Preparation and Characterization of Self-Assembled Poly(l-Lactide) on the Surface of β-Tricalcium Diphosphate(V) for Bone Tissue Theranostics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of β-tricalcium diphosphates (β-TCP) Doped with Pr3+and Co-Doped with Ce3+ Ions
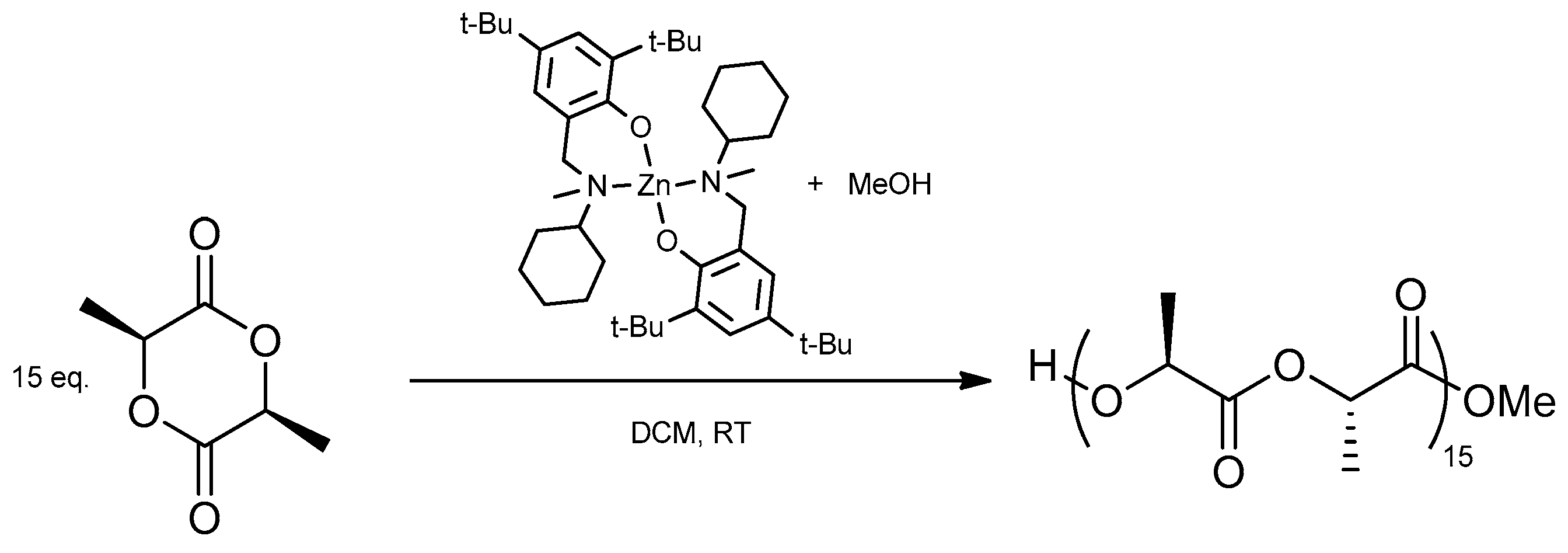
2.3. Synthesis of poly(l-lactide) (PLLA)
2.3.1. Synthesis of Initiator
2.3.2. Representative Procedure for Solution Polymerization
2.4. Representative Procedure for Preparation of β-TCP@PLLA Composites
2.5. Physicochemical Characterization
2.6. Evaluation of Biological Properties
2.6.1. Human Chondrocytes Cell Line
2.6.2. Influence of Obtained Composites on Chondrocytes Proliferation Rate
2.6.3. Hemolysis Assay
3. Results and Discussion
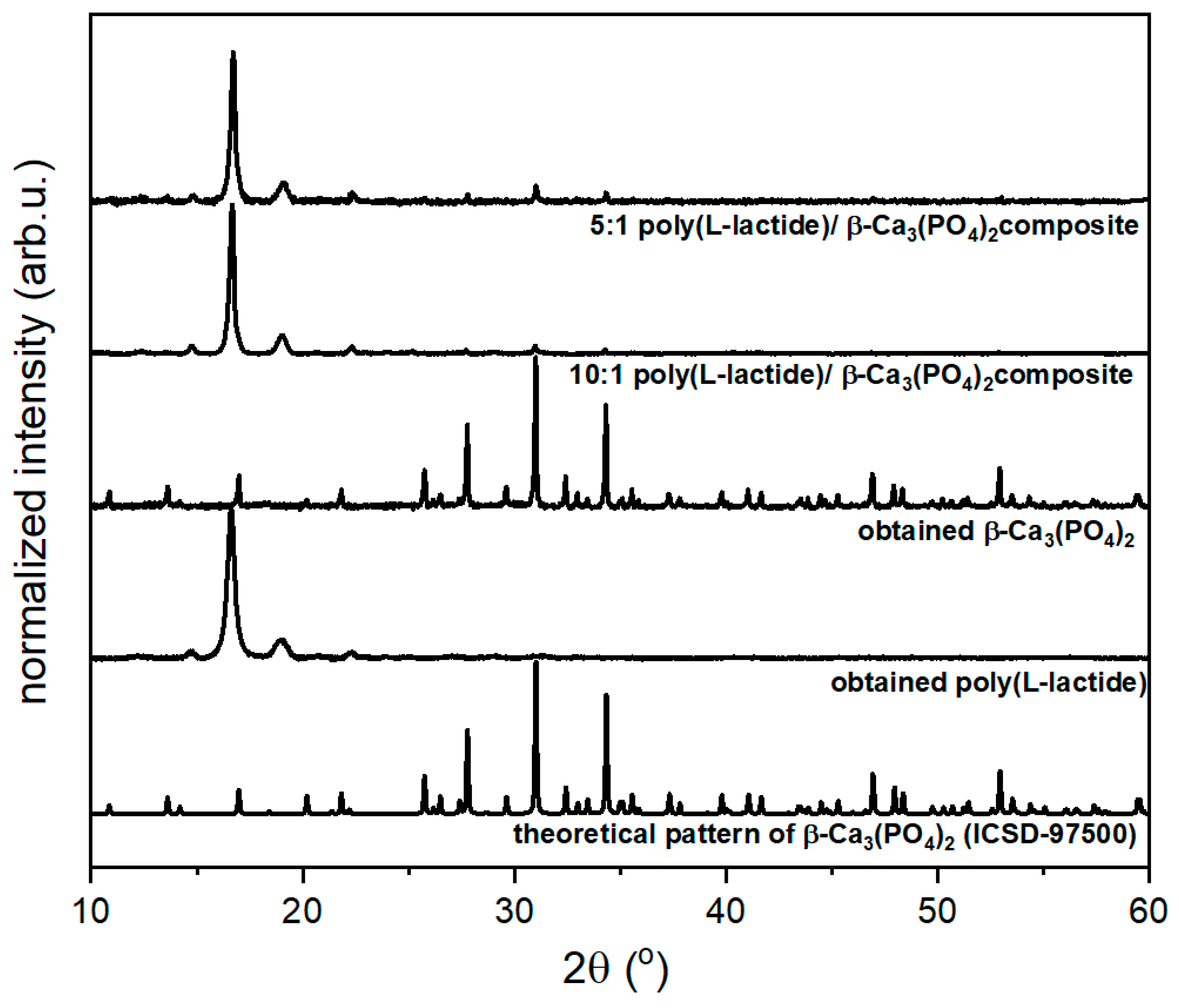
3.1. Structural Properties
3.1.1. β-tricalcium diphosphate (β-TCP)
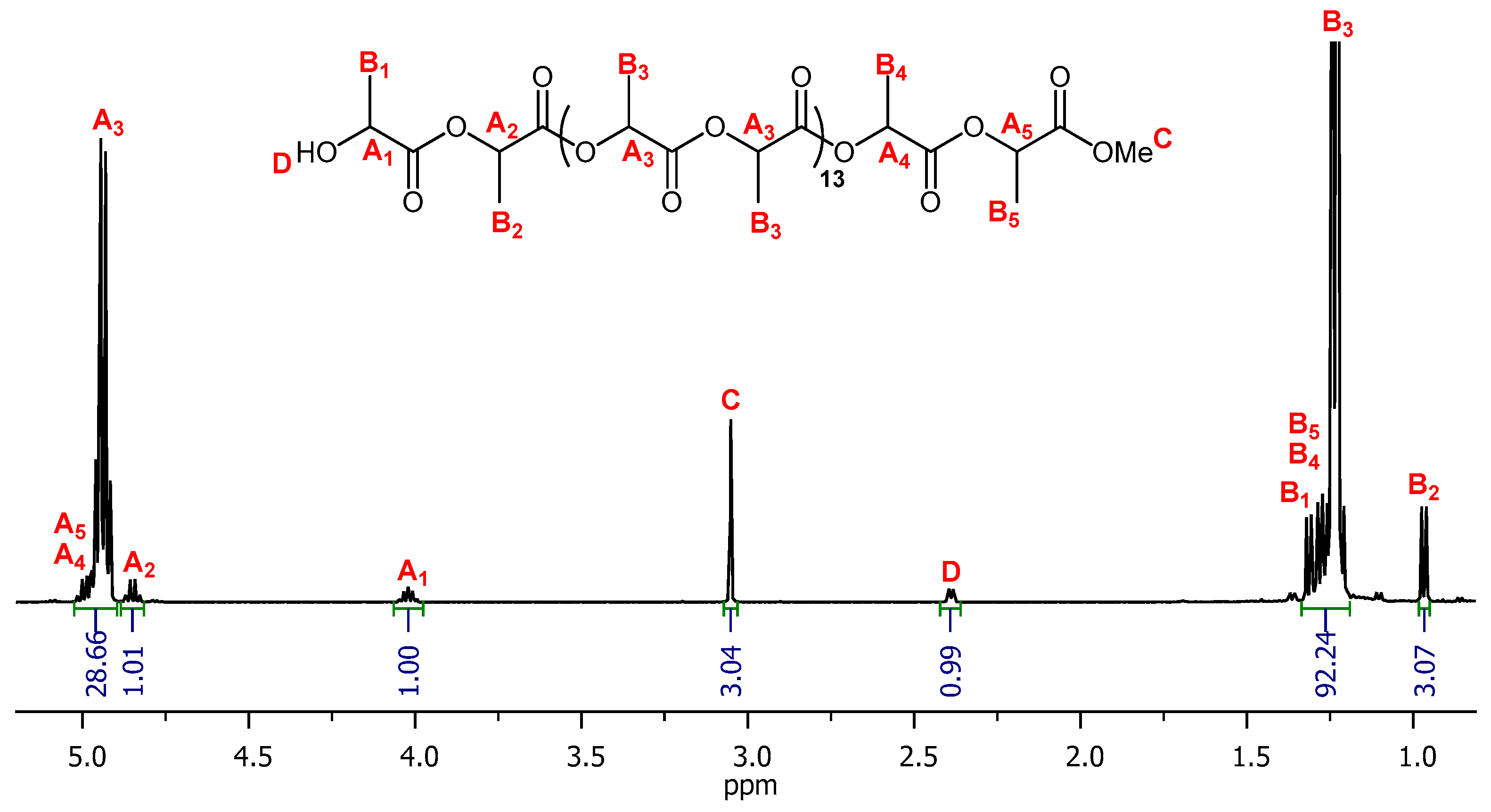
3.1.2. Poly(l-lactide) (PLLA)
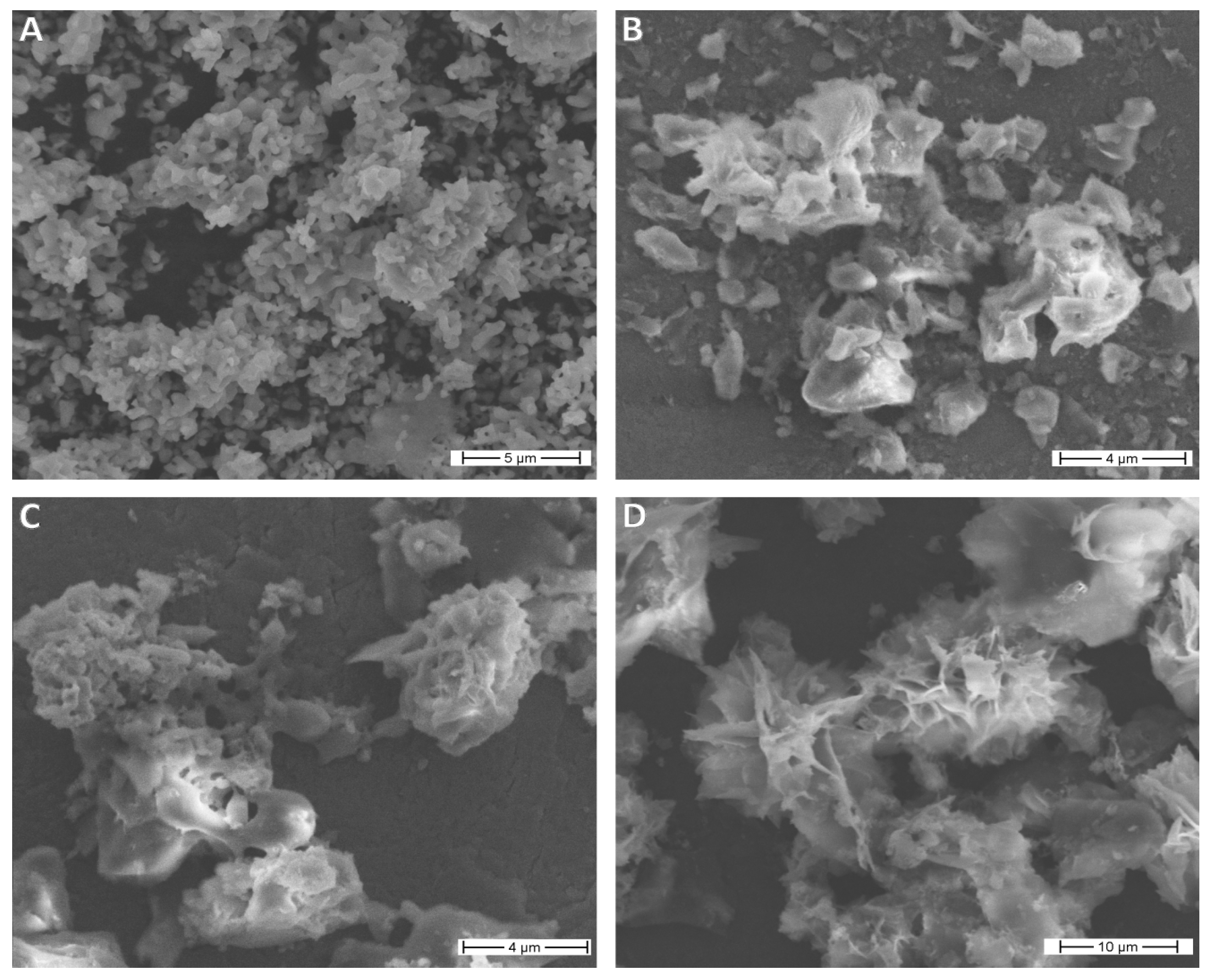
3.2. Morfological Properties
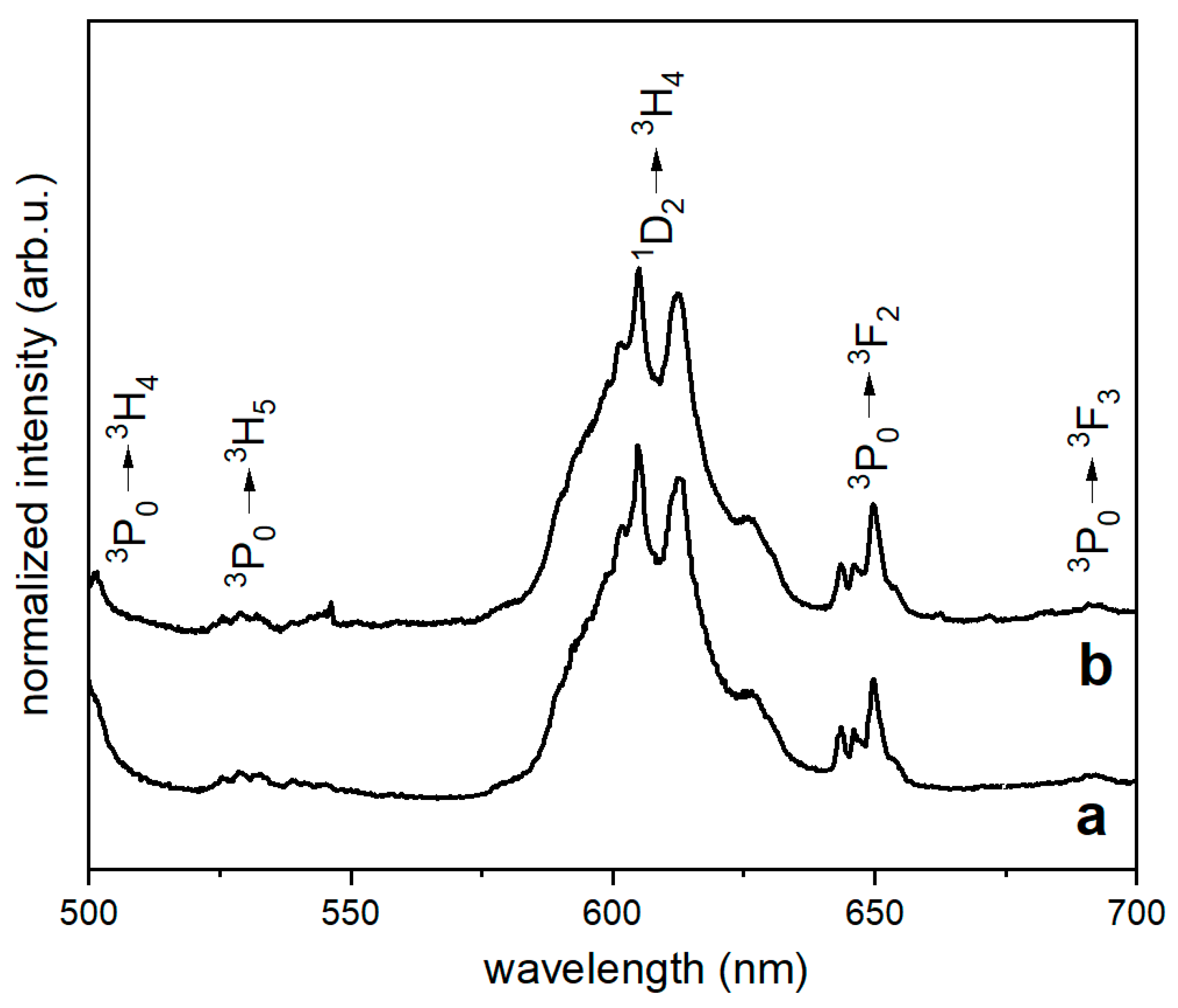
3.3. Spectroscopic Properties
3.3.1. Emission Spectra
3.3.2. Luminescence Kinetics
3.4. Biological Features
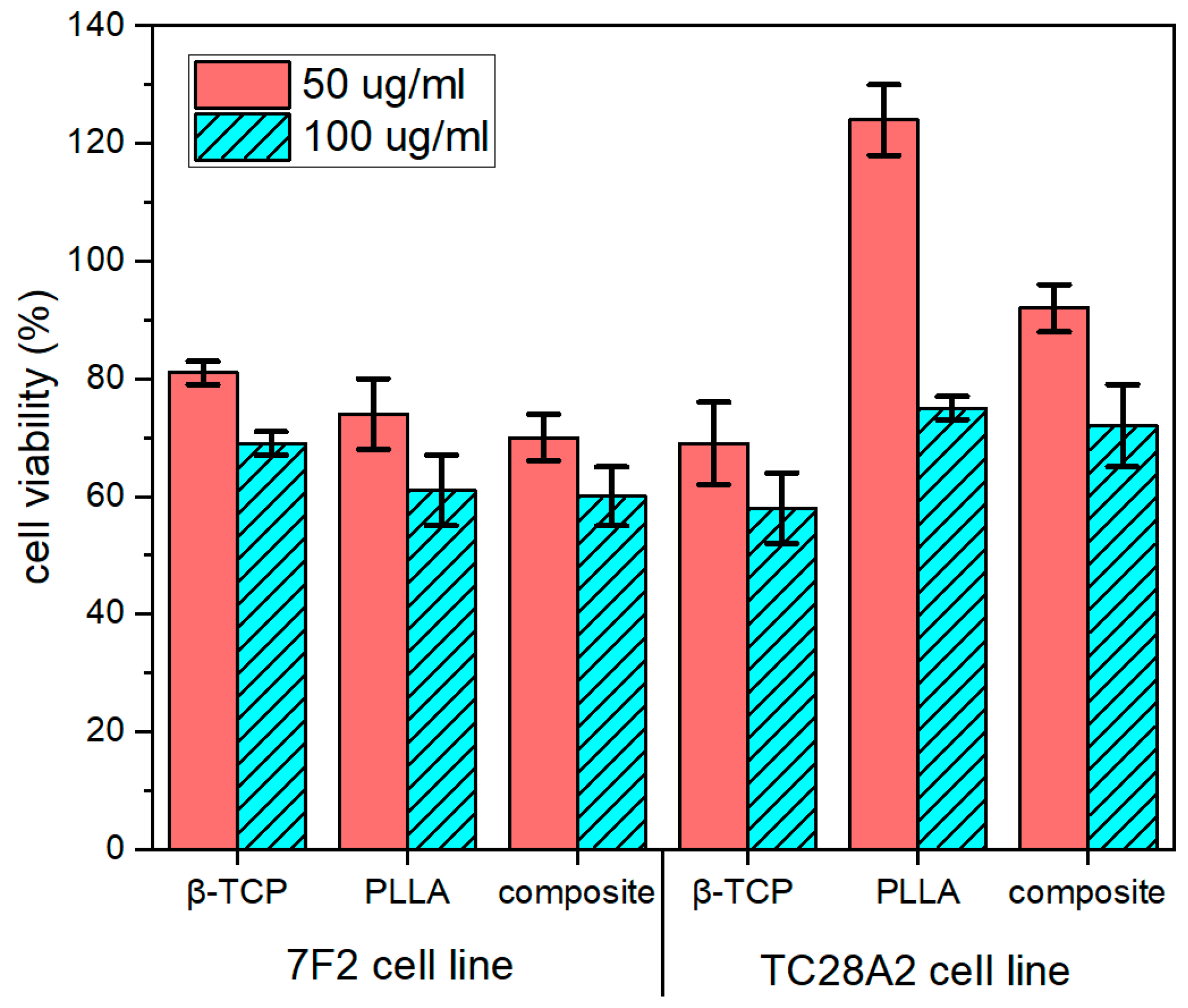
3.4.1. Cytotoxicity Assay
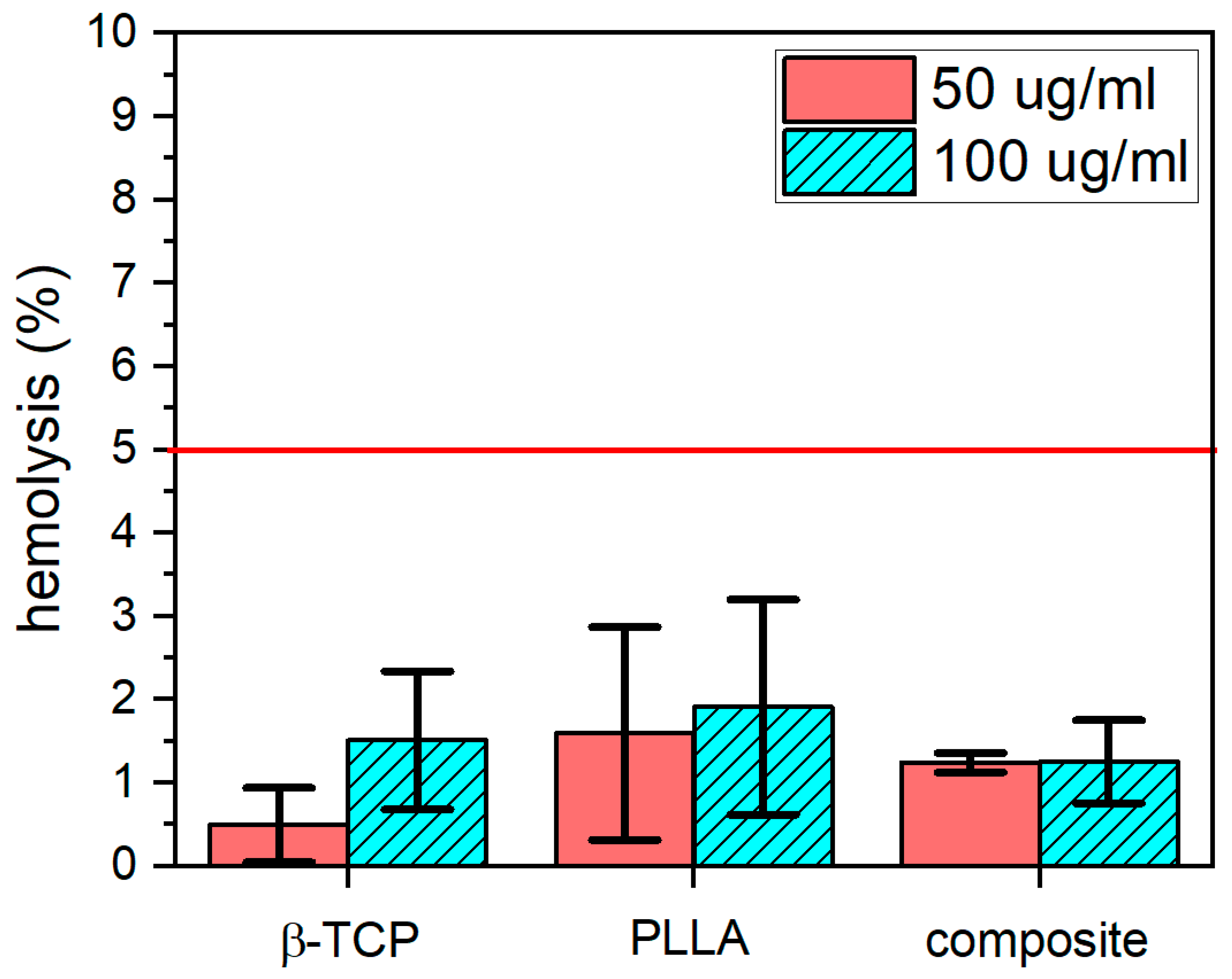
