Using an Eco-Friendly Leaf Extract of Calotropis-gigantean-Mediated Silver Nanoparticles" Nanomaterials 10, no. 3: 422. https://doi.org/10.3390/nano10030422
APA StyleAli, E. M., & Abdallah, B. M. (2020). Effective Inhibition of Candidiasis Using an Eco-Friendly Leaf Extract of Calotropis-gigantean-Mediated Silver Nanoparticles. Nanomaterials, 10(3), 422. https://doi.org/10.3390/nano10030422





