1. Introduction
Cardiovascular disease is the leading cause of morbidity and mortality in industrialized nations and the largest cause of disability-adjusted life years globally [
1]. Atherosclerosis is an inflammatory disease with the mainstay of treatment focusing on decreasing lipid burden and, for advanced disease, focusing on interventions such as angioplasty, stenting, or bypass grafting. Recently, the focus of lowering atherosclerotic disease burden has turned to methods of decreasing low-density lipoprotein-induced immune activation, albeit through in vitro studies and animal models. The notion of atherosclerosis as an immune-mediated disorder is based on evidence of immune activation and cytokine signaling within atherosclerotic lesions, and interference with immune signaling and inflammatory mediators inhibiting the pathogenesis of atheroma development in mouse models of atherosclerosis [
2].
Cardiovascular disease interventions for the treatment of advanced atherosclerosis are limited by the development of neointimal hyperplasia, leading to a re-occlusive process that is multifactorial and involves inflammation, cellular proliferation and migration, and other processes [
3,
4,
5]. Balloon dilation of the arterial wall provokes denudation of the endothelium, leading to exposure of vascular smooth muscle cells (VSMC) to serum containing pro-inflammatory cytokines, of which elevated levels are found in post-intervention patients [
6]. Neointimal hyperplasia is mediated by a series of complex interactions of sustained autocrine and paracrine growth factor and cytokine expression on cells in the vascular wall, including VSMC and adventitial fibroblasts [
7,
8,
9,
10,
11]. This cascade of events after arterial injury ultimately leads to narrowing of the arterial lumen and introduces further difficulty in re-intervention. Development of a therapy that effectively prevents atherosclerosis and inhibits the development of neointimal hyperplasia if vascular intervention is required is a significant unmet clinical need in cardiovascular medicine. Fractalkine is one such target involved in both disease processes.
Fractalkine (CX3CL1) is a unique chemokine that exists as both a membrane-bound and soluble chemokine and is present on activated endothelium, dendritic cells, and VSMC [
10]. It is anchored to vascular wall cells by an extended mucin stalk linked to a transmembrane domain and acts as an adhesion molecule for leukocytes (
Figure S1A) [
11]. Fractalkine and its receptor (CX3CR1) regulate leukocyte adhesion and extravasation at the leukocyte–endothelial cell interface. More importantly, accumulating evidence suggests that fractalkine is involved in the pathogenesis of atherosclerosis, is highly expressed in early atherosclerotic lesions, and is expressed after arterial injury [
12,
13,
14,
15]. Mice with the ApoE and CX3CR1 genes deleted were shown to have less atheroma formation, and polymorphisms in these genes are associated with a significantly reduced risk of coronary artery disease [
14,
16]. Furthermore, inhibition of fractalkine by intraperitoneal injection of alpha-lipoic acid in rats after carotid artery balloon angioplasty has been shown to decrease neointimal hyperplasia development, mediated by the inhibition of the NF-kappaB pathway [
15].
Current therapies approved by the United States Food and Drug Administration (FDA) to prevent neointimal hyperplasia are limited to drug-eluting stents and balloons, which deliver drugs locally to the site of injury [
16,
17]. A systemically delivered therapy that is targeted specifically to the site of interest in the vasculature would offer several advantages: (1) the opportunity for drug delivery without the long-term presence of a foreign body material; (2) the ability to deliver multiple doses of a drug and delivery of high local drug concentrations not possible with stents, which are limited by their drug carrying surface area; and (3) the avoidance of systemic side effects due to the targeted nature of the therapeutic. In this context, fractalkine is an ideal therapeutic target, as it would have targeting capability at the site of both the atheroma and neointimal hyperplasia development. The goal of the study was to develop a systemically delivered supramolecular nanostructure that targets the site of arterial injury. We hypothesized that a fractalkine-targeted nanofiber will localize to the site of arterial injury in a dose dependent fashion.
2. Materials and Methods
2.1. PA Synthesis and Labeling
Peptide amphiphile (PA) molecules were synthesized via standard 9-fluorenyl methoxycarbonyl (Fmoc) solid-phase peptide chemistry on Rink amide 4-methylbenzhydrylamine resin using a CEM Liberty Blue automated microwave peptide synthesizer (CEM Corporation; Matthews, NC, USA). Automated coupling reactions were performed using 4 equivalents of Fmoc-protected amino acid, 4 equivalents of N,N’-diisopropylcarbodiimide (DIC), and 8 equivalents of ethyl(hydroxyimino)cyanoacetate (Oxyma pure). Removal of the Fmoc groups was achieved with 20% 4-methylpiperidine in dimethylformamide (DMF). Peptides were cleaved from the resin using standard solutions of 95% trifluoroacetic acid (TFA), 2.5% water, and 2.5% triisopropylsilane (TIPS). Cysteine containing peptides included 3% 2,2′-(Ethylenedioxy)diethanethiol (DODT) and 92% TFA with the same TIPS/H2O mixture. Peptides were precipitated with cold ether and then purified by reverse-phase high-performance liquid chromatography on a Waters Prep150 (Milford, MA, USA) or Shimadzu Prominence high-performance liquid chromatograph (HPLC, West Chicago, IL, USA) using a water/acetonitrile (each containing 0.1% v/v TFA or 0.1% NH4OH) gradient. Eluting fractions containing the desired peptide were confirmed by mass spectrometry using an Agilent 6520 QTOF liquid chromatograph/mass spectrometer (LCMS; Santa Clara, CA, USA). Confirmed fractions were pooled and the acetonitrile was removed by rotary evaporation before freezing and lyophilization. Purity of lyophilized products was tested by LCMS. For PAs labeled with 5-carboxytetramethyrhodamine (TAMRA), the methtrityl (Mtt) protecting group was first removed from the lysine on-resin using 1% TFA in dichloromethane (DCM) with 5% TIS. After washing with DCM and DMF, TAMRA was then coupled to the now free epsilon amine of lysine using 1.2 equivalents of TAMRA, 1.2 equivalents of PyBOP (benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate), and 8 equivalents of N,N-diisopropylethylamine for approximately 18 h.