3.4.2. Hemolysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zarrin, A.; Sadighian, S.; Rostamizadeh, K.; Firuzi, O.; Hamidi, M.; Mohammadi-Samani, S.; Miri, R. Design, preparation, and in vitro characterization of a trimodally-targeted nanomagnetic onco-theranostic system for cancer diagnosis and therapy. Int. J. Pharm. 2016, 500, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, H. Nanostructure-based theranostic systems. Theranostics 2016, 6, 1274–1276. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 11th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2006; ISBN 0721602401. [Google Scholar]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, Y.-S. Energy transfer-based spectral properties of Tb-, Pr-, or Sm-codoped YAG:Ce nanocrystalline phosphors. J. Lumin. 2008, 128, 1570–1576. [Google Scholar] [CrossRef]
- Murariu, M.; Doumbia, A.; Bonnaud, L.; Dechief, A.; Paint, Y.; Ferreira, M.; Campagne, C.; Devaux, E.; Dubois, P. High-Performance Polylactide/ZnO Nanocomposites Designed for Films and Fibers with Special End-Use Properties. Biomacromolecules 2011, 12, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.; Lee, J.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Lee, K.C.; Youn, Y.S. Highly porous large poly(lactic-co-glycolic acid) microspheres adsorbed with palmityl-acylated exendin-4 as a long-acting inhalation system for treating diabetes. Biomaterials 2011, 32, 1685–1693. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, H.; Wu, H.; Lù, Z.; Deng, C.; Hong, Z.; Jing, X.; Chen, X. RGD-Conjugated Copolymer Incorporated into Composite of Poly(lactide-co-glycotide) and Poly(l-lactide)-Grafted Nanohydroxyapatite for Bone Tissue Engineering. Biomacromolecules 2011, 12, 2667–2680. [Google Scholar] [CrossRef]
- Bertrand, A.; Hillmyer, M.A. Nanoporous Poly(lactide) by Olefin Metathesis Degradation. J. Am. Chem. Soc. 2013, 135, 10918–10921. [Google Scholar] [CrossRef]
- Dusselier, M.; van Wouwe, P.; Dewaele, A.; Jacobs, P.A.; Sels, B.F. Shape-selective zeolite catalysis for bioplastics production. Science 2015, 349, 78–80. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.J.; Dove, A.P. Stereocontrolled ring-opening polymerisation of lactide. Chem. Soc. Rev. 2010, 39, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Sarazin, Y.; Carpentier, J.F. Discrete Cationic Complexes for Ring-Opening Polymerization Catalysis of Cyclic Esters and Epoxides. Chem. Rev. 2015, 115, 3564–3614. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, M.H.; Eilerts, N.W. Single-site metal alkoxide catalysts for ring-opening polymerizations. Poly(dilactide) synthesis employing {HB(3-ButPz)3}Mg(OEt). Chem. Commun. 1996, 853–854. [Google Scholar] [CrossRef]