2.2. Conventional Transmission Electron Microscopy (TEM)
Conventional TEM images were taken on a FEI Tecnai T-12 TEM (ThermoFisher Scientific; Hillsboro, OR, USA) at 80 kV with an Orius® 2k × 2k CCD camera (Gatan, Inc.; Pleasanton, CA, USA). PAs at 0.5 mg/mL in Hanks Balanced Salt Solution (HBSS) were prepared for TEM by negative staining. Briefly, 8 µL samples were incubated onto 400-mesh copper grids covered with a thin carbon film and previously treated with glow discharge. After 3 min, samples were stained with 2% uranyl acetate for 2–3 min and air-dried before imaging.
2.3. Circular Dichroism (CD) Spectroscopy
CD measurements were performed at a concentration of 0.125 mg/mL in HBSS using a Jasco J-815 CD spectrophotometer (Jasco Analytic Instruments; Easton, MD, USA) at 25 °C using a 1-mm path length demountable quartz cuvette. The far-ultraviolet (UV) spectral region (190–250 nm) was observed to determine if the assemblies contained secondary structure. Background subtraction of the HBSS buffer was performed. The data represent an average of two scans. Data points were taken every 0.2 nm with an analysis time per data point of 120 ms.
2.4. Study Approval
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication 85-23, 1996) and approved by the Animal Care and Use Committee at University of North Carolina Chapel Hill. The number of rats randomized to each treatment group (n = 3) was calculated using a power of 0.8, difference in means of 0.2, standard deviation of 0.1, and alpha of 0.05 using Stata/SE 15.1 (StataCorp LLC; College Station, TX, USA).
2.5. Animal Experiments
Adult male Sprague-Dawley rats weighing 300–350 g underwent carotid artery balloon injury as previously described [
18]. After balloon injury, the arteriotomy was ligated and the animal was allowed to recover. Twenty-four hours after balloon injury, the different nanostructures were dissolved in 1 mL of 1× phosphate buffered saline (PBS) and administered via tail vein injection. Rats were euthanized at 5 h and 1, 2, 3, and 14 days post-injection, based on the experiment being conducted. The number of animals per treatment group for the different experiments were as follows: for localization of the nanofibers 5 h after injection,
n = 4/treatment group; for localization duration study,
n = 3/group; for concentration study,
n = 3/treatment group.
Low-density lipoprotein receptor knockout (LDLR-/-) mice at 4 weeks of age were fed high fat diet consisting of 20% fat, 0.2% cholesterol, and 34% sucrose for 18 weeks. LDLR-/- mice develop severe hypercholesterolemia (>800 mg/dL) and hypertriglyceridemia (>300 mg/dL) as early as 2 weeks and atherosclerotic lesions after 12 weeks on high fat diet. At 16 weeks of age, aortic roots were harvested as previously described [
19]. Tissue was harvested at 16 weeks of age to identify maximal atherosclerotic burden and later stages of atherosclerosis.
2.6. Tissue Processing
2.6.1. Tissue Harvest
After euthanasia via isoflurane overdose, bilateral thoracotomies were performed, followed by in situ perfusion with 1× PBS (250 mL) or until the liver appeared clear. Carotid arteries and viscera were harvested and then placed in 2% paraformaldehyde for 2 h at 4 °C, followed by 30% sucrose overnight at 4 °C. Tissue was processed as previously described [
18]. Arterial tissue for light sheet microscopy was harvested after in situ perfusion with 1× PBS (250 mL) or until liver appeared clear and then placed in 4% paraformaldehyde at 4 °C for 2 h. Arterial tissue was then warmed in a 37 °C incubator for 30 min, removed from paraformaldehyde, and embedded in 1% agarose prepared in tri-acetate ethylenediaminetetraacetic acid EDTA buffer. Once agarose solidified, arteries were dehydrated by placing them in progressive methanol: DI H
2O solutions, from 20% to 100%, at 20% intervals and 1 h incubation time per concentration. Tissue blocks were then removed, incubated in 66:33% (
v/
v) dichloromethane:methanol solution for 3 h at room temperature, and then transferred to 100% dichloromethane for 15 min. Lastly, arterial blocks were placed in 100% dibenzylether (DBE) until ready for imaging.
2.6.2. Immunofluorescent Staining
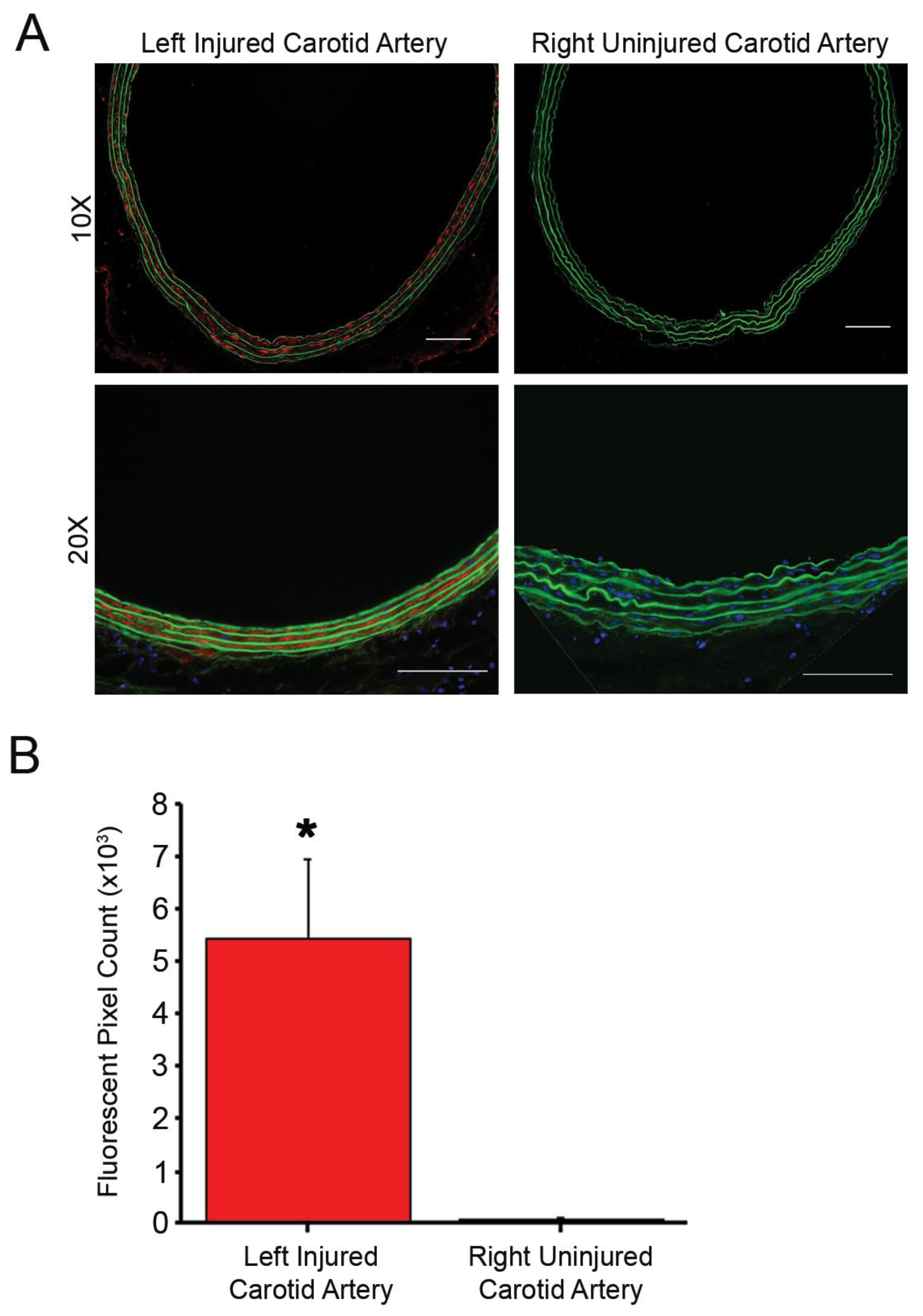
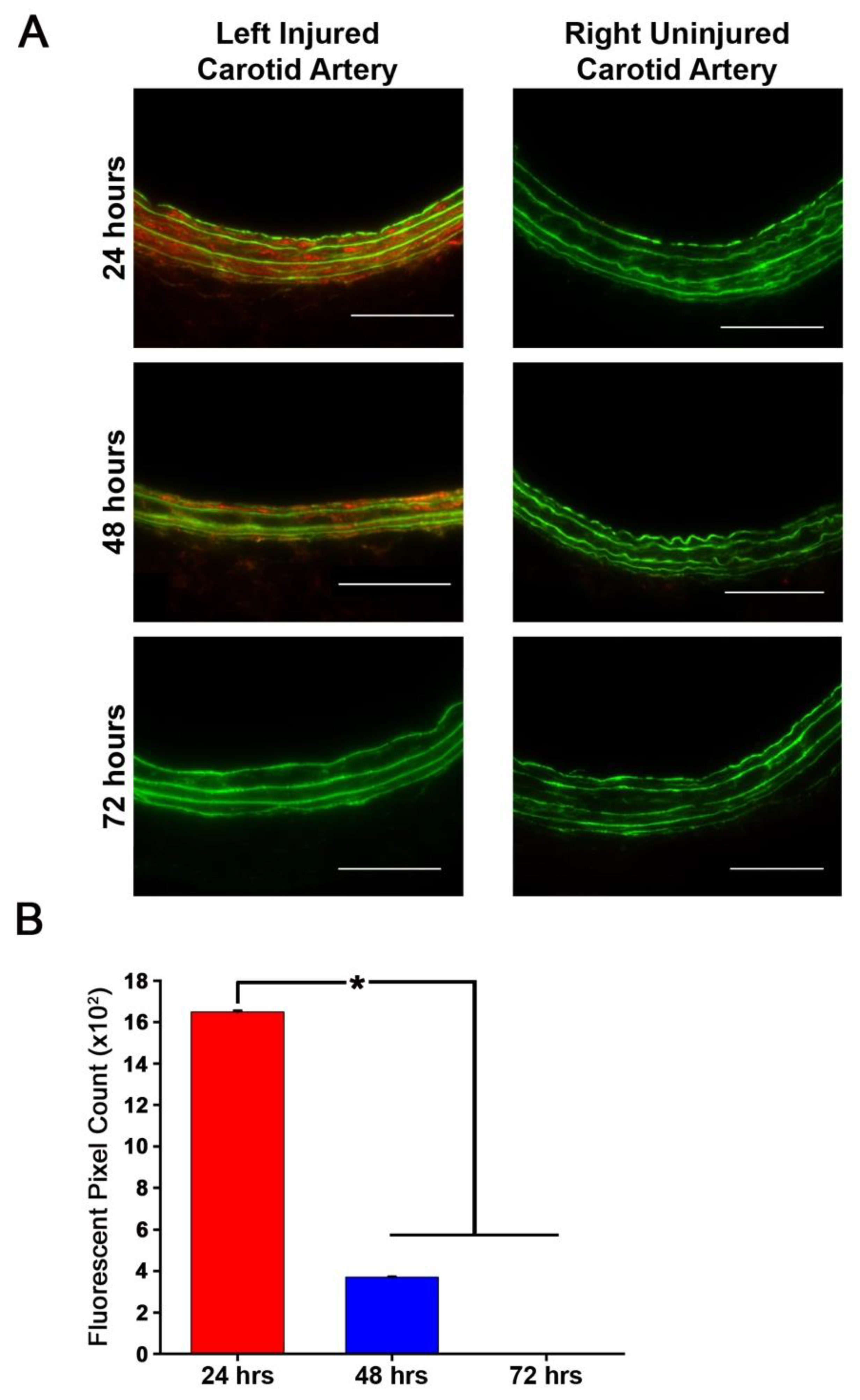
Rat carotid arteries were harvested 24 h after injury and stained for CX3CL1 to determine presence of the protein. Primary antibody (rabbit polyclonal to CX3CL1, Abcam: ab25088) and secondary antibody (goat anti-rabbit IgG highly cross-adsorbed Alexa 647) dilutions were 1:200 and 1:3000, respectively. Aortic roots of mice fed a high-fat diet for 16 weeks were stained for CX3CR1 to determine presence of the protein in the atherosclerotic plaque. Primary antibody (rabbit polyclonal to CX3CR1, Abcam: ab8021) and secondary antibody (goat anti-rabbit IgG Alexa 555, Invitrogen or Alexa 647 highly cross-adsorbed, Invitrogen A32733) dilutions were 1:200 and 1:3000, respectively. Quantification of CX3CL1 staining data are presented as number of fluorescent pixels, which are the average of 5 images per animal, n = 3 animals per treatment group. Fluorescent imaging of CX3CR1 in arteries from atherosclerotic mice was obtained but not quantified due to low sample numbers. Results are expressed as mean number of fluorescent pixels ± the standard error of the mean (SEM).