- Chamberlain, B.M.; Cheng, M.; Moore, D.R.; Ovitt, T.M.; Lobkovsky, E.B.; Coates, G.W. Polymerization of lactide with zinc and magnesium β-diiminate complexes: Stereocontrol and mechanism. J. Am. Chem. Soc. 2001, 123, 3229–3238. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Gallucci, J.C.; Phomphrai, K. Well-defined calcium initiators for lactide polymerization. Inorg. Chem. 2004, 43, 6717–6725. [Google Scholar] [CrossRef]
- Wu, J.-C.; Huang, B.-H.; Hsueh, M.-L.; Lai, S.-L.; Lin, C.-C. Ring-opening polymerization of lactide initiated by magnesium and zinc alkoxides. Polymer 2005, 46, 9784–9792. [Google Scholar] [CrossRef]
- Chuang, H.J.; Weng, S.F.; Chang, C.C.; Lin, C.C.; Chen, H.Y. Synthesis, characterization and catalytic activity of magnesium and zinc aminophenoxide complexes: Catalysts for ring-opening polymerization of l-lactide. Dalton Trans. 2011, 40, 9601–9607. [Google Scholar] [CrossRef]
- Huang, M.; Pan, C.; Ma, H. Ring-opening polymerization of rac-lactide and α-methyltrimethylene carbonate catalyzed by magnesium and zinc complexes derived from binaphthyl-based iminophenolate ligands. Dalton Trans. 2015, 44, 12420–12431. [Google Scholar] [CrossRef]
- Williams, C.K.; Breyfogle, L.E.; Choi, S.K.; Nam, W.; Young, V.G.; Hillmyer, M.A.; Tolman, W.B. A Highly Active Zinc Catalyst for the Controlled Polymerization of Lactide. J. Am. Chem. Soc. 2003, 125, 11350–11359. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; Ma, H.; Yang, Y. Zinc complexes supported by claw-type aminophenolate ligands: Synthesis, characterization and catalysis in the ring-opening polymerization of rac-lactide. Dalton Trans. 2012, 41, 3266. [Google Scholar] [CrossRef]
- Jędrzkiewicz, D.; Adamus, G.; Kwiecień, M.; John, Ł.; Ejfler, J. Lactide as the Playmaker of the ROP Game: Theoretical and Experimental Investigation of Ring-Opening Polymerization of Lactide Initiated by Aminonaphtholate Zinc Complexes. Inorg. Chem. 2017, 56, 1349–1365. [Google Scholar] [CrossRef]
- Ejfler, J.; Kobyłka, M.; Jerzykiewicz, L.B.; Sobota, P. Highly efficient magnesium initiators for lactide polymerization. Dalton Trans. 2005, 2047–2050. [Google Scholar] [CrossRef]
- Wojtaszak, J.; Mierzwicki, K.; Szafert, S.; Gulia, N.; Ejfler, J. Homoleptic aminophenolates of Zn, Mg and Ca. Synthesis, structure, DFT studies and polymerization activity in ROP of lactides. Dalton Trans. 2014, 43, 2424–2436. [Google Scholar] [CrossRef]
- Jędrzkiewicz, D.; Czeluśniak, I.; Wierzejewska, M.; Szafert, S.; Ejfler, J. Well-controlled, zinc-catalyzed synthesis of low molecular weight oligolactides by ring opening reaction. J. Mol. Catal. A Chem. 2015, 396, 155–163. [Google Scholar] [CrossRef]