Tissue sections from LDL KO mice were stained for CX3CR1 (Abcam ab8021) using primary dilution of 1:200 and secondary goat anti-rabbit Alexa 555 dilution of 1:3000 (Life Technologies A21425). Nuclear staining was carried out using DAPI at 1:500 dilution. Digital images were acquired as mentioned above.
2.7. Fluorescent Imaging
Carotid arteries harvested at respective time points underwent fluorescent imaging. Digital images were acquired using a Zeiss Axio Imager.A2 microscope (Hallbergmoos, Germany) with a 20× objective, HE Cy3 filter (Zeiss filter #43), using excitation and emission wavelengths of 550–575 nm and 605–670 nm, respectively, to assess presence of TAMRA-labeled PA fluorescence and immunohistofluorescence of stained arteries. The green fluorescent protein filter (Zeiss filter #38) using excitation and emission wavelengths of 470–495 and 525–550 nm, respectively, was used to assess tissue autofluorescence. To assess localization of the nanostructures, the fluorescent pixels of the arterial cross-sections were quantified in high power field (10× objective) images using ImageJ software (v.1.51, National Institutes of Health; Bethesda, MD, USA). Quantification data are presented as number of fluorescent pixels, which are the average of 5 images per animal, n ≥ 3 animals per treatment group. Results are expressed as mean ± SEM.