- Guan, R.; Johnson, I.; Cui, T.; Zhao, T.; Zhao, Z.; Li, X.; Liu, H. Electrodeposition of hydroxyapatite coating on Mg-4.0Zn-1.0Ca-0.6Zr alloy and in vitro evaluation of degradation, hemolysis, and cytotoxicity. J. Biomed. Mater. Res. Part A 2012, 100, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Tadrous, P.J.; Siegel, J.; French, P.M.W.; Shousha, S.; Lalani, E.-N.; Stamp, G.W.H. Fluorescence lifetime imaging of unstained tissues: Early results in human breast cancer. J. Pathol. 2003, 199, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Chiang, C.-P.; You, C.; Hsiao, T.-C.; Wang, C.-Y. Time-resolved autofluorescence spectroscopy for classifying normal and premalignant oral tissues. Lasers Surg. Med. 2005, 37, 37–45. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Wilson, M.C. The monocarboxylate transporter family-Role and regulation. IUBMB Life 2012, 64, 109–119. [Google Scholar] [CrossRef]
- Esen, E.; Long, F. Aerobic Glycolysis in Osteoblasts Aerobic Glycolysis in Osteoblasts. Curr. Osteoporos. Rep. 2015, 12, 433–438. [Google Scholar] [CrossRef]
- Aditya, N.P.; Chimote, G.; Gunalan, K.; Banerjee, R.; Patankar, S.; Madhusudhan, B. Experimental Parasitology Curcuminoids-loaded liposomes in combination with arteether protects against Plasmodium berghei infection in mice. Exp. Parasitol. 2012, 131, 292–299. [Google Scholar] [CrossRef]