2.8. Light Sheet Fluorescent Microscopy Imaging
Imaging was performed using a LaVision BioTec Ultramicroscope II (LaVision BioTec GmbH; Bielefeld, Germany) as previously described [
20], with the following modifications. Cleared agarose-artery blocks mounted in a custom sample holder were submerged in 100% DBE. Artery images were acquired at 1.26× mag (0.63× zoom), using the three-light-sheet configuration with both left and right light sheets, with the horizontal focus centered in the middle of the field of view, a numerical aperture of 0.026 (sheet thickness at horizontal focus = 28 µm), and a light sheet width of 100%. Pixel size was 4.96 µm and Z-slice spacing was 14 µm. Two channels were imaged: arterial autofluorescence with 488 nm laser excitation and a Chroma ET525/50m emission filter and TAMRA channel with 561 nm laser excitation and a Chroma ET600/50m emission filter. Focus in both channels was ensured by use of the chromatic correction module on the instrument. Sample bleaching during initial imaging setup was minimized by use of low laser power and long exposure times. For image acquisition, higher laser power and shorter exposure times were used.
2.9. Statistical Analysis
JMP (SAS Institute, Inc.; Cary, NC, USA) was used to determine differences between groups depending on the study being conducted using an analysis of variance (ANOVA) followed by a Student’s t-test. Results are expressed as number of fluorescent pixels ± SEM.
4. Discussion
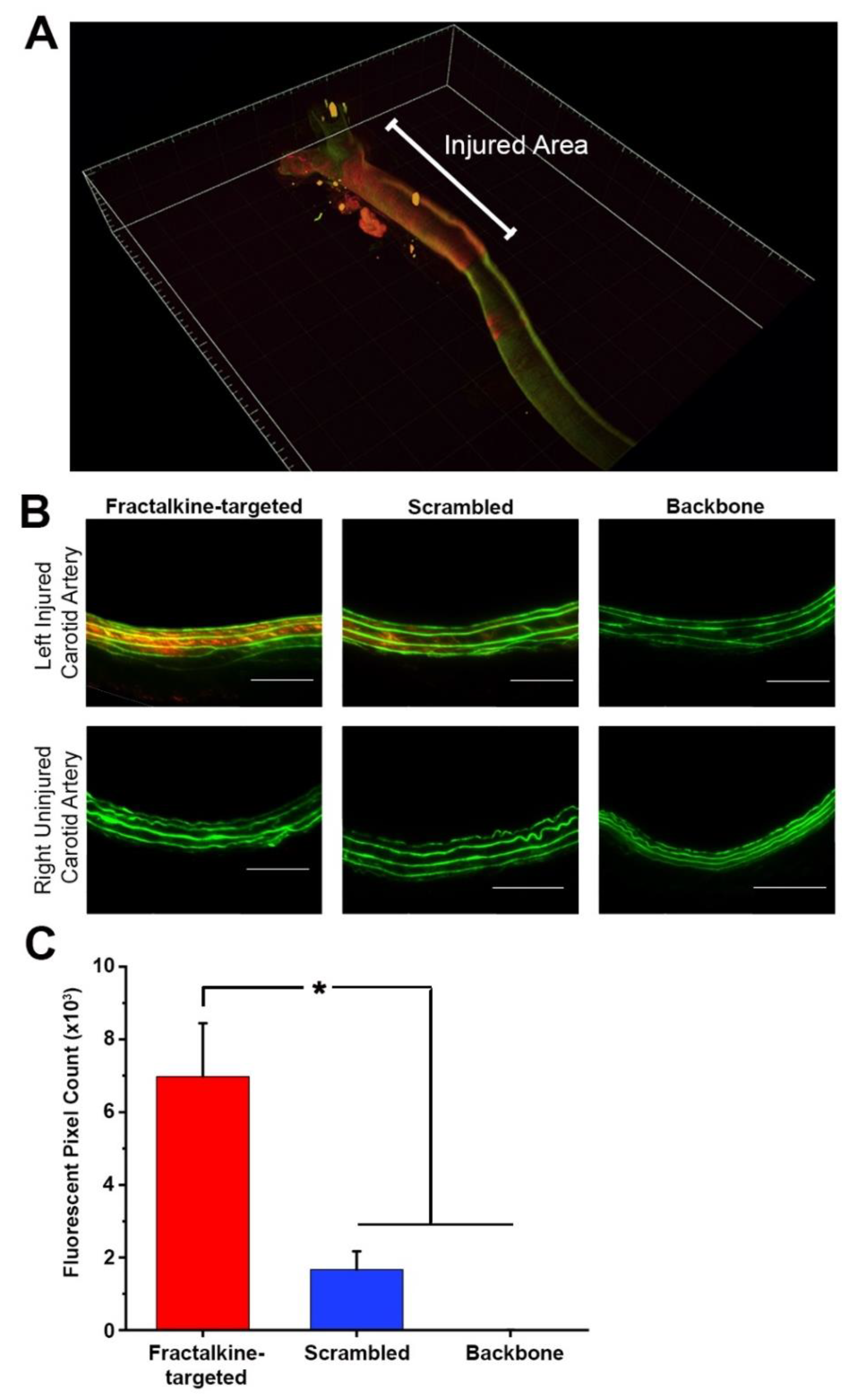
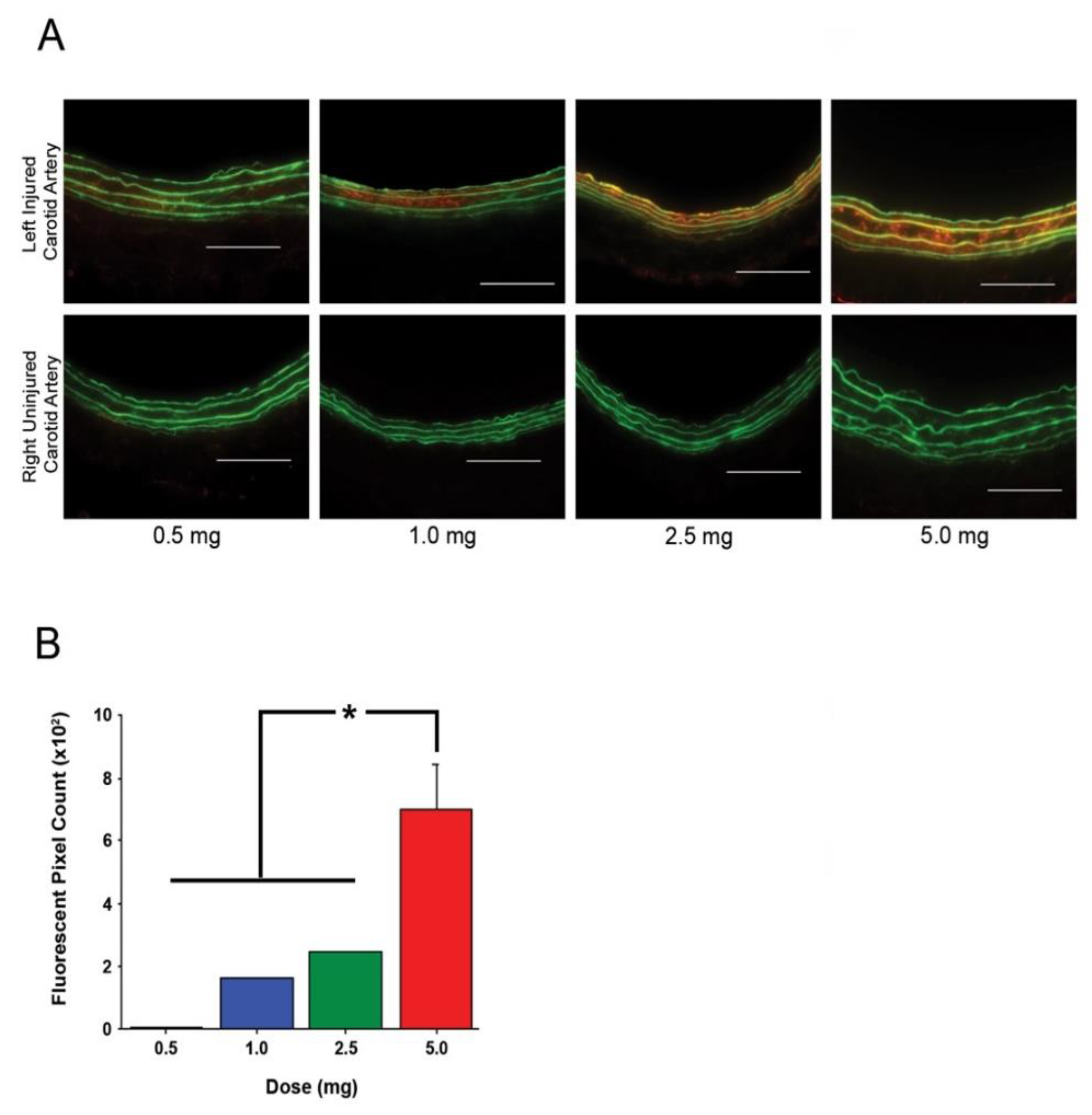
We describe here the presence of fractalkine in the arterial media after vascular injury and within the plaque in an atherosclerotic artery. Further, we describe the development and evaluation of a novel delivery vehicle designed to target fractalkine present in the artery after balloon injury. We show that our fractalkine-targeted PA nanofiber localizes to the area of arterial injury when administered by systemic intravenous injection. This targeted nanofiber can be administered over a range of dosages, with localization observed after injection of as little as 1.0 mg, and the targeted nanofiber remaining localized to the area of interest for up to two days. As such, these data represent the foundation for the development of a therapeutic that has potential to target both restenosis and atherosclerosis.
We previously reported on a targeted therapy for the treatment of neointimal hyperplasia and atherosclerosis using a similar PA delivery vehicle, but with different targeting sequences [
23,
24]. In these studies, PAs with amino acid sequences that interact with type IV collagen or ApoA1 were used to target the area of neointimal hyperplasia and atherosclerotic plaque, respectively. The fractalkine-targeted PA is novel given that the protein is present in both these disease models and therefore one PA can be used to target the pathologic area in both diseases. It has previously been reported that fractalkine is present in the arterial media after arterial injury, albeit this was only accomplished through the evaluation of the effects of alpha-lipoic acid administration after balloon angioplasty [
15]. Moreover, the effects of fractalkine in the development of atherosclerosis have been extensively studied. A polymorphism of CX3CR1, thereby resulting in the presence but lack of function of the fractalkine receptor, leads to the inability for leukocyte attachment and confers a reduced risk of acute coronary events [
14,
25]. Deficiency of the fractalkine receptor in ApoE-/- animal models provides significant protection from both macrophage recruitment to the vessel wall and subsequent development of atherosclerotic lesions [
12]. While all these studies are promising, no literature exists on the development of a delivery vehicle targeting fractalkine in both a neointimal hyperplasia and atherosclerotic model. Furthermore, no literature exists on targeting the fractalkine receptor itself as a potential therapy in these two disease models.
Our fractalkine-targeted PA has great potential for clinical use in the prevention of neointimal hyperplasia development after arterial injury. The ability to target the area of injury via intravenous injection, to be re-dosed if necessary, and the apparent safety profile of PAs makes this a great delivery vehicle for therapeutic agents [
26]. In our previous study, a single 5.0 mg dose of S-nitrosylated collagen PA (collagen-SNO PA) was given immediately after injury and the therapeutic effects were seen up to seven months later. Animals exhibited a 51% decrease in intima/media ratio and a continued 45% decrease in percent occlusion of the injured artery [
26]. We are also working to better quantify the dose–response observed with the fractalkine-targeted PA by continuing to expand the use of light-sheet fluorescence microscopy in our lab, which will allow for more accurate volume and area determinations. The ability of our fractalkine-targeted PA to remain localized at the area of injury for up to 48 h after systemic delivery further enhances its therapeutic potential. Previous studies have investigated different therapies to decrease expression of the fractalkine receptor but none have looked at the receptor itself as a therapeutic target [
15]. Just as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can target the LDL receptor, we envision a similar therapeutic agent targeting fractalkine, thereby preventing leukocyte recruitment and adhesion. Moreover, the involvement of fractalkine in both disease processes is due to the recruitment of inflammatory cells at the area of disease. By blocking the receptor and delivering a therapeutic agent with anti-inflammatory activity, we would anticipate a multiplicative therapeutic effect. Secondly, the specificity of our fractalkine-targeted PA would bypass any systemic side effects, contrary to those seen with statins and other medications, when used for primary and secondary prevention of atherosclerosis. Perhaps most importantly, the long-term therapeutic effects seen in our collagen-targeted SNO PA may translate to our fractalkine-targeted therapeutic PA.
There are several limitations of our current study. First, our scrambled PA nanofiber exhibits a small degree of localization in the arterial media. This nanofiber possesses the same net charge (−7 at physiological pH) as the fractalkine-targeted nanofiber, and this charge may be the reason for the fluorescence that is observed in the injured artery. Preliminary studies conducted in the same animal model and injecting a nonsensical, negatively-charged (−7) nanofiber also exhibited a small amount of fluorescence (data not shown), indicating that both the charge and the fractalkine-targeted sequence are required for robust localization to the injured site. Second, while fractalkine is present in atherosclerotic arteries and is involved in the development of atherosclerosis, we have not tested our fractalkine-targeted PA in an atherosclerotic model, and we may not observe similar localization as in the arterial injury model. Third, while we have not determined the critical aggregation concentration for the fractalkine-targeted PA, we have previously shown that similar PA nanofibers can form in solutions containing serum [
23].