- Li, Z.; Chee, P.L.; Owh, C.; Lakshminarayanan, R.; Loh, X.J. Safe and efficient membrane permeabilizing polymers based on PLLA for antibacterial applications. RSC Adv. 2016, 6, 28947–28955. [Google Scholar] [CrossRef]
- Wei, Z.; Tian, P.; Liu, X.; Zhou, B. In vitro degradation, hemolysis, and cytocompatibility of PEO / PLLA composite coating on biodegradable AZ31 alloy. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2015, 103, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kannan, S. Synthesis, Structural analysis, Mechanical, antibacterial and Hemolytic activity of Mg2+ and Cu2+ co-substitutions in β-Ca3(PO4)2. Mater. Sci. Eng. C 2014, 45, 530–538. [Google Scholar] [CrossRef]
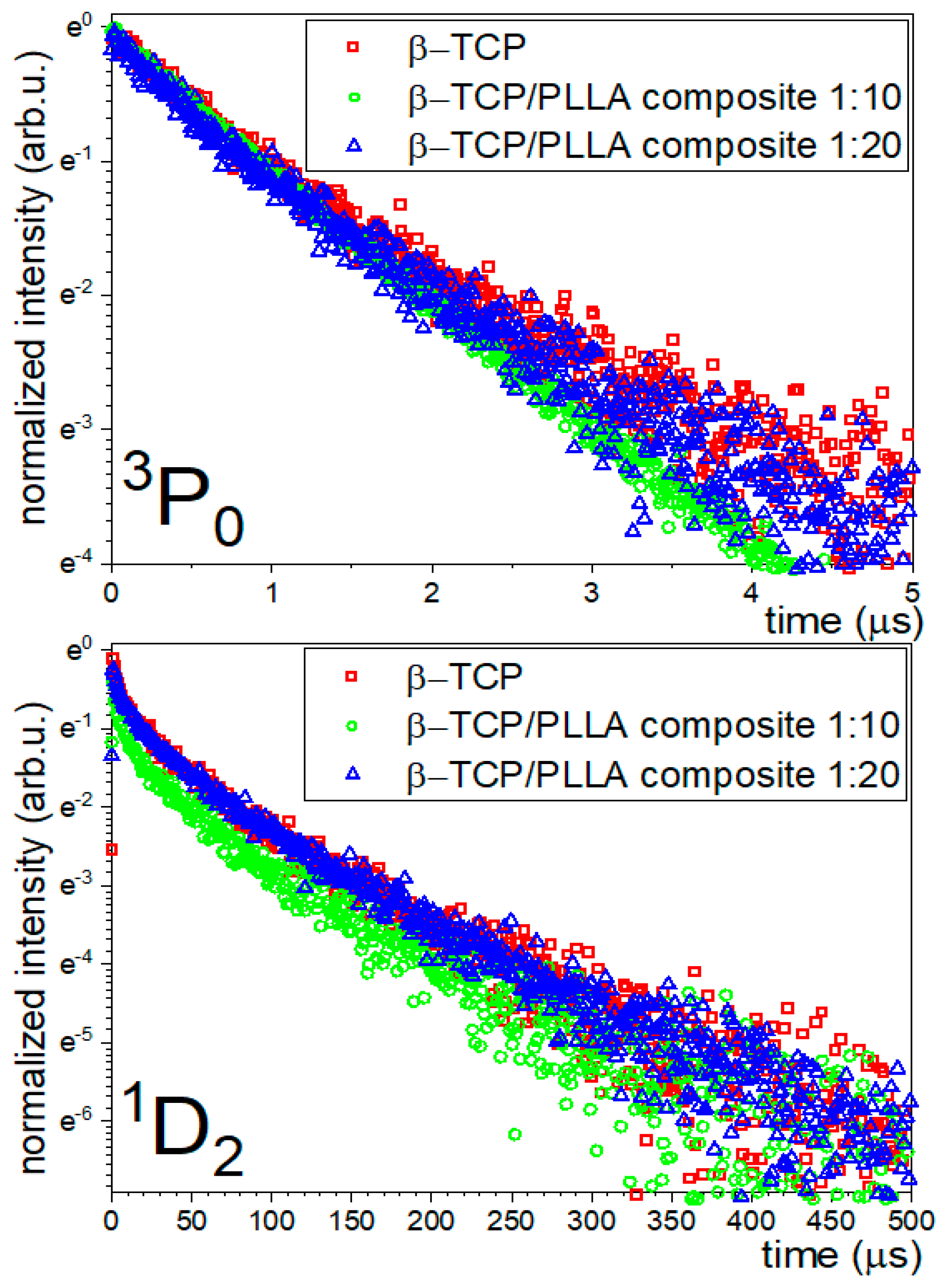
| Materials | State | Observed Wavelength [nm] | Lifetime [µs] (τavg) |
|---|---|---|---|
| β-TCP:0.5%Ce/0.5% Pr, 900 °C | 3P0 | 650 | 1.31 |
| 1D2 | 605 | 84.39 | |
| β-TCP/PLLA composite 1:20 | 3P0 | 650 | 1.28 |
| 1D2 | 605 | 84.50 | |
| β-TCP/PLLA composite 1:10 | 3P0 | 650 | 1.34 |