Fourth, we did not determine the biodistribution of the fractalkine-targeted PA, although we can qualitatively say that the PA traffics to the typical organs of metabolism (liver and kidney). We have seen this previously with other PAs, and did not observe long-term adverse effects on a variety of biochemical markers (blood count, coagulation factors, liver enzymes, etc.) [
26]. Biodistribution is the subject of ongoing research in the lab, and we have recently completed a large pharmacokinetic and biodistribution study of a targeted PA and observed a three-compartment model with rapid clearance from the intravascular space (manuscript in preparation). Of note, the fractalkine-targeted peptide sequence alone would likely be too small to target the luminal surface of the blood vessel, since the fatty acid tail of the amphiphile would not be present to encourage formation of long fibers (
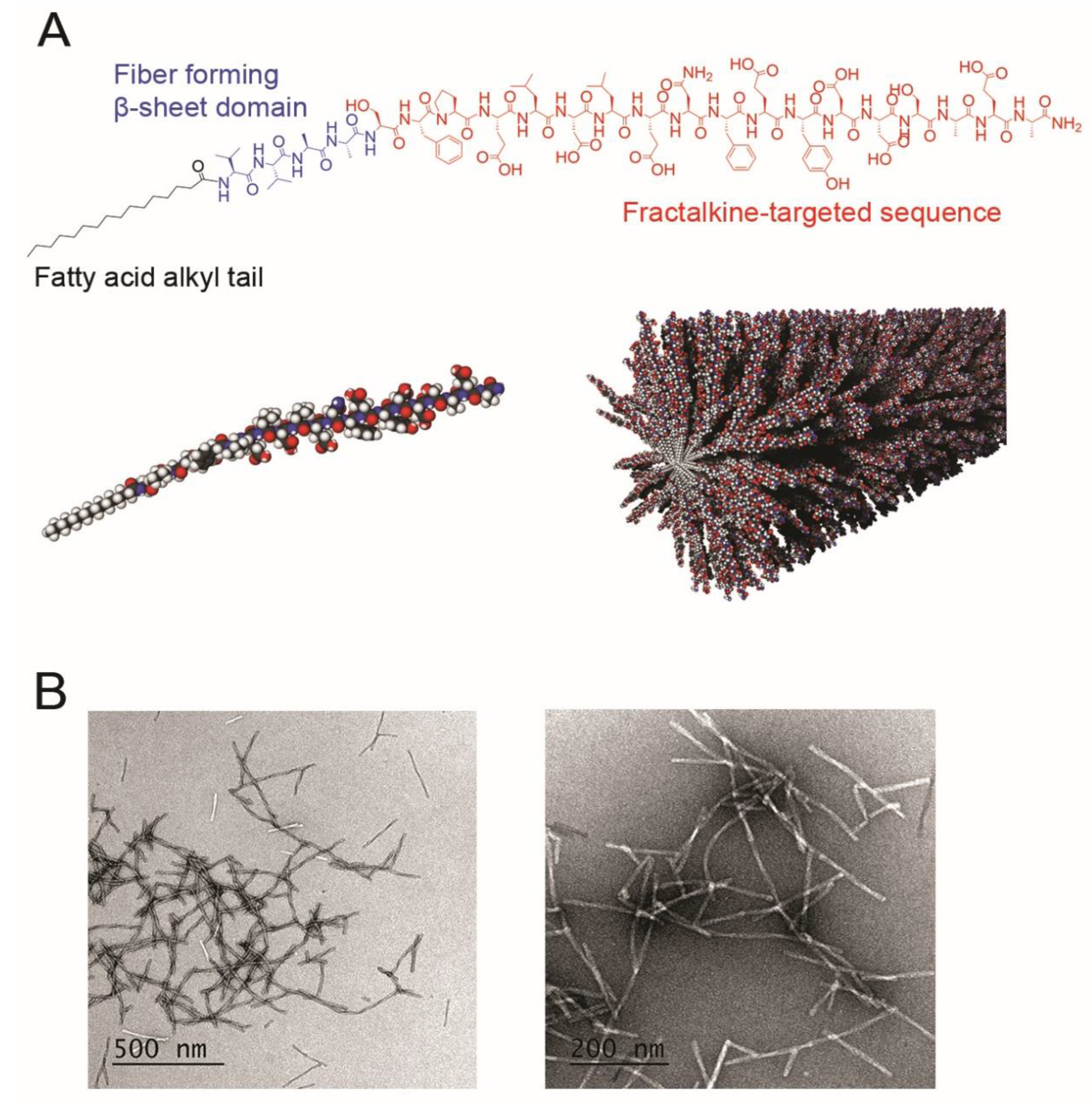
Figure 2B). The surface of the PA is also much larger, allowing for more epitope exposure, as well as margination of the long fibers to the low-flow regions near the vessel wall. Inclusion of the acyl tail allows for the ability to incorporate a therapeutic in the hydrophobic core of the nanofibers or covalently attach a therapeutic to a PA that could co-assemble with fractalkine-targeted PA. This would not be possible with the fractalkine-targeted peptide sequence alone.
Lastly, we have not tested the therapeutic effects of our fractalkine-targeted PA. Attaching a therapeutic agent may change the overall structure of our fractalkine-targeted PA that could lead to a lack of localization or, given the long targeting sequence, the inability to attach a therapeutic agent. In the latter case, we can attempt co-assembly of our fractalkine-targeted PA with a therapeutic PA attached to a backbone structure, thereby allowing for development of a therapeutic fractalkine-targeted nanofiber, as done in our previous work [
21]. Optimizing the ratio of fractalkine-targeted to backbone PA in the supramolecular nanostructure that will still allow for targeting and drug delivery is ongoing in our lab. Future work will also include verifying the localization of the fractalkine-targeted PA in a CX3CR1-/- rat model, although this must be genetically engineered, as it does not currently exist. Finally, while direct head-to-head comparisons are difficult due to changes in backbone, epitope, charge, fluorophore, and time of PA administration, work to compare and contrast the properties of the various PAs our lab has used over the years will be the subject of future studies.