| 1D2 | 605 | 77.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zienkiewicz, J.A.; Strzep, A.; Jedrzkiewicz, D.; Nowak, N.; Rewak-Soroczynska, J.; Watras, A.; Ejfler, J.; Wiglusz, R.J. Preparation and Characterization of Self-Assembled Poly(l-Lactide) on the Surface of β-Tricalcium Diphosphate(V) for Bone Tissue Theranostics. Nanomaterials 2020, 10, 331. https://doi.org/10.3390/nano10020331
Zienkiewicz JA, Strzep A, Jedrzkiewicz D, Nowak N, Rewak-Soroczynska J, Watras A, Ejfler J, Wiglusz RJ. Preparation and Characterization of Self-Assembled Poly(l-Lactide) on the Surface of β-Tricalcium Diphosphate(V) for Bone Tissue Theranostics. Nanomaterials. 2020; 10(2):331. https://doi.org/10.3390/nano10020331
Chicago/Turabian StyleZienkiewicz, Jan A., Adam Strzep, Dawid Jedrzkiewicz, Nicole Nowak, Justyna Rewak-Soroczynska, Adam Watras, Jolanta Ejfler, and Rafal J. Wiglusz. 2020. "Preparation and Characterization of Self-Assembled Poly(l-Lactide) on the Surface of β-Tricalcium Diphosphate(V) for Bone Tissue Theranostics" Nanomaterials 10, no. 2: 331. https://doi.org/10.3390/nano10020331
APA StyleZienkiewicz, J. A., Strzep, A., Jedrzkiewicz, D., Nowak, N., Rewak-Soroczynska, J., Watras, A., Ejfler, J., & Wiglusz, R. J. (2020). Preparation and Characterization of Self-Assembled Poly(l-Lactide) on the Surface of β-Tricalcium Diphosphate(V) for Bone Tissue Theranostics. Nanomaterials, 10(2), 331. https://doi.org/10.3390/nano10020331